Barley Leaf Ameliorates Citrobacter rodentium-Induced Colitis through Preventive Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of BL Powder
2.2. Animals
2.3. CR Infection
2.4. Quantification of CR in Mouse Feces and Tissues
2.5. Disease Activity Index (DAI)
2.6. Histological Staining
2.7. Immunofluorescence Staining
2.8. Inflammatory Cytokine Analysis
2.9. 16S rRNA Gene Sequencing
2.10. Statistics
3. Results
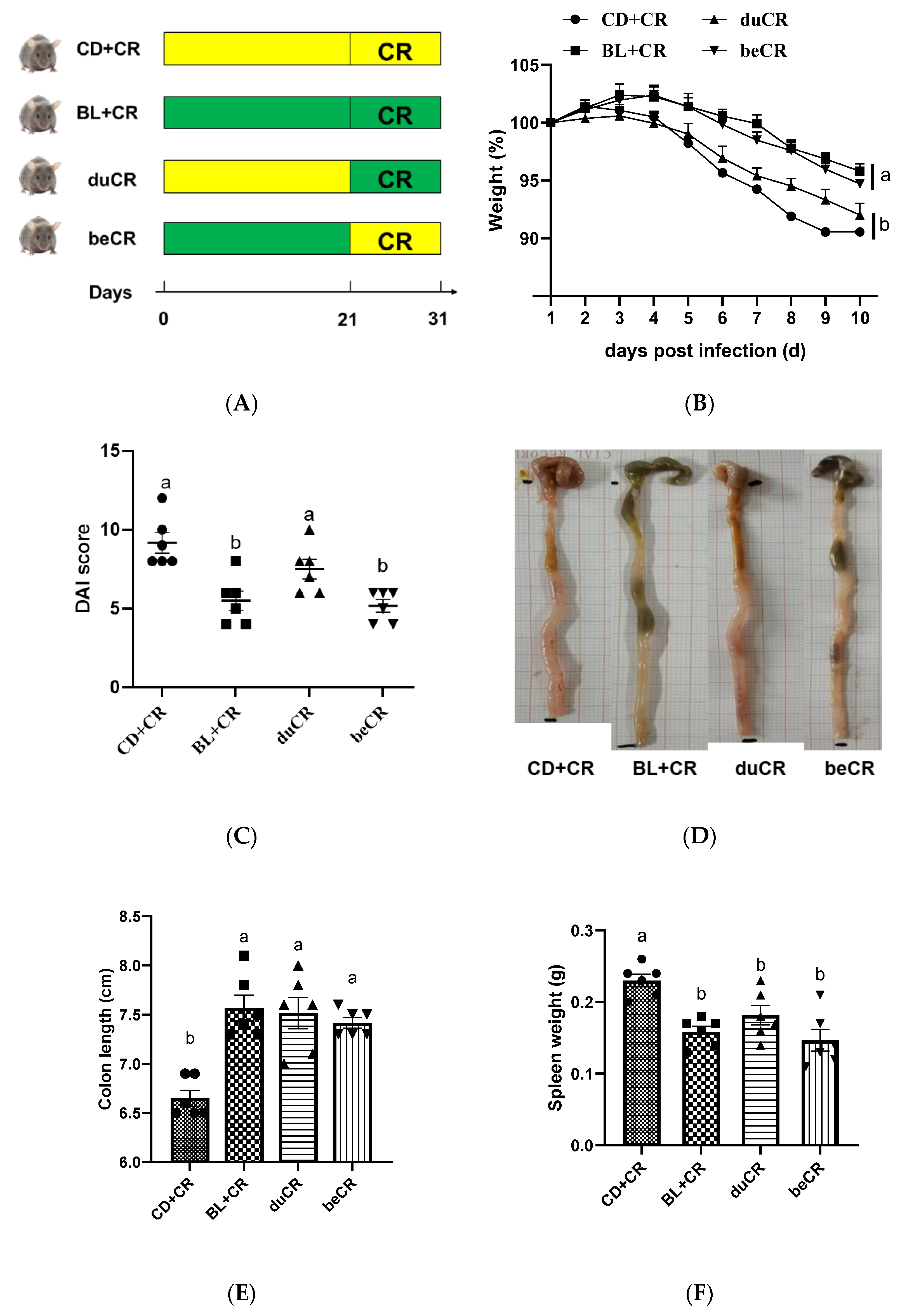
3.1. Effect of beCR and duCR on CR-Induced Colitis
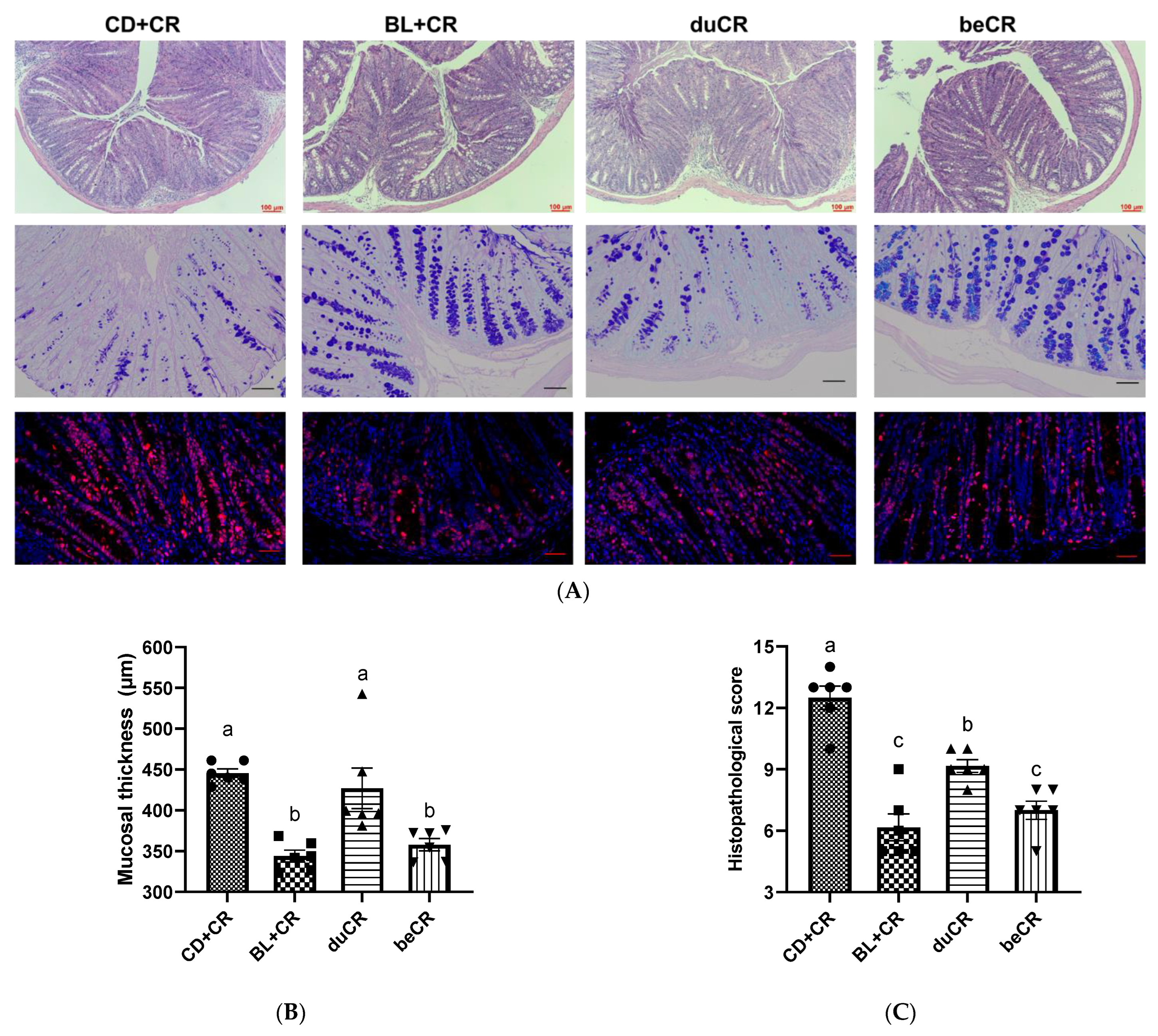
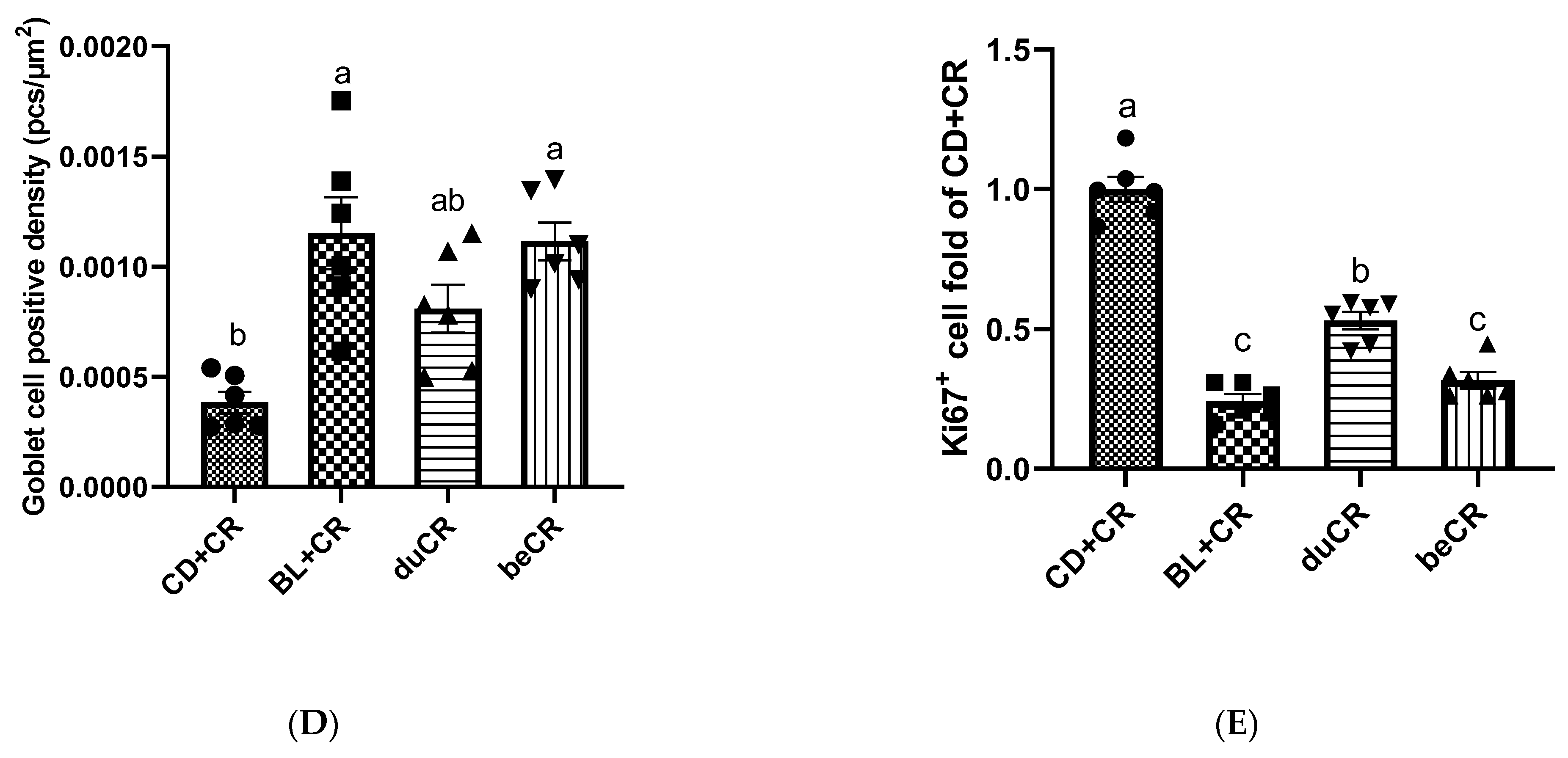
3.2. Effect of beCR and duCR on CR-Induced Intestinal Pathology
3.3. Effect of beCR and duCR on Inflammatory Cytokines in Colon
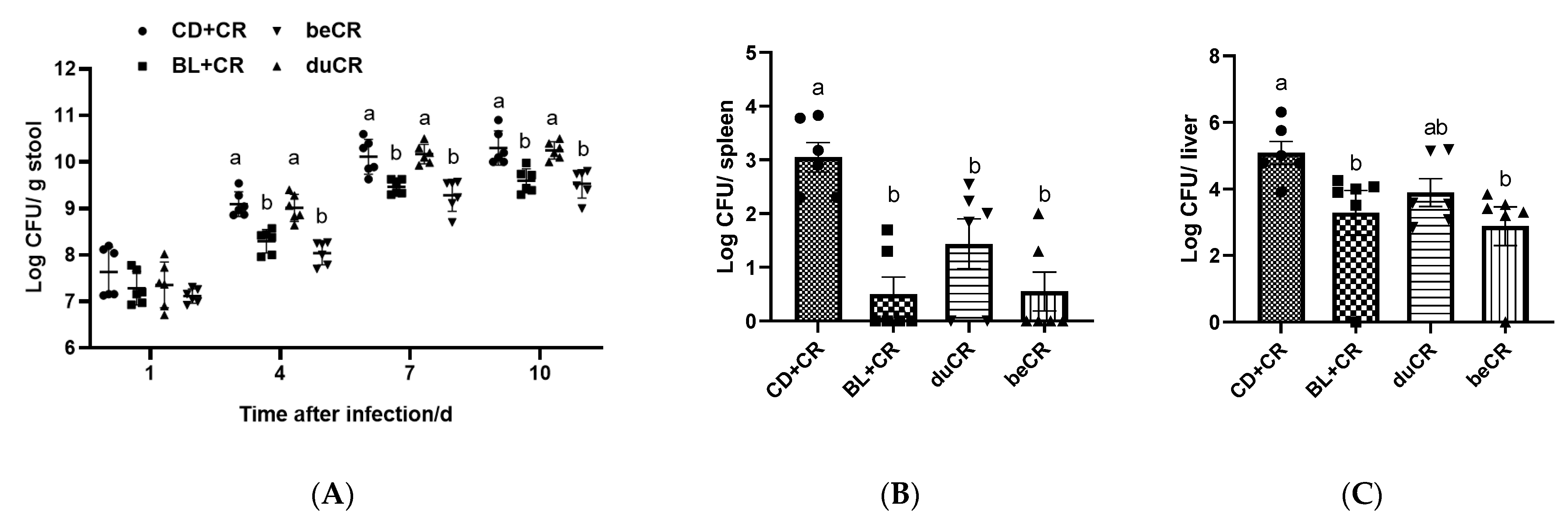
3.4. Effect of beCR and duCR on CR Colonization
3.5. Effect of beCR and duCR on Modulating Alpha and Beta Diversity of Gut Microbiota
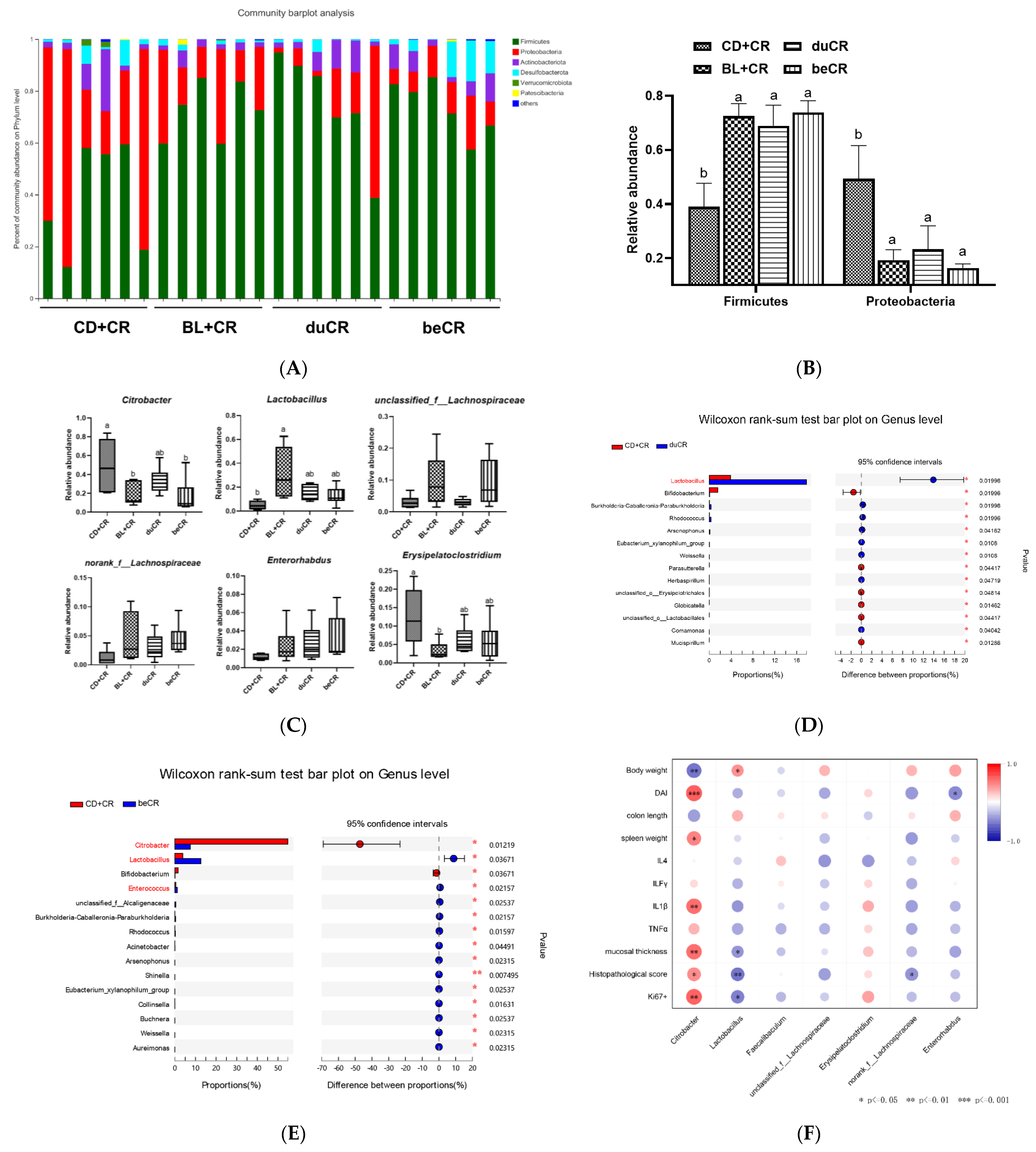
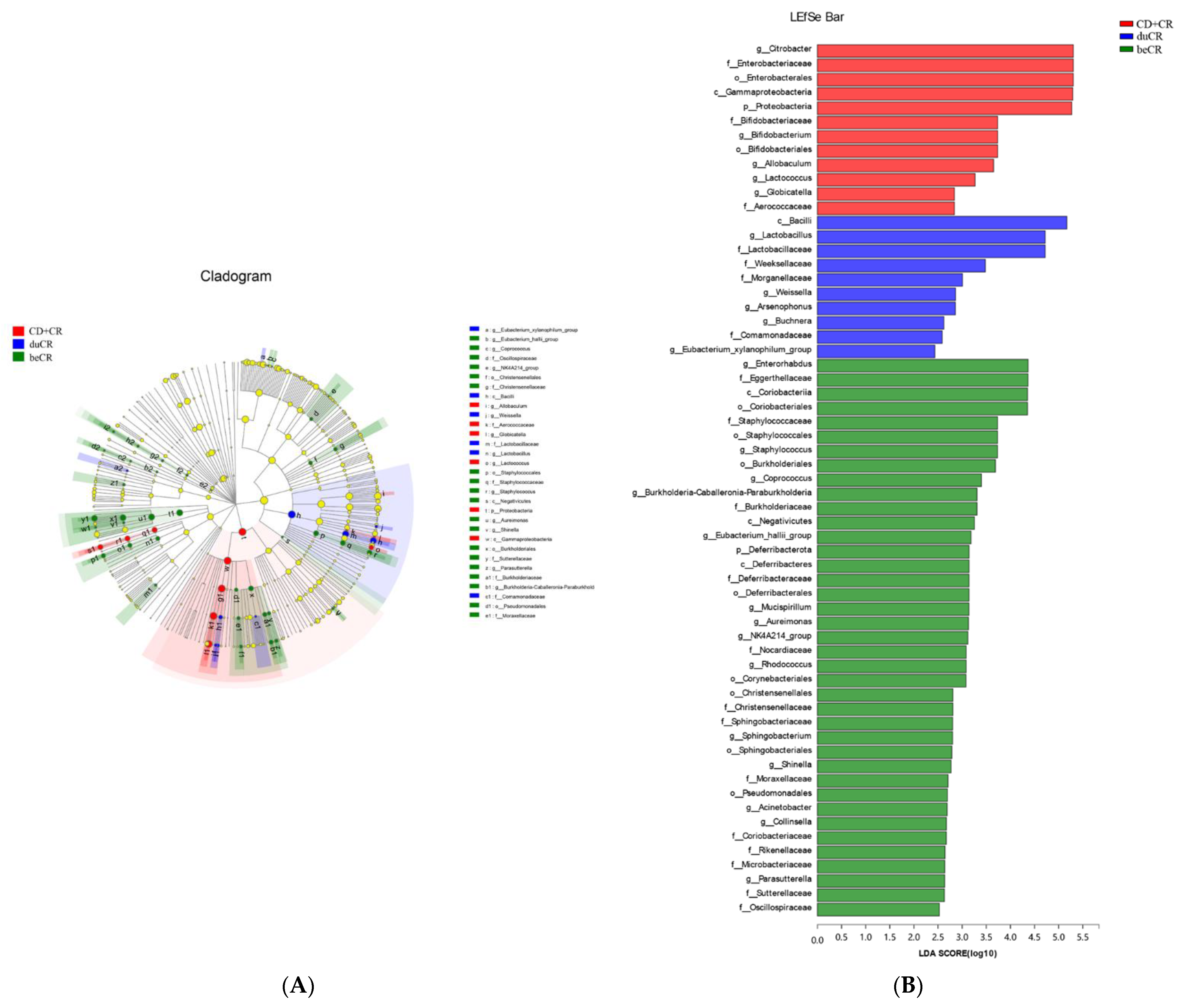
3.6. Effect of beCR and duCR on Regulating Taxonomic Microbial Community Profiles
3.7. Effect of beCR and duCR on Key Microbial Phylotypes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Seyed Tabib, N.S.; Madgwick, M.; Sudhakar, P.; Verstockt, B.; Korcsmaros, T.; Vermeire, S. Big data in IBD: Big progress for clinical practice. Gut 2020, 69, 1520–1532. [Google Scholar] [CrossRef]
- Kotze, P.G.; Underwood, F.E.; Damião, A.O.M.C.; Ferraz, J.G.P.; Saad-Hossne, R.; Toro, M.; Iade, B.; Bosques-Padilla, F.; Teixeira, F.V.; Juliao-Banos, F.; et al. Progression of Inflammatory Bowel Diseases Throughout Latin America and the Caribbean: A Systematic Review. Clin. Gastroenterol. Hepatol. 2020, 18, 304–312. [Google Scholar] [CrossRef]
- Ye, Y.; Manne, S.; Treem, W.R.; Bennett, D. Prevalence of Inflammatory Bowel Disease in Pediatric and Adult Populations: Recent Estimates from Large National Databases in the United States, 2007–2016. Inflamm. Bowel Dis. 2020, 26, 619–625. [Google Scholar] [CrossRef]
- Bruscoli, S.; Febo, M.; Riccardi, C.; Migliorati, G. Glucocorticoid Therapy in Inflammatory Bowel Disease: Mechanisms and Clinical Practice. Front. Immunol. 2021, 12, 691480. [Google Scholar] [CrossRef]
- Zuo, T.; Ng, S.C. The Gut Microbiota in the Pathogenesis and Therapeutics of Inflammatory bowel disease. Front. Microbiol. 2018, 9, 2247. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Pizzoferrato, M.; Gasbarrini, A.; Miggiano, G.A.D. Nutrition, IBD and Gut Microbiota: A Review. Nutrients 2020, 12, 944. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Montrose, D.C.; Nishiguchi, R.; Basu, S.; Staab, H.A.; Zhou, X.K.; Wang, H.; Meng, L.; Johncilla, M.; Cubillos-Ruiz, J.R.; Morales, D.K.; et al. Dietary Fructose Alters the Composition, Localization, and Metabolism of Gut Microbiota in Association With Worsening Colitis. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 525–550. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef]
- Ferrer-Picón, E.; Dotti, I.; Corraliza, A.M.; Mayorgas, A.; Esteller, M.; Perales, J.C.; Ricart, E.; Masamunt, M.C.; Carrasco, A.; Tristán, E.; et al. Intestinal Inflammation Modulates the Epithelial Response to Butyrate in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.R.; Khoruts, A.; Staley, C.; Sadowsky, M.J.; Abd, M.; Alani, M.; Bakow, B.; Curran, P.; McKenney, J.; Tisch, A.; et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent clostridium difficile infection a randomized trial. Ann. Intern. Med. 2016, 165, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Crepin, V.F.; Collins, J.W.; Habibzay, M.; Frankel, G. Citrobacter rodentium mouse model of bacterial infection. Nat. Protoc. 2016, 11, 1851–1876. [Google Scholar] [CrossRef]
- Collins, J.W.; Keeney, K.M.; Crepin, V.F.; Rathinam, V.A.K.; Fitzgerald, K.A.; Finlay, B.B.; Frankel, G. Citrobacter rodentium: Infection, inflammation and the microbiota. Nat. Rev. Microbiol. 2014, 12, 612–623. [Google Scholar] [CrossRef]
- Wiles, S.; Clare, S.; Harker, J.; Huett, A.; Young, D.; Dougan, G.; Frankel, G. Organ specificity, colonization and clearance dynamics in vivo following oral challenges with the murine pathogen Citrobacter rodentium. Cell. Microbiol. 2004, 6, 963–972. [Google Scholar] [CrossRef]
- Kamada, N.; Kim, Y.G.; Sham, H.P.; Vallance, B.A.; Puente, J.L.; Martens, E.C.; Núñez, G. Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science 2012, 336, 1325–1329. [Google Scholar] [CrossRef]
- Ferreres, F.; Kršková, Z.; Gonçalves, R.F.; Valentão, P.; Pereira, J.A.; Dušek, J.; Martin, J.; Andrade, P.B. Free water-soluble phenolics profiling in barley (Hordeum vulgare L.). J. Agric. Food Chem. 2009, 57, 2405–2409. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-H.; Ha, H.; Kim, R.; Cho, C.-W.; Song, Y.-R.; Hong, H.-D.; Kim, T. Protective effects of a polysaccharide BLE0 isolated from barley leaf on bone loss in ovariectomized mice. Int. J. Biol. Macromol. 2019, 123, 314–321. [Google Scholar] [CrossRef]
- Yu, Y.M.; Chang, W.C.; Chang, C.T.; Hsieh, C.L.; Tsai, C.E. Effects of young barley leaf extract and antioxidative vitamins on LDL oxidation and free radical scavenging activities in type 2 diabetes. Diabetes Metab. 2002, 28, 107–114. [Google Scholar]
- Yamaura, K.; Shimada, M.; Fukata, H.; Nakayama, N.; Bi, Y.; Ueno, K. Antidepressant-like effects of young green barley leaf (Hordeum vulgare L.) in the mouse forced swimming test. Pharmacogn. Res. 2012, 4, 22–26. [Google Scholar] [CrossRef]
- Kamiyama, M.; Shibamoto, T. Flavonoids with potent antioxidant activity found in young green barley leaves. J. Agric. Food Chem. 2012, 60, 6260–6267. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Feng, Y.; Tian, M.; Hu, X.; Zheng, R.; Chen, F. Dietary barley leaf mitigates tumorigenesis in experimental colitis-associated colorectal cancer. Nutrients 2021, 13, 3487. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Li, D.; Ma, C.; Feng, Y.; Hu, X.; Chen, F. Barley leaf insoluble dietary fiber alleviated dextran sulfate sodium-induced mice colitis by modulating gut microbiota. Nutrients 2021, 13, 846. [Google Scholar] [CrossRef]
- Li, D.; Feng, Y.; Tian, M.; Ji, J.; Hu, X.; Chen, F. Gut microbiota-derived inosine from dietary barley leaf supplementation attenuates colitis through PPARγ signaling activation. Microbiome 2021, 9, 83. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, D.; Jin, Y.; Jia, H.; Yang, Y.; Kim, I.H.; Dai, Z.; Zhang, J.; Ren, F.; Wu, Z. Glycine Attenuates Citrobacter rodentium-Induced Colitis by Regulating ATF6-Mediated Endoplasmic Reticulum Stress in Mice. Mol. Nutr. Food Res. 2021, 65, 2001065. [Google Scholar] [CrossRef]
- Yu, Y.M.; Chang, W.C.; Liu, C.S.; Tsai, C.M. Effect of young barley leaf extract and adlay on plasma lipids and LDL oxidation in hyperlipidemic smokers. Biol. Pharm. Bull. 2004, 27, 802–805. [Google Scholar] [CrossRef]
- Lopez, C.A.; Miller, B.M.; Rivera-Chávez, F.; Velazquez, E.M.; Byndloss, M.X.; Chávez-Arroyo, A.; Lokken, K.L.; Tsolis, R.M.; Winter, S.E.; Bäumler, A.J. Virulence factors enhance Citrobacter rodentium expansion through aerobic respiration. Science 2016, 353, 1249–1253. [Google Scholar] [CrossRef]
- Bergstrom, K.S.B.; Kissoon-Singh, V.; Gibson, D.L.; Ma, C.; Montero, M.; Sham, H.P.; Ryz, N.; Huang, T.; Velcich, A.; Finlay, B.B.; et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010, 6, e1000902. [Google Scholar] [CrossRef]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD—What role do Proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef]
- Wu, H.; Xie, S.; Miao, J.; Li, Y.; Wang, Z.; Wang, M.; Yu, Q. Lactobacillus reuteri maintains intestinal epithelial regeneration and repairs damaged intestinal mucosa. Gut Microbes 2020, 11, 997–1014. [Google Scholar] [CrossRef]
- Waki, N.; Kuwabara, Y.; Yoshikawa, Y.; Suganuma, H.; Koide, H.; Oku, N.; Ohashi, N. Amelioration of Citrobacter rodentium proliferation in early stage of infection in mice by pre-treatment with lactobacillus brevis KB290 and verification using in vivo bioluminescence imaging. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.H.; Park, J.H.; Choi, S.Y.; Jeon, H.Y.; Park, J.-I.; Kim, J.Y.; Ham, S.H.; Choi, Y.K. The probiotic Lactobacillus prevents citrobacter rodentium-induced murine colitis in a TLR2-dependent manner. J. Microbiol. Biotechnol. 2016, 26, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Anbazhagan, A.N.; Coffing, H.; Chatterjee, I.; Priyamvada, S.; Gujral, T.; Saksena, S.; Gill, R.K.; Alrefai, W.A.; Borthakur, A.; et al. Lactobacillus acidophilus counteracts inhibition of NHE3 and DRA expression and alleviates diarrheal phenotype in mice infected with Citrobacter rodentium. Am. J. Physiol.-Gastrointest. Liver Physiol. 2016, 311, G817–G826. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Louie, S.; Shi, H.N.; Walker, W.A. Preinoculation with the probiotic Lactobacillus acidophilus early in life effectively inhibits murine Citrobacter rodentium colitis. Pediatr. Res. 2005, 58, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Bai, R.; Zhou, W.; Yao, Z.; Liu, Y.; Tang, S.; Ge, X.; Luo, L.; Luo, C.; Hu, G.F.; et al. Angiogenin maintains gut microbe homeostasis by balancing α-Proteobacteria and Lachnospiraceae. Gut 2021, 70, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.L.; Zhao, Q.; Reif, M.; Rosenberg, A.F.; Mannon, P.J.; Duck, L.W.; Elson, C.O. Human Microbiota Flagellins Drive Adaptive Immune Responses in Crohn’s Disease. Gastroenterology 2021, 161, 522–535. [Google Scholar] [CrossRef]
- Brown, K.; Abbott, D.W.; Uwiera, R.R.E.; Inglis, G.D. Removal of the cecum affects intestinal fermentation, enteric bacterial community structure, and acute colitis in mice. Gut Microbes 2018, 9, 218–235. [Google Scholar] [CrossRef]
- Milosavljevic, M.N.; Kostic, M.; Milovanovic, J.; Zaric, R.Z.; Stojadinovic, M.; Jankovic, S.M.; Stefanovic, S.M. Antimicrobial treatment of erysipelatoclostridium ramosum invasive infections: A systematic review. Rev. Inst. Med. Trop. Sao Paulo 2021, 63, e30. [Google Scholar] [CrossRef]
- Nagayama, M.; Yano, T.; Atarashi, K.; Tanoue, T.; Sekiya, M.; Kobayashi, Y.; Sakamoto, H.; Miura, K.; Sunada, K.; Kawaguchi, T.; et al. TH1 cell-inducing Escherichia coli strain identified from the small intestinal mucosa of patients with Crohn’s disease. Gut Microbes 2020, 12, 1788898. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Wang, P.; Hu, X.; Chen, F. Gut microbiota promotes production of aromatic metabolites through degradation of barley leaf fiber. J. Nutr. Biochem. 2018, 58, 49–58. [Google Scholar] [CrossRef]
- Hwang, H.; Lee, S.R.; Yoon, J.; Moon, H.; Zhang, J.; Park, E.; Yoon, S.; Cho, J.A. Ferulic Acid as a Protective Antioxidant of Human Intestinal Epithelial Cells. Antioxidants 2022, 11, 1448. [Google Scholar] [CrossRef] [PubMed]
- Jiminez, J.A.; Uwiera, T.C.; Abbott, D.W.; Uwiera, R.R.E.; Inglis, G.D. Butyrate Supplementation at High Concentrations Alters Enteric Bacterial Communities and Reduces Intestinal Inflammation in Mice Infected with Citrobacter rodentium. mSphere 2017, 2, e00243-17. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yu, T.; Huang, X.; Bilotta, A.J.; Xu, L.; Lu, Y.; Sun, J.; Pan, F.; Zhou, J.; Zhang, W.; et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 2020, 11, 4457. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Li, D.; Ma, C.; Tian, M.; Hu, X.; Chen, F. Barley Leaf Ameliorates Citrobacter rodentium-Induced Colitis through Preventive Effects. Nutrients 2022, 14, 3833. https://doi.org/10.3390/nu14183833
Feng Y, Li D, Ma C, Tian M, Hu X, Chen F. Barley Leaf Ameliorates Citrobacter rodentium-Induced Colitis through Preventive Effects. Nutrients. 2022; 14(18):3833. https://doi.org/10.3390/nu14183833
Chicago/Turabian StyleFeng, Yu, Daotong Li, Chen Ma, Meiling Tian, Xiaosong Hu, and Fang Chen. 2022. "Barley Leaf Ameliorates Citrobacter rodentium-Induced Colitis through Preventive Effects" Nutrients 14, no. 18: 3833. https://doi.org/10.3390/nu14183833
APA StyleFeng, Y., Li, D., Ma, C., Tian, M., Hu, X., & Chen, F. (2022). Barley Leaf Ameliorates Citrobacter rodentium-Induced Colitis through Preventive Effects. Nutrients, 14(18), 3833. https://doi.org/10.3390/nu14183833





