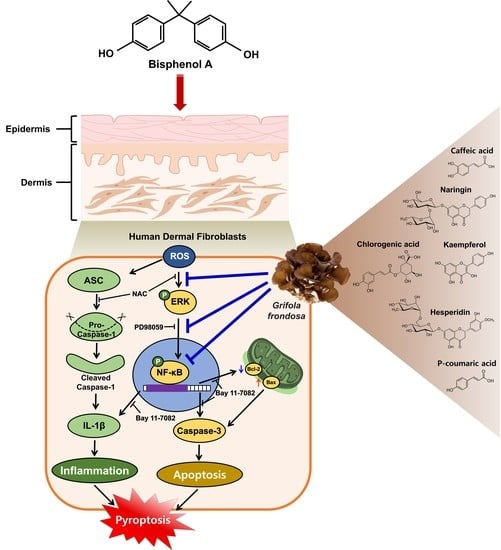
Grifola frondosa Extract Containing Bioactive Components Blocks Skin Fibroblastic Inflammation and Cytotoxicity Caused by Endocrine Disrupting Chemical, Bisphenol A
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Grifola frondosa Extraction
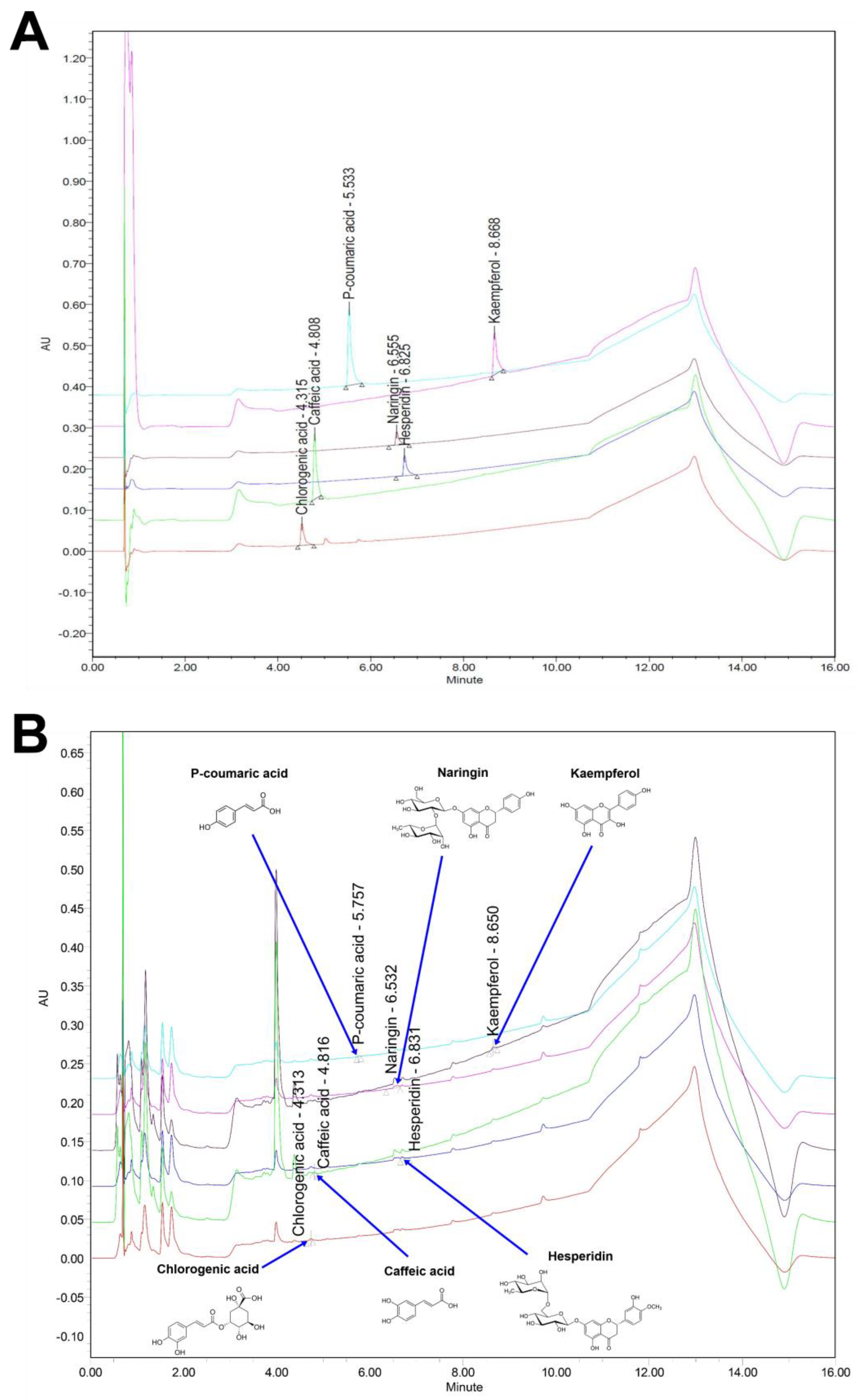
2.3. Ultra-Performance Liquid Chromatography (UPLC)
2.4. Cells
2.5. Measurement of Total Polyphenols Content
2.6. Measurement of Total Flavonoids Content
2.7. Cell Proliferation Assay
2.8. Intracellular Reactive Oxygen Species (ROS) Detection
2.9. Western Blot Analysis
2.10. Flow Cytometry
2.11. Determination of Adenosine Triphosphate (ATP) Level
2.12. Real-Time PCR
2.13. Enzyme-Linked Immunosorbent Assay (ELISA)
2.14. Immunofluorescence
2.15. Statistical Analysis
3. Results
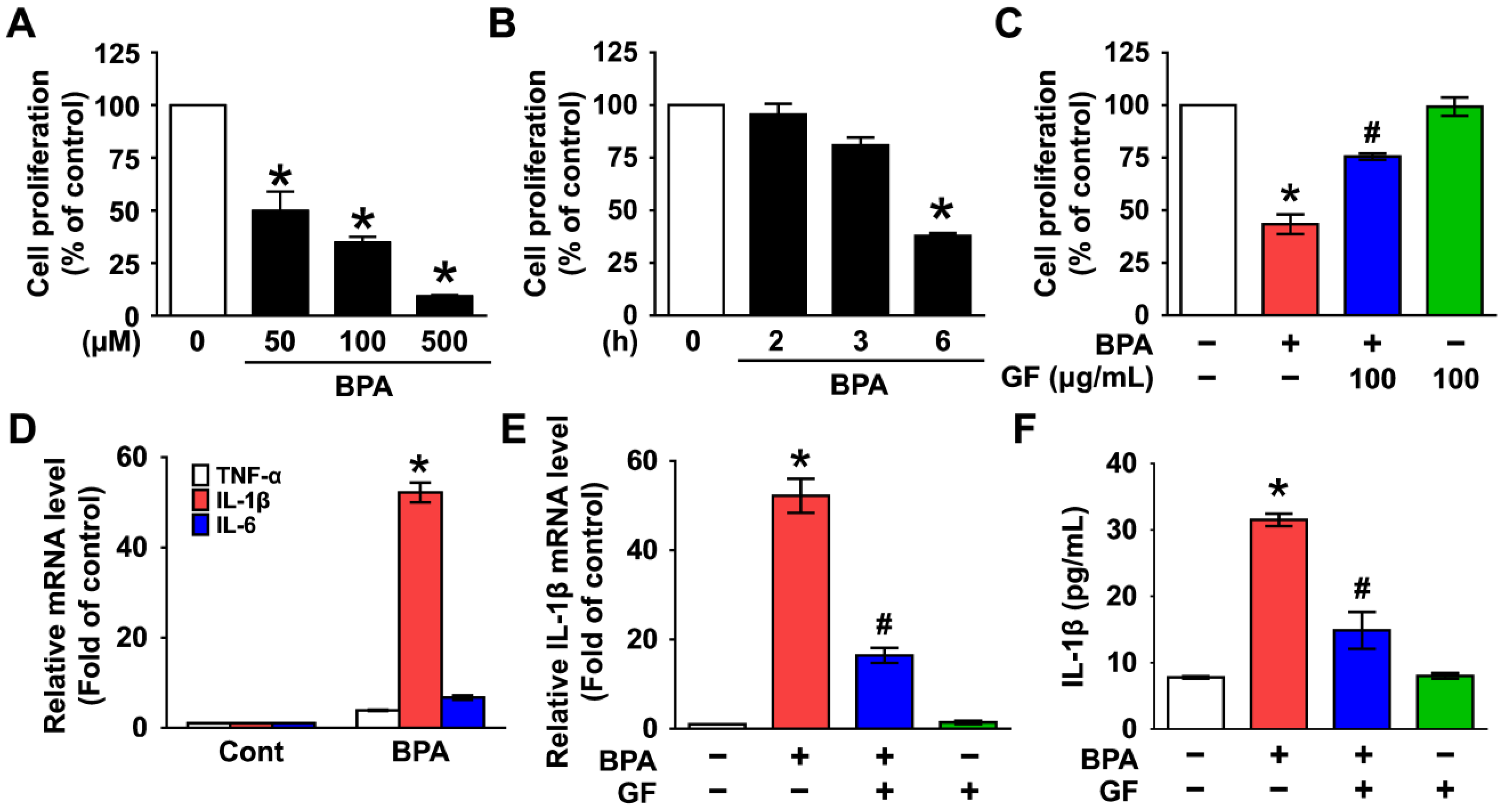
3.1. Inhibitory Effect of Grifola frondosa (GF) on Skin Cytotoxicity and Inflammation Stimulated by Bisphenol A (BPA)
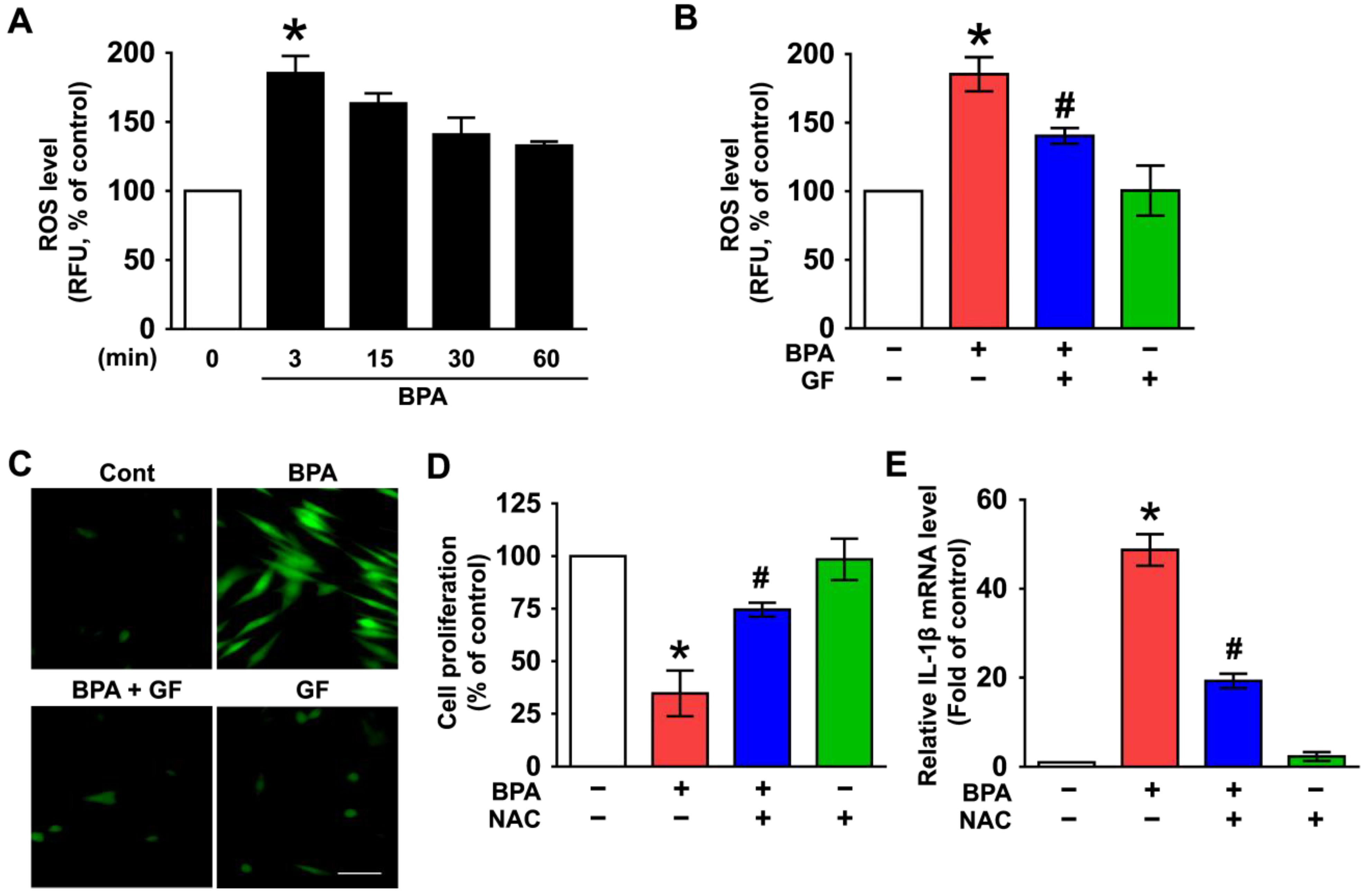
3.2. GF Contains Anti-Oxidative Components That Scavenge Intracellular ROS Caused by BPA
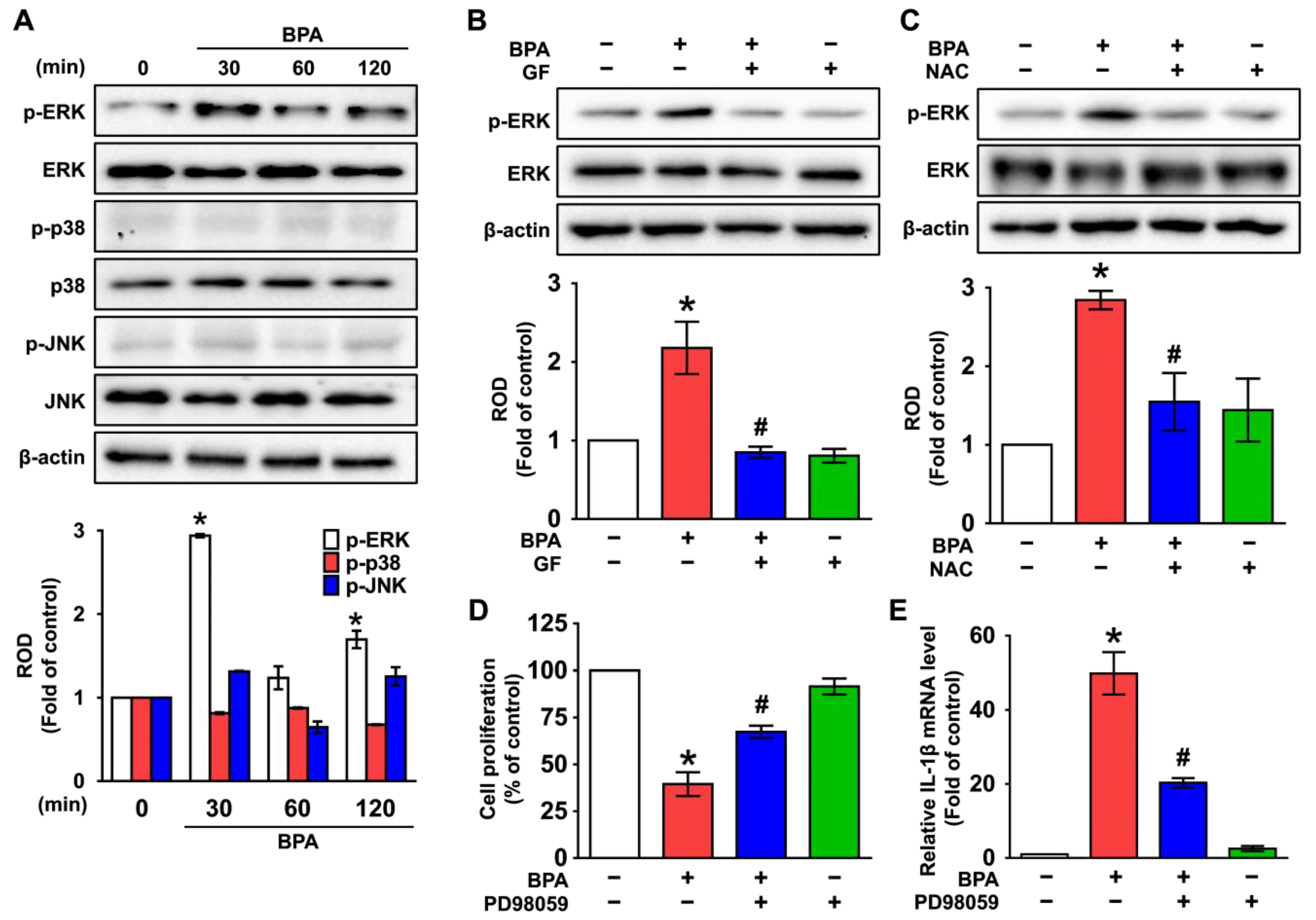
3.3. GF Inhibits the Phosphorylation of ERK in BPA-Induced NHDFs
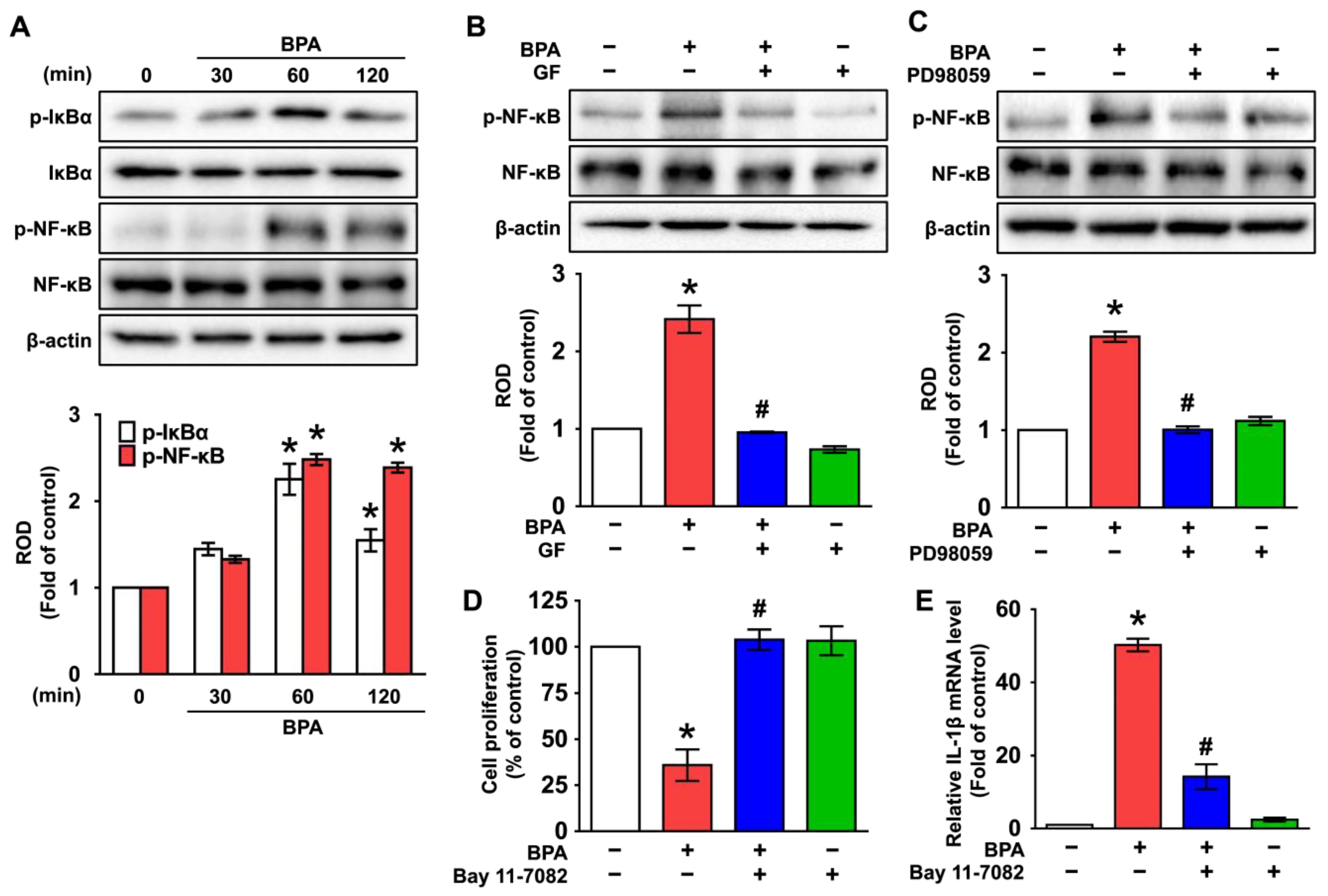
3.4. GF Regulates the Activation of NF-κB Responsible for the IL-1β Expression Triggered by BPA
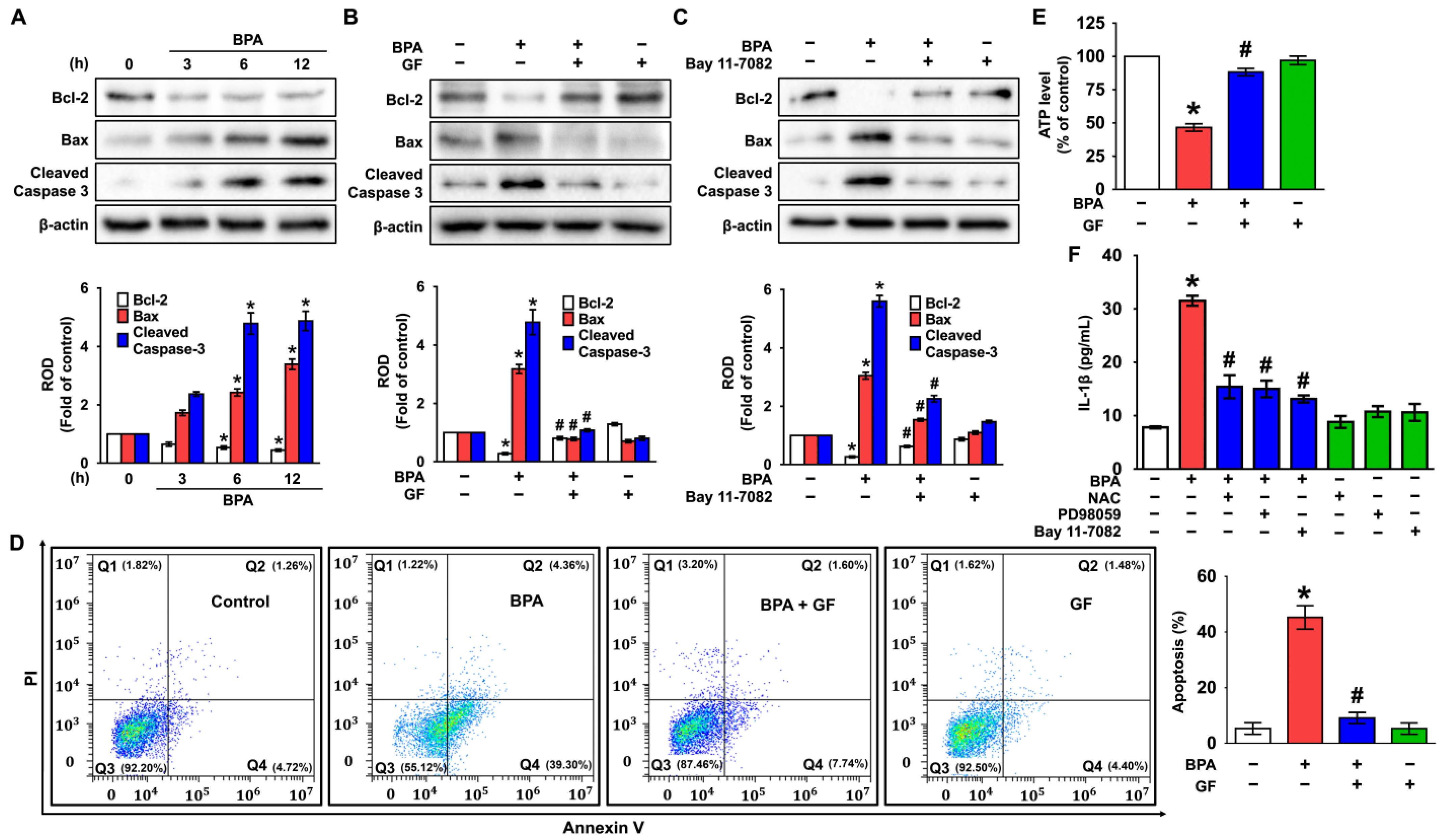
3.5. GF Blocks Dermal Fibroblastic Apoptosis and Inflammation Caused by BPA
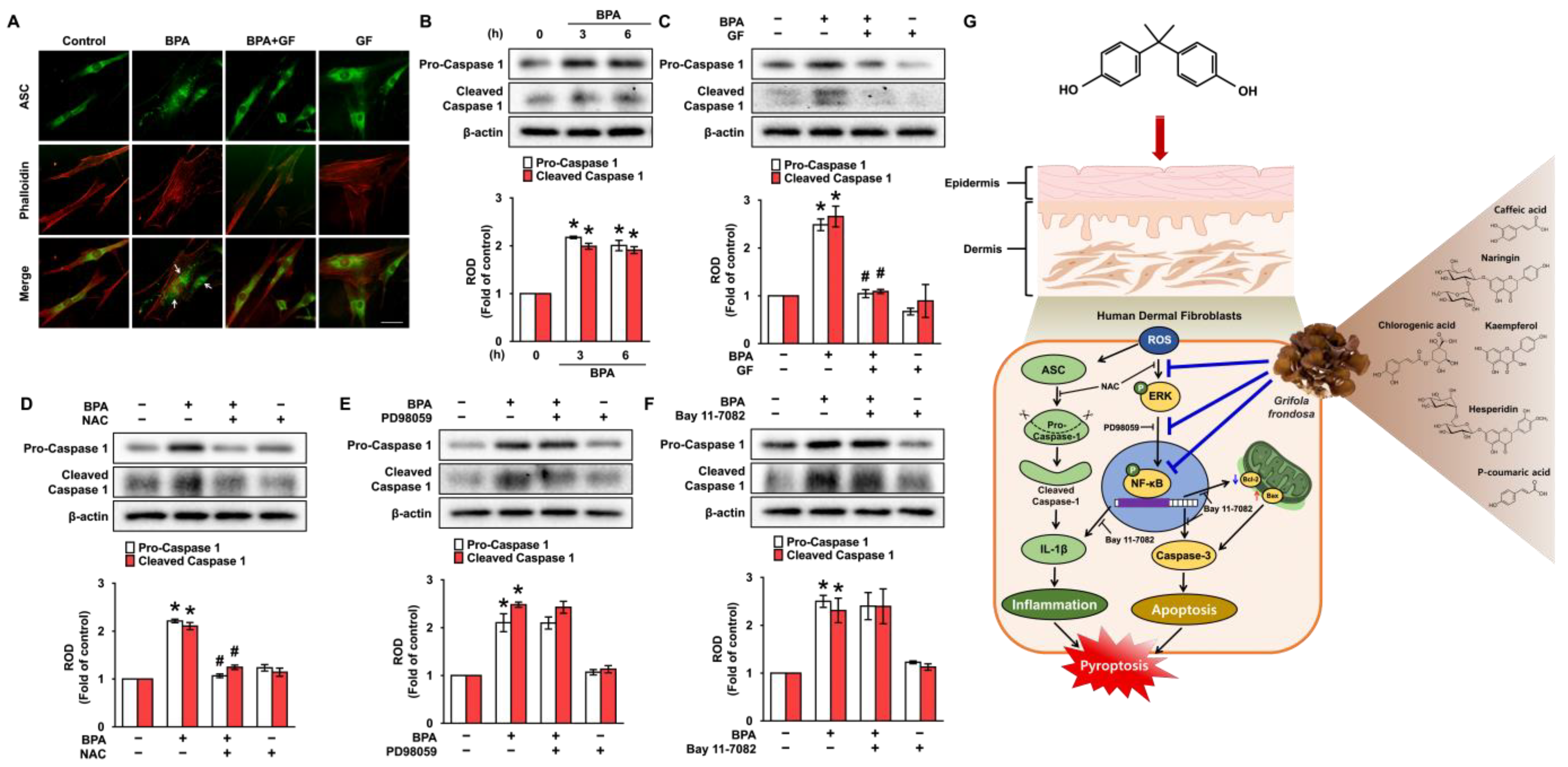
3.6. GF Inhibits Dermal Fibroblastic Pyroptosis Caused by BPA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, Y.; Qu, X.; Ming, Z.; Yao, Y.; Zhang, Y. The correlation between exposure to BPA and the decrease of the ovarian reserve. Int. J. Clin. Exp. Pathol. 2018, 11, 3375–3382. [Google Scholar] [PubMed]
- Fenichel, P.; Chevalier, N.; Brucker-Davis, F. Bisphenol A: An endocrine and metabolic disruptor. Ann. Endocrinol. 2013, 74, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Zalko, D.; Jacques, C.; Duplan, H.; Bruel, S.; Perdu, E. Viable skin efficiently absorbs and metabolizes bisphenol A. Chemosphere 2011, 82, 424–430. [Google Scholar] [CrossRef]
- Geens, T.; Aerts, D.; Berthot, C.; Bourguignon, J.P.; Goeyens, L.; Lecomte, P.; Maghuin-Rogister, G.; Pironnet, A.M.; Pussemier, L.; Scippo, M.L.; et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol. 2012, 50, 3725–3740. [Google Scholar] [CrossRef]
- Wickett, R.R.; Visscher, M.O. Structure and function of the epidermal barrier. Am. J. Infect. Control 2006, 34, S98–S110. [Google Scholar] [CrossRef]
- Shin, J.W.; Kwon, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Choi, H.R.; Park, K.C. Molecular mechanisms of dermal aging and antiaging approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Uitto, J. Connective tissue biochemistry of the aging dermis. Age-related alterations in collagen and elastin. Dermatol. Clin. 1986, 4, 433–446. [Google Scholar] [CrossRef]
- Lv, Y.; Lu, S.; Dai, Y.; Rui, C.; Wang, Y.; Zhou, Y.; Li, Y.; Pang, Q.; Fan, R. Higher dermal exposure of cashiers to BPA and its association with DNA oxidative damage. Environ. Int. 2017, 98, 69–74. [Google Scholar] [CrossRef]
- Ju, Q.; Zouboulis, C.C. Endocrine-disrupting chemicals and skin manifestations. Rev. Endocr. Metab. Disord. 2016, 17, 449–457. [Google Scholar] [CrossRef]
- Kaya Ozden, H.; Karadag, A.S. Could endocrine disruptors be a new player for acne pathogenesis? The effect of bisphenol A on the formation and severity of acne vulgaris: A prospective, case-controlled study. J. Cosmet. Dermatol. 2021, 20, 3573–3579. [Google Scholar] [CrossRef]
- Huang, Y.F.; Wang, P.W.; Huang, L.W.; Lai, C.H.; Yang, W.; Wu, K.Y.; Lu, C.A.; Chen, H.C.; Chen, M.L. Prenatal nonylphenol and Bisphenol A exposures and inflammation are determinants of oxidative/nitrative stress: A Taiwanese cohort study. Environ. Sci. Technol. 2017, 51, 6422–6429. [Google Scholar] [CrossRef] [PubMed]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta 2016, 1862, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.; Grzybowski, A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef]
- Yuan, J.; Kong, Y.; Ommati, M.M.; Tang, Z.; Li, H.; Li, L.; Zhao, C.; Shi, Z.; Wang, J. Bisphenol A-induced apoptosis, oxidative stress and DNA damage in cultured rhesus monkey embryo renal epithelial Marc-145cells. Chemosphere 2019, 234, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Ashida, H.; Mimuro, H.; Ogawa, M.; Kobayashi, T.; Sanada, T.; Kim, M.; Sasakawa, C. Cell death and infection: A double-edged sword for host and pathogen survival. J. Cell Biol. 2011, 195, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Saluja, M.; Bansal, M.P. Bisphenol A induced oxidative stress and apoptosis in mice testes: Modulation by selenium. Andrologia 2018, 50, e12834. [Google Scholar] [CrossRef]
- Filip, G.A.; Postescu, I.D.; Bolfa, P.; Catoi, C.; Muresan, A.; Clichici, S. Inhibition of UVB-induced skin phototoxicity by a grape seed extract as modulator of nitrosative stress, ERK/NF-κB signaling pathway and apoptosis, in SKH-1 mice. Food Chem. Toxicol. 2013, 57, 296–306. [Google Scholar] [CrossRef]
- Fullard, N.; Moles, A.; O’Reilly, S.; van Laar, J.M.; Faini, D.; Diboll, J.; Reynolds, N.J.; Mann, D.A.; Reichelt, J.; Oakley, F. The c-Rel subunit of NF-κB regulates epidermal homeostasis and promotes skin fibrosis in mice. Am. J. Pathol. 2013, 182, 2109–2120. [Google Scholar] [CrossRef]
- Jensen, L.E. Targeting the IL-1 family members in skin inflammation. Curr. Opin. Investig. Drugs 2010, 11, 1211–1220. [Google Scholar]
- Mapoung, S.; Umsumarng, S.; Semmarath, W.; Arjsri, P.; Thippraphan, P.; Yodkeeree, S.; Dejkriengkraikul, P.L. Skin wound-healing potential of polysaccharides from medicinal mushroom Auricularia auricula-judae (Bull.). J. Fungi 2021, 7, 247. [Google Scholar] [CrossRef]
- Nowakowski, P.; Markiewicz-Zukowska, R.; Bielecka, J.; Mielcarek, K.; Grabia, M.; Socha, K. Treasures from the forest: Evaluation of mushroom extracts as anti-cancer agents. Biomed. Pharmacother. 2021, 143, 112106. [Google Scholar] [CrossRef] [PubMed]
- Ajith, T.A.; Janardhanan, K. Indian medicinal mushrooms as a source of antioxidant and antitumor agents. J. Clin. Biochem. Nutr. 2007, 40, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [PubMed]
- Wu, J.Y.; Siu, K.C.; Geng, P. Bioactive ingredients and medicinal values of Grifola frondosa (Maitake). Foods 2021, 10, 95. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Sarker, M.M.R.; Yan, X.; Yang, C.; Zhao, L.; Lv, X.; Liu, B.; Zhao, C. Structural characterization and antidiabetic potential of a novel heteropolysaccharide from Grifola frondosa via IRS1/PI3K-JNK signaling pathways. Carbohydr. Polym. 2018, 198, 452–461. [Google Scholar] [CrossRef]
- Wang, C.L.; Meng, M.; Liu, S.B.; Wang, L.R.; Hou, L.H.; Cao, X.H. A chemically sulfated polysaccharide from Grifola frondos induces HepG2 cell apoptosis by notch1-NF-κB pathway. Carbohydr. Polym. 2013, 95, 282–287. [Google Scholar] [CrossRef]
- Zhao, C.; Gao, L.; Wang, C.; Liu, B.; Jin, Y.; Xing, Z. Structural characterization and antiviral activity of a novel heteropolysaccharide isolated from Grifola frondosa against enterovirus 71. Carbohydr. Polym. 2016, 144, 382–389. [Google Scholar] [CrossRef]
- Jiang, X.; Hao, J.; Liu, Z.; Ma, X.; Feng, Y.; Teng, L.; Li, Y.; Wang, D. Anti-obesity effects of Grifola frondosa through the modulation of lipid metabolism via ceramide in mice fed a high-fat diet. Food Funct. 2021, 12, 6725–6739. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, Y.M.; Kim, D.W.; Min, T.; Lee, S.J. Nanosphere loaded with curcumin inhibits the gastrointestinal cell death signaling pathway induced by the foodborne pathogen Vibrio vulnificus. Cells 2020, 9, 631. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, K.; Zhang, Y.; Xie, L.; Chen, P. Bisphenol A interferes with redox balance and the Nrf2 signaling pathway in Xenopus tropicalis during embryonic development. Animals 2022, 12, 937. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef] [PubMed]
- Shindo, R.; Kakehashi, H.; Okumura, K.; Kumagai, Y.; Nakano, H. Critical contribution of oxidative stress to TNFα-induced necroptosis downstream of RIPK1 activation. Biochem. Biophys. Res. Commun. 2013, 436, 212–216. [Google Scholar] [CrossRef]
- Ichijo, H.; Nishida, E.; Irie, K.; Dijke, P.T.; Saitoh, M.; Moriguchi, T.; Takagi, M.; Matsumoto, K.; Miyazono, K.; Gotoh, Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 1997, 275, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.R.; Kim, D.W.; Sung, J.; Kim, T.H.; Choi, C.H.; Lee, S.J. Astaxanthin inhibits autophagic cell death induced by Bisphenol A in human dermal fibroblasts. Antioxidants 2021, 10, 1273. [Google Scholar] [CrossRef]
- Taruno, A.; Niisato, N.; Marunaka, Y. Hypotonicity stimulates renal epithelial sodium transport by activating JNK via receptor tyrosine kinases. Am. J. Physiol. Ren. Physiol. 2007, 293, F128–F138. [Google Scholar] [CrossRef]
- Wu, D.; Luo, N.; Wang, L.; Zhao, Z.; Bu, H.; Xu, G.; Yan, Y.; Che, X.; Jiao, Z.; Zhao, T.; et al. Hydrogen sulfide ameliorates chronic renal failure in rats by inhibiting apoptosis and inflammation through ROS/MAPK and NF-κB signaling pathways. Sci. Rep. 2017, 7, 455. [Google Scholar] [CrossRef]
- Hildesheim, J.; Bulavin, D.V.; Anver, M.R.; Alvord, W.G.; Hollander, M.C.; Vardanian, L.; Fornace, A.J., Jr. Gadd45a protects against UV irradiation-induced skin tumors, and promotes apoptosis and stress signaling via MAPK and p53. Cancer Res. 2002, 62, 7305–7315. [Google Scholar]
- Muthusamy, V.; Piva, T.J. The UV response of the skin: A review of the MAPK, NFκB and TNFα signal transduction pathways. Arch. Dermatol. Res. 2010, 302, 5–17. [Google Scholar] [CrossRef]
- Liu, F.L.; Chen, C.H.; Chu, S.J.; Chen, J.H.; Lai, J.H.; Sytwu, H.K.; Chang, D.M. Interleukin (IL)-23 p19 expression induced by IL-1β in human fibroblast-like synoviocytes with rheumatoid arthritis via active nuclear factor-kappaB and AP-1 dependent pathway. Rheumatology 2007, 46, 1266–1273. [Google Scholar] [CrossRef]
- Montaseri, A.; Busch, F.; Mobasheri, A.; Buhrmann, C.; Aldinger, C.; Rad, J.S.; Shakibaei, M. IGF-1 and PDGF-bb suppress IL-1β-induced cartilage degradation through down-regulation of NF-κB signaling: Involvement of Src/PI-3K/AKT pathway. PLoS ONE 2011, 6, e28663. [Google Scholar] [CrossRef]
- Mahemuti, L.; Chen, Q.; Coughlan, M.C.; Qiao, C.; Chepelev, N.L.; Florian, M.; Dong, D.; Woodworth, R.G.; Yan, J.; Cao, X.L.; et al. Bisphenol A induces DSB-ATM-p53 signaling leading to cell cycle arrest, senescence, autophagy, stress response, and estrogen release in human fetal lung fibroblasts. Arch. Toxicol. 2018, 92, 1453–1469. [Google Scholar] [CrossRef]
- Bhandari, R.K.; Deem, S.L.; Holliday, D.K.; Jandegian, C.M.; Kassotis, C.D.; Nagel, S.C.; Tillitt, D.E.; Vom Saal, F.S.; Rosenfeld, C.S. Effects of the environmental estrogenic contaminants bisphenol A and 17α-ethinyl estradiol on sexual development and adult behaviors in aquatic wildlife species. Gen. Comp. Endocrinol. 2015, 214, 195–219. [Google Scholar] [CrossRef]
- Jiang, W.; Zhao, H.; Zhang, L.; Wu, B.; Zha, Z. Maintenance of mitochondrial function by astaxanthin protects against bisphenol A-induced kidney toxicity in rats. Biomed. Pharmacother. 2020, 121, 109629. [Google Scholar] [CrossRef]
- Mao, G.; Zou, Y.; Feng, W.; Wang, W.; Zhao, T.; Ye, C.; Zhu, Y.; Wu, X.; Yang, L.; Wu, X. Extraction, preliminary characterization and antioxidant activity of Se-enriched Maitake polysaccharide. Carbohydr. Polym. 2014, 101, 213–219. [Google Scholar] [CrossRef]
- Yang, B.K.; Gu, Y.A.; Jeong, Y.T.; Jeong, H.; Song, C.H. Chemical characteristics and immuno-modulating activities of exo-biopolymers produced by Grifola frondosa during submerged fermentation process. Int. J. Biol. Macromol. 2007, 41, 227–233. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, S.Y.; Thapa, D.; Choi, M.K.; Chung, I.M.; Park, Y.J.; Yong, C.S.; Choi, H.G.; Kim, J.A. Grifola frondosa water extract alleviates intestinal inflammation by suppressing TNF-α production and its signaling. Exp. Mol. Med. 2010, 42, 143–154. [Google Scholar] [CrossRef]
- Pan, Y.; Wan, X.; Zeng, F.; Zhong, R.; Guo, W.; Lv, X.C.; Zhao, C.; Liu, B. Regulatory effect of Grifola frondosa extract rich in polysaccharides and organic acids on glycolipid metabolism and gut microbiota in rats. Int. J. Biol. Macromol. 2020, 155, 1030–1039. [Google Scholar] [CrossRef]
- Pan, Y.Y.; Zeng, F.; Guo, W.L.; Li, T.T.; Jia, R.B.; Huang, Z.R.; Lv, X.C.; Zhang, J.; Liu, B. Effect of Grifola frondosa 95% ethanol extract on lipid metabolism and gut microbiota composition in high-fat diet-fed rats. Food Funct. 2018, 9, 6268–6278. [Google Scholar] [CrossRef]
- Yeh, J.Y.; Hsieh, L.H.; Wu, K.T.; Tsai, C.F. Antioxidant properties and antioxidant compounds of various extracts from the edible basidiomycete Grifola frondosa (Maitake). Molecules 2011, 16, 3197–3211. [Google Scholar] [CrossRef]
- Man, M.Q.; Yang, B.; Elias, P.M. Benefits of hesperidin for cutaneous functions. Evid. Based Complement Altern. Med. 2019, 2019, 2676307. [Google Scholar] [CrossRef]
- Raja Kumar, S.; Mohd Ramli, E.S.; Abdul Nasir, N.A.; Ismail, N.H.M.; Mohd Fahami, N.A. Preventive effect of naringin on metabolic syndrome and its mechanism of action: A systematic review. Evid. Based Complement Altern. Med. 2019, 2019, 9752826. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.; Hassani, A.; Alirezalu, A.; Maleki, R. Phenolic and flavonoid compounds and antioxidant activity in flowers of nine endemic Verbascum species from Iran. J. Sci. Food Agric. 2021, 102, 3250–3258. [Google Scholar] [CrossRef]
- Awad, A.M.; Kumar, P.; Ismail-Fitry, M.R.; Jusoh, S.; Ab Aziz, M.F.; Sazili, A.Q. Green extraction of bioactive compounds from plant biomass and their application in meat as natural antioxidant. Antioxidants 2021, 10, 1465. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, D.W.; Lim, S.R.; Sung, J.; Kim, T.H.; Min, I.S.; Choi, C.H.; Lee, S.J. Kaempferol blocks the skin fibroblastic interleukin 1β expression and cytotoxicity induced by 12-O-tetradecanoylphorbol-13-acetate by suppressing c-Jun N-terminal Kinase. Nutrients 2021, 13, 3079. [Google Scholar] [CrossRef]
- de Freitas, E.N.; Bubna, G.A.; Brugnari, T.; Kato, C.G.; Nolli, M.; Rauen, T.G.; Moreira, R.D.F.P.M.; Peralta, R.A.; Bracht, A.; de Souza, C.G.; et al. Removal of bisphenol A by laccases from Pleurotus ostreatus and Pleurotus pulmonarius and evaluation of ecotoxicity of degradation products. Chem. Eng. J. 2017, 330, 1361–1369. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, B.C.; Ko, Y.J.; Choi, M.K.; Choi, H.G.; Yong, C.S.; Lee, J.S.; Kim, J.A. Grifola frondosa (maitake mushroom) water extract inhibits vascular endothelial growth factor-induced angiogenesis through inhibition of reactive oxygen species and extracellular signal-regulated kinase phosphorylation. J. Med. Food 2008, 11, 643–651. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, W.; Sun, X.; Wang, Y.; Zhou, M. Kaempferol ameliorates Cisplatin induced nephrotoxicity by modulating oxidative stress, inflammation and apoptosis via ERK and NF-κB pathways. AMB Express 2020, 10, 58. [Google Scholar] [CrossRef]
- Lidke, D.S.; Huang, F.; Post, J.N.; Rieger, B.; Wilsbacher, J.; Thomas, J.L.; Pouyssegur, J.; Jovin, T.M.; Lenormand, P. ERK nuclear translocation is dimerization-independent but controlled by the rate of phosphorylation. J. Biol. Chem. 2010, 285, 3092–3102. [Google Scholar] [CrossRef]
- Lee, Y.J.; Chung, J.G.; Chien, Y.T.; Lin, S.S.; Hsu, F.T. Suppression of ERK/NF-κB activation is associated with amentoflavone-inhibited osteosarcoma progression in vivo. Anticancer Res. 2019, 39, 3669–3675. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hu, Y.C.; Dong, S.; Fan, M.; Tamae, D.; Ozeki, M.; Gao, Q.; Gius, D.; Li, J.J. Co-activation of ERK, NF-κB, and GADD45β in response to ionizing radiation. J. Biol. Chem. 2005, 280, 12593–12601. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, K.C.; Makena, M.R.; Bhowmick, K.; Pandey, M.K. Advancement of NF-κB signaling pathway: A novel target in pancreatic cancer. Int. J. Mol. Sci. 2018, 19, 3890. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, K.; Kowshik, J.; Kishore, T.K.; Baba, A.B.; Nagini, S. Astaxanthin inhibits NF-κB and Wnt/β-catenin signaling pathways via inactivation of Erk/MAPK and PI3K/Akt to induce intrinsic apoptosis in a hamster model of oral cancer. Biochim. Biophys. Acta 2013, 1830, 4433–4444. [Google Scholar] [CrossRef]
- Weng, M.C.; Wang, M.H.; Tsai, J.J.; Kuo, Y.C.; Liu, Y.C.; Hsu, F.T.; Wang, H.E. Regorafenib inhibits tumor progression through suppression of ERK/NF- κB activation in hepatocellular carcinoma bearing mice. Biosci. Rep. 2018, 38, BSR20171264. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Q.; Dai, W.; Li, S.; Feng, J.; Li, J.; Liu, T.; Xu, S.; Wang, W.; Lu, X.; et al. Quercetin pretreatment attenuates hepatic ischemia reperfusion-induced apoptosis and autophagy by inhibiting ERK/NF-κB pathway. Gastroenterol. Res. Pract. 2017, 2017, 9724217. [Google Scholar] [CrossRef]
- Liu, Q.; Song, J.; Li, H.; Dong, L.; Dai, S. Schizandrin B inhibits the cis-DDP-induced apoptosis of HK-2 cells by activating ERK/NF-κB signaling to regulate the expression of survivin. Int. J. Mol. Med. 2018, 41, 2108–2116. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Zhang, J.; Qi, X.; Cui, Y.; Yin, K.; Lin, H. Cadmium induced inflammation and apoptosis of porcine epididymis via activating RAF1/MEK/ERK and NF-κB pathways. Toxicol. Appl. Pharmacol. 2021, 415, 115449. [Google Scholar] [CrossRef]
- Nakagami, H.; Morishita, R.; Yamamoto, K.; Yoshimura, S.I.; Taniyama, Y.; Aoki, M.; Matsubara, H.; Kim, S.; Kaneda, Y.; Ogihara, T. Phosphorylation of p38 mitogen-activated protein kinase downstream of bax-caspase-3 pathway leads to cell death induced by high D-glucose in human endothelial cells. Diabetes 2001, 50, 1472–1481. [Google Scholar] [CrossRef]
- Wang, C.; He, J.; Xu, T.; Han, H.; Zhu, Z.; Meng, L.; Pang, Q.; Fan, R. Bisphenol A(BPA), BPS and BPB-induced oxidative stress and apoptosis mediated by mitochondria in human neuroblastoma cell lines. Ecotoxicol. Environ. Saf. 2021, 207, 111299. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, Z.; Ji, W. Bisphenol A induces apoptosis, oxidative stress and inflammatory response in colon and liver of mice in a mitochondria-dependent manner. Biomed. Pharmacother. 2019, 117, 109182. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Vande Walle, L.; Kanneganti, T.D. Deregulated inflammasome signaling in disease. Immunol. Rev. 2011, 243, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed]
| Time (min) | 0.1% FA/Water (%) | 0.1% FA/Acetonitrile (%) |
|---|---|---|
| 0 | 98 | 2 |
| 1.5 | 98 | 2 |
| 2.0 | 90 | 10 |
| 4.0 | 70 | 30 |
| 6.0 | 70 | 30 |
| 7.0 | 60 | 40 |
| 9.0 | 30 | 70 |
| 10.0 | 5 | 95 |
| 14.0 | 98 | 2 |
| 16.0 | 98 | 2 |
| Gene | Identification | Primer Sequence, 5′-3′ |
|---|---|---|
| IL-1β | Forward | TTCGAGGCACAAGGCACAAC |
| Reverse | GTGGTGGTCGGAGATTCGTA | |
| TNF-α | Forward | CTCCTCACCCACACCATCA |
| Reverse | GGAAGACCCCTCCCAGATAG | |
| IL-6 | Forward | CAATAACCACCCCTGACCCAA |
| Reverse | ACCAGAAGAAGGAATGCCCA | |
| β-actin | Forward | AACCGCGAGAAGATGACCCAGATCATGTTT |
| Reverse | AGCAGCCGTGGCCATCTCTTGCTCGAAGTC |
| Total Polyphenol | Total Flavonoid |
|---|---|
| 53.30 μg/mL | 59.28 μg/mL |
| Compound | Area (mV × s) | Height (mm) | Content (mg/L) |
|---|---|---|---|
| Naringin | 31,172 | 3025 | 18.183 ± 0.962 |
| Hesperidin | 17,915 | 3335 | 7.488 ± 0.270 |
| P-coumaric acid | 5679 | 1763 | 4.794 ± 0.176 |
| Chlorogenic acid | 16,961 | 5422 | 4.688 ± 0.440 |
| Kaempferol | 24,594 | 5920 | 4.169 ± 0.227 |
| Caffeic acid | 5690 | 1720 | 1.887 ± 0.148 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Lim, S.-R.; Jung, D.-H.; Kim, E.-J.; Sung, J.; Kim, S.C.; Choi, C.-H.; Kang, J.-W.; Lee, S.-J. Grifola frondosa Extract Containing Bioactive Components Blocks Skin Fibroblastic Inflammation and Cytotoxicity Caused by Endocrine Disrupting Chemical, Bisphenol A. Nutrients 2022, 14, 3812. https://doi.org/10.3390/nu14183812
Kim J-H, Lim S-R, Jung D-H, Kim E-J, Sung J, Kim SC, Choi C-H, Kang J-W, Lee S-J. Grifola frondosa Extract Containing Bioactive Components Blocks Skin Fibroblastic Inflammation and Cytotoxicity Caused by Endocrine Disrupting Chemical, Bisphenol A. Nutrients. 2022; 14(18):3812. https://doi.org/10.3390/nu14183812
Chicago/Turabian StyleKim, Ju-Ha, Seong-Ryeong Lim, Dae-Hwa Jung, Eun-Ju Kim, Junghee Sung, Sang Chan Kim, Chang-Hyung Choi, Ji-Woong Kang, and Sei-Jung Lee. 2022. "Grifola frondosa Extract Containing Bioactive Components Blocks Skin Fibroblastic Inflammation and Cytotoxicity Caused by Endocrine Disrupting Chemical, Bisphenol A" Nutrients 14, no. 18: 3812. https://doi.org/10.3390/nu14183812
APA StyleKim, J.-H., Lim, S.-R., Jung, D.-H., Kim, E.-J., Sung, J., Kim, S. C., Choi, C.-H., Kang, J.-W., & Lee, S.-J. (2022). Grifola frondosa Extract Containing Bioactive Components Blocks Skin Fibroblastic Inflammation and Cytotoxicity Caused by Endocrine Disrupting Chemical, Bisphenol A. Nutrients, 14(18), 3812. https://doi.org/10.3390/nu14183812