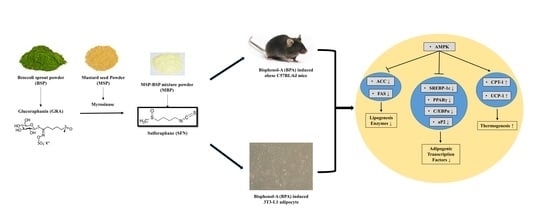
Anti-Obesogenic Effects of Sulforaphane-Rich Broccoli (Brassica oleracea var. italica) Sprouts and Myrosinase-Rich Mustard (Sinapis alba L.) Seeds In Vitro and In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of MBP, BSP, and MSP
2.3. Cell Culture
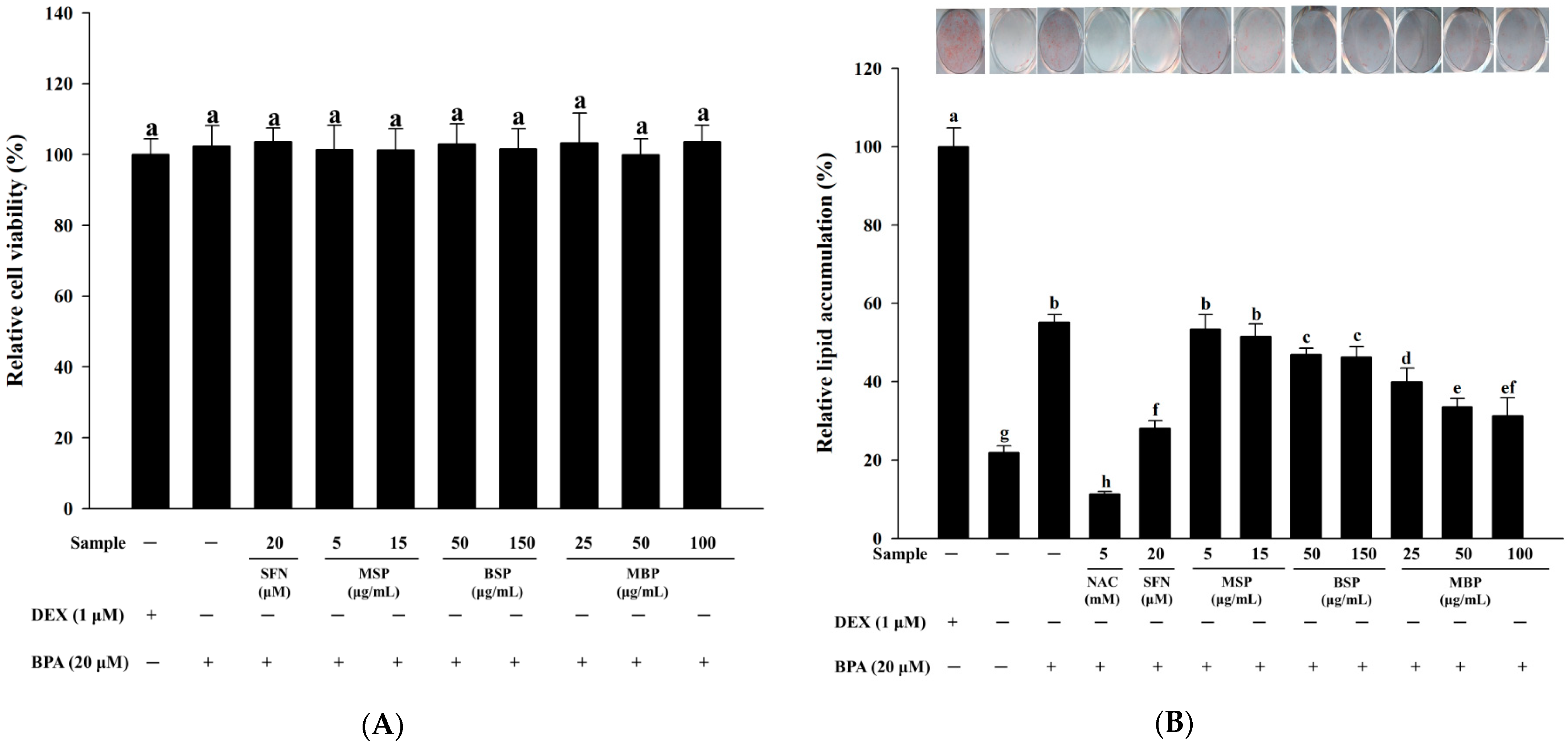
2.4. Cell Viability Assay
2.5. Oil Red O Staining Assay
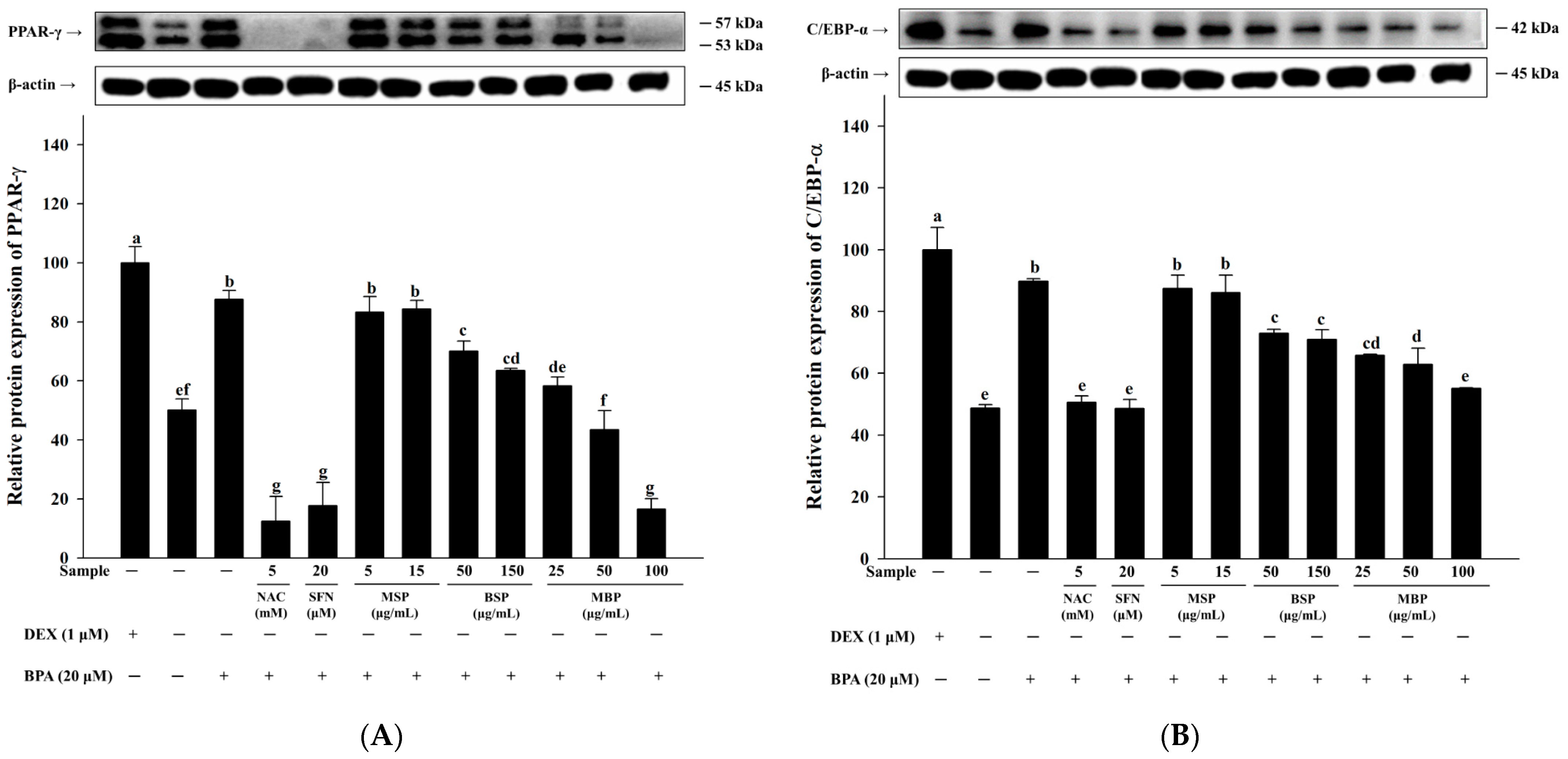
2.6. Western Blot Analysis
2.7. Animals
2.8. Experimental Design
2.9. Biochemical Analysis
2.10. Histological Analysis
2.11. Statistical Analysis
3. Results
3.1. Effect of MBP, BSP, and MSP on Cell Viability and Lipid Accumulation in BPA-Induced 3T3-L1 Cells
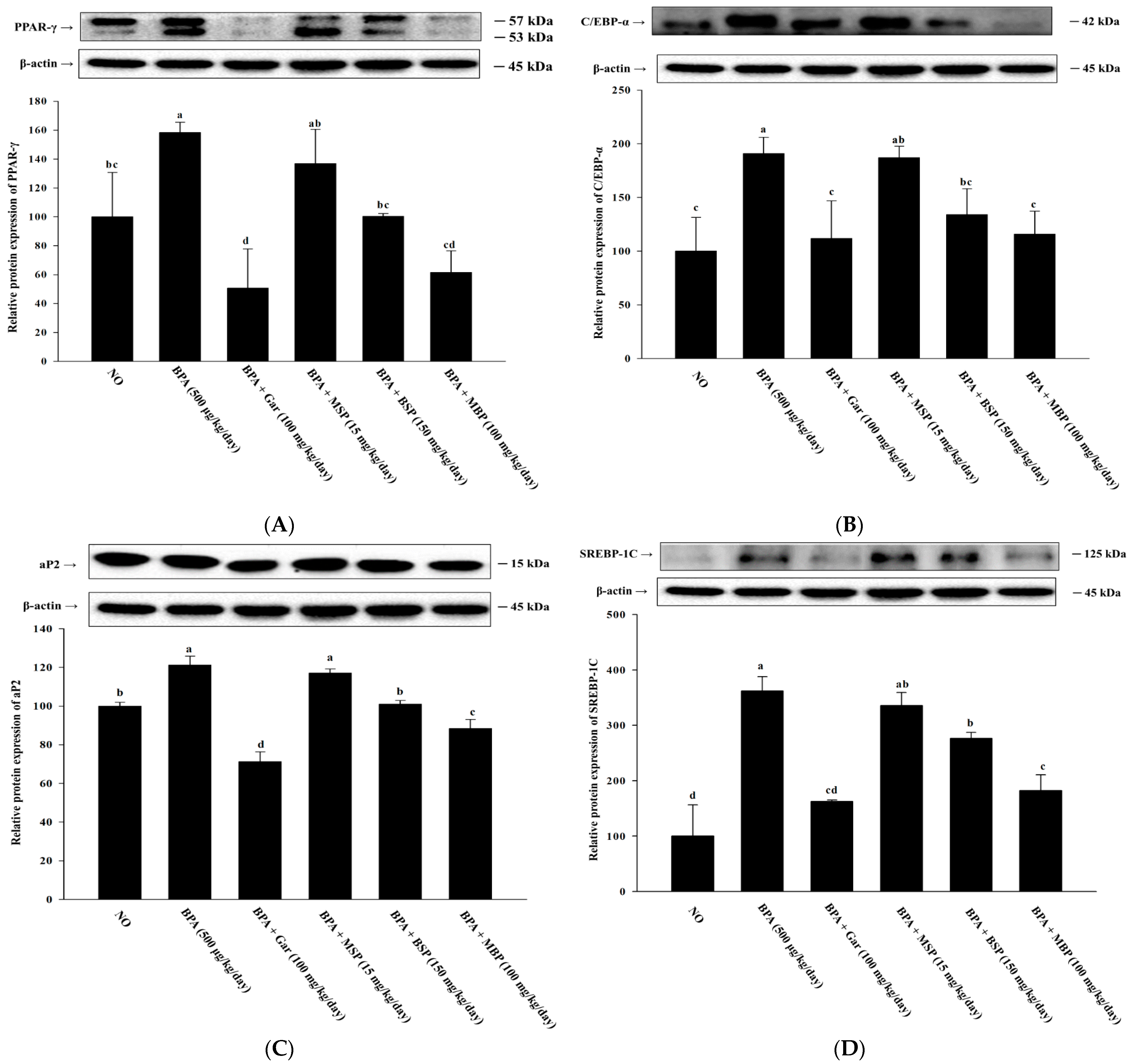
3.2. MBP, BSP, and MSP Reduce the Expression of Adipogenic-Related Proteins in BPA-Induced 3T3-L1 Cells
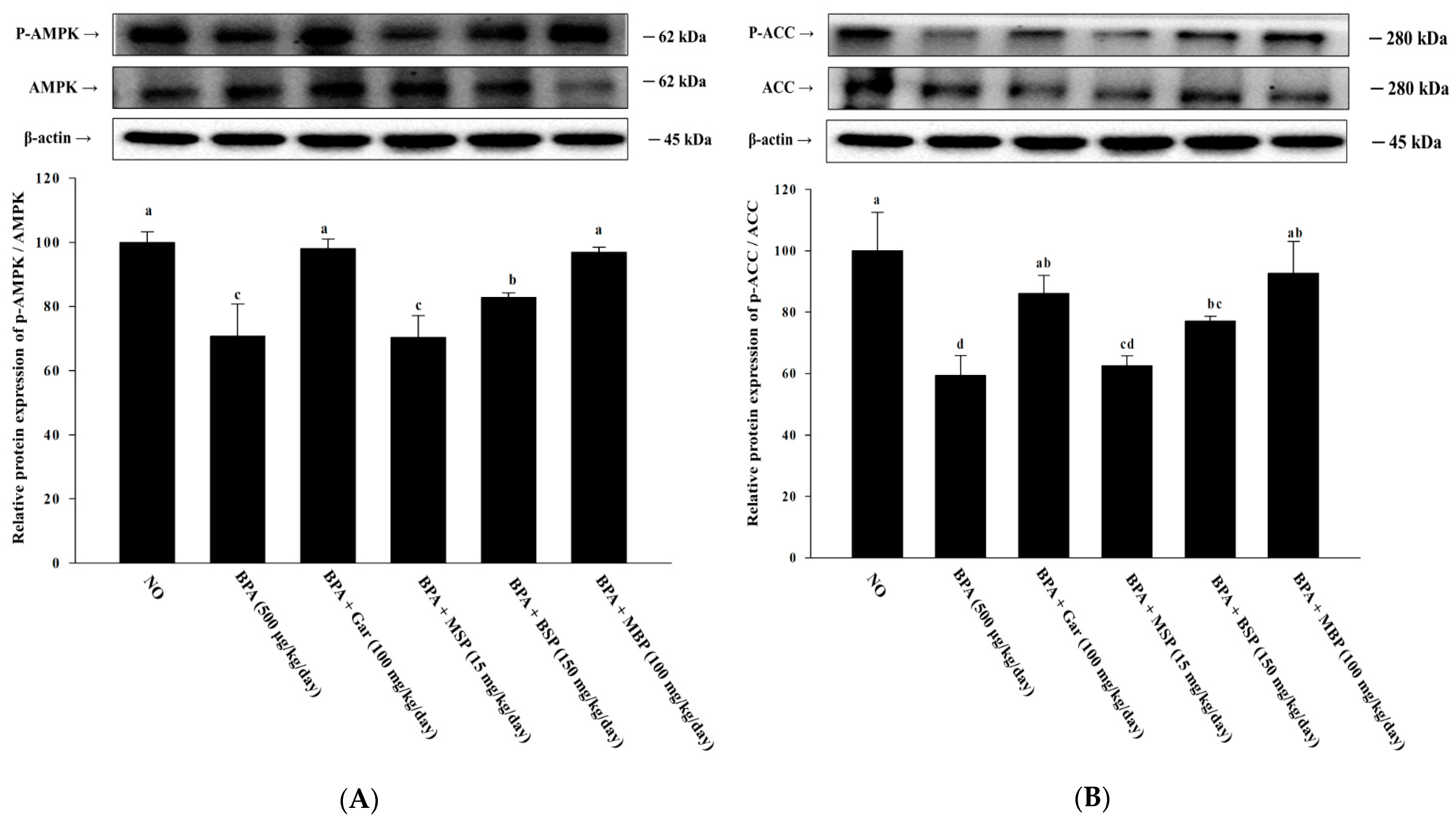
3.3. Effects of MBP, BSP, and MSP on AMPK Phosphorylation in BPA-Induced 3T3-L1 Cells
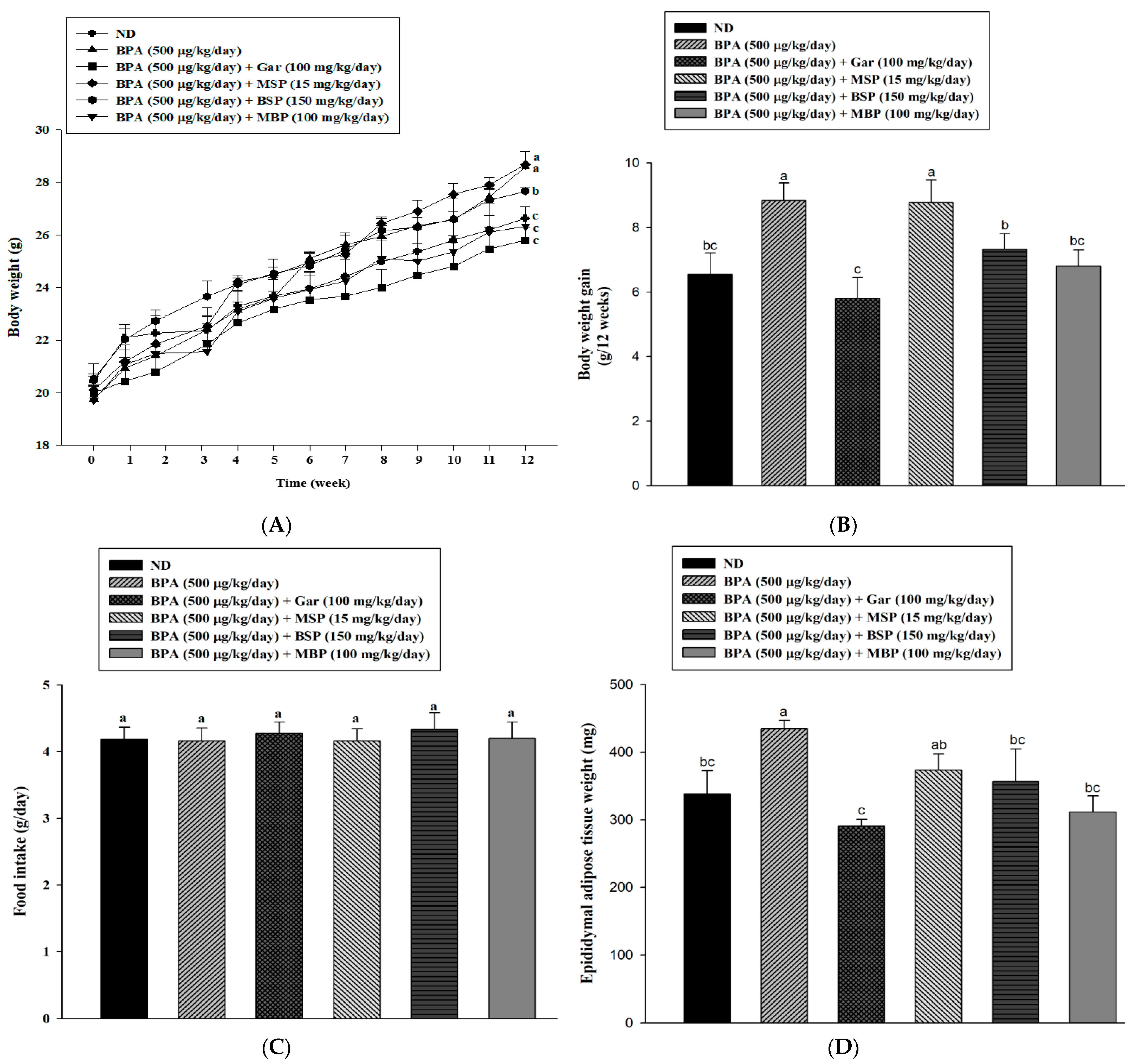
3.4. Effects of MBP, BSP, and MSP on Adipose Tissue Structure, Tissue Weight, and Body Weight in C57BL/6J Mice with BPA-Induced Obesity
3.5. Effects of MBP, BSP, and MSP on Serum Biochemical Indexes in C57BL/6J Mice with BPA-Induced Obesity
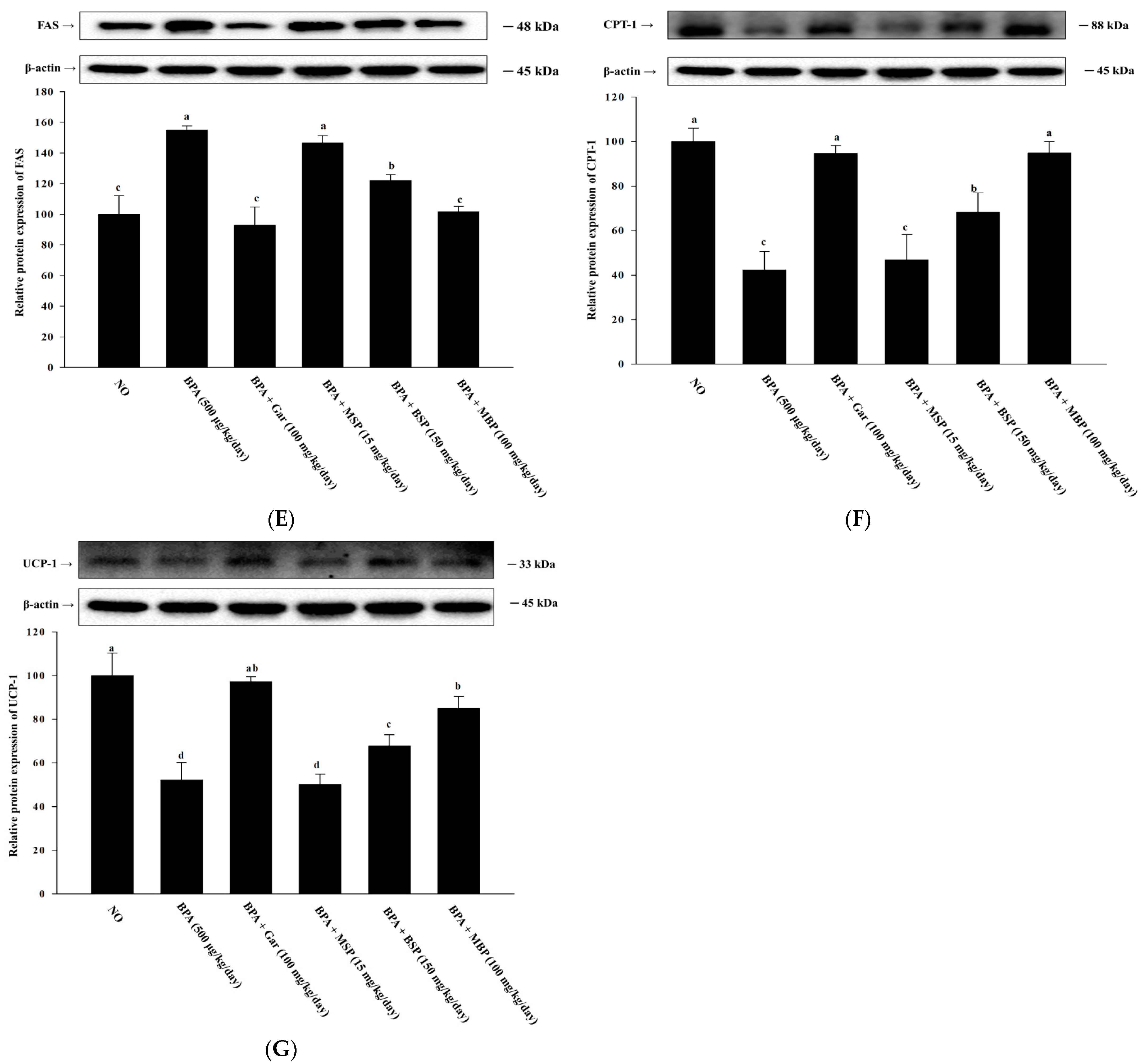
3.6. Effects of MBP, BSP, and MSP on Lipid Metabolism-Related Proteins in BPA-Induced Obese Mice
3.7. Effects of MBP, BSP, and MSP on AMPK Phosphorylation in C57BL/6J Mice with BPA-Induced Obesity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, P. Obesogenic environment and childhood obesity. Obes. Rev. 2020, 22, e13158. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; He, J.; Wang, H.; Zhu, D.; Bi, Y. Adipose morphology: A critical factor in regulation of human metabolic diseases and adipose tissue dysfunction. Obes. Surg. 2020, 30, 5086–5100. [Google Scholar] [CrossRef]
- Kowalska, K.; Olejnik, A.; Rychlik, J.; Grajek, W. Cranberries (Oxycoccus quadripetalus) inhibit adipogenesis and lipogenesis in 3T3-L1 cells. Food Chem. 2014, 148, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Liébana-García, R.; Olivares, M.; Bullich-Vilarrubias, C.; López-Almela, I.; Romaní-Pérez, M.; Sanz, Y. The gut microbiota as a versatile immunomodulator in obesity and associated metabolic disorders. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101542. [Google Scholar] [CrossRef] [PubMed]
- Hebebrand, J.; Gearhardt, A. The concept of “food addiction” helps inform the understanding of overeating and obesity: NO. Am. J. Clin. Nutr. 2021, 113, 268–273. [Google Scholar] [CrossRef] [PubMed]
- González-Muniesa, P.; Mártinez-González, M.A.; Hu, F.B.; Després, J.P.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A.; Martinez, J.A. Obesity. Nat. Rev. Dis. Primers 2017, 3, 17034. [Google Scholar] [CrossRef]
- Newbold, R.R. Impact of environmental endocrine disrupting chemicals on the development of obesity. Hormones 2010, 9, 206–217. [Google Scholar] [CrossRef]
- Sargis, R.M.; Johnson, D.N.; Choudhury, R.A.; Brady, M.J. Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation. Obesity 2010, 18, 1283–1288. [Google Scholar] [CrossRef]
- Ariemma1, F.; D’Esposito, V.; Liguoro, D.; Oriente, F.; Cabaro, S.; Liotti, A.; Cimmino, I.; Longo, M.; Beguinot, F.; Formisano, P.; et al. Low-dose bisphenol-A impairs adipogenesis and generates dysfunctional 3T3-L1 adipocytes. PLoS ONE 2016, 11, 0150762. [Google Scholar] [CrossRef]
- Darbre, P.D. Endocrine disruptors and obesity. Curr. Obes. Rep. 2017, 6, 18–27. [Google Scholar] [CrossRef]
- Combarnous, Y.; Nguyen, T.M.D. Comparative overview of the mechanisms of action of hormones and endocrine disruptor compounds. Toxics 2019, 7, 5. [Google Scholar] [CrossRef]
- Swedenborg, E.; Rüegg, J.; Mäkelä, S.; Pongratz, I. Endocrine disruptive chemicals: Mechanisms of action and involvement in metabolic disorders. J. Mol. Endocrinol. 2009, 43, 1–10. [Google Scholar] [CrossRef]
- Gupta, R.; Kumar, P.; Fahmi, N.; Garg, B.; Dutta, S.; Sachar, S.; Matharu, A.S.; Vimaleswaran, K.S. Endocrine disruption and obesity: A current review on environmental obesogens. Curr. Res. Green Sustain. Chem. 2020, 3, 100009. [Google Scholar] [CrossRef]
- Choi, S.I.; Lee, J.S.; Lee, S.; Sim, W.S.; Kim, Y.C.; Lee, O.H. Potentilla rugulosa Nakai Extract Attenuates Bisphenol A-, S-and F-Induced ROS Production and Differentiation of 3T3-L1 Preadipocytes in the Absence of Dexamethasone. Antioxidants 2020, 9, 113. [Google Scholar] [CrossRef]
- Holtcamp, W. Obesogens: An environmental link to obesity. Environ. Health Perspect. 2012, 120, 2. [Google Scholar]
- Veiga-Lopez, A.; Pu, Y.; Gingrich, J.; Padmanabhan, V. Obesogenic endocrine disrupting chemicals: Identifying knowledge gaps. Trends Endocrinol. Metab. 2018, 29, 607–625. [Google Scholar] [CrossRef]
- Nadal, A. Fat from plastics? Linking bisphenol A exposure and obesity. Nat. Rev. Endocrinol. 2013, 9, 9–10. [Google Scholar] [CrossRef]
- Oppeneer, S.J.; Robien, K. Bisphenol A exposure and associations with obesity among adults: A critical review. Public Health Nutr. 2015, 18, 1847–1863. [Google Scholar] [CrossRef]
- MacKay, H.; Patterson, Z.R.; Abizaid, A. Perinatal exposure to low-dose bisphenol-A disrupts the structural and functional development of the hypothalamic feeding circuitry. Endocrinology 2017, 158, 768–777. [Google Scholar] [CrossRef]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or sulforaphane: Is it the source or dose that matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef]
- Gu, Y.; Guo, Q.; Zhang, L.; Chen, Z.; Han, Y.; Gu, Z. Physiological and biochemical metabolism of germinating broccoli seeds and sprouts. J. Agric. Food Chem. 2012, 60, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Mamun, A.A.; Jakaria, M.; Thangapandiyan, S.; Ahmad, J.; Rahman, M.A.; Mathew, B.; Abdel-Daim, M.M.; Aleya, L. Emerging promise of sulforaphane-mediated Nrf2 signaling cascade against neurological disorders. Sci. Total Environ. 2020, 707, 135624. [Google Scholar] [CrossRef] [PubMed]
- Ruhee, R.T.; Suzuki, K. The integrative role of sulforaphane in preventing inflammation, oxidative stress and fatigue: A review of a potential protective phytochemical. Antioxidants 2020, 9, 521. [Google Scholar] [CrossRef]
- Kamal, M.M.; Akter, S.; Lin, C.N.; Nazzal, S. Sulforaphane as an anticancer molecule: Mechanisms of action, synergistic effects, enhancement of drug safety, and delivery systems. Arch. Pharm. Res. 2020, 43, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Zhang, G.; Huang, E.C.; Huang, J.; Cai, J.; Cai, L.; Wang, S.; Keller, B.B. Sulforaphane prevents right ventricular injury and reduces pulmonary vascular remodeling in pulmonary arterial hypertension. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H853–H866. [Google Scholar] [CrossRef]
- Choi, K.M.; Lee, Y.S.; Sin, D.M.; Lee, S.; Lee, M.K.; Lee, Y.M.; Hong, J.T.; Yun, Y.P.; Yoo, H.S. Sulforaphane inhibits mitotic clonal expansion during adipogenesis through cell cycle arrest. Obesity 2012, 20, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Q.; Chen, S.Y.; Wang, A.S.; Yao, A.J.; Fu, J.F.; Zhao, J.S.; Chen, F.; Zou, Z.Q.; Zhang, X.H.; Shan, Y.J.; et al. Sulforaphane induces adipocyte browning and promotes glucose and lipid utilization. Mol. Nutr. Food Res. 2016, 60, 2185–2197. [Google Scholar] [CrossRef]
- Yao, A.; Shen, Y.; Wang, A.; Chen, S.; Zhang, H.; Chen, F.; Chen, Z.; Wei, H.; Zou, Z.; Shan, Y.; et al. Sulforaphane induces apoptosis in adipocytes via Akt/p70s6k1/Bad inhibition and ERK activation. Biochem. Biophys. Res. Commun. 2015, 465, 696–701. [Google Scholar] [CrossRef]
- Azizi, S.N.; Amiri-Beshelib, B.; Sharifi-Mehra, S. The isolation and determination of sulforaphane from broccoli tissues by reverse phase-High Performance Liquid Chromatography. J. Chin. Chem. Soc. 2011, 58, 906–910. [Google Scholar] [CrossRef]
- Shen, L.; Su, G.; Wang, X.; Du, Q.; Wang, K. Endogenous and exogenous enzymolysis of vegetable-sourced glucosinolates and influencing factors. Food Chem. 2010, 119, 987–994. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Klopsch, R.; Oliviero, T.; Schreiner, M.; Verkerk, R.; Dekker, M. Optimizing isothiocyanate formation during enzymatic glucosinolate breakdown by adjusting pH value, temperature and dilution in Brassica vegetables and Arabidopsis thaliana. Sci. Rep. 2017, 7, 40807. [Google Scholar] [CrossRef] [PubMed]
- Men, X.; Han, X.; Lee, S.J.; Park, K.T.; Han, J.K.; Choi, S.I.; Lee, O.H. Anti-adipogenic effects of sulforaphane-rich ingredient with broccoli sprout and mustard seed in 3T3-L1 preadipocytes. Planta Med. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Zilberfarb, V.; Siquier, K.; Strosberg, A.D.; Issad, T. Effect of dexamethasone on adipocyte differentiation markers and tumour necrosis factor-α expression in human PAZ6 cells. Diabetologia 2001, 44, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Calzadilla, P.; Sapochnik, D.; Cosentino, S.; Diz, V.; Dicelio, L.; Calvo, J.C.; Guerra, L.N. N-acetylcysteine reduces markers of differentiation in 3T3-L1 adipocytes. Int. J. Mol. Sci. 2011, 12, 6936–6951. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Tinahones, F.J. Impaired adipose tissue expandability and lipogenic capacities as ones of the main causes of metabolic disorders. J. Diabetes Res. 2015, 2015, 970375. [Google Scholar] [CrossRef]
- Orci, L.; Cook, W.S.; Ravazzola, M.; Unger, R.H. Rapid transformation of white adipocytes into fat-oxidizing machines. Proc. Natl. Acad. Sci. USA 2004, 101, 2058–2063. [Google Scholar] [CrossRef]
- Carling, D.; Mayer, F.V.; Sanders, M.J.; Gamblin, S.J. AMP-activated protein kinase: Nature’s energy sensor. Nat. Chem. Biol. 2011, 7, 512–518. [Google Scholar] [CrossRef]
- Kim, K.Y.; Lee, H.N.; Kim, Y.J.; Park, T. Garcinia cambogia extract ameliorates visceral adiposity in C57BL/6J mice fed on a high-fat diet. Biosci. Biotechnol. Biochem. 2008, 72, 1772–1780. [Google Scholar] [CrossRef]
- Lee, H.G.; Lu, Y.A.; Li, X.; Hyun, J.M.; Kim, H.S.; Lee, J.J.; Kim, T.H.; Kim, H.M.; Kang, M.C.; Jeon, Y.J. Anti-Obesity effects of Grateloupia elliptica, a red seaweed, in mice with high-fat diet-induced obesity via suppression of adipogenic factors in white adipose tissue and increased thermogenic factors in brown adipose tissue. Nutrients 2020, 12, 208. [Google Scholar] [CrossRef]
- Moon, J.; Do, H.J.; Kim, O.Y.; Shin, M.J. Antiobesity effects of quercetin-rich onion peel extract on the differentiation of 3T3-L1 preadipocytes and the adipogenesis in high fat-fed rats. Food Chem. Toxicol. 2013, 58, 347–354. [Google Scholar] [CrossRef]
- Cariou, B.; Postic, C.; Boudou, P.; Burcelin, R.; Kahn, C.R.; Girard, J.; Burnol, A.F.; Mauvais-Jarvis, F. Cellular and molecular mechanisms of adipose tissue plasticity in muscle insulin receptor knockout mice. Endocrinology 2004, 145, 1926–1932. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Q.A.; Scherer, P.E.; Gupta, R.K. Improved methodologies for the study of adipose biology: Insights gained and opportunities ahead. J. Lipid Res. 2014, 55, 605–624. [Google Scholar] [CrossRef] [PubMed]
- Klop, B.; Elte, J.W.F.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef]
- Ko, G.T.C.; Cockram, C.S.; Woo, J.; Chan, J.C.N. Obesity, insulin resistance and isolated low high-density-lipoprotein cholesterol in Chinese subjects. Diabet. Med. 2001, 18, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Q.; Otto, T.C.; Lane, M.D. Mitotic clonal expansion: A synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Hsu, C.H.; Wang, X.; Sakai, S.; Freeman, M.W.; Gonzalez, F.J.; Spiegelman, B.M. C/EBPα induces adipogenesis through PPARγ: A unified pathway. Genes Dev. 2002, 16, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, L.; Li, B.; Jiang, H.; Duan, Y.; Xie, Z.; Shuai, L.; Li, J.; Li, J. AMP-activated protein kinase (AMPK) regulates energy metabolism through modulating thermogenesis in adipose tissue. Front. Physiol. 2018, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Day, E.A.; Ford, R.J.; Steinberg, G.R. AMPK as a therapeutic target for treating metabolic diseases. Trends Endocrinol. Metab. 2017, 28, 545–560. [Google Scholar] [CrossRef]
- Fullerton, M.D.; Galic, S.; Marcinko, K.; Sarah Sikkema, S.; Pulinilkunnil, T.; Chen, Z.P.; O’Neill, H.M.; Ford, R.J.; Palanivel, R.; O’Brien, M.; et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 2013, 19, 1649–1654. [Google Scholar] [CrossRef]
- Ali, F.; Ismail, A.; Esa, N.M.; Pei, C. Cocoa polyphenols treatment ameliorates visceral obesity by reduction lipogenesis and promoting fatty acid oxidation genes in obese rats through interfering with AMPK pathway. Eur. J. Lipid Sci. Technol. 2016, 118, 564–575. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, J.; Hamzah, S.S.; Ge, S.; Lin, Y.; Zheng, B.; Zeng, S.; Lin, S. n-Butanol extract of lotus seeds exerts antiobesity effects in 3T3-L1 preadipocytes and high-fat diet-fed mice via activating adenosine monophosphate-activated protein kinase. J. Agric. Food Chem. 2019, 67, 1092–1103. [Google Scholar] [CrossRef] [PubMed]


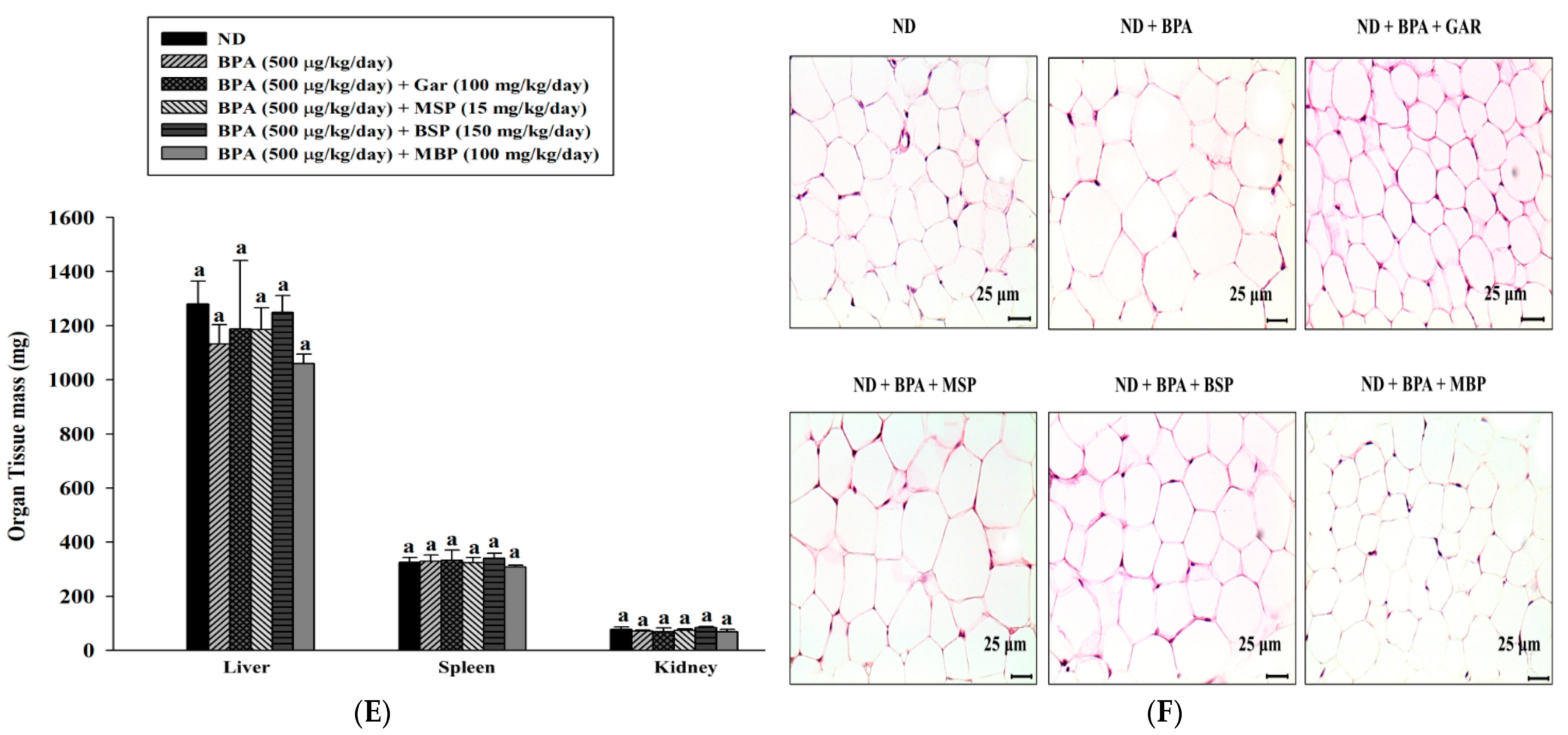
| Groups | ND | BPA (500 μg/kg/day) | BPA + Gar (100 mg/kg/day) | BPA + MSP (15 mg/kg/day) | BPA + BSP (150 mg/kg/day) | BPA + MBP (100 mg/kg/day) |
|---|---|---|---|---|---|---|
| TG (mg/dL) | 29.60 ± 6.86 ab | 36.42 ± 18.20 a | 23.04 ± 2.29 ab | 26.17 ± 4.16 ab | 19.82 ± 3.24 b | 18.84 ± 1.17 b |
| TC (mg/dL) | 60.11 ± 4.41 a | 55.53 ± 8.59 a | 48.66 ± 2.76 a | 58.90 ± 4.44 a | 55.06 ± 12.55 a | 60.09 ± 6.32 a |
| HDL-C (mg/dL) | 57.95 ± 4.48 a | 45.38 ± 2.89 b | 56.95 ± 1.14 a | 57.03 ± 4.13 a | 57.17 ± 0.02 a | 60.09 ± 5.37 a |
| LDL-C (mg/dL) | 9.53 ± 0.64 a | 9.82 ± 5.20 a | 8.98 ± 0.93 a | 10.47 ± 0.51 a | 10.73 ± 0.66 a | 10.31 ± 2.33 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Men, X.; Han, X.; Lee, S.-J.; Oh, G.; Park, K.-T.; Han, J.-K.; Choi, S.-I.; Lee, O.-H. Anti-Obesogenic Effects of Sulforaphane-Rich Broccoli (Brassica oleracea var. italica) Sprouts and Myrosinase-Rich Mustard (Sinapis alba L.) Seeds In Vitro and In Vivo. Nutrients 2022, 14, 3814. https://doi.org/10.3390/nu14183814
Men X, Han X, Lee S-J, Oh G, Park K-T, Han J-K, Choi S-I, Lee O-H. Anti-Obesogenic Effects of Sulforaphane-Rich Broccoli (Brassica oleracea var. italica) Sprouts and Myrosinase-Rich Mustard (Sinapis alba L.) Seeds In Vitro and In Vivo. Nutrients. 2022; 14(18):3814. https://doi.org/10.3390/nu14183814
Chicago/Turabian StyleMen, Xiao, Xionggao Han, Se-Jeong Lee, Geon Oh, Keun-Tae Park, Jong-Kwon Han, Sun-Il Choi, and Ok-Hwan Lee. 2022. "Anti-Obesogenic Effects of Sulforaphane-Rich Broccoli (Brassica oleracea var. italica) Sprouts and Myrosinase-Rich Mustard (Sinapis alba L.) Seeds In Vitro and In Vivo" Nutrients 14, no. 18: 3814. https://doi.org/10.3390/nu14183814
APA StyleMen, X., Han, X., Lee, S.-J., Oh, G., Park, K.-T., Han, J.-K., Choi, S.-I., & Lee, O.-H. (2022). Anti-Obesogenic Effects of Sulforaphane-Rich Broccoli (Brassica oleracea var. italica) Sprouts and Myrosinase-Rich Mustard (Sinapis alba L.) Seeds In Vitro and In Vivo. Nutrients, 14(18), 3814. https://doi.org/10.3390/nu14183814