Abstract
Photodynamic therapy is an unconventional yet increasingly common method of treating dermatological diseases and cancer that is implemented more often in adults than in children. Current clinical uses include treatment of actinic keratosis, superficial basal cell carcinomas, and acne. Despite its high efficiency, photodynamic therapy support supplements have recently been reported in the literature, including calcitriol (1,25-dihydroxycholecalciferol), the active form of vitamin D, and vitamin D3 cholecalciferol. In clinical trials, photodynamic therapy enhanced with vitamin D or D3 supplementation has been reported for treatment of squamous cell skin cancers, actinic keratosis, and psoriasis. Experimental research on the effect of photodynamic therapy with vitamin D or D3 has also been carried out in breast cancer cell lines and in animal models. The aim of this review is to evaluate the usefulness and effectiveness of vitamin D and D3 as supports for photodynamic therapy. For this purpose, the Pubmed and Scopus literature databases were searched. The search keyword was: “vitamin D in photodynamic therapy”. In the analyzed articles (1979–2022), the authors found experimental evidence of a positive effect of vitamin D and D3 when used in conjunction with photodynamic therapy. An average of 6–30% (in one case, up to 10 times) increased response to photodynamic therapy was reported in combination with vitamin D and D3 as compared to photodynamic therapy alone. Implementing vitamin D and D3 as a supplement to photodynamic therapy is promising and may lead to further clinical trials and new clinical methodologies.
1. Introduction
Photodynamic therapy (PDT) is a minimally invasive and modern therapeutic method that is clinically approved for the treatment of some oncological skin diseases, such as Basal Cell carcinoma [1,2,3], actinic keratosis [4], Bowen’s disease [5], cutaneous lymphomas [6], and cutaneous Kaposi sarcoma [7]. It has been approved for the treatment of several types of cancer, including those of the prostate [8], lung [9], breast [10], larynx [11], and bladder [12], and for the treatment of non-oncological dermatological diseases such as psoriasis [13], acne [14,15], herpes simplex labialis [16], cutaneous leishmaniasis [17], Darier disease (Keratosis follicularis) [18], and port-wine stains [19].
The main determinant of PDT is the application of a photosensitizer (PS) (which accumulates in tissue or is present in the bloodstream at the time of illumination), local illumination of a targeted site with laser light, and molecular oxygen [20,21,22,23].
Photodynamic therapy is a two-step process: 1. administration of a PS (on the surface of a lesion for skin diseases, or intravenously) [24]; 2. local illumination of the tumor or lesion with light that corresponds to the PS absorption wavelength [25]. Light is absorbed by the PS and results in the generation of reactive oxygen species (ROS) such as hydroxyl radicals or singlet oxygen (1O2), which are highly reactive and cytotoxic species [26,27,28,29].
Photodynamic pathways are divided into two main types: Type I and Type II [30]. Type I involves photoinduced electron transfer, leading to local formation of superoxide, hydroperoxyl, and hydroxyl radicals. Type II is characterized by energy transfer from the PS in its excited triplet state to oxygen, producing cytotoxic 1O2 [31]. Figure 1 shows a generalized scheme for the production of singlet oxygen in vitro. The in vitro research methodology allows for a better understanding of the mechanism of action of PDT and its more effective use in in vivo research.
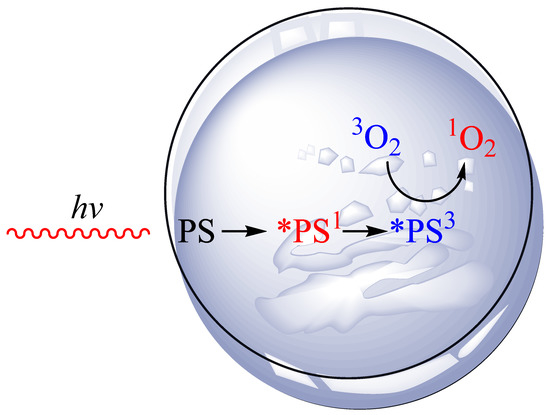
Figure 1.
Singlet oxygen generation in vitro.
For the photodynamic effect to take place, three elements are necessary: an administered photosensitizer (PS), a light source (hv), and oxygen (O2). An example of an in vitro Type II process is presented above. Cancer tissue has been placed in a petri dish. A specifically selected PS with an appropriate concentration is applied. The tissue is then exposed to laser light of a given wavelength. Absorption of a laser light quantum by PS produces a short-term singlet excited state (*PS1). This state can lose energy through fluorescence, internal conversion to heat, or through an intersystem transition to a long-term triplet excited state (*PS3). The (*PS3) transfers energy to the triplet oxygen. As a result of this process, the triplet oxygen (3O2) is converted into singlet oxygen (1O2) via triplet–triplet energy transfer, which can induce cancer cell death.
Improvements to PDT are currently addressing the difficulty in treating areas located in deeper tissue due to the limited penetration of light [32,33]. Research is also being conducted on the modification of photosensitizers in order to minimize their impact on a specific organ and eliminate the potentially harmful effect of PDT on healthy tissue [33]. Enhanced PDT is also practiced through the use of nanoparticles bearing PS [34], chemotherapeutic agents [35], or in conjunction with calcipotriol (a synthetic derivative of calcitriol) [36].
Vitamin D3 and each of its analogues is a steroid hormone involved in the metabolism of calcium. It has been shown in experimental studies that this supplement increases the production of protoporphyrin IX in mitochondria, supporting the destruction of cancer cells during PDT [37]. It is noteworthy that the mechanisms by which vitamins D and D3 (and their analogues) may influence PDT response are not fully understood. Understanding how high doses of vitamins D and D3 affect PDT reactivity continues. The analysis of the influence of calcitriol on the physiology of the organism and cells is very often analyzed in in vitro studies. It is now known that calcitriol promotes the repair of UV-induced mutations in keratinocytes by increasing functional P53 and has several anti-tumor effects on epidermal tumors through the immune system. The transcriptional profile of calcitriol-treated healthy keratinocytes was examined, showing upregulation of approximately 82 genes and downregulation of 16 other genes [38]. One example of the analysis of the effect of calcitriol on the patient’s body is the study conducted by Anand et al. Tumors pretreated with calcitriol showed increased apoptotic cell death following PDT. Tumors pre-conditioned with calcitriol before receiving ALA-PDT showed greater activation of the external apoptotic pathway (greater caspase-8 cleavage and increased TNFα production) [39]. There are also known cases of in vitro experiments in which cells treated with calcitriol increased the degree of DNA fragmentation compared to untreated cells [40]. For example, treatment with calcitriol can reduce stemness to varying extents in a panel of glioblastoma stem-like cells, and that it effectively hinders tumor growth of responding glioblastoma stem-like cells ex vivo [41].
The aim of this review was to evaluate the reports on the usefulness and effectiveness of topical synthetic vitamin D in support of PDT in both adults and children.
2. Materials and Methods
2.1. Search Strategy and Select Criteria
A search focused on the effect of synthetic vitamin D and D3 on the effectiveness of PDT was conducted on Pubmed and Scopus from inception (1979) to June 2022. This review was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [42]. The search term included the phrase: “vitamin D in photodynamic therapy”. Two authors undertook the task of identifying relevant data. Any discrepancies between the reviewers were resolved by a third author. The authors of this review worked on the basis of an agreed scheme, selecting articles based on their title, language (English), abstract, and access. Duplicate records were removed.
Full-text and accessible articles were reviewed. In order to minimize the selection bias, the inclusion and exclusion criteria were established as follows:
Inclusion criteria:
- vitamin D supplemented or administered during PDT treatment;
- analysis of the effect of synthetic vitamin D supplementation on the effectiveness of PDT;
- clinical and experimental studies of the effect of vitamin D supplementation on the effectiveness of PDT in animal studies or in in vitro studies on cell lines;
- dermatological diseases, cancer treatment, and treatment of internal organ tumors
Exclusion criteria:
- no analysis of the relationship between vitamin D supplementation and PDT effectiveness
- conducting photochemotherapy
2.2. Data Extraction
Two authors identified relevant information according to the inclusion and exclusion criteria. From the qualified articles, they listed the publication year, type of disease, type of examination, and the vitamin D or D3 used as a supplement to PDT.
3. Results
3.1. Study Selection
We searched 231 articles, and 24 articles were selected for this review (Figure 2). Among the included, 19 were research articles and 5 were reviews.
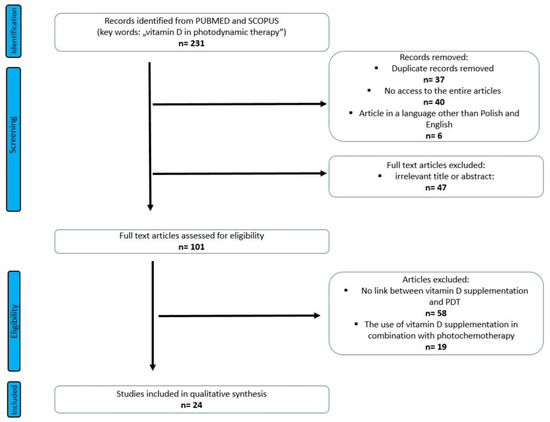
Figure 2.
PRISMA flow diagram of included studies.
3.2. Study Characteristics
The 24 articles included in the review were analyzed in terms of the type of study (clinical study/animal study/cell lines), type of disease, type of application, and effect of synthetic vitamin D administration on PDT efficacy. An additional analyzed parameter was the type of PS used. Table 1 shows a description of study characteristics.

Table 1.
Description of study characteristics.
3.3. Results of Studies
Of the 20 studies analyzed, 45% were clinical human studies, 30% were in vitro studies on cell lines, and 25% were animal studies. In all clinical trials, patients were adults aged ≥18 years. The most common type of diseases treated was actinic keratoses (25%), followed by squamous cell skin cancers (15%). Cell line in vitro treatment on breast, glioma, and prostate cells represented 25%. Overall, 55% of synthetic vitamin D or D3 was administered topically (using a cream or ointment), 30% was added to cell cultures, and 15% was added systemically or intraperitoneally—in two cases, orally (as a drug or dietary). For PDT, the most frequently used PS was 5-ALA (in 60% of cases), followed by MAL (in 30%). Table 2 presents a summary of the studies reported in this review.

Table 2.
Summary of studies reviewed.
4. Discussion
Vitamin D is a fat-soluble vitamin that has a significant impact on human functioning and health [59]. A natural source of vitamin D for humans is its synthesis in the skin under the influence of ultraviolet B (UVB) radiation (290–315 nm) [60]. Previtamin D occurs mainly in the spinous and basal layers of the epidermis, with fewer resources in the outer layers of the epidermis or dermis [61,62,63].
Vitamin D therapy using calcitriol, calcipotriol, and tacalcitol has been used to treat skin conditions including psoriasis, atopic dermatitis, and vitiligo [64]. Effective treatment of these cases with synthetic vitamin D or D3 continues to be clinically relevant [65]. The possibility of an enhanced PDT treatment is provided by vitamin D and D3 supplementation. A common hypothesis is that PDT with adjuvant vitamin D therapy is more effective than PDT alone [66]. It has been suggested that vitamin D and D3 modifies the tumor environment, allowing for a reduction of effective dose of the administered PS [67]. The motivation to analyze correlations between vitamin D and D3 and the effectiveness of PDT was the reported differentiated tumor responses to PDT in the presence of vitamin D and D3 [68,69,70]. In order to confirm the hypothesis, over the last 43 years, research and review articles have been written assessing the effect of vitamin D and D3 on the effectiveness of PDT. Researchers introduced vitamin D-fortified PDT in cases such as squamous cell carcinoma [39,46], actinic keratosis [37,52,53,55,56,57,58], human psoriasis [36], and follicular mucinosis of the scalp [54]. Research has also been carried out on human glioma cell lines U87 and T98 [44,45], prostate cancer cells [47], and human breast cancer cell lines MCF7 and MDA-MB-231 [43,49]. Furthermore, in experimental research, animal models such as murine models [39,48,49,51], germ-free fetal rat keratinocytes [40], and precancerous lesions in the buccal pouch of hamsters [50] have been used.
The authors of studies assessing the effect of vitamin D and D3 administration (in the form of topical administration) during PDT reported enhanced results compared to un-adjuvant therapy. Response to treatment was variable. Responses depended on the type of case and the patients’ state of health (applies to clinical trials). Most studies focused on dermatological diseases (65%). This may be due to the common use of vitamin D and D3 therapy alone in dermatological cases. It should also be noted that in all clinical trials, the patients were adults over 18 years of age, with people over 50 being qualified more often. Although the therapy itself was a safe procedure, with a reduced number of potential side effects, children and adolescents were not qualified to be included in research groups. In this review, 55% of all studies were experimental (preclinical) in cell lines and animal models. This demonstrates that vitamin D-assisted PDT is a relatively new methodology that is still being studied and remains in the experimental stage. Articles from inception (1979) to 2022 were included in the review. The oldest article is from 2002; i.e., the research spans 20 years.
Our review shows that a much more frequent form of application is topical application of vitamin D and D3 (in 55% of cases, cream or ointment was used). The reason for this may be the number of dermatological cases in which topical application is easier and safer. For example, one clinical case (actinic keratosis) was analyzed in which oral administration was used [37]; in other cases, topical treatment was applied [52,53,55,56,57,58]. Authors who administered oral synthetic vitamin D observed that pre-treatment with high doses improved lesion clearance [37], with few side effects besides a feeling of warmth and a mild tickling sensation during illumination. In the topical application, the response to treatment was high (varying from 6 to even 45% lesion reduction), with good tolerance of tacalcitol. It is worth noting that long-term consumption of a vitamin may itself be toxic to the body and potentially life threatening [71]. Hence, in our opinion, increasing the use of topical application is safer. This information was also taken into account by the authors of [37]; however, they reported that, in fact, many physicians recommend vitamin supplementation in large amounts without risk of toxicity.
This review also included a case of analyzing the effectiveness of a cream compared to an ointment in psoriasis [36]. The authors observed clinical improvement 2 weeks after PDT was present after the application of ointment, which was not observed after the application of cream [36]. Pre-treatment with ointment aided in recovery. The choice of the carrier in which synthetic vitamin D and D3 is delivered is important because the greater the penetration rate of the drug, the higher the increase in the level of PS and the increase in PDT efficiency.
The third case in human clinical trials was follicular mucinosis of the scalp [54]. A single description of a 59-year-old woman treated with tacalcitol was reported. The ointment application itself was started a month before PDT and continued throughout the therapy period without any side effects. The authors observed a progressive regrowth of hair, suggesting increased PDT efficacy. However, in their final conclusion, they suggested PDT therapy in combination with tacalcitol ointment in diseases are refractory to standard forms of treatment, i.e., using it following classical techniques.
The review attempted to analyze the stage of administration of vitamin D or D3 regarding its positive or negative impact. In most studies (95%) included in the review, vitamins D and D3 (and their analogues) were administered before phototherapy. Additionally, in one case, an attempt was made to continue supplementation during therapy [54], and in another case, the process was continued also after therapy [40]. In a study in which supplementation was carried out both before and during PDT, no side effects were observed in the treated woman. At the end of the therapy, there was a gradual reduction of cuticles and new hair growth. The application of tacalcitol was well tolerated by the patient. The study in which the application of calcitriol was used before and after therapy was an in vitro study [40]. The addition of calcitriol to the cell solution after irradiation allowed the observation of the effect of the active form of vitamin D3 on PDT-induced apoptosis. It was observed in the study that treatment of cells with calcitriol after PDT increased the degree of DNA fragmentation compared to untreated cells. The enhanced DNA fragmentation effect was observed in cells that had been injected with calcitriol immediately after irradiation. The supplementation and applications of vitamins D and D3 (and their analogues) were most often implemented before phototherapy; one of the reasons for this phenomenon could be the fact that most of the studies included in the review were human studies. Application or supplementation was carried out locally on the skin; therefore, doctors recommended the application of the ointment/cream in advance (on average 14–15 days). One of the advantages of such a solution is that the patient can apply the preparation to the skin themself, without the supervision of a doctor/nurse. In the case of application after therapy on reddened areas, medical care would be required. It is worth noting that this is a moot point because in the articles, the authors of the research did not provide an explanation of the reason for the time of application. Conducting clinical trials in which the application of vitamin D is implemented after PDT would be able to confirm or contradict our assumptions. Additionally, it would be possible to assess the advantages and disadvantages of each stage of administration in a clear and lucid way.
The review also analyzed the selection of the research group in terms of gender. In the analyzed human studies, both women and men were qualified for research groups in most cases (in 56% of trials). In three cases, male selection was the qualifying criterion. One article was a case report of a woman treated with PDT and vitamin D/D3. The authors did not analyze the effects of vitamin D or D3 supplementation in combination with PDT in relation to the gender of the patients in the evaluation of the results of their studies. In [36], the results of the therapy were similar in both groups when using a cream, ointment, or a different light source. Similar conclusions were presented in [52], where the research group consisted of 10 men and 1 woman. In another study [53], 75% of the research group were men and 25% were women. The description of treatment effects included the characteristics of changes, without taking into account the gender criterion. In the studies presented in [37,55], the authors noted that there were no significant differences in gender in the assessment of treatment results. In studies [56,57,58], male gender was one of the qualifying criteria for the therapy. Based on the above description, it can be concluded that significantly fewer women were qualified for PDT in combination with vitamin D or D3 supplementation compared to the number of men. Perhaps, of the entire group of patients, the majority of patients with dermatological problems are men. Another reason for this phenomenon could be the fact that in all studies, an exclusion criterion was pregnancy. Therefore, a safer variant was the choice of men for therapy than women in whom pregnancy could not be confirmed or excluded, e.g., due to the phase of the menstrual cycle. Nevertheless, the assessment of the gender effects of PDT supplemented with vitamin D or D3 may be a pioneering study that will allow the study protocol to be tailored to each patient (both women and men).
In the clinical trials reviewed, some limitations related to vitamin D and D3 supplementation or application were observed. One of them was the long period of ointment application before PDT. In some cases, this procedure was introduced a month before [54]. Another limitation was the side effects of the therapy, such as facial erythema, scales, and scabs [52]. Additionally, although a given procedure is applicable in a given group of patients, it may not be applicable to all patients.
Animal studies covered 25% of all articles included in the review. The results presented by the authors suggest a positive effect of vitamin D on the effectiveness of PDT. The results presented by the authors of [48] are promising, with the possibility of applying the developed procedures to human research. According to the authors, supplementation with a diet rich in vitamin D may prove to be a much easier method for patients than supplements with a high vitamin content. Researchers in PDT for breast cancer suggest including vitamin D-assisted PDT in human clinical trials as an alternative form of therapy for patients who have not had success with other techniques [49]. The use of the methodologies presented by the authors of [39,50,51] in in vivo studies is an open issue due to the risk of side effects after administration of calcitriol. The method provides hope for patients with non-melanoma skin cancer and squamous cell skin cancer after surgery, radiotherapy, and chemotherapy. We believe that further studies will confirm this in the coming years.
In vitro studies in cell lines covered 30% of articles included in the review [40,43,44,45,46,47]. This type of test is a precursor to in vivo testing. Although the specific test scheme and its effects are satisfactory, it does not determine similar effectiveness in in vivo tests. The cited experimental work indicates a greater effectiveness of PDT after the application of synthetic vitamin D derivatives; this topic must be researched further, perhaps first in animal models, and then in human patients (especially in relation to prostate [47], breast [43], or glioblastoma [44,45] cancers). In in vitro studies (being tested), the dose escalation/reduction process is not as risky as in human clinical studies. The development of an effective in vitro methodology provides the opportunity to increase the effectiveness of the therapy in in vivo clinical studies.
The presented positive effect of vitamin D supplementation in PDT can be assessed in relation to therapy assisted with other vitamins. An example of such therapies is vitamin A [72], E [73], K [74], and C [75] therapy. Vitamins A, E, and C are natural antioxidants which are used as anti-cancer supplements. Similarly to vitamins D and D3 (and their analogues), their influence on the effectiveness of PDT has been studied. In a study by Mahmood et al., the effect of vitamin A in photodynamic therapy of rhabdomyosarcoma cells was evaluated [72]. Vitamin A analogues were applied prior to the photosensitive application and irradiation. The analogues used were retinyl acetate, retinoic acid, and retinyl palmitate, which were applied at various concentrations.
Studies show that pretreatment with vitamin A derivatives reduced the tumor response to PDT. The reason for this phenomenon is the fact that vitamin A has antioxidant properties. PDT, in turn, initiates oxidative stress in cells, leading to the formation of reactive oxygen species. Antioxidants disrupt the action of free radicals, reducing the effectiveness of PDT.
In a study by Mielnikova et al., the effect of the vitamin E analogue on the enhancement of the photodynamic effect was assessed. The studies were carried out on human tumor xenografts [73]. The vitamin E analogue was the water-soluble Trolox when applied with the drug. In the study, a significant delay in tumor growth was observed after the application of Trolox and PDT. The authors made an attempt to explain the cause of this phenomenon. One of the reasons could be that Trolox, which mediated the radical pathway, could also act as a singlet oxygen transmitter, the concentration of which decreases with the progression of PDT. Another explanation may be the fact that Trolox as an antioxidant inhibited the growth of cancer cells after the action of PDT, which initiated an increase in the permeability of blood vessels to Trolox [73].
Another example of assisted therapy is the application of vitamin K3; in [74], rhabdomyosarcoma cells were used. A significant cell destruction effect was observed in this study after vitamin K3 was administered as a neoadjuvant [74]. The combination of a chemotherapeutic drug (CDDP-cisplatin) with vitamin K3 and PDT was also observed to increase the combined efficacy.
In the literature, there are also known cases of vitamin C application before the irradiation process. In [75], the authors used melanoma cells that were incubated at various concentrations one hour before irradiation. It was observed in the experiment that vitamin C supplementation increased cell viability by 10%. The authors showed that vitamin C supplementation caused a significant reduction in cytotoxicity induced by photodynamic treatment in melanoma cells [75]. Pre-treatment with vitamin C reduced the rate of cell death following PDT.
From the review of other forms of PDT enhancement (with vitamins A, K, E, and C), which do not always positively affect the effectiveness of PDT, therapy with vitamins D and D3 (and their analogues) is much more effective.
5. Conclusions
In order to improve the quality of PDT research, combination therapies that can improve the PDT method by increasing its effectiveness have been sought for several decades. An example of such a combination therapy is the use of calcitriol, calcipotriol, and tacalcitol on lesions or diseased cells. This review of the studies that has been conducted shows that the combination of vitamin D or D3 with PDT may improve the effectiveness of the method by increasing the accumulation of protoporphyrin via 5-ALA. In addition, PDT supplemented with vitamin D or D3 can enhance cell differentiation, thereby increasing the effectiveness of the treatment. These findings suggest that PDT enhanced with vitamin D and D3 yields a more effective and selective therapeutic response. Due to the fact that all the presented clinical trials used adult patients, the analysis confirming the possibility and effectiveness of using this therapy in young patients remains an open field for doctors and researchers to explore. The proposed therapy model should be further investigated in future clinical trials.
Author Contributions
Conceptualization, A.M., K.D., D.A. and D.B.-A.; methodology, K.K. and K.D.; validation, A.M., K.D., D.A. and D.B.-A.; formal analysis, D.B.-A.; resources, K.K. and D.B.-A.; writing—original draft preparation, A.M., K.D., D.A. and K.K.; writing—review and editing, A.M., K.D. and D.B.-A.; supervision, D.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study is available by request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitiz-ers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- De Albuquerque, I.O.; Nunes, J.; Figueiró Longo, J.P.; Muehlmann, L.A.; Azevedo, R.B. Photodynamic therapy in super-ficial basal cell carcinoma treatment. Photodiagn. Photodyn. Ther. 2019, 27, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, S.R.; Brianti, P.; Dattola, A.; Bennardo, L.; Silvestri, M.; Schipani, G.; Nisticò, S.P. CO2 laser and photodynamic therapy: Study of efficacy in periocular BCC. Dermatol. Ther. 2018, 31, e12616. [Google Scholar] [CrossRef] [PubMed]
- Fusano, M.; Zane, C.; Calzavara-Pinton, P.; Bencini, P.L. Photodynamic therapy for actinic keratosis in vegan and omnivore patients: The role of diet on skin healing. J. Dermatolog. Treat. 2021, 32, 78–83. [Google Scholar] [CrossRef]
- Calin, M.A.; Diaconeasa, A.; Savastru, D.; Tautan, M. Photosensitizers and light sources for photodynamic therapy of the Bow-en’s disease. Arch. Dermatol. Res. 2011, 303, 145–151. [Google Scholar] [CrossRef]
- Umegaki, N.; Moritsugu, R.; Katoh, S.; Harada, K.; Nakano, H.; Tamai, K.; Hanada, K.; Tanaka, M. Photodynamic therapy may be useful in debulking cutaneous lymphoma prior to radiotherapy. Clin. Exp. Dermatol. 2004, 29, 42–45. [Google Scholar] [CrossRef]
- Fonda-Pascual, P.; Fernandez-Gonzalez, P.; Sanchez-Los Arcos, L.; Alcantara-Nicolas, F.; Lopez-Galan, C.; Canseco-Martin, M.; Vidal-Asensi, S. Treatment of cutaneous Kaposi sarcoma with methylaminolevulinate photodynamic therapy: A case series. Photodermatol. Photoimmunol. Photomed. 2020, 36, 392–395. [Google Scholar] [CrossRef]
- Osuchowski, M.; Osuchowski, F.; Latos, W.; Kawczyk-Krupka, A. The Use of Upconversion Nanoparticles in Prostate Cancer Photodynamic Therapy. Life 2021, 11, 360. [Google Scholar] [CrossRef]
- Ikeda, N.; Usuda, J.; Maehara, S. Photodynamic therapy for central-type early-stage lung cancer. Gen. Thorac. Cardiovasc. Surg. 2020, 68, 679–683. [Google Scholar] [CrossRef]
- Ostańska, E.; Aebisher, D.; Bartusik-Aebisher, D. The potential of photodynamic therapy in current breast cancer treatment methodologies. Biomed. Pharmacother. 2021, 137, 111302. [Google Scholar] [CrossRef]
- Bartusik-Aebisher, D.; Ożóg, Ł.; Domka, W.; Aebisher, D. Rose Bengal and Future Directions in Larynx Tumor Photodynamic Therapy. Photochem. Photobiol. 2021, 97, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Railkar, R.; Agarwal, P.K. Photodynamic Therapy in the Treatment of Bladder Cancer: Past Challenges and Current Innova-tions. Eur. Urol. Focus. 2018, 4, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Adelzadeh, L.; Wu, J.J. Photodynamic therapy for psoriasis. J Dermatolog. Treat. 2015, 26, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Boen, M.; Brownell, J.; Patel, P.; Tsoukas, M.M. The Role of Photodynamic Therapy in Acne: An Evidence-Based Review. Am. J. Clin. Dermatol. 2017, 18, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Del Duca, E.; Manfredini, M.; Petrini, N.; Farnetani, F.; Chester, J.; Bennardo, L.; Schipani, G.; Tamburi, F.; Sannino, M.; Canna-rozzo, G.; et al. Daylight photodynamic therapy with 5-aminolevulinic acid 5% gel for the treatment of mild-to-moderate inflammatory acne. Ital. J. Dermatol. Venerol. 2021, 156, 46–50. [Google Scholar] [CrossRef]
- Marotti, J.; Aranha, A.C.; Eduardo Cde, P.; Ribeiro, M.S. Photodynamic therapy can be effective as a treatment for herpes sim-plex labialis. Photomed. Laser Surg. 2009, 27, 357–363. [Google Scholar] [CrossRef]
- Akilov, O.E.; Kosaka, S.; O’Riordan, K.; Hasan, T. Parasiticidal effect of delta-aminolevulinic acid-based photodynamic therapy for cutaneous leishmaniasis is indirect and mediated through the killing of the host cells. Exp. Dermatol. 2007, 16, 651–660. [Google Scholar] [CrossRef]
- Exadaktylou, D.; Kurwa, H.A.; Calonje, E.; Barlow, R.J. Treatment of Darier’s disease with photodynamic therapy. Br. J. Derma-tol. 2003, 149, 606–610. [Google Scholar] [CrossRef]
- Zhao, Y.; Tu, P.; Zhou, G.; Zhou, Z.; Lin, X.; Yang, H.; Lu, Z.; Gao, T.; Tu, Y.; Xie, H.; et al. Hemo-porfin Photodynamic Therapy for Port-Wine Stain: A Randomized Controlled Trial. PLoS ONE 2016, 11, e0156219. [Google Scholar] [CrossRef]
- Acedo, P.; Stockert, J.C.; Cañete, M.; Villanueva, A. Two combined photosensitizers: A goal for more effective photodynamic therapy of cancer. Cell Death Dis. 2014, 5, e1122. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, A.F.; Terra, L.F.; Wailemann, R.A.; Oliveira, T.C.; Gomes, V.M.; Mineiro, M.F.; Meotti, F.C.; Bruni-Cardoso, A.; Baptista, M.S.; Labriola, L. Methylene blue photodynamic therapy induces selective and massive cell death in human breast can-cer cells. BMC Cancer 2017, 17, 194. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kes-sel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Marcus, S.L. Photodynamic therapy. Eur. J. Cancer 1992, 28A, 1734–1742. [Google Scholar] [CrossRef]
- Czarnecka-Czapczyńska, M.; Aebisher, D.; Oleś, P.; Sosna, B.; Krupka-Olek, M.; Dynarowicz, K.; Latos, W.; Cieślar, G.; Kaw-czyk-Krupka, A. The role of photodynamic therapy in breast cancer-A review of in vitro research. Biomed. Pharmacother. 2021, 144, 112342. [Google Scholar] [CrossRef]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Braathen, L.R.; Morton, C.A.; Basset-Seguin, N.; Bissonnette, R.; Gerritsen, M.J.; Gilaberte, Y.; Calzavara-Pinton, P.; Sidoroff, A.; Wulf, H.C.; Szeimies, R.M. Photodynamic therapy for skin field cancerization: An international consensus. International Soci-ety for Photodynamic Therapy in Dermatology. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1063–1066. [Google Scholar] [CrossRef]
- Collier, N.J.; Haylett, A.K.; Wong, T.H.; Morton, C.A.; Ibbotson, S.H.; McKenna, K.E.; Mallipeddi, R.; Moseley, H.; Seukeran, D.; Ward, K.A.; et al. Conventional and combination topical photodynamic therapy for basal cell carcinoma: Systematic review and meta-analysis. Br. J. Dermatol. 2018, 179, 1277–1296. [Google Scholar] [CrossRef]
- O’Connell, K.A.; Okhovat, J.P.; Zeitouni, N.C. Photodynamic therapy for Bowen’s Disease (squamous cell carcinoma in situ) current review and update. Photodiagnosis Photodyn. Ther. 2018, 24, 109–114. [Google Scholar] [CrossRef]
- Ming, L.; Cheng, K.; Chen, Y.; Yang, R.; Chen, D. Enhancement of tumor lethality of ROS in photodynamic therapy. Cancer Med. 2021, 10, 257–268. [Google Scholar] [CrossRef]
- Kubrak, T.; Karakuła, M.; Czop, M.; Kawczyk-Krupka, A.; Aebisher, D. Advances in Management of Bladder Cancer-The Role of Photodynamic Therapy. Molecules 2022, 27, 731. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhang, Y.; He, Y.; Xiong, M.; Huang, H.; Pei, S.; Liao, J.; Wang, Y.; Shao, D. Green synthesis of carrier-free curcumin nanodrugs for light-activated breast cancer photodynamic therapy. Colloids Surf. B Biointerfaces 2019, 180, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, M.; Sun, W.; Fan, J.; Du, J.; Peng, X. An estrogen receptor targeted ruthenium complex as a two-photon photody-namic therapy agent for breast cancer cells. Chem. Commun. 2018, 54, 7038–7041. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Song, X.; Dong, X.; Li, B. Nano-photosensitizers for enhanced photodynamic therapy. Photodiagn. Photodyn. Ther. 2021, 36, 102597. [Google Scholar] [CrossRef]
- Zhu, T.; Shi, L.; Yu, C.; Dong, Y.; Qiu, F.; Shen, L.; Qian, Q.; Zhou, G.; Zhu, X. Ferroptosis Promotes Photodynamic Therapy: Supramolecular Photosensitizer-Inducer Nanodrug for Enhanced Cancer Treatment. Theranostics 2019, 9, 3293–3307. [Google Scholar] [CrossRef]
- Maytin, E.V.; Honari, G.; Khachemoune, A.; Taylor, C.R.; Ortel, B.; Pogue, B.W.; Sznycer-Taub, N.; Hasan, T. Vitamin D Com-bined with Aminolevulinate (ALA)-Mediated Photodynamic Therapy (PDT) for Human Psoriasis: A Proof-of-Principle Study. Isr. J. Chem. 2012, 52, 767–775. [Google Scholar] [CrossRef]
- Bullock, T.A.; Negrey, J.; Hu, B.; Warren, C.B.; Hasan, T.; Maytin, E.V. Significant improvement of facial actinic keratoses after blue light photodynamic therapy with oral vitamin D pretreatment: An interventional cohort-controlled trial. J. Am. Acad. Der-matol. 2022, 87, 80–86. [Google Scholar] [CrossRef]
- Moreno, R.; Nájera, L.; Mascaraque, M.; Juarranz, Á.; González, S.; Gilaberte, Y. Influence of Serum Vitamin D Level in the Response of Actinic Keratosis to Photodynamic Therapy with Methylaminolevulinate. J. Clin. Med. 2020, 9, 398. [Google Scholar] [CrossRef]
- Anand, S.; Wilson, C.; Hasan, T.; Maytin, E.V. Vitamin D3 enhances the apoptotic response of epithelial tumors to ami-nolevulinate-based photodynamic therapy. Cancer Res. 2011, 71, 6040–6050. [Google Scholar] [CrossRef]
- Matsuyama, A.; Nakano, H.; Harada, K.; Yamazaki, T.; Kanno, T.; Wakui, M.; Hanada, K. Enhancement of photodynamic effect in normal rat keratinocytes by treatment with 1,25 dihydroxy vitamin D3. Photodermatol. Photoimmunol. Photomed. 2003, 19, 303–308. [Google Scholar] [CrossRef]
- Gerstmeier, J.; Possmayer, A.L.; Bozkurt, S.; Hoffmann, M.E.; Dikic, I.; Herold-Mende, C.; Burger, M.C.; Munch, C.; Kogel, D.; Linder, B. Calcitriol promotes differentiation of glioma stem-like cells and increases their susceptibility to Temozolomide. Cancers 2021, 13, 3577. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Liu, R.; Li, Y.; Zhang, Q.; Cai, X.; Li, L. Calcitriol enhances the effect of photodynamic therapy in human breast can-cer. Off. J. Balk. Union Oncol. 2016, 21, 1068–1075. [Google Scholar]
- Chen, X.; Wang, C.; Teng, L.; Liu, Y.; Chen, X.; Yang, G.; Wang, L.; Liu, H.; Liu, Z.; Zhang, D.; et al. Calcitriol enhances 5-aminolevulinic acid-induced fluorescence and the effect of photodynamic therapy in human glioma. Acta Oncol. 2014, 53, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Parry, P.V.; Engh, J.A. Calcitriol enhances 5-aminolevulinic acid-induced fluorescence and the effect of photodynamic therapy in human glioma. Neurosurgery 2014, 74, N8–N9. [Google Scholar] [CrossRef]
- Cicarma, E.; Tuorkey, M.; Juzeniene, A.; Ma, L.W.; Moan, J. Calcitriol treatment improves methyl aminolaevulinate-based photodynamic therapy in human squamous cell carcinoma A431 cells. Br. J. Dermatol. 2009, 161, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Ortel, B.; Sharlin, D.; O’Donnell, D.; Sinha, A.K.; Maytin, E.V.; Hasan, T. Differentiation enhances aminolevulinic ac-id-dependent photodynamic treatment of LNCaP prostate cancer cells. Br. J. Cancer 2002, 87, 1321–1327. [Google Scholar] [CrossRef]
- Anand, S.; Rollakanti, K.R.; Horst, R.L.; Hasan, T.; Maytin, E.V. Combination of oral vitamin D3 with photodynamic therapy enhances tumor cell death in a murine model of cutaneous squamous cell carcinoma. Photochem. Photobiol. 2014, 90, 1126–1135. [Google Scholar] [CrossRef]
- Rollakanti, K.R.; Anand, S.; Maytin, E.V. Vitamin D enhances the efficacy of photodynamic therapy in a murine model of breast cancer. Cancer Med. 2015, 4, 633–642. [Google Scholar] [CrossRef]
- Yang, D.F.; Chen, J.H.; Chiang, C.P.; Huang, Z.; Lee, J.W.; Liu, C.J.; Chang, J.L.; Hsu, Y.C. Improve efficacy of topical ALA-PDT by calcipotriol through up-regulation of coproporphyrinogen oxidase. Photodiagn. Photodyn. Ther. 2014, 11, 331–341. [Google Scholar] [CrossRef]
- Rollakanti, K.; Anand, S.; Maytin, E.V. Topical calcitriol prior to photodynamic therapy enhances treatment efficacy in non-melanoma skin cancer mouse models. Proc. SPIE Int. Soc. Opt. Eng. 2015, 9308, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Galimberti, G.N. Calcipotriol as pretreatment prior to daylight-mediated photodynamic therapy in patients with actinic keratosis: A case series. Photodiagn. Photodyn. Ther. 2018, 21, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Borgia, F.; Riso, G.; Catalano, F.; Coppola, M.; Giuffrida, R.; Cannavò, S.P. Topical tacalcitol as neoadjuvant for photodynamic therapy of acral actinic keratoses: An intra-patient randomized study. Photodiagn. Photodyn. Ther. 2020, 31, 101803. [Google Scholar] [CrossRef]
- Giuffrida, R.; Borgia, F.; Marafioti, I.; Riso, G.; Cannavò, S.P. Combination of tacalcitol ointment and photodynamic therapy for the treatment of follicular mucinosis of the scalp. Photodiagn. Photodyn. Ther. 2019, 27, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.W.; Song, K.H. Topical calcipotriol before ablative fractional laser-assisted photodynamic therapy enhances treatment outcomes for actinic keratosis in Fitzpatrick grades III-V skin: A prospective randomized clinical trial. J. Am. Acad. Dermatol. 2018, 78, 795–797. [Google Scholar] [CrossRef]
- Piaserico, S.; Piccioni, A.; Gutiérrez Garcìa-Rodrigo, C.; Sacco, G.; Pellegrini, C.; Fargnoli, M.C. Sequential treatment with calcitriol and methyl aminolevulinate-daylight photodynamic therapy for patients with multiple actinic keratoses of the upper extremities. Photodiagn. Photodyn. Ther. 2021, 34, 102325. [Google Scholar] [CrossRef]
- Torezan, L.; Grinblat, B.; Haedersdal, M.; Festa-Neto, C.; Szeimies, R.M. A 12-month follow-up split-scalp study comparing calcipotriol-assisted MAL-PDT with conventional MAL-PDT for the treatment of actinic keratosis: A randomized controlled trial. Eur. J. Dermatol. 2021, 31, 638–644. [Google Scholar] [CrossRef]
- Torezan, L.; Grinblat, B.; Haedersdal, M.; Valente, N.; Festa-Neto, C.; Szeimies, R.M. A randomized split-scalp study compar-ing calcipotriol-assisted methyl aminolaevulinate photodynamic therapy (MAL-PDT) with conventional MAL-PDT for the treatment of actinic keratosis. Br. J. Dermatol. 2018, 179, 829–835. [Google Scholar] [CrossRef]
- Albahrani, A.A.; Greaves, R.F. Fat-Soluble Vitamins: Clinical Indications and Current Challenges for Chromatographic Measurement. Clin. Biochem. Rev. 2016, 37, 27–47. [Google Scholar]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Dermato-Endocrinology 2013, 5, 51–108. [Google Scholar] [CrossRef]
- Marcinowska-Suchowierska, E.; Walicka, M.; Tałałaj, M.; Horst-Sikorska, W.; Ignaszak-Szczepaniak, M.; Sewerynek, E. Vitamin D supplementation in adults-guidelines. Endokrynol. Pol. 2010, 61, 723–729. [Google Scholar] [PubMed]
- Karczmarewicz, E.; Łukaszkiewicz, J.; Lorenc, R. Vitamin D-metabolism, action, requirements and treatment strategies. Stand. Med. 2007, 4, 137–142. [Google Scholar]
- Jones, G. Expanding role for vitamin D in chronic kidney disease: Importance of blood 25-OH-D levels and extra-renal 1alpha-hydroxylase in the classical and nonclassical actions of 1alpha,25-dihydroxyvitamin D(3). Semin. Dial. 2007, 20, 316–324. [Google Scholar] [CrossRef]
- Kechichian, E.; Ezzedine, K. Vitamin D and the Skin: An Update for Dermatologists. Am. J. Clin. Dermatol. 2018, 19, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Savanelli, M.C.; Di Somma, C.; Napolitano, M.; Megna, M.; Colao, A.; Savastano, S. Vitamin D and its role in psori-asis: An overview of the dermatologist and nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Maytin, E.V.; Hasan, T. Vitamin D and Other Differentiation-promoting Agents as Neoadjuvants for Photodynamic Therapy of Cancer. Photochem. Photobiol. 2020, 96, 529–538. [Google Scholar] [CrossRef]
- Garland, C.F.; Garland, F.C.; Gorham, E.D.; Lipkin, M.; Newmark, H.; Mohr, S.B.; Holick, M.F. The role of vitamin D in cancer prevention. Am. J. Public Health 2006, 96, 252–261. [Google Scholar] [CrossRef]
- Ortel, B.; Jabeen, S.; Greer, A. Adjuvants that Empower the Action of Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 725–727. [Google Scholar] [CrossRef]
- Anand, S.; Ortel, B.J.; Pereira, S.P.; Hasan, T.; Maytin, E.V. Biomodulatory approaches to photodynamic therapy for solid tu-mors. Cancer Lett. 2012, 326, 8–16. [Google Scholar] [CrossRef]
- Hasan, T. Using cellular mechanisms to develop effective combinations of photodynamic therapy and targeted therapies. J. Natl. Compr. Canc. Netw. 2012, 10, S23–S26. [Google Scholar] [CrossRef]
- Marcinowska-Suchowierska, E.; Kupisz-Urbańska, M.; Łukaszkiewicz, J.; Płudowski, P.; Jones, G. Vitamin D Toxicity-A Clini-cal Perspective. Front. Endocrinol. 2018, 9, 550. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, R.; Khurshid, A.; Yousaf, M.S.; Aalam, M.; Salman, M.; Ikram, M. Effect of vitamin A as a neoadjuvant agent in chemotherapy and photodynamic therapy of Rhabdomyosarcoma cells. Photodiagn. Photodyn. Ther. 2020, 32, 102088. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, V.O.; Bezdetnaya, L.N.; Brault, D.; Potapenko, A.Y.; Guillemin, F. Enhancement of meta-tetrahydroxyphenylchlorin-sensitized photodynamic treatment on human tumor xenografts using a water-soluble vitamin E analogue, Trolox. Int. J. Cancer 2000, 88, 798–803. [Google Scholar] [CrossRef]
- Mahmood, R.; Khurshid, A.; Khan, J.A.; Rafi, M.; Yousaf, M.S.; Maqsood, M.; Aalam, M.; Salman, M.; Ikram, M. Enhanced efficacy of chemo-photodynamic therapy of rhabdomyosarcoma cells by using vitamin K3 as a neoadjuvant agent. Laser Phys. 2018, 29, 015603. [Google Scholar] [CrossRef]
- Grimm, S.; Mvondo, D.; Grune, T.; Breusing, N. The outcome of 5-ALA-mediated photodynamic treatment in melanoma cells is influenced by vitamin C and heme oxygenase-1. Biofactors 2011, 37, 17–24. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).