Effects of Genistein on Common Kidney Diseases
Abstract
1. Introduction
| Foods | Content (μg/100 g Hydrated Portion) | Reference |
|---|---|---|
| Soybean | 26,800–102,500 | [27] |
| Kidney bean | 18.0–518.0 | [27] |
| Chickpea | 69.0–214.0 | [27] |
| Pea | 0–49.7 | [27] |
| Lentil | 7.0–19.0 | [27] |
| Kudzu leaf | 2520 | [27] |
| Kudzu root | 12600 | [27] |
| Black gram | 1900 | [28] |
| Alfalfa | 5.0 | [28] |
| Peanut | 8.0 | [29] |
| Caraway seed | 64.0 | [28] |
| Sunflower seed | 13.9 | [30] |
| Barley | 7.7 | [28] |
| Broccoli | 8.0 | [28] |
| Cauliflower | 9.0 | [29] |
2. The Role of Genistein on Pathologies of Kidney Cells
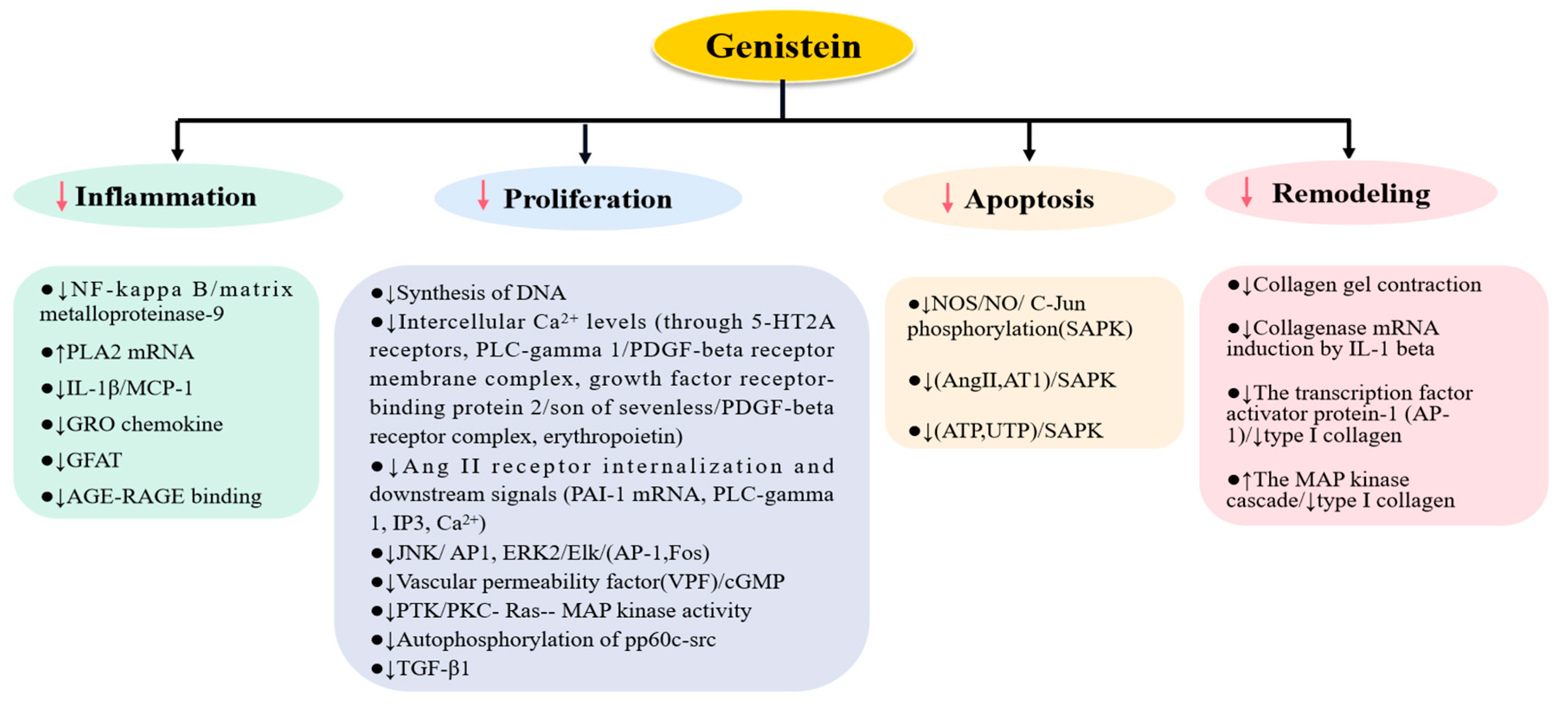
2.1. The Effects of Genistein on Mesangial Cells
2.2. The Effects of Genistein on Endothelial Cells
2.3. The Effects of Genistein on Podocytes
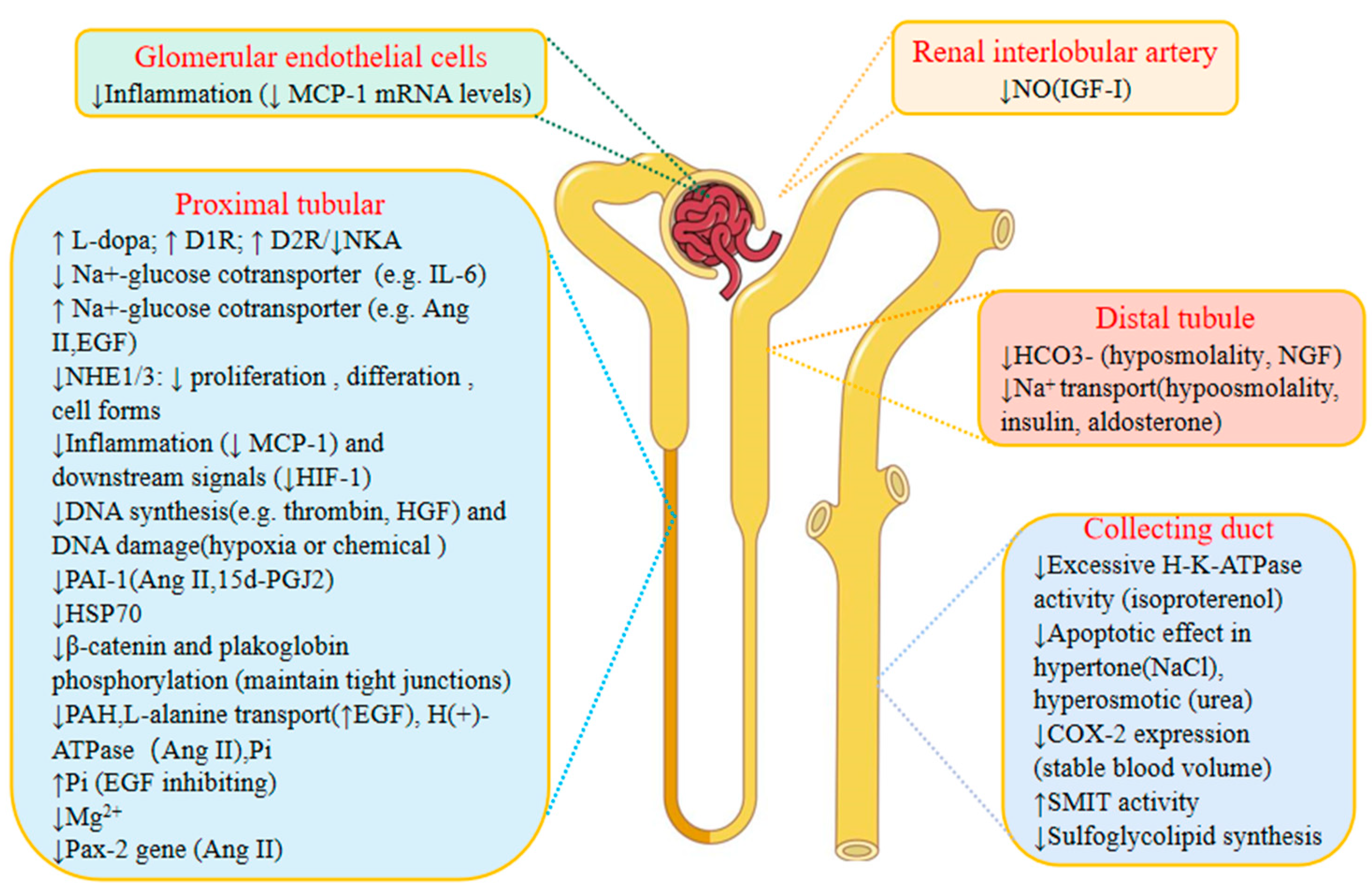
3. The Effects of Genistein on Kidney Physiology (Figure 3)
3.1. The Effects of Genistein on Renin
3.2. The Effects of Genistein on Regulating Calcium and Phosphate
3.3. The Diuretic Effect of Genistein
3.4. The Effects of Genistein on Nephron Barrier
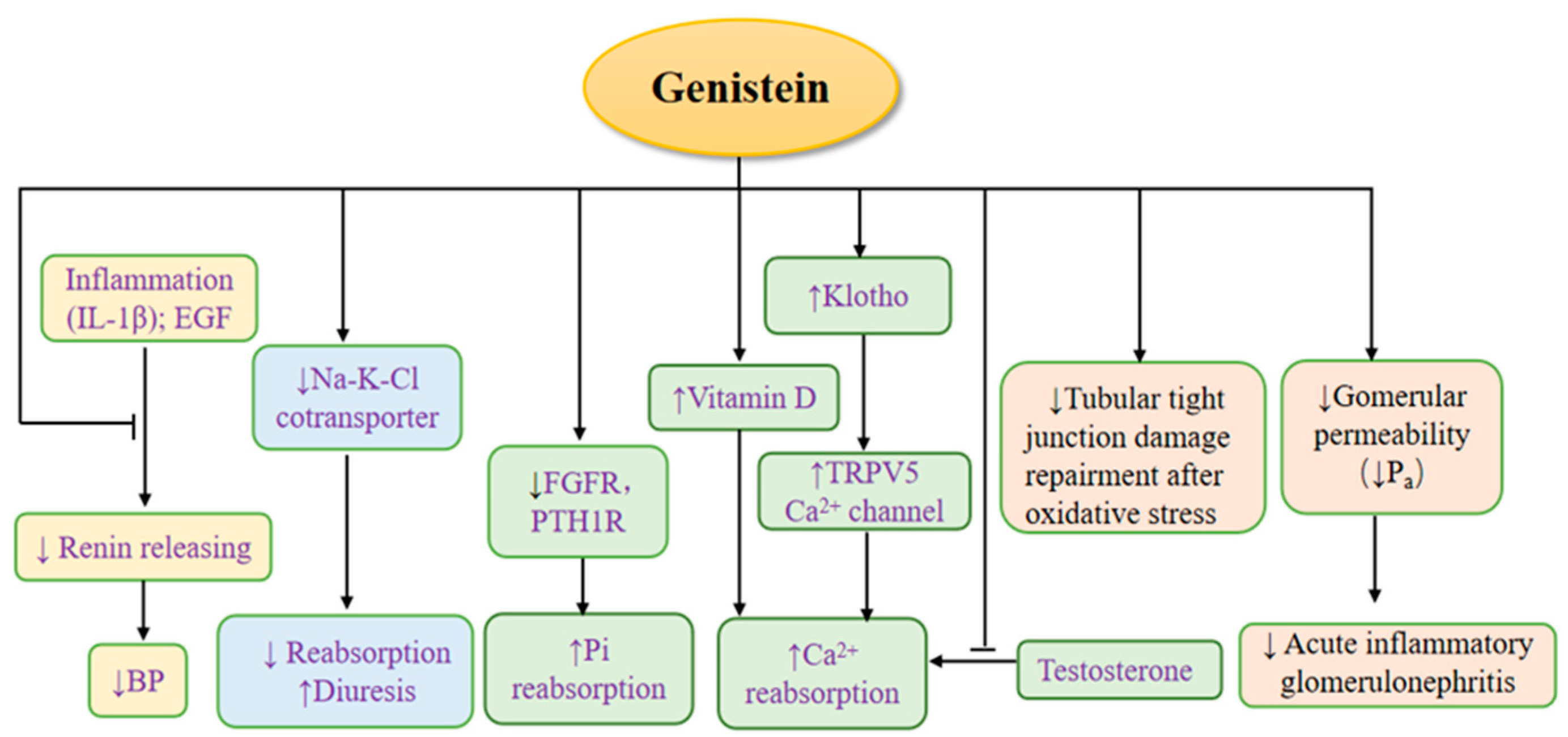
4. The Effects of Genistein on Common Kidney Diseases
4.1. The Effects of Genistein on Acute Kidney Injuries
4.1.1. LPS
4.1.2. The Effects of Genistein on Kidney Ischemia/Reperfusion Injury
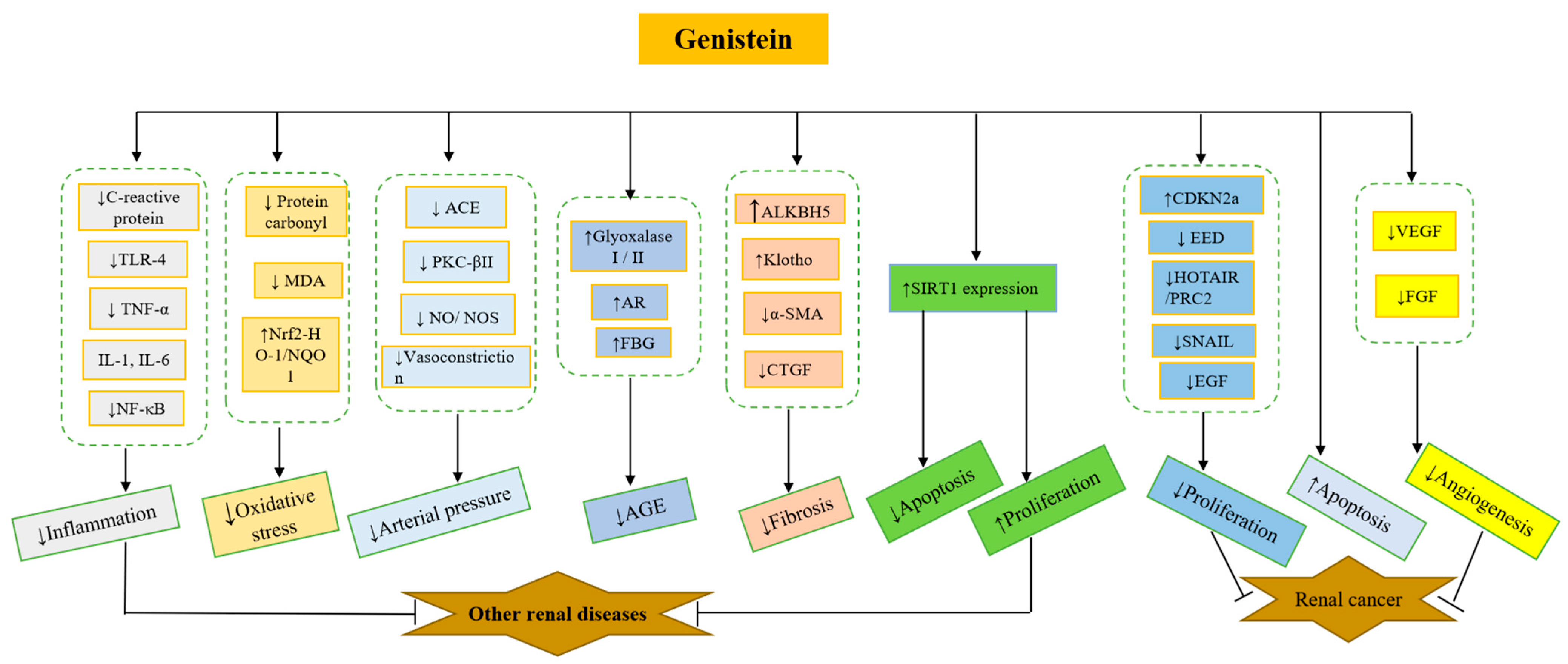
4.2. The Effects of Genistein on Kidney Cancer Cells
4.3. The Effects of Genistein on Diabetic Nephropathy
| Animal | Diabetes Models | Treatments (Genistein) | Effects and Mechanisms | Ref. |
|---|---|---|---|---|
| C57BL/6J mice | High-fat diet | 0.25% genistein in diet for 18 weeks | Inhibiting AGE formation by trapping MGO to form adducts and upregulating the expression of glyoxalase I and II and aldose reductase in kidney to detoxify MGO | [172] |
| Albino rats | Alloxan-induceddiabetes | 20 mg/kg/day for 30 d | Normalizing kidney function (biomarkers: creatinine and BUN) by downregulating inflammatory responses (↓IL-6, TNF-α, and C-reactive protein in serum) | [173] |
| Mice | Streptozotocin-induced diabetes | 10 mg/kg, i.p. three times a week for 10 weeks | Reducing kidney inflammation, oxidative stress, and apoptosis | [174] |
| ICR mice | Alloxan-induced diabetes | 0.25 and 1 mg/g in diet for 2 weeks | Improving levels of FBG and attenuated kidney oxidative stress; decreasing inflammatory and fibrosis-related markers | [175] |
| Wistar rats | STZ-induced diabetes | 4 mg/kg b.w/day, i.p. for 7 d | Protecting against kidney dysfunction, lowering blood glucose levels, and protecting against kidney dysfunction | [176] |
| KKAy mouse | Type 2 diabetes | 12 mg /kg, oral gavage, once a day for 3 months | Inhibiting inflammatory responses, repressing HGA-induced activator protein 1 activation and oxidase stress generation, and reducing NADPH oxidase (NOX) gene expression | [177] |
| Wistar rats | STZ-induced diabetes | 1.5 mg/kg/alt diem for 4 weeks | Normalizing vasoconstriction induced by agonist (norepinephrine, endothelin-1, and Ang II) | [178] |
4.4. The Effects of Genistein on Hypertensive Kidney Disease
4.5. The Effects of Genistein on Kidney Injury by Medications and Irradiation (Table 6)
| Animal | Model | Treatments (Genistein) | Effects and Mechanisms | Ref. |
|---|---|---|---|---|
| LLC-PK1 | Cephaloridine-induced kidney injury | 25 µg/mL preincubated for 2 h | Inhibiting increases in LDH leakage and lipid peroxidation in LLC-PK1 cells exposed to cephaloridine | [189] |
| Sprague-Dawley rats | p-Nonylphenol-induced polycystic kidneys | 0.005 μM/10 μL for 35 d | Modulating the development of PKD induced by dietary NP in rats | [197] |
| Mice | Cisplatin-induced kidney injury and cisplatin-treated normal human kidney HK-2 cells | 10 mg/kg orally once a day for 3 d | Decreasing oxidative stress (reactive oxygen species), inflammation (ICAM, MCP-1, and NF-κB), and apoptosis (regulating p53 induction) | [193] |
| Wistar albino rats | Gentamicin-induced acute kidney injury rats | 10 mg/kg/day, i.p, one week before gentamicin treatment, for 17 d | Decreasing serum levels of Kim-1, cystatin C, LDH, and GGT | [190] |
| Swiss albino mice | A single dose of 6 Gy γ-radiation (Co60) | 200 mg/kg, subcutaneous injection, for 24 weeks | Decreasing the incidence of kidney tubular atrophy and the level of MDA | [198] |
4.6. The Effects of Genistein on Kidney Fibrosis
4.7. The Effects of Genistein on Nephrotic Syndrome
4.8. The Effects of Genistein on Menopausal Kidney Injury
4.9. The Effects of Genistein on Aging-Induced Kidney Injury
5. The Mechanism of Genistein Actions in Kidney
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AP-1 | Activating protein-1 |
| Ang II | Angiotensin II |
| ATP | Adenosine-triphosphate |
| AR | Aldose reductase |
| ACE | Angiotensin-converting enzyme |
| AKI | Acute kidney injury |
| ACE | Angiotensin-converting enzyme |
| α-SMA | α-Smooth muscle actin |
| BP | Blood pressure |
| BUN | Blood urea nitrogen |
| Ca2+ | Calcium |
| cGMP | Cyclic guanosine monophosphate |
| COX-2 | Cyclooxygenase-2 |
| CDKN2a | Cyclin-dependent kinase inhibitor 2a |
| CKD | Chronic kidney disease |
| CTGF | Connective tissue growth factor |
| DIR | D1 receptor |
| D2R | D1 receptor |
| DIC | Disseminated intravascular coagulation |
| ER | Estrogen receptor |
| EGF | Epidermal growth factor |
| ERK2 | Extracellular signal-regulated kinase 2 |
| Evs | Extracellular vesicles |
| ECM | Extracellular matrix |
| EPO | Erythropoietin |
| EED | Embryonic ectoderm development |
| ESRD | End-stage renal disease |
| eNOS | Endothelial NO synthase |
| Fos | c-Fos gene |
| FAK | Focal adhesion kinase |
| FGF | Fibroblast growth factor |
| FBG | Fasting blood glucose |
| GFAT | Glutamine:fructose-6-phosphate amidotransferase |
| GGT | Gamma-glutamyl transferase |
| HIF-1 | Hypoxia-inducible factor 1 |
| HGF | Hepatocyte growth factor |
| HSP70 | Heat shock protein 70 |
| HG | High glucose |
| HOTAIR | HOX transcript antisense RNA |
| HO-1 | Heme oxygenase-1 |
| IL-1β | Interleukin-1 beta |
| IP3 | Inositol triphosphate |
| IGF-I | Insulin-like growth factor-I |
| IRF3 | Interferon regulatory factor 3 |
| IL-6 | Interleukin-6 |
| I/R | Ischemia/reperfusion |
| ICAM | Intercellular adhesion molecule-1 |
| L-dopa | l-Dihydroxyphenylalanine |
| LysoPC | Lysophosphatidylcholine |
| LPS | Lipopolysaccharide |
| LDH | Lactate dehydrogenase |
| MC | Glomerular mesangial cells |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MAPK | Mitogen-activated protein kinases |
| MAP | Mitogen-activated protein |
| mTOR | Mammalian target of rapamycin |
| MyD88 | Myeloid differentiation primary response 88 |
| MVD | Microvessel density |
| MGO | Methylglyoxal |
| MDCK | Madin-Darby canine kidney |
| MDA | Malondialdehyde |
| NF-κB | Nuclear factor NF-kappaB |
| NO | Nitric oxide |
| NOS | Nitric oxide synthesis |
| NKA | Na (+), K (+)-ATPase |
| NHE | Na+/H+ exchanger |
| NGF | Nerve growth factor |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NP | p-Nonylphenol |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NQO1 | NAD(P)H:Quinone Oxidoreductase 1 |
| PDGF | Platelet-derived growth factor |
| PGE2 | Prostaglandin E2 |
| PLA2 | Phospholipase A2 transcription |
| PLC-γ1 | Phospholipase C-γ1 |
| PAI-1 | Plasminogen activator inhibitor-1 |
| PTK | Phototherapeutic keratectomy |
| PKC | Protein kinase C |
| PAH | Phenylalanine hydroxylase |
| Pax-2 | Paired homeobox-2 gene |
| Pi | Phosphate |
| PTH1R | Parathyroid hormone 1 receptor |
| Pa | Albumin permeability |
| PRC2 | Polycomb repressive complex 2 |
| PKC-βII | Protein kinase C-βII |
| PTH | Parathyroid hormone |
| RAGE | Receptor for advanced glycation end products |
| Ras | Renin–angiotensin system |
| RCC | Renal cell carcinoma |
| ROS | Reactive oxygen species |
| RT | Radiation therapy |
| SAPK | c-Jun phosphorylation |
| SMIT | Sodium/myo-inositol cotransporter |
| SNAP | s-Nitroso-N-acetyl-penicillamine |
| SMARCB1 | Subfamily B member 1 |
| SHR-SPs | Stroke-prone spontaneously hypertensive rats |
| TGF-β | Transforming growth factor-β |
| TRPV5 | Transient receptor potential vanilloid 5 |
| TLR4-MD2 | Toll-like receptor 4- myeloid differentiation 2 |
| TRIF | TIR domain-containing adapter-inducing interferon-beta |
| TNF-α | Tumor necrosis factor α |
| TLR-4 | Toll-like-receptor-4 |
| UTP | Uridine triphosphate |
| UUO | Unilateral ureteral obstruction |
| VPF | Vascular permeability factor |
| VEGF | Vascular endothelial growth factor |
| 5-HT2A | 5-Hydroxytryptamine 2A |
| 15d-PGJ2 | 15-Deoxy-delta12,14-prostaglandin J2 |
References
- Walter, E.D. Genistin (an Isoflavone Glucoside) and its Aglucone, Genistein, from Soybeans. J. Am. Chem.Soc. 1941, 63, 3273–3276. [Google Scholar] [CrossRef]
- Coward, L.; Barnes, N.C.; Setchell, K.D.R.; Barnes, S. Genistein, daidzein, and their.beta.-glycoside conjugates: Antitumor isoflavones in soybean foods from American and Asian diets. J. Agr. Food Chem. 1993, 41, 1961–1967. [Google Scholar] [CrossRef]
- Kaufman, P.B.; Duke, J.A.; Brielmann, H.; Boik, J.; Hoyt, J.E. A comparative survey of leguminous plants as sources of the isoflavones, genistein and daidzein: Implications for human nutrition and health. J. Altern. Complement. Med. 1997, 3, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Rao, H. Isoflavones from Flemingia vestita. Fitoterapia 1991, 62, 458. [Google Scholar]
- Rao, K.N.; Srimannarayana, G. Fleminone, a flavanone from the stems of Flemingia macrophylla. Phytochemistry. 1983, 22, 2287–2290. [Google Scholar] [CrossRef]
- Wang, B.-S.; Juang, L.-J.; Yang, J.-J.; Chen, L.-Y.; Tai, H.-M.; Huang, M.-H. Antioxidant and Antityrosinase Activity of Flemingia macrophylla and Glycine tomentella Roots. Evid. Based Complement. Altern. Med. 2012, 20, 431081. [Google Scholar]
- Fedoreyev, S.A.; Pokushalova, T.V.; Veselova, M.V.; Glebko, L.I.; Kulesh, N.I.; Muzarok, T.I.; Seletskaya, L.D.; Bulgakov, V.P.; Zhuravlev, Y.N. Isoflavonoid production by callus cultures of Maackia amurensis. Fitoterapia 2000, 71, 365–372. [Google Scholar] [CrossRef]
- Popiolkiewicz, J.; Polkowski, K.; Skierski, J.S.; Mazurek, A.P. In vitro toxicity evaluation in the development of new anticancer drugs-genistein glycosides. Cancer Lett. 2005, 22, 67–75. [Google Scholar] [CrossRef]
- Walsh, K.R.; Haak, S.J.; Bohn, T.; Tian, Q.; Schwartz, S.J.; Failla, M.L. Isoflavonoid glucosides are deconjugated and absorbed in the small intestine of human subjects with ileostomies. Am. J. Clin. Nutr. 2007, 85, 1050–1056. [Google Scholar] [CrossRef]
- Bokkenheuser, V.D.; Shackleton, C.H.; Winter, J. Hydrolysis of dietary flavonoid glycosides by strains of intestinal Bacteroides from humans. Biochem. J. 1987, 248, 953–956. [Google Scholar] [CrossRef]
- Hu, M. Commentary: Bioavailability of flavonoids and polyphenols: Call to arms. Mol. Pharm. 2007, 4, 803–806. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, W.; Gao, S.; Xu, H.; Wu, B.; Kulkarni, K.; Singh, R.; Tang, L.; Hu, M. Simultaneous determination of genistein and its four phase II metabolites in blood by a sensitive and robust UPLC-MS/MS method: Application to an oral bioavailability study of genistein in mice. J. Pharm. Biomed. Anal. 2010, 53, 81–89. [Google Scholar] [CrossRef]
- Kurkela, M.; García-Horsman, J.A.; Luukkanen, L.; Mörsky, S.; Taskinen, J.; Baumann, M.; Kostiainen, R.; Hirvonen, J.; Finel, M. Expression and characterization of recombinant human UDP-glucuronosyltransferases (UGTs). UGT1A9 is more resistant to detergent inhibition than other UGTs and was purified as an active dimeric enzyme. J. Biol. Chem. 2003, 278, 3536–3544. [Google Scholar] [CrossRef]
- Rozman, K.K.; Bhatia, J.; Calafat, A.M.; Chambers, C.; Culty, M.; Etzel, R.A.; Flaws, J.A.; Hansen, D.K.; Hoyer, P.B.; Jeffery, E.H.; et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of genistein. Birth Defects Res. B Dev. Reprod Toxicol. 2006, 77, 485–638. [Google Scholar] [CrossRef]
- Andersen, O.M.; Markham, K.R. Flavonoids: Chemistry, Biochemistry and Applications; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Morito, K.; Hirose, T.; Kinjo, J.; Hirakawa, T.; Okawa, M.; Nohara, T.; Ogawa, S.; Inoue, S.; Muramatsu, M.; Masamune, Y. Interaction of Phytoestrogens with Estrogen Receptors & alpha; and & beta. Biol. Pharam. Bull. 2001, 24, 351–356. [Google Scholar]
- Sakamoto, T.; Horiguchi, H.; Oguma, E.; Kayama, F. Effects of diverse dietary phytoestrogens on cell growth, cell cycle and apoptosis in estrogen-receptor-positive breast cancer cells. J. Nutr. Biochem. 2010, 21, 856–864. [Google Scholar] [CrossRef]
- De Lemos, M.L. Effects of Soy Phytoestrogens Genistein and Daidzein on Breast Cancer Growth. Ann. Pharmacother. 2001, 35, 1118–1121. [Google Scholar] [CrossRef]
- Das, A.; Banik, N.L.; Ray, S.K. Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes. Cancer. 2010, 116, 164–176. [Google Scholar] [CrossRef]
- Hwang, Y.W.; Kim, S.Y.; Jee, S.H.; Kim, Y.N.; Nam, C.M. Soy Food Consumption and Risk of Prostate Cancer: A Meta-Analysis of Observational Studies. Nutr. Cancer. 2009, 61, 598–606. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, S.-H.; Kim, Y.-B.; Jeon, Y.-T.; Lee, S.-C.; Song, Y.-S. Genistein Inhibits Cell Growth by Modulating Various Mitogen-Activated Protein Kinases and AKT in Cervical Cancer Cells. Ann. N.Y. Acad. Sci. 2009, 117, 495–500. [Google Scholar] [CrossRef]
- Atteritano, M.; Marini, H.; Minutoli, L.; Polito, F.; Bitto, A.; Altavilla, D.; Mazzaferro, S.; D’anna, R.; Cannata, M.L.; Gaudio, A.; et al. Effects of the phytoestrogen genistein on some predictors of cardiovascular risk in osteopenic, postmenopausal women: A two-year randomized, double-blind, placebo-controlled study. J. Clin. Endocrinol. Metab. 2007, 92, 3068–3075. [Google Scholar] [CrossRef]
- Szkudelska, K.; Nogowski, L. Genistein—A dietary compound inducing hormonal and metabolic changes. J. Steroid. Biochem. Mol. Biol. 2007, 105, 37–45. [Google Scholar] [CrossRef]
- Choi, M.S.; Jung, U.J.; Yeo, J.; Kim, M.J.; Lee, M.K. Genistein and daidzein prevent diabetes onset by elevating insulin level and altering hepatic gluconeogenic and lipogenic enzyme activities in non-obese diabetic (NOD) mice. Diabetes Metab. Res. Rev. 2008, 24, 74–81. [Google Scholar] [CrossRef]
- Rodríguez-Landa, J.F.; Cueto-Escobedo, J.; Puga-Olguín, A.; Rivadeneyra-Domínguez, E.; Bernal-Morales, B.; Herrera-Huerta, E.V.; Santos-Torres, A. The Phytoestrogen Genistein Produces Similar Effects as 17β-Estradiol on Anxiety-Like Behavior in Rats at 12 Weeks after Ovariectomy. Biomed. Res. Int. 2017, 2017, 9073816. [Google Scholar] [CrossRef]
- Velasquez, M.T.; Bhathena, S.J. Dietary phytoestrogens: A possible role in renal disease protection. Am. J. Kidney Dis. 2001, 37, 1056–1068. [Google Scholar] [CrossRef]
- Mazur, W.M.; Duke, J.A.; Wähälä, K.; Rasku, S.; Adlercreutz, H. Isoflavonoids and Lignans in Legumes: Nutritional and Health Aspects in Humans. J. Nutr. Biochem. 1998, 9, 193–200. [Google Scholar] [CrossRef]
- Adlercreutz, H.; Mazur, W. Phyto-oestrogens and Western diseases. Ann. Med. 1997, 29, 95–120. [Google Scholar] [CrossRef]
- Mazur, W.; Adlercreutz, H. Naturally occurring oestrogens in food. Pure Appl. Chem. 1998, 70, 1759–1776. [Google Scholar] [CrossRef]
- Mazur, W.; Fotsis, T.; Wähälä, K.; Ojala, S.; Salakka, A.; Adlercreutz, H. Isotope dilution gas chromatographic-mass spectrometric method for the determination of isoflavonoids, coumestrol, and lignans in food samples. Anal. Biochem. 1996, 233, 169–180. [Google Scholar] [CrossRef]
- Schlondorff, D. The glomerular mesangial cell: An expanding role for a specialized pericyte. Faseb. J. 1987, 1, 272–281. [Google Scholar] [CrossRef]
- Kitching, A.R.; Hutton, H.L. The Players: Cells Involved in Glomerular Disease. Clin. J. Am. Soc. Nephrol. 2016, 11, 1664–1674. [Google Scholar] [CrossRef] [PubMed]
- Yokoo, T.; Kitamura, M. Dual regulation of IL-1 beta-mediated matrix metalloproteinase-9 expression in mesangial cells by NF-kappa B and AP-1. Am. J. Physiol. 1996, 270 Pt 2, F123–F130. [Google Scholar] [CrossRef] [PubMed]
- Mühl, H.; Geiger, T.; Pignat, W.; Märki, F.; Van Den Bosch, H.; Vosbeck, K.; Pfeilschifter, J. PDGF suppresses the activation of group II phospholipase A2 gene expression by interleukin 1 and forskolin in mesangial cells. FEBS Lett. 1991, 291, 249–252. [Google Scholar] [CrossRef]
- Rovin, B.H.; Tan, L.C. Role of protein kinase pathways in IL-1-induced chemoattractant expression by human mesangial cells. Kidney Int. 1994, 46, 1059–1068. [Google Scholar] [CrossRef]
- Wu, X.; Dolecki, G.J.; Lefkowith, J.B. GRO chemokines: A transduction, integration, and amplification mechanism in acute renal inflammation. Am. J. Physiol. 1995, 269 Pt 2, F248–F256. [Google Scholar] [CrossRef]
- James, L.R.; Ingram, A.; Ly, H.; Thai, K.; Cai, L.; Scholey, J.W. Angiotensin II activates the GFAT promoter in mesangial cells. Am. J. Physiol. Renal. Physiol. 2001, 281, F151–F162. [Google Scholar] [CrossRef]
- Jung, D.H.; Kim, Y.S.; Kim, J.S. Screening system of blocking agents of the receptor for advanced glycation endproducts in cells using fluorescence. Biol. Pharm. Bull. 2012, 35, 1826–1830. [Google Scholar] [CrossRef]
- Grandaliano, G.; Choudhury, G.G.; Biswas, P.; Abboud, H.E. Mitogenic signaling of thrombin in mesangial cells: Role of tyrosine phosphorylation. Am. J. Physiol. 1994, 267 Pt 2, F528–F536. [Google Scholar] [CrossRef]
- Wenzel, U.O.; Fouqueray, B.; Biswas, P.; Grandaliano, G.; Choudhury, G.G.; Abboud, H.E. Activation of mesangial cells by the phosphatase inhibitor vanadate. Potential implications for diabetic nephropathy. J. Clin. Investig. 1995, 95, 1244–1252. [Google Scholar] [CrossRef]
- Goppelt-Struebe, M.; Stroebel, M. Mechanisms of serotonin-induced Ca2+ responses in mesangial cells. Naunyn Schmiedebergs Arch. Pharmacol. 1997, 356, 240–247. [Google Scholar] [CrossRef]
- Marrero, M.B.; Venema, R.C.; Ma, H.; Ling, B.N.; Eaton, D.C. Erythropoietin receptor-operated Ca2+ channels: Activation by phospholipase C-gamma 1. Kidney Int. 1998, 53, 1259–1268. [Google Scholar] [CrossRef][Green Version]
- Ma, H.; Matsunaga, H.; Li, B.; Marrero, M.B.; Ling, B.N. Regulation of PDGF-beta receptor-operated Ca2+ channels by phospholipase C-gamma 1 in glomerular mesangial cells. Am. J. Physiol. 1996, 271 Pt 2, F994–F1003. [Google Scholar] [CrossRef]
- Ma, H.; Matsunaga, H.; Li, B.; Schieffer, B.; Marrero, M.B.; Ling, B.N. Ca2+ channel activation by platelet-derived growth factor-induced tyrosine phosphorylation and Ras guanine triphosphate-binding proteins in rat glomerular mesangial cells. J. Clin. Investig. 1996, 97, 2332–2341. [Google Scholar] [CrossRef][Green Version]
- Narisawa-Saito, M.; Kimura, S.; Fujiwara, N.; Oite, T.; Shimoji, K.; Shimizu, F. Thy-1-mediated phosphatidylinositol turnover in cultured rat glomerular mesangial cell. J. Cell. Physiol. 1996, 168, 705–710. [Google Scholar] [CrossRef]
- Motojima, M.; Kakuchi, J.; Yoshioka, T. Association of TGF-beta signaling in angiotensin II-induced PAI-1 mRNA upregulation in mesangial cells: Role of PKC. Biochim. Biophys. Acta 1999, 1449, 217–226. [Google Scholar] [CrossRef]
- Marrero, M.B.; Schieffer, B.; Ma, H.; Bernstein, K.E.; Ling, B.N. ANG II-induced tyrosine phosphorylation stimulates phospholipase C-gamma 1 and Cl-channels in mesangial cells. Am. J. Physiol. 1996, 270 Pt 1, C1834–C1842. [Google Scholar] [CrossRef]
- Becker, B.N.; Kondo, S.; Chen, J.K.; Harris, R.C. Tyrosine kinase inhibition affects type 1 angiotensin II receptor internalization. J. Recept. Signal Transduct. Res. 1999, 19, 975–993. [Google Scholar] [CrossRef]
- Yuan, H.T.; Yang, S.P.; Woolf, A.S. Hypoxia up-regulates angiopoietin-2, a Tie-2 ligand, in mouse mesangial cells. Kidney Int. 2000, 58, 1912–1919. [Google Scholar] [CrossRef]
- Wu, Z.L.; Wang, Y.C.; Zhou, Q.; Ge, Y.Q.; Lan, Y. Oxidized LDL induces transcription factor activator protein-1 in rat mesangial cells. Cell. Biochem. Funct. 2003, 21, 249–256. [Google Scholar] [CrossRef]
- El-Dahr, S.S.; Dipp, S.; Baricos, W.H. Bradykinin stimulates the ERK-->Elk-1-->Fos/AP-1 pathway in mesangial cells. Am. J. Physiol. 1998, 275, F343–F352. [Google Scholar] [CrossRef]
- Gruden, G.; Thomas, S.; Burt, D.; Lane, S.; Chusney, G.; Sacks, S.; Viberti, G. Mechanical stretch induces vascular permeability factor in human mesangial cells: Mechanisms of signal transduction. Proc. Natl. Acad. Sci. USA 1997, 94, 12112–12116. [Google Scholar] [CrossRef]
- Alric, C.; Pecher, C.; Tack, I.; Schanstra, J.P.; Bascands, J.L.; Girolami, J.P. Inhibition of cGMP accumulation in mesangial cells by bradykinin and tyrosine kinase inhibitors. Int. J. Mol. Med. 1999, 4, 557–564. [Google Scholar] [CrossRef]
- Bassa, B.V.; Roh, D.D.; Vaziri, N.D.; Kirschenbaum, M.A.; Kamanna, V.S. Lysophosphatidylcholine activates mesangial cell PKC and MAP kinase by PLCgamma-1 and tyrosine kinase-Ras pathways. Am. J. Physiol. 1999, 277, F328–F337. [Google Scholar]
- Simonson, M.S.; Herman, W.H. Protein kinase C and protein tyrosine kinase activity contribute to mitogenic signaling by endothelin-1. Cross-talk between G protein-coupled receptors and pp60c-src. J. Biol. Chem. 1993, 268, 9347–9357. [Google Scholar] [CrossRef]
- Hirakata, M.; Kaname, S.; Chung, U.G.; Joki, N.; Hori, Y.; Noda, M.; Takuwa, Y.; Okazaki, T.; Fujita, T.; Katoh, T.; et al. Tyrosine kinase dependent expression of TGF-beta induced by stretch in mesangial cells. Kidney Int. 1997, 51, 1028–1036. [Google Scholar] [CrossRef]
- Yuan, W.J.; Jia, F.Y.; Meng, J.Z. Effects of genistein on secretion of extracellular matrix components and transforming growth factor beta in high-glucose-cultured rat mesangial cells. J. Artif. Organs. 2009, 12, 242–246. [Google Scholar] [CrossRef]
- Tetsuka, T.; Morrison, A.R. Tyrosine kinase activation is necessary for inducible nitric oxide synthase expression by interleukin-1 beta. Am. J. Physiol. 1995, 269 Pt 1, C55–C59. [Google Scholar] [CrossRef]
- Pfeilschifter, J.; Huwiler, A. Nitric oxide stimulates stress-activated protein kinases in glomerular endothelial and mesangial cells. FEBS Lett. 1996, 396, 67–70. [Google Scholar] [CrossRef]
- Huwiler, A.; Van Rossum, G.; Wartmann, M.; Pfeilschifter, J. Angiotensin II stimulation of the stress-activated protein kinases in renal mesangial cells is mediated by the angiotensin AT1 receptor subtype. Eur. J. Pharmacol. 1998, 343, 297–302. [Google Scholar] [CrossRef]
- Huwiler, A.; Van Rossum, G.; Wartmann, M.; Pfeilschifter, J. Stimulation by extracellular ATP and UTP of the stress-activated protein kinase cascade in rat renal mesangial cells. Br. J. Pharmacol. 1997, 120, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Kagami, S.; Urushihara, M.; Kondo, S.; Löster, K.; Reutter, W.; Tamaki, T.; Yoshizumi, M.; Kuroda, Y. Requirement for tyrosine kinase-ERK1/2 signaling in alpha 1 beta 1 integrin-mediated collagen matrix remodeling by rat mesangial cells. Exp. Cell Res. 2001, 268, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Daphna-Iken, D.; Morrison, A.R. Interleukin-1 beta induces interstitial collagenase gene expression and protein secretion in renal mesangial cells. Am. J. Physiol. 1995, 269 Pt 2, F831–F837. [Google Scholar] [CrossRef] [PubMed]
- Neugarten, J.; Acharya, A.; Lei, J.; Silbiger, S. Selective estrogen receptor modulators suppress mesangial cell collagen synthesis. Am. J. Physiol. Renal Physiol. 2000, 279, F309–F318. [Google Scholar] [CrossRef] [PubMed]
- Neugarten, J.; Medve, I.; Lei, J.; Silbiger, S.R. Estradiol suppresses mesangial cell type I collagen synthesis via activation of the MAP kinase cascade. Am. J. Physiol. 1999, 277, F875–F881. [Google Scholar] [CrossRef] [PubMed]
- Mizobata, R. Effects of lysophosphatidylcholine on expression of monocyte chemoattractant protein-1 in glomerular endothelial cells. Nihon. Jinzo. Gakkai. Shi. 2003, 45, 76–83. [Google Scholar] [PubMed]
- Carranza, A.; Musolino, P.L.; Villar, M.; Nowicki, S. Signaling cascade of insulin-induced stimulation of L-dopa uptake in renal proximal tubule cells. Am. J. Physiol. Cell Physiol. 2008, 295, C1602–C1609. [Google Scholar] [CrossRef] [PubMed]
- Soares-Da-Silva, P.; Serrão, M.P. Molecular modulation of inward and outward apical transporters of L-dopa in LLC-PK(1) cells. Am. J. Physiol. Renal Physiol. 2000, 279, F736–F746. [Google Scholar] [CrossRef]
- Banday, A.A.; Fazili, F.R.; Lokhandwala, M.F. Insulin causes renal dopamine D1 receptor desensitization via GRK2-mediated receptor phosphorylation involving phosphatidylinositol 3-kinase and protein kinase C. Am. J. Physiol. Renal Physiol. 2007, 293, F877–F884. [Google Scholar] [CrossRef]
- Narkar, V.; Hussain, T.; Lokhandwala, M. Role of tyrosine kinase and p44/42 MAPK in D(2)-like receptor-mediated stimulation of Na(+), K(+)-ATPase in kidney. Am. J. Physiol. Renal Physiol. 2002, 282, F697–F702. [Google Scholar] [CrossRef]
- Lee, Y.J.; Heo, J.S.; Suh, H.N.; Lee, M.Y.; Han, H.J. Interleukin-6 stimulates alpha-MG uptake in renal proximal tubule cells: Involvement of STAT3, PI3K/Akt, MAPKs, and NF-kappaB. Am. J. Physiol. Renal Physiol. 2007, 293, F1036–F1046. [Google Scholar] [CrossRef][Green Version]
- Han, H.J.; Park, S.H.; Lee, Y.J. Signaling cascade of ANG II-induced inhibition of alpha-MG uptake in renal proximal tubule cells. Am. J. Physiol. Renal Physiol. 2004, 286, F634–F642. [Google Scholar] [CrossRef]
- Jae Han, H.; Yeong Park, J.; Jung Lee, Y.; Taub, M. Epidermal growth factor inhibits 14C-alpha-methyl-D-glucopyranoside uptake in renal proximal tubule cells: Involvement of PLC/PKC, p44/42 MAPK, and cPLA2. J. Cell Physiol. 2004, 199, 206–216. [Google Scholar] [CrossRef]
- Girardi, A.C.; Knauf, F.; Demuth, H.U.; Aronson, P.S. Role of dipeptidyl peptidase IV in regulating activity of Na+/H+ exchanger isoform NHE3 in proximal tubule cells. Am. J. Physiol. Cell Physiol. 2004, 287, C1238–C1245. [Google Scholar] [CrossRef]
- Carraro-Lacroix, L.R.; Ramirez, M.A.; Zorn, T.M.; Rebouças, N.A.; Malnic, G. Increased NHE1 expression is associated with serum deprivation-induced differentiation in immortalized rat proximal tubule cells. Am. J. Physiol. Renal Physiol. 2006, 291, F129–F139. [Google Scholar] [CrossRef]
- Miyata, Y.; Asano, Y.; Muto, S. Hyperosmotic urea activates basolateral NHE in proximal tubule from P-gp null and wild-type mice. Am. J. Physiol. Renal Physiol. 2002, 283, F771–F783. [Google Scholar] [CrossRef]
- Grandaliano, G.; Monno, R.; Ranieri, E.; Gesualdo, L.; Schena, F.P.; Martino, C.; Ursi, M. Regenerative and proinflammatory effects of thrombin on human proximal tubular cells. J. Am. Soc. Nephrol. 2000, 11, 1016–1025. [Google Scholar] [CrossRef]
- Sandau, K.B.; Zhou, J.; Kietzmann, T.; Brüne, B. Regulation of the hypoxia-inducible factor 1alpha by the inflammatory mediators nitric oxide and tumor necrosis factor-alpha in contrast to desferroxamine and phenylarsine oxide. J Biol. Chem. 2001, 276, 39805–39811. [Google Scholar] [CrossRef]
- Hagar, H.; Ueda, N.; Shah, S.V. Tyrosine phosphorylation in DNA damage and cell death in hypoxic injury to LLC-PK1 cells. Kidney Int. 1997, 51, 1747–1753. [Google Scholar] [CrossRef][Green Version]
- Fintha, A.; Sebe, A.; Masszi, A.; Terebessy, T.; Huszár, T.; Rosivall, L.; Mucsi, I. Angiotensin II activates plasminogen activator inhibitor-I promoter in renal tubular epithelial cells via the AT1 receptor. Acta Physiol. Hung. 2007, 94, 19–30. [Google Scholar] [CrossRef]
- Kimura, H.; Li, X.; Torii, K.; Okada, T.; Takahashi, N.; Fujii, H.; Ishihara, S.; Yoshida, H. A natural PPAR-gamma agonist, 15-deoxy-delta 12,14-prostaglandin J2, may act as an enhancer of PAI-1 in human proximal renal tubular cells under hypoxic and inflammatory conditions. Nephrol. Dial. Transplant. 2008, 23, 2496–2503. [Google Scholar] [CrossRef][Green Version]
- Yamamoto, N.; Smith, M.W.; Maki, A.; Berezesky, I.K.; Trump, B.F. Role of cytosolic Ca2+ and protein kinases in the induction of the hsp70 gene. Kidney Int. 1994, 45, 1093–1104. [Google Scholar] [CrossRef]
- Schwartz, J.H.; Shih, T.; Menza, S.A.; Lieberthal, W. ATP depletion increases tyrosine phosphorylation of beta-catenin and plakoglobin in renal tubular cells. J. Am. Soc. Nephrol. 1999, 10, 2297–2305. [Google Scholar] [CrossRef]
- Gabriëls, G.; Werners, A.; Mauss, S.; Greven, J. Evidence for differential regulation of renal proximal tubular p-aminohippurate and sodium-dependent dicarboxylate transport. J. Pharmacol. Exp. Ther. 1999, 290, 710–715. [Google Scholar]
- Nishida, M.; Kawakatsu, H.; Matsumura, T.; Ishiwari, K.; Tamai, M.; Sawada, T. Effect of epidermal growth factor on sodium-dependent L-alanine transport in LLC-PK1 cells. Kidney Blood Press. Res. 2000, 23, 10–13. [Google Scholar] [CrossRef]
- Carraro-Lacroix, L.R.; Girardi, A.C.; Malnic, G. Long-term regulation of vacuolar H(+)-ATPase by angiotensin II in proximal tubule cells. Pflugers. Arch. 2009, 458, 969–979. [Google Scholar] [CrossRef]
- Caverzasio, J.; Bonjour, J.P. Tyrosine phosphorylation selectively regulates renal cellular phosphate transport. Evidence that it mediates the stimulatory effect of insulin-like growth factor-1. Endocrinology 1992, 130, 373–380. [Google Scholar] [CrossRef]
- Han, H.J.; Park, J.Y.; Lee, Y.J.; Park, S.H. Effect of epidermal growth factor on phosphate uptake in renal proximal tubule cells: Involvement of PKC, MAPK, and cPLA2. Kidney Blood Press. Res. 2003, 26, 315–324. [Google Scholar] [CrossRef]
- Ikari, A.; Nakajima, K.; Taki, S.; Suketa, Y. Up-regulation of Na+-dependent Mg2+ transport by nitric oxide and cyclic GMP pathway in renal epithelial cells. Eur. J. Pharmacol. 2002, 451, 133–139. [Google Scholar] [CrossRef]
- Record, R.D.; Johnson, M.; Lee, S.; Blazer-Yost, B.L. Aldosterone and insulin stimulate amiloride-sensitive sodium transport in A6 cells by additive mechanisms. Am. J. Physiol. 1996, 27 Pt 1, C1079–C1084. [Google Scholar] [CrossRef]
- Niisato, N.; Van Driessche, W.; Liu, M.; Marunaka, Y. Involvement of protein tyrosine kinase in osmoregulation of Na(+) transport and membrane capacitance in renal A6 cells. J. Membr. Biol. 2000, 175, 63–77. [Google Scholar] [CrossRef]
- Niisato, N.; Marunaka, Y. Activation of the Na+-K+ pump by hyposmolality through tyrosine kinase-dependent Cl- conductance in Xenopus renal epithelial A6 cells. J. Physiol. 1999, 518 Pt 2, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Good, D.W. Nerve growth factor regulates HCO3- absorption in thick ascending limb: Modifying effects of vasopressin. Am. J. Physiol. 1998, 274, C931–C939. [Google Scholar] [CrossRef] [PubMed]
- Watts, B.A., 3rd; Good, D.W. Hyposmolality stimulates apical membrane Na(+)/H(+) exchange and HCO(3)(-) absorption in renal thick ascending limb. J. Clin. Investig. 1999, 104, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tian, W.; Cohen, D.M. Urea protects from the proapoptotic effect of NaCl in renal medullary cells. Am. J. Physiol. Renal Physiol. 2000, 279, F345–F352. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Schnermann, J.B.; Briggs, J.P. Regulation of cyclooxygenase-2 expression in renal medulla by tonicity in vivo and in vitro. Am. J. Physiol. 1999, 277, F1–F9. [Google Scholar] [CrossRef] [PubMed]
- Atta, M.G.; Dahl, S.C.; Kwon, H.M.; Handler, J.S. Tyrosine kinase inhibitors and immunosuppressants perturb the myo-inositol but not the betaine cotransporter in isotonic and hypertonic MDCK cells. Kidney Int. 1999, 55, 956–962. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsukahara, H.; Gordienko, D.V.; Tonshoff, B.; Gelato, M.C.; Goligorsky, M.S. Direct demonstration of insulin-like growth factor-I-induced nitric oxide production by endothelial cells. Kidney Int. 1994, 45, 598–604. [Google Scholar] [CrossRef]
- Zhang, S.L.; Guo, J.; Moini, B.; Ingelfinger, J.R. Angiotensin II stimulates Pax-2 in rat kidney proximal tubular cells: Impact on proliferation and apoptosis. Kidney Int. 2004, 66, 2181–2192. [Google Scholar] [CrossRef]
- Niimura, Y.; Moue, T.; Takahashi, N.; Nagai, K. Medium osmolarity-dependent biosynthesis of renal cellular sulfoglycolipids is mediated by the MAPK signaling pathway. Biochim. Biophys. Acta 2010, 1801, 1155–1162. [Google Scholar] [CrossRef]
- Löwik, M.M.; Groenen, P.J.; Levtchenko, E.N.; Monnens, L.A.; Van Den Heuvel, L.P. Molecular genetic analysis of podocyte genes in focal segmental glomerulosclerosis—A review. Eur. J. Pediatr. 2009, 168, 1291–1304. [Google Scholar] [CrossRef]
- Kim, Y.H.; Goyal, M.; Kurnit, D.; Wharram, B.; Wiggins, J.; Holzman, L.; Kershaw, D.; Wiggins, R. Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int. 2001, 60, 957–968. [Google Scholar] [CrossRef]
- Maezawa, Y.; Takemoto, M.; Yokote, K. Cell biology of diabetic nephropathy: Roles of endothelial cells, tubulointerstitial cells and podocytes. J. Diabetes Investig. 2015, 6, 3–15. [Google Scholar] [CrossRef]
- Bariéty, J.; Nochy, D.; Mandet, C.; Jacquot, C.; Glotz, D.; Meyrier, A. Podocytes undergo phenotypic changes and express macrophagic-associated markers in idiopathic collapsing glomerulopathy. Kidney Int. 1998, 53, 918–925. [Google Scholar] [CrossRef]
- Hong, J.; Bhat, O.M.; Li, G.; Dempsey, S.K.; Zhang, Q.; Ritter, J.K.; Li, W.; Li, P.L. Lysosomal regulation of extracellular vesicle excretion during d-ribose-induced NLRP3 inflammasome activation in podocytes. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 849–860. [Google Scholar] [CrossRef]
- Chen, C.A.; Hwang, J.C.; Guh, J.Y.; Tsai, J.C.; Chen, H.C. TGF-beta1 and integrin synergistically facilitate the differentiation of rat podocytes by increasing alpha-smooth muscle actin expression. Transl. Res. 2006, 148, 134–141. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Zhang, T.; Chi, Y.; Liu, M.; Liu, Y. Genistein and Myd88 Activate Autophagy in High Glucose-Induced Renal Podocytes In Vitro. Med. Sci. Monit. 2018, 24, 4823–4831. [Google Scholar] [CrossRef]
- Chen, C.A.; Tsai, J.C.; Su, P.W.; Lai, Y.H.; Chen, H.C. Signaling and regulatory mechanisms of integrinalpha3beta1 on the apoptosis of cultured rat podocytes. J. Lab. Clin. Med. 2006, 147, 274–280. [Google Scholar] [CrossRef]
- Friedrich, C.; Endlich, N.; Kriz, W.; Endlich, K. Podocytes are sensitive to fluid shear stress in vitro. Am. J. Physiol. Renal Physiol. 2006, 291, F856–F865. [Google Scholar] [CrossRef]
- Liu, X.; Shi, Q.; Sigmund, C.D. Interleukin-1beta attenuates renin gene expression via a mitogen-activated protein kinase kinase-extracellular signal-regulated kinase and signal transducer and activator of transcription 3-dependent mechanism in As4.1 cells. Endocrinology 2006, 147, 6011–6018. [Google Scholar] [CrossRef]
- Antonipillai, I. Epidermal growth factor is a potent inhibitor of renin secretion. Hypertension 1993, 21, 654–659. [Google Scholar] [CrossRef]
- Endo, M. Calcium ion as a second messenger with special reference to excitation-contraction coupling. J. Pharmacol. Sci. 2006, 100, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Živanović, J.; Jarić, I.; Ajdžanović, V.; Miler, M.; Stanković, S.; Milošević, V.; Filipović, B. Genistein regulates calcium and phosphate homeostasis without activation of MEK 1/2 signalling pathway in an animal model of the andropause. Ann. Anat. 2022, 239, 151836. [Google Scholar] [CrossRef] [PubMed]
- Couchourel, D.; Leclerc, M.; Filep, J.; Brunette, M.G. Testosterone enhances calcium reabsorption by the kidney. Mol. Cell Endocrinol. 2004, 222, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Giménez, I.; Martinez, R.M.; Lou, M.; Mayoral, J.A.; Garay, R.P.; Alda, J.O. Salidiuretic action by genistein in the isolated, perfused rat kidney. Hypertension 1998, 31, 706–711. [Google Scholar] [CrossRef]
- Lou, J.M.; Giménez, I.; Martinez, R.M.; Alda, J.O.; Garay, R.P. Natriuretic effect of equol. J. Med. Food 1999, 2, 257–260. [Google Scholar] [CrossRef]
- Li, B.; Yao, J.; Morioka, T.; Oite, T. Nitric oxide increases albumin permeability of isolated rat glomeruli via a phosphorylation-dependent mechanism. J. Am. Soc. Nephrol. 2001, 12, 2616–2624. [Google Scholar] [CrossRef]
- Meyer, T.N.; Schwesinger, C.; Ye, J.; Denker, B.M.; Nigam, S.K. Reassembly of the tight junction after oxidative stress depends on tyrosine kinase activity. J. Biol. Chem. 2001, 276, 22048–22055. [Google Scholar] [CrossRef]
- Steimle, A.; Autenrieth, I.B.; Frick, J.S. Structure and function: Lipid A modifications in commensals and pathogens. Int. J. Med. Microbiol. 2016, 306, 290–301. [Google Scholar] [CrossRef]
- Glaros, T.G.; Chang, S.; Gilliam, E.A.; Maitra, U.; Deng, H.; Li, L. Causes and consequences of low grade endotoxemia and inflammatory diseases. Front. Biosci. 2013, 5, 754–765. [Google Scholar] [CrossRef]
- Gutierrez-Ramos, J.C.; Bluethmann, H. Molecules and mechanisms operating in septic shock: Lessons from knockout mice. Immunol. Today 1997, 18, 329–334. [Google Scholar] [CrossRef]
- Asakura, H. Classifying types of disseminated intravascular coagulation: Clinical and animal models. J. Intensive Care 2014, 2, 20. [Google Scholar] [CrossRef]
- Catorce, M.N.; Gevorkian, G. LPS-induced Murine Neuroinflammation Model: Main Features and Suitability for Pre-clinical Assessment of Nutraceuticals. Curr. Neuropharmacol. 2016, 14, 155–164. [Google Scholar] [CrossRef]
- Liang, Y.; Shen, T.; Ming, Q.; Han, G.; Zhang, Y.; Liang, J.; Zhu, D. Alpinetin ameliorates inflammatory response in LPS-induced endometritis in mice. Int. Immunopharmacol. 2018, 62, 309–312. [Google Scholar] [CrossRef]
- Zakaria, R.; Wan Yaacob, W.M.; Othman, Z.; Long, I.; Ahmad, A.H.; Al-Rahbi, B. Lipopolysaccharide-induced memory impairment in rats: A model of Alzheimer’s disease. Physiol. Res. 2017, 66, 553–565. [Google Scholar] [CrossRef]
- Stasi, A.; Intini, A.; Divella, C.; Franzin, R.; Montemurno, E.; Grandaliano, G.; Ronco, C.; Fiaccadori, E.; Pertosa, G.B.; Gesualdo, L.; et al. Emerging role of Lipopolysaccharide binding protein in sepsis-induced acute kidney injury. Nephrol. Dial. Transpl. 2017, 32, 24–31. [Google Scholar] [CrossRef]
- Park, B.S.; Song, D.H.; Kim, H.M.; Choi, B.S.; Lee, H.; Lee, J.O. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 2009, 458, 1191–1195. [Google Scholar] [CrossRef]
- Sung, M.J.; Kim, D.H.; Davaatseren, M.; Hur, H.J.; Kim, W.; Jung, Y.J.; Park, S.K.; Kwon, D.Y. Genistein suppression of TNF-alpha-induced fractalkine expression in endothelial cells. Cell Physiol. Biochem. 2010, 26, 431–440. [Google Scholar] [CrossRef]
- Chen, X.; Tan, J.; Yang, M.; Liao, Z.K.; Lu, C.; Huang, Y.; Wu, L.C. Genistein has the function of alleviating and treating disseminated intravascular coagulation caused by lipopolysaccharide. J. Nat. Med. 2018, 72, 846–856. [Google Scholar] [CrossRef]
- Asmis, R.; Stevens, J.; Begley, J.G.; Grimes, B.; Van Zant, G.; Fanti, P. The isoflavone genistein inhibits LPS-stimulated TNFalpha, but not IL-6 expression in monocytes from hemodialysis patients and healthy subjects. Clin. Nephrol. 2006, 65, 267–275. [Google Scholar] [CrossRef]
- Gourgoutis, G.; Das, G. Gastrointestinal manifestations of cocaine addiction. Int. J. Clin. Pharmacol. Ther. 1994, 32, 136–141. [Google Scholar]
- Zhang, F.; Lu, Z.; Wang, F. Advances in the pathogenesis and prevention of contrast-induced nephropathy. Life Sci. 2020, 259, 118379. [Google Scholar] [CrossRef]
- Ghionzoli, N.; Sciaccaluga, C.; Mandoli, G.E.; Vergaro, G.; Gentile, F.; D’ascenzi, F.; Mondillo, S.; Emdin, M.; Valente, S.; Cameli, M. Cardiogenic shock and acute kidney injury: The rule rather than the exception. Heart Fail Rev. 2021, 26, 487–496. [Google Scholar] [CrossRef]
- Sukkummee, W.; Jittisak, P.; Wonganan, P.; Wittayalertpanya, S.; Chariyavilaskul, P.; Leelahavanichkul, A. The prominent impairment of liver/intestinal cytochrome P450 and intestinal drug transporters in sepsis-induced acute kidney injury over acute and chronic renal ischemia, a mouse model comparison. Ren. Fail. 2019, 41, 314–325. [Google Scholar] [CrossRef]
- Jeong, E.K.; Jang, H.J.; Kim, S.S.; Lee, S.Y.; Oh, M.Y.; Kim, H.J.; Eom, D.W.; Ham, J.Y.; Han, D.J. Protective Effect of Polydeoxyribonucleotide Against Renal Ischemia-Reperfusion Injury in Mice. Transpl. Proc. 2016, 48, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.K.; Jorgensen, T.M.; Ravlo, K.; Nielsen, T.K.; Olsen, L.H.; Stolle, L.B. Microdialysis for detection of renal ischemia after experimental renal transplantation. J. Urol. 2009, 182, 1854–1859. [Google Scholar] [CrossRef] [PubMed]
- Bonventre, J.V.; Yang, L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Investig. 2011, 121, 4210–4221. [Google Scholar] [CrossRef]
- Schefold, J.C.; Filippatos, G.; Hasenfuss, G.; Anker, S.D.; Von Haehling, S. Heart failure and kidney dysfunction: Epidemiology, mechanisms and management. Nat. Rev. Nephrol. 2016, 12, 610–623. [Google Scholar] [CrossRef] [PubMed]
- Gonsalez, S.R.; Cortês, A.L.; Silva, R.C.D.; Lowe, J.; Prieto, M.C.; Silva Lara, L.D. Acute kidney injury overview: From basic findings to new prevention and therapy strategies. Pharmacol. Ther. 2019, 200, 1–12. [Google Scholar] [CrossRef]
- Praddaude, F.; Marchetti, J.; Alhenc-Gelas, F.; Ader, J. Dissimilar mechanisms of Ca(2+) response to bradykinin in different types of juxtamedullary glomerular arterioles. Am. J. Physiol. 1999, 277, F697–F705. [Google Scholar]
- Salomonsson, M.; Arendshorst, W.J. Effect of tyrosine kinase blockade on norepinephrine-induced cytosolic calcium response in rat afferent arterioles. Am. J. Physiol. Renal Physiol. 2004, 286, F866–F874. [Google Scholar] [CrossRef]
- Ji, E.S.; Zhang, L.H.; Wang, Y.H.; Yue, H.; He, R.R. Responses of regional vascular beds to local injection of genistein in rats. Sheng Li Xue Bao 2003, 55, 255–259. [Google Scholar]
- Fetscher, C.; Chen, H.; Schäfers, R.F.; Wambach, G.; Heusch, G.; Michel, M.C. Modulation of noradrenaline-induced microvascular constriction by protein kinase inhibitors. Naunyn Schmiedebergs Arch. Pharmacol. 2001, 363, 57–65. [Google Scholar] [CrossRef]
- Sun, C.W.; Falck, J.R.; Harder, D.R.; Roman, R.J. Role of tyrosine kinase and PKC in the vasoconstrictor response to 20-HETE in renal arterioles. Hypertension 1999, 33 Pt 2, 414–418. [Google Scholar] [CrossRef]
- Kurt, A.H.; Buyukafsar, K. Vasoconstriction induced by G1, a G-protein-coupled oestrogen receptor1 (GPER-1) agonist, in the isolated perfused rat kidney. Eur. J. Pharmacol. 2013, 702, 71–78. [Google Scholar] [CrossRef]
- Algin, M.C.; Hacioglu, A.; Yaylak, F.; Gulcan, E.; Aydin, T.; Hacioglu, B.A.; Ilhan, D.; Cevik, A.A.; Ates, E. The role of erythropoietin in hemorrhagic shock-induced liver and renal injury in rats. Adv. Ther. 2008, 25, 1353–1374. [Google Scholar] [CrossRef]
- Li, W.F.; Yang, K.; Zhu, P.; Zhao, H.Q.; Song, Y.H.; Liu, K.C.; Huang, W.F. Genistein Ameliorates Ischemia/Reperfusion-Induced Renal Injury in a SIRT1-Dependent Manner. Nutrients 2017, 9, 403. [Google Scholar] [CrossRef]
- Gholampour, F.; Mohammadi, Z.; Karimi, Z.; Owji, S.M. Protective effect of genistein in a rat model of ischemic acute kidney injury. Gene 2020, 753, 144789. [Google Scholar] [CrossRef]
- Ates, E.; Yalcin, A.U.; Yilmaz, S.; Koken, T.; Tokyol, C. Protective effect of erythropoietin on renal ischemia and reperfusion injury. ANZ J. Surg. 2005, 75, 1100–1105. [Google Scholar] [CrossRef]
- Linehan, W.M.; Schmidt, L.S.; Crooks, D.R.; Wei, D.; Srinivasan, R.; Lang, M.; Ricketts, C.J. The Metabolic Basis of Kidney Cancer. Cancer Discov. 2019, 9, 1006–1021. [Google Scholar] [CrossRef]
- Capitanio, U.; Montorsi, F. Renal cancer. Lancet 2016, 387, 894–906. [Google Scholar] [CrossRef]
- Balbaa, M.; Honke, K.; Makita, A. Regulation of glycolipid sulfotransferase by tyrosine kinases in human renal cancer cells. Biochim. Biophys. Acta 1996, 1299, 141–145. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.T.; Liu, F.; Zhang, S.; Wang, W.; Li, Y.M. Effect of genistein on proliferation of renal cell carcinoma cell line GRC-1 and its influence to p27 expression. Ai Zheng 2003, 22, 1272–1275. [Google Scholar]
- Sasamura, H.; Takahashi, A.; Miyao, N.; Yanase, M.; Masumori, N.; Kitamura, H.; Itoh, N.; Tsukamoto, T. Inhibitory effect on expression of angiogenic factors by antiangiogenic agents in renal cell carcinoma. Br. J. Cancer 2002, 86, 768–773. [Google Scholar] [CrossRef]
- Majid, S.; Dar, A.A.; Ahmad, A.E.; Hirata, H.; Kawakami, K.; Shahryari, V.; Saini, S.; Tanaka, Y.; Dahiya, A.V.; Khatri, G.; et al. BTG3 tumor suppressor gene promoter demethylation, histone modification and cell cycle arrest by genistein in renal cancer. Carcinogenesis 2009, 30, 662–670. [Google Scholar] [CrossRef]
- Sasamura, H.; Takahashi, A.; Yuan, J.; Kitamura, H.; Masumori, N.; Miyao, N.; Itoh, N.; Tsukamoto, T. Antiproliferative and antiangiogenic activities of genistein in human renal cell carcinoma. Urology 2004, 64, 389–393. [Google Scholar] [CrossRef]
- Hirata, H.; Ueno, K.; Nakajima, K.; Tabatabai, Z.L.; Hinoda, Y.; Ishii, N.; Dahiya, R. Genistein downregulates onco-miR-1260b and inhibits Wnt-signalling in renal cancer cells. Br. J. Cancer 2013, 108, 2070–2078. [Google Scholar] [CrossRef]
- Imai-Sumida, M.; Dasgupta, P.; Kulkarni, P.; Shiina, M.; Hashimoto, Y.; Shahryari, V.; Majid, S.; Tanaka, Y.; Dahiya, R.; Yamamura, S. Genistein Represses HOTAIR/Chromatin Remodeling Pathways to Suppress Kidney Cancer. Cell Physiol. Biochem. 2020, 54, 53–70. [Google Scholar]
- Ji, Z.; Huo, C.; Yang, P. Genistein inhibited the proliferation of kidney cancer cells via CDKN2a hypomethylation: Role of abnormal apoptosis. Int. Urol. Nephrol. 2020, 52, 1049–1055. [Google Scholar] [CrossRef]
- Zaman, M.S.; Shahryari, V.; Deng, G.; Thamminana, S.; Saini, S.; Majid, S.; Chang, I.; Hirata, H.; Ueno, K.; Yamamura, S.; et al. Up-regulation of microRNA-21 correlates with lower kidney cancer survival. PLoS ONE 2012, 7, e31060. [Google Scholar] [CrossRef]
- Oh, H.Y.; Kwon, S.M.; Kim, S.I.; Jae, Y.W.; Hong, S.J. Antiangiogenic effect of ZD1839 against murine renal cell carcinoma (RENCA) in an orthotopic mouse model. Urol. Int. 2005, 75, 159–166. [Google Scholar] [CrossRef]
- Valencia, W.M.; Florez, H. How to prevent the microvascular complications of type 2 diabetes beyond glucose control. BMJ 2017, 356, i6505. [Google Scholar] [CrossRef] [PubMed]
- Saran, R.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.C.; Bragg-Gresham, J.; Balkrishnan, R.; Bhave, N.; Dietrich, X.; Ding, Z.; Eggers, P.W.; et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2019, 73 (Suppl. S1), A7–A8. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, L.; He, J.; Bi, Y.; Li, M.; Wang, T.; Wang, L.; Jiang, Y.; Dai, M.; Lu, J.; et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013, 310, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Sasser, J.M.; Sullivan, J.C.; Hobbs, J.L.; Yamamoto, T.; Pollock, D.M.; Carmines, P.K.; Pollock, J.S. Endothelin A receptor blockade reduces diabetic renal injury via an anti-inflammatory mechanism. J. Am. Soc. Nephrol. 2007, 18, 143–154. [Google Scholar] [CrossRef]
- Chen, J.; Chen, K.-H.; Xiao, F.; Dai, H.-Z.; Yang, J.; Wang, L.-M.; Wang, X.-Y.; Zhang, J.-G.; He, Y.-N. Decoy receptor 2 mediation of the senescent phenotype of tubular cells by interacting with peroxiredoxin 1 presents a novel mechanism of renal fibrosis in diabetic nephropathy. Kidney Int. 2020, 98, 645–662. [Google Scholar]
- Demir, Y.; Durmaz, L.; Taslimi, P.; Gulcin, I. Antidiabetic properties of dietary phenolic compounds: Inhibition effects on alpha-amylase, aldose reductase, and alpha-glycosidase. Biotechnol. Appl. Biochem. 2019, 66, 781–786. [Google Scholar] [CrossRef]
- Chu, C.; Lu, F.J.; Yeh, R.H.; Li, Z.L.; Chen, C.H. Synergistic antioxidant activity of resveratrol with genistein in high-glucose treated Madin-Darby canine kidney epithelial cells. Biomed. Rep. 2016, 4, 349–354. [Google Scholar] [CrossRef]
- Li, P.; Cao, Y.; Song, G.; Zhao, B.; Ma, Q.; Li, Z.; He, C. Anti-diabetic properties of genistein-chromium (III) complex in db/db diabetic mice and its sub-acute toxicity evaluation in normal mice. J. Trace Elem. Med. Biol. 2020, 62, 126606. [Google Scholar] [CrossRef]
- Palanisamy, N.; Viswanathan, P.; Anuradha, C.V. Effect of genistein, a soy isoflavone, on whole body insulin sensitivity and renal damage induced by a high-fructose diet. Ren. Fail. 2008, 30, 645–654. [Google Scholar] [CrossRef]
- Guo, T.L.; Germolec, D.R.; Zheng, J.F.; Kooistra, L.; Auttachoat, W.; Smith, M.J.; White, K.L.; Elmore, S.A. Genistein protects female nonobese diabetic mice from developing type 1 diabetes when fed a soy- and alfalfa-free diet. Toxicol. Pathol. 2015, 43, 435–448. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, P.; Sang, S. Dietary Genistein Inhibits Methylglyoxal-Induced Advanced Glycation End Product Formation in Mice Fed a High-Fat Diet. J. Nutr. 2019, 149, 776–787. [Google Scholar] [CrossRef]
- Rehman, K.; Ali, M.B.; Akash, M.S.H. Genistein enhances the secretion of glucagon-like peptide-1 (GLP-1) via downregulation of inflammatory responses. Biomed. Pharmacother. 2019, 112, 108670. [Google Scholar] [CrossRef]
- Elmarakby, A.A.; Ibrahim, A.S.; Faulkner, J.; Mozaffari, M.S.; Liou, G.I.; Abdelsayed, R. Tyrosine kinase inhibitor, genistein, reduces renal inflammation and injury in streptozotocin-induced diabetic mice. Vascul. Pharmacol. 2011, 55, 149–156. [Google Scholar] [CrossRef]
- Kim, M.J.; Lim, Y. Protective effect of short-term genistein supplementation on the early stage in diabetes-induced renal damage. Mediat. Inflamm. 2013, 2013, 510212. [Google Scholar] [CrossRef]
- Rauter, A.P.; Martins, A.; Borges, C.; Mota-Filipe, H.; Pinto, R.; Sepodes, B.; Justino, J. Antihyperglycaemic and protective effects of flavonoids on streptozotocin-induced diabetic rats. Phytother. Res. 2010, 24 (Suppl. 2), S133–S138. [Google Scholar] [CrossRef]
- Jheng, H.F.; Hayashi, K.; Matsumura, Y.; Kawada, T.; Seno, S.; Matsuda, H.; Inoue, K.; Nomura, W.; Takahashi, H.; Goto, T. Anti-Inflammatory and Antioxidative Properties of Isoflavones Provide Renal Protective Effects Distinct from Those of Dietary Soy Proteins against Diabetic Nephropathy. Mol. Nutr. Food Res. 2020, 64, e2000015. [Google Scholar] [CrossRef]
- Benter, I.F.; Yousif, M.H.; Hollins, A.J.; Griffiths, S.M.; Akhtar, S. Diabetes-induced renal vascular dysfunction is normalized by inhibition of epidermal growth factor receptor tyrosine kinase. J. Vasc. Res. 2005, 42, 284–291. [Google Scholar] [CrossRef]
- Stompór, T.; Perkowska-Ptasińska, A. Hypertensive kidney disease: A true epidemic or rare disease? Pol. Arch. Intern. Med. 2020, 130, 130–139. [Google Scholar] [CrossRef]
- Wenzel, R.R. Renal protection in hypertensive patients: Selection of antihypertensive therapy. Drugs. 2005, 65 (Suppl. 2), 29–39. [Google Scholar] [CrossRef]
- Palanisamy, N.; Venkataraman, A.C. Beneficial effect of genistein on lowering blood pressure and kidney toxicity in fructose-fed hypertensive rats. Br. J. Nutr. 2013, 109, 1806–1812. [Google Scholar] [CrossRef]
- Li, J.; Xie, Z.Z.; Tang, Y.B. Genistein prevents myocardial hypertrophy in 2-kidney 1-clip renal hypertensive rats by restoring eNOS pathway. Pharmacology 2010, 86, 240–248. [Google Scholar] [CrossRef]
- Cho, T.M.; Peng, N.; Clark, J.T.; Novak, L.; Roysommuti, S.; Prasain, J.; Wyss, J.M. Genistein attenuates the hypertensive effects of dietary NaCl in hypertensive male rats. Endocrinology 2007, 148, 5396–5402. [Google Scholar] [CrossRef]
- Mehta, R.L.; Pascual, M.T.; Soroko, S.; Savage, B.R.; Himmelfarb, J.; Ikizler, T.A.; Paganini, E.P.; Chertow, G.M. Spectrum of acute renal failure in the intensive care unit: The PICARD experience. Kidney Int. 2004, 66, 1613–1621. [Google Scholar] [CrossRef]
- Hoste, E.A.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef]
- Moffett, B.S.; Goldstein, S.L. Acute kidney injury and increasing nephrotoxic-medication exposure in noncritically-ill children. Clin. J. Am. Soc. Nephrol. 2011, 6, 856–863. [Google Scholar] [CrossRef]
- Atkinson, R.M.; Currie, J.P.; Davis, B.; Pratt, D.A.; Sharpe, H.M.; Tomich, E.G. Acute toxicity of cephaloridine, an antibiotic derived from cephalosporin C. Toxicol. Appl. Pharmacol. 1966, 8, 398–406. [Google Scholar] [CrossRef]
- Foord, R.D. Cephaloridine, cephalothin and the kidney. J. Antimicrob. Chemother. 1975, 1, 119–133. [Google Scholar] [CrossRef]
- Kawai, Y.; Kohda, Y.; Kodawara, T.; Gemba, M. Protective effect of a protein kinase inhibitor on cellular injury induced by cephaloridine in the porcine kidney cell line LLC-PK(1). J. Toxicol. Sci. 2005, 30, 157–163. [Google Scholar] [CrossRef][Green Version]
- Abd El-Lateef, S.M.; El-Sayed, E.M.; Mansour, A.M.; Salama, S.A. The protective role of estrogen and its receptors in gentamicin-induced acute kidney injury in rats. Life Sci. 2019, 239, 117082. [Google Scholar] [CrossRef]
- Lebwohl, D.; Canetta, R. Clinical development of platinum complexes in cancer therapy: An historical perspective and an update. Eur. J. Cancer 1998, 34, 1522–1534. [Google Scholar] [CrossRef]
- Levi, J.; Jacobs, C.; Kalman, S.M.; Mctigue, M.; Weiner, M.W. Mechanism of cis-platinum nephrotoxicity: I. Effects of sulfhydryl groups in rat kidneys. J. Pharmacol. Exp. Ther. 1980, 213, 545–550. [Google Scholar] [PubMed]
- Sung, M.J.; Kim, D.H.; Jung, Y.J.; Kang, K.P.; Lee, A.S.; Lee, S.; Kim, W.; Davaatseren, M.; Hwang, J.T.; Kim, H.J.; et al. Genistein protects the kidney from cisplatin-induced injury. Kidney Int. 2008, 74, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Robbins, M.E.; Bonsib, S.M. Radiation nephropathy: A review. Scanning Microsc. 1995, 2, 535–560. [Google Scholar]
- Cohen, E.P.; Robbins, M.E. Radiation nephropathy. Semin. Nephrol. 2003, 23, 486–499. [Google Scholar] [CrossRef]
- Vijayalaxmi; Meltz, M.L.; Reiter, R.J.; Herman, T.S.; Kumar, K.S. Melatonin and protection from whole-body irradiation: Survival studies in mice. Mutat. Res. 1999, 425, 21–27. [Google Scholar] [CrossRef]
- Cooper, S.; Latendresse, J.R.; Doerge, D.R.; Twaddle, N.C.; Fu, X.; Delclos, K.B. Dietary modulation of p-nonylphenol-induced polycystic kidneys in male Sprague-Dawley rats. Toxicol. Sci. 2006, 91, 631–642. [Google Scholar] [CrossRef]
- Canyilmaz, E.; Uslu, G.H.; Bahat, Z.; Kandaz, M.; Mungan, S.; Haciislamoglu, E.; Mentese, A.; Yoney, A. Comparison of the effects of melatonin and genistein on radiation-induced nephrotoxicity: Results of an experimental study. Biomed. Rep. 2016, 4, 45–50. [Google Scholar] [CrossRef]
- Meguid El Nahas, A.; Bello, A.K. Chronic kidney disease: The global challenge. Lancet 2005, 365, 331–340. [Google Scholar] [CrossRef]
- Nogueira, A.; Pires, M.J.; Oliveira, P.A. Pathophysiological Mechanisms of Renal Fibrosis: A Review of Animal Models and Therapeutic Strategies. In Vivo 2017, 31, 1–22. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, Q.Q.; Tu, J.K.; Tang, W.B.; Yuan, X.N.; Xie, Y.Y.; Wang, W.; Peng, Z.Z.; Huang, L.; Xu, H.; et al. Involvement of hydrogen sulfide in the progression of renal fibrosis. Chin. Med. J. 2019, 132, 2872–2880. [Google Scholar] [CrossRef]
- Lovisa, S.; Zeisberg, M.; Kalluri, R. Partial Epithelial-to-Mesenchymal Transition and Other New Mechanisms of Kidney Fibrosis. Trends Endocrinol. Metab. 2016, 27, 681–695. [Google Scholar] [CrossRef]
- Ning, Y.; Chen, J.; Shi, Y.; Song, N.; Yu, X.; Fang, Y.; Ding, X. Genistein Ameliorates Renal Fibrosis Through Regulation Snail via m6A RNA Demethylase ALKBH5. Front. Pharmacol. 2020, 11, 579265. [Google Scholar] [CrossRef]
- Jia, Q.; Yang, R.; Liu, X.F.; Ma, S.F.; Wang, L. Genistein attenuates renal fibrosis in streptozotocin-induced diabetic rats. Mol. Med. Rep. 2019, 19, 423–431. [Google Scholar] [CrossRef]
- Li, Y.; Chen, F.; Wei, A.; Bi, F.; Zhu, X.; Yin, S.; Lin, W.; Cao, W. Klotho recovery by genistein via promoter histone acetylation and DNA demethylation mitigates renal fibrosis in mice. J. Mol. Med. 2019, 97, 541–552. [Google Scholar] [CrossRef]
- Palanisamy, N.; Kannappan, S.; Anuradha, C.V. Genistein modulates NF-κB-associated renal inflammation, fibrosis and podocyte abnormalities in fructose-fed rats. Eur. J. Pharmacol. 2011, 667, 355–364. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, A.; Ding, Y.; Wang, Y.; Yuan, W. Genistein ameliorates parathyroid hormone-induced epithelial-to-mesenchymal transition and inhibits expression of connective tissue growth factor in human renal proximal tubular cells. Arch. Med. Sci. 2013, 9, 724–730. [Google Scholar] [CrossRef]
- Ghodake, S.R.; Suryakar, A.N.; Ankush, R.D.; Katkam, R.V.; Shaikh, K.; Katta, A.V. Role of free radicals and antioxidant status in childhood nephrotic syndrome. Indian J. Nephrol. 2011, 21, 37–40. [Google Scholar] [CrossRef]
- Javanbakht, M.H.; Sadria, R.; Djalali, M.; Derakhshanian, H.; Hosseinzadeh, P.; Zarei, M.; Azizi, G.; Sedaghat, R.; Mirshafiey, A. Soy protein and genistein improves renal antioxidant status in experimental nephrotic syndrome. Nefrologia 2014, 34, 483–490. [Google Scholar]
- Bryzgalova, G.; Gao, H.; Ahren, B.; Zierath, J.R.; Galuska, D.; Steiler, T.L.; Dahlman-Wright, K.; Nilsson, S.; Gustafsson, J.A.; Efendic, S.; et al. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: Insulin sensitivity in the liver. Diabetologia 2006, 49, 588–597. [Google Scholar] [CrossRef]
- Mohamed, M.K.; Abdel-Rahman, A.A. Effect of long-term ovariectomy and estrogen replacement on the expression of estrogen receptor gene in female rats. Eur. J. Endocrinol. 2000, 142, 307–314. [Google Scholar] [CrossRef]
- Choi, J.S.; Song, J. Effect of genistein on insulin resistance, renal lipid metabolism, and antioxidative activities in ovariectomized rats. Nutrition 2009, 25, 676–685. [Google Scholar] [CrossRef]
- Bowen, R.L.; Atwood, C.S. Living and Dying for Sex. Gerontology 2004, 50, 265–290. [Google Scholar] [CrossRef]
- Willems, H.M.E.; Van Den Heuvel, E.; Schoemaker, R.J.W.; Klein-Nulend, J.; Bakker, A.D. Diet and Exercise: A Match Made in Bone. Curr. Osteoporos. Rep. 2017, 15, 555–563. [Google Scholar] [CrossRef]
- Duan, X.; Li, Y.; Xu, F.; Ding, H. Study on the neuroprotective effects of Genistein on Alzheimer’s disease. Brain Behav. 2021, 11, e02100. [Google Scholar] [CrossRef]
- Kim, J.M.; Uehara, Y.; Choi, Y.J.; Ha, Y.M.; Ye, B.H.; Yu, B.P.; Chung, H.Y. Mechanism of attenuation of pro-inflammatory Ang II-induced NF-κB activation by genistein in the kidneys of male rats during aging. Biogerontology 2011, 12, 537–550. [Google Scholar] [CrossRef]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N. Advanced glycation endproducts--role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21. [Google Scholar] [CrossRef] [PubMed]
| Cells | Treatments | Effects and Mechanisms | Ref. |
|---|---|---|---|
| Mouse podocyte cells | 20 μM, 30 min prior to treatment for 24 h | Decreasing D-ribose-induced ceramide accumulation, EV release and IL-1β secretion, and NLRP3 inflammasome | [105] |
| Rat podocytes | 200 µM for 4 d | Decreasing the expression of a-SMA protein and the percentage of a-SMA-positive cells stimulated by TGF-β1 | [106] |
| Mouse podocyte cell line, H-2Kb-tsA58, with high D-glucose | 20 µM for 6 h | Maintaining the level of autophagy by inactivating mTOR signaling and the level of MyD88 siRNA | [107] |
| Rat primary podocytes | 200 µM for 4 h | Causing apoptosis of podocytes | [108] |
| Mouse podocyte cell line | 60 μM for 20 h | Increasing cell loss under fluid flow stress | [109] |
| Kidney Cancer Cell Lines | Treatments (Genistein) | Effects and Mechanisms | Ref. |
|---|---|---|---|
| SMKT-R3 (human) | 50 g/mL for 15 min | Inhibiting tyrosine kinases and glycolipid sulfotransferase | [152] |
| GRC-1 (human) | 20 and 40 mM/L for 72 h | Inhibiting the proliferation of kidney cell carcinoma cells; causing cell cycle arrest at the G1/M and G2/S phases | [153] |
| kidney carcinoma cells SMKT-R-1,3 (human) | 4, 40, and 100 μg/mL under hypoxic conditions for 12 h | Suppressing the expression of the angiogenic factors vascular endothelial growth factor and basic FGF | [154] |
| A498, ACHN, and HEK-293 (human) | 10, 25, and 50 μ mol/L for 3 d | Inhibiting proliferation by decreasing DNA Methyltransferase and methyl-CpG-binding domain 2 activity and increasing HAT activity and induction of cell cycle arrest | [155] |
| SMKT R-1, 2, 3, 4 lines (human) | 50 and 100 mg/mL for 48 h | Inhibiting cell proliferation, inducing apoptosis, and suppressing in vivo angiogenesis | [156] |
| A-498; ATCC numbers: HTB44, HTB-47, 786-O, CRL-1932, and Caki-2 (human) | 25 µM for 4 d | Inhibiting Wnt signaling by regulating miR-1260b expression | [157] |
| Human clear cell kidney carcinoma cell lines (ccRCC) (human) | 25 µM for 96 h | Reducing cell proliferation and migration by suppressing EED levels in PRC2 HOTAIR/PRC2 interaction, HOTAIR /PRC2 recruitment to the ZO-1 promoter, and enhancing ZO-1 transcription; inhibiting SNAIL transcription by reducing HOTAIR/SMARCB1 interaction | [158] |
| HEK293, HK-2, 786-O, CAKI-1, 769-P, and CAKI-2 cell lines (human) | 25, 50, and 100 µM for 5 d | Inducing cell apoptosis and inhibiting cell proliferation of kidney cancer cells by increasing the expression of CDKN2a and decreasing CDKN2a methylation | [159] |
| A-498 cells in nude mice (mouse) | 25 µM for 4 d | Inhibiting the expression of miR-21 in A-498 cells and in the tumors | [160] |
| Kidney carcinoma cell (mouse) | 0.2 mL, 80 mg/kg/day, injected once a day for 14 d | Suppressing tumor growth and decreasing MVD and VEGF levels | [161] |
| Animal | Model | Treatments (Genistein) | Effects and Mechanisms | Ref. |
|---|---|---|---|---|
| Wistar rats | Fructose-fed hypertensive | 1 mg/kg/day in diet for 60 d | Lowering BP by restoring ACE, PKC-βII, and eNOS expression and preserving kidney ultrastructural integrity | [181] |
| Sprague-Dawley rats | 2-kidney 1-clip kidney hypertensive | 5.0 mg/kg/day for 8 weeks | Restoring nitric oxide, NOS activity, phosphorylated eNOS expression, and cGMP | [182] |
| SHR-SPs | Dietary NaCl with hypertension | 0.6 mg/g diet for 9 weeks | Blunting a dose-related increase in arterial pressure | [183] |
| Wistar rats | Isolated perfused rat kidney | 15 mg/kg for 24 h | Reducing kidney vascular resistance relative to vehicle in isolated perfused kidney | [115] |
| Animal | Model | Treatments (Genistein) | Effects and Mechanisms | Ref. |
|---|---|---|---|---|
| C57BL mice | UUO-induced kidney interstitial fibrosis | 10 mg/kg/body weight i.p. 24 h prior to the UUO for 7 d | Increasing kidney ALKBH5 expression, reducing RNA m6A levels, and ameliorating kidney damage. | [203] |
| Sprague-Dawley rats | Streptozotocin-induced diabetic | 5 and 25 mg/kg, daily gavage for 8 weeks | Inhibiting oxidative stress by activating the Nrf2-HO-1/NQO1 pathway and alleviating kidney fibrosis by inhibiting the TGF-β1/Smad3 pathway | [204] |
| C57BL/6 mice | Kidney fibrosis, UUO-induced | 10 mg/kg, intraperitoneal injection daily administered 1 day before UUO | Restoring Klotho via epigenetic histone acetylation and DNA demethylation | [205] |
| Wistar rats | Standard pelletdiet (fructose-fed) | 1 mg/kg/day for 45 d | Decreasing α-SMA expression and mitigating proliferation of connective tissue collagen deposition in perivascular and intraglomerular regions | [206] |
| Human kidney tubular epithelial HK-2 cells | PTH-induced kidney interstitial fibrosis | 1, 25, 50, and 100 µM for 30 min | Inhibiting PTH-induced α-SMA expression, restoring E-cadherin expression, decreasing mRNA, protein expression, and activity of CTGF | [207] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Q.; Li, Y.; Shang, J.; Huang, H.; Zhang, Y.; Ding, Y.; Liang, Y.; Xie, Z.; Chen, C. Effects of Genistein on Common Kidney Diseases. Nutrients 2022, 14, 3768. https://doi.org/10.3390/nu14183768
Peng Q, Li Y, Shang J, Huang H, Zhang Y, Ding Y, Liang Y, Xie Z, Chen C. Effects of Genistein on Common Kidney Diseases. Nutrients. 2022; 14(18):3768. https://doi.org/10.3390/nu14183768
Chicago/Turabian StylePeng, Qianwen, Yuanyuan Li, Jia Shang, Haitao Huang, Yiming Zhang, Yueming Ding, Yipei Liang, Zhenxing Xie, and Chaoran Chen. 2022. "Effects of Genistein on Common Kidney Diseases" Nutrients 14, no. 18: 3768. https://doi.org/10.3390/nu14183768
APA StylePeng, Q., Li, Y., Shang, J., Huang, H., Zhang, Y., Ding, Y., Liang, Y., Xie, Z., & Chen, C. (2022). Effects of Genistein on Common Kidney Diseases. Nutrients, 14(18), 3768. https://doi.org/10.3390/nu14183768






