A Hot Water Extract of Curcuma longa L. Improves Fasting Serum Glucose Levels in Participants with Low-Grade Inflammation: Reanalysis of Data from Two Randomized, Double-Blind, Placebo-Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Enrolment of Participants
2.2.1. Study 1
2.2.2. Study 2
2.3. Study Agent
2.4. Intervention
2.5. Measurement of Serum hsCRP Level
2.6. Measurement of Fasting Serum Glucose Level
2.7. Sample Size
2.8. Stratified Analysis
2.9. Statistical Analysis
3. Results
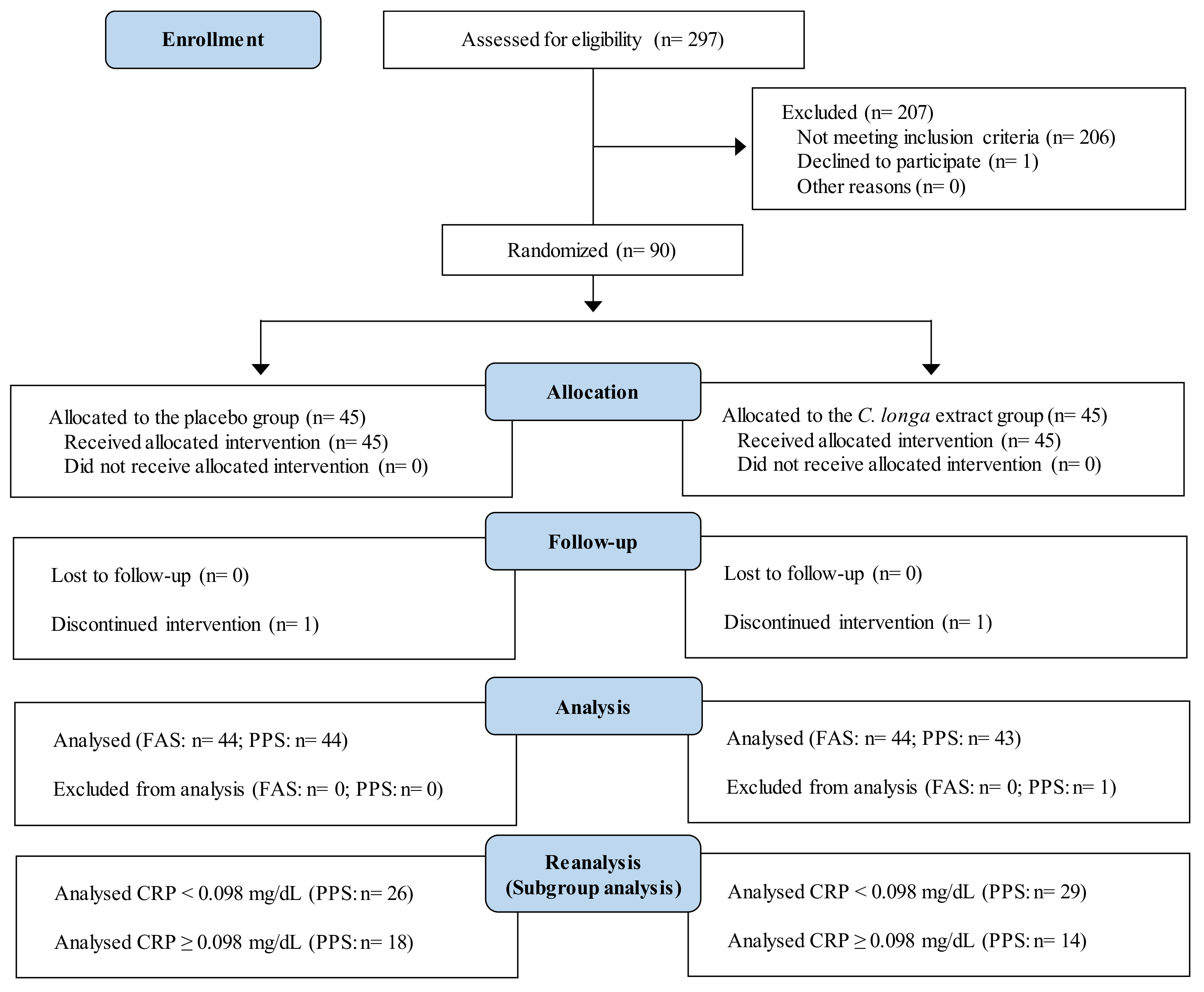
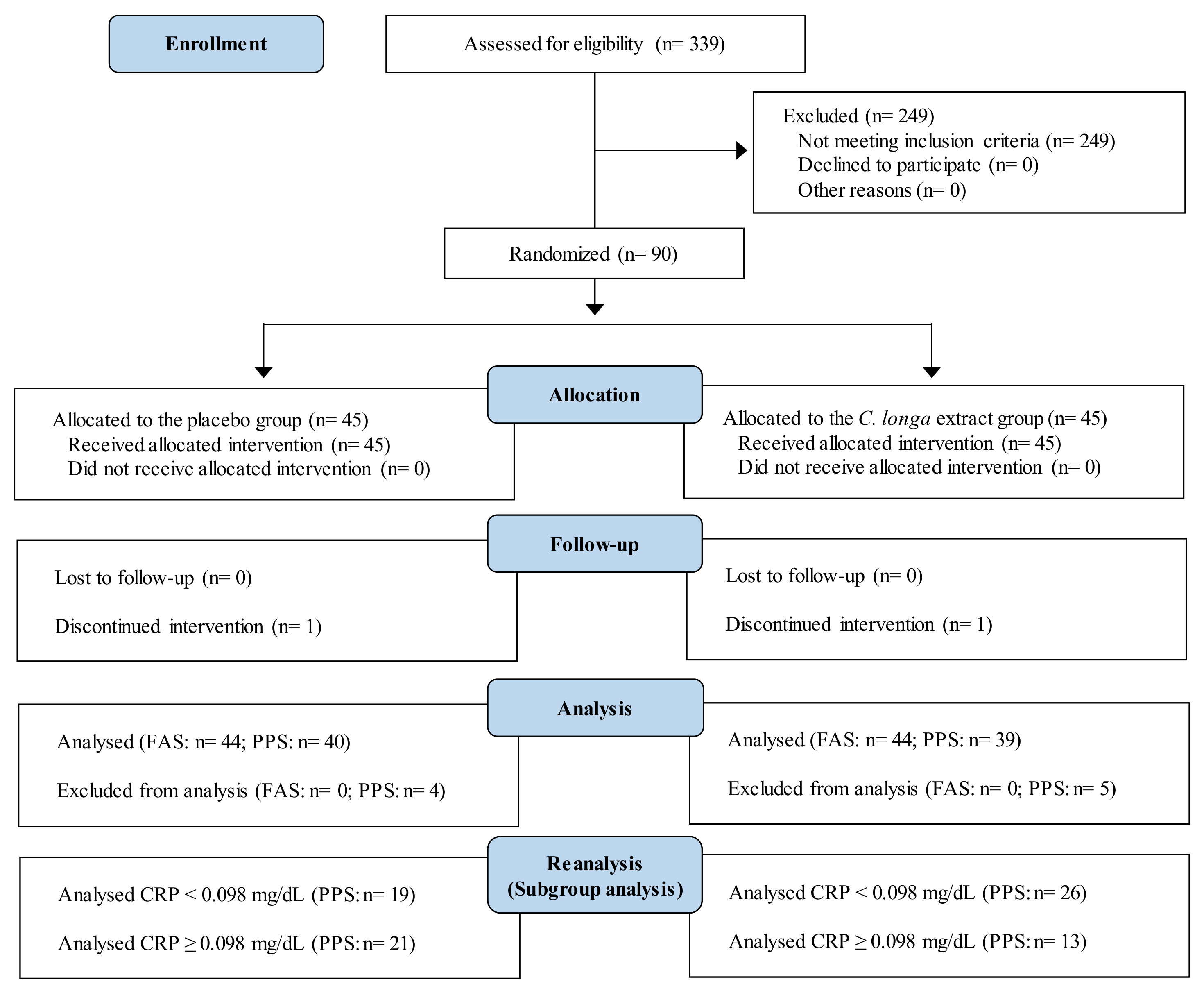
3.1. Subjects
3.1.1. Study 1
3.1.2. Study 2
3.2. Effect of C. longa Extract on Serum hsCRP Levels
3.2.1. Study 1
3.2.2. Study 2
3.3. Effect of C. longa Extract on Fasting Serum Glucose Levels
3.3.1. Study 1
3.3.2. Study 2
3.4. Safety of the Intervention
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petersen, M.C.; Vatner, D.F.; Shulman, G.I. Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 2017, 13, 572–587. [Google Scholar] [CrossRef] [PubMed]
- Szablewski, L. Glucose Homeostasis–Mechanism and Defects. Diabetes-Damages and Treatments; IntechOpen Ltd.: London, UK, 2011; Volume 2. [Google Scholar] [CrossRef]
- Röder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic regulation of glucose homeostasis. Exp. Mol. Med. 2016, 48, e219. [Google Scholar] [CrossRef] [PubMed]
- Roh, E.; Kim, M.-S. Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp. Mol. Med. 2016, 48, e216. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A guiding map for inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Calder, P.C.; Bosco, N.; Bourdet-Sicard, R.; Capuron, L.; Delzenne, N.; Dore, J.; Franceschi, C.; Lehtinen, M.J.; Recker, T.; Salvioli, S.; et al. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res. Rev. 2017, 40, 95–119. [Google Scholar] [CrossRef]
- Leon-Pedroza, J.I.; Gonzalez-Tapia, L.A.; del Olmo-Gil, E.; Castellanos-Rodriguez, D.; Escobedo, G.; Gonzalez-Chavez, A. Low-grade systemic inflammation and the development of metabolic diseases: From the molecular evidence to the clinical practice. Cirugia Y Cirujanos 2015, 83, 543–551. [Google Scholar] [CrossRef]
- Kolb, H.; Mandrup-Poulsen, T. The global diabetes epidemic as a consequence of lifestyle-induced low-grade inflammation. Diabetologia 2010, 53, 10–20. [Google Scholar] [CrossRef]
- Kushner, I.; Samols, D.; Magrey, M. A unifying biologic explanation for “high-sensitivity” C-reactive protein and “low-grade” inflammation. Arthritis Care Res. 2010, 62, 442–446. [Google Scholar] [CrossRef]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Donath, M.Y. Targeting inflammation in the treatment of type 2 diabetes: Time to start. Nat. Rev. Drug Discov. 2014, 13, 465–476. [Google Scholar] [CrossRef]
- Matsuo, Y.; Hashizume, T.; Shioji, S.; Akasaka, T. Metabolic syndrome is strongly associated with chronic subclinical inflammation in patients achieving optimal low-density lipoprotein-cholesterol levels in secondary prevention of cardiovascular disease. Circ. J. 2008, 72, 2046. [Google Scholar] [CrossRef]
- Tamakoshi, K.; Yatsuya, H.; Kondo, T.; Hori, Y.; Ishikawa, M.; Zhang, H.; Murata, C.; Otsuka, R.; Zhu, S.; Toyoshima, H. The metabolic syndrome is associated with elevated circulating C-reactive protein in healthy reference range, a systemic low-grade inflammatory state. Int. J. Obes. 2003, 27, 443–449. [Google Scholar] [CrossRef]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives–A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Yuan, W.; Li, S.; Gupta, S.C. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: Identification of novel components of turmeric. Mol. Nutr. Food Res. 2013, 57, 1529–1542. [Google Scholar] [CrossRef]
- Kawasaki, K.; Muroyama, K.; Yamamoto, N.; Murosaki, S. A hot water extract of Curcuma longa inhibits adhesion molecule protein expression and monocyte adhesion to TNF-alpha-stimulated human endothelial cells. Biosci. Biotechnol. Biochem. 2015, 79, 1654–1659. [Google Scholar] [CrossRef]
- Kawasaki, K.; Okuda-Hanafusa, C.; Aoyagi, M.; Taoka, K.; Yamamoto, N.; Muroyama, K.; Murosaki, S.; Yamamoto, Y. Inhibitory effect of the compounds from the water extract of Curcuma longa on the production of PGE2 and NO in a macrophage cell line stimulated by LPS. Biosci. Biotechnol. Biochem. 2018, 82, 2109–2117. [Google Scholar] [CrossRef]
- Uchio, R.; Higashi, Y.; Kohama, Y.; Kawasaki, K.; Hirao, T.; Muroyama, K.; Murosaki, S. A hot water extract of turmeric (Curcuma longa) suppresses acute ethanol-induced liver injury in mice by inhibiting hepatic oxidative stress and inflammatory cytokine production. J. Nutr. Sci. 2017, 6, e3. [Google Scholar] [CrossRef]
- Kim, S.; Kim, K.; Park, J.; Jun, W. Curcuma longa L. Water Extract Improves Dexamethasone-Induced Sarcopenia by Modulating the Muscle-Related Gene and Oxidative Stress in Mice. Antioxidants 2021, 10, 1000. [Google Scholar] [CrossRef]
- Asada, K.; Ohara, T.; Muroyama, K.; Yamamoto, Y.; Murosaki, S. Effects of hot water extract of Curcuma longa on human epidermal keratinocytes in vitro and skin conditions in healthy participants: A randomized, double-blind, placebo-controlled trial. J. Cosmet. Dermatol. 2019, 18, 1866–1874. [Google Scholar] [CrossRef]
- Mehra, K.S.; Mikuni, I.; Gupta, U.; Gode, K.D. Curcuma longa (Linn) drops in corneal wound healing. Tokai J. Exp. Clin. Med. 1984, 9, 27–31. [Google Scholar]
- Kawasaki, K.; Muroyama, K.; Murosaki, S. Effect of a water extract of Curcuma longa on emotional states in healthy participants. Biosci. Microbiota Food Health 2018, 37, 25–29. [Google Scholar] [CrossRef]
- Anandakumar, S.; Joseph, J.A.; Bethapudi, B.; Agarwal, A.; Jung, E.-B. Anti-inflammatory effects of turmeric (Curcuma longa L.) extract on acute and chronic inflammation models. J. Korean Soc. Food Sci. Nutr. 2014, 43, 612–617. [Google Scholar] [CrossRef]
- Sengupta, M.; Sharma, G.D.; Chakraborty, B. Hepatoprotective and immunomodulatory properties of aqueous extract of Curcuma longa in carbon tetra chloride intoxicated Swiss albino mice. Asian Pac. J. Trop. Biomed. 2011, 1, 193–199. [Google Scholar] [CrossRef]
- Uchio, R.; Murosaki, S.; Ichikawa, H. Hot water extract of turmeric (Curcuma longa) prevents non-alcoholic steatohepatitis in mice by inhibiting hepatic oxidative stress and inflammation. J. Nutr. Sci. 2018, 7, e36. [Google Scholar] [CrossRef]
- Uchio, R.; Muroyama, K.; Okuda-Hanafusa, C.; Kawasaki, K.; Yamamoto, Y.; Murosaki, S. Hot Water Extract of Curcuma longa L. Improves Serum Inflammatory Markers and General Health in Subjects with Overweight or Prehypertension/Mild Hypertension: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 1822. [Google Scholar] [CrossRef]
- Uchio, R.; Kawasaki, K.; Okuda-Hanafusa, C.; Saji, R.; Muroyama, K.; Murosaki, S.; Yamamoto, Y.; Hirose, Y. Curcuma longa extract improves serum inflammatory markers and mental health in healthy participants who are overweight: A randomized, double-blind, placebo-controlled trial. Nutr. J. 2021, 20, 91. [Google Scholar] [CrossRef]
- Hussain, H.E.M.A. Hypoglycemic, hypolipidemic and antioxidant properties of combination of Curcumin from Curcuma longa, Linn, and partially purified product from Abroma augusta, Linn in streptozotocin induced diabetes. Indian J. Clin. Biochem. 2002, 17, 33–43. [Google Scholar] [CrossRef]
- Lee, S.-J.; Han, J.-M.; Lee, J.-S.; Son, C.-G.; Im, H.-J.; Jo, H.-K.; Yoo, H.-R.; Kim, Y.-S.; Seol, I.-C. ACE reduces metabolic abnormalities in a high-fat diet mouse model. Evid. Based Complement. Alternat. Med. 2015, 2015, 352647. [Google Scholar] [CrossRef]
- Shiwaku, K.; Anuurad, E.; Enkhmaa, B.; Nogi, A.; Kitajima, K.; Shimono, K.; Yamane, Y.; Oyunsuren, T. Overweight Japanese with body mass indexes of 23.0-24.9 have higher risks for obesity-associated disorders: A comparison of Japanese and Mongolians. Int. J. Obes. 2004, 28, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Katabami, T.; Tsukiyama, H.; Tanabe, M.; Matsuba, R.; Murakami, M.; Nishine, A.; Shimizu, S.; Sakai, K.; Tanaka, Y.; Yanase, T. Development of a simple prediction model for adrenal crisis diagnosis. Sci. Rep. 2020, 10, 13546. [Google Scholar] [CrossRef]
- Muramoto, A.; Tsushita, K.; Kato, A.; Ozaki, N.; Tabata, M.; Endo, M.; Oike, Y.; Oiso, Y. Angiopoietin-like protein 2 sensitively responds to weight reduction induced by lifestyle intervention on overweight Japanese men. Nutr. Diabetes 2011, 1, e20. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Tochio, T.; Kadota, Y.; Takahashi, M.; Kitaura, Y.; Ishikawa, H. Supplementation of 1-Kestose Modulates the Gut Microbiota Composition to Ameliorate Glucose Metabolism in Obesity-Prone Hosts. Nutrients 2021, 13, 2983. [Google Scholar] [CrossRef] [PubMed]
- Bogdanski, P.; Suliburska, J.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr. Res. 2012, 32, 421–427. [Google Scholar] [CrossRef]
- Howell, D.C. Statistical Methods for Psychology; Cengage Learning: Belmont, CA, USA, 2009; pp. 461–483. [Google Scholar]
- Paschalis, V.; Theodorou, A.A.; Kyparos, A.; Dipla, K.; Zafeiridis, A.; Panayiotou, G.; Vrabas, I.S.; Nikolaidis, M.G. Low vitamin C values are linked with decreased physical performance and increased oxidative stress: Reversal by vitamin C supplementation. Eur. J. Nutr. 2016, 55, 45–53. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kitada, Y.; Naito, Y. Endothelial function is improved by inducing microbial polyamine production in the gut: A randomized placebo-controlled trial. Nutrients 2019, 11, 1188. [Google Scholar] [CrossRef]
- Hu, F.B.; Meigs, J.B.; Li, T.Y.; Rifai, N.; Manson, J.E. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes 2004, 53, 693–700. [Google Scholar] [CrossRef]
- Hayashino, Y.; Hennekens, C.H.; Kurth, T. Aspirin use and risk of type 2 diabetes in apparently healthy men. Am. J. Med. 2009, 122, 374–379. [Google Scholar] [CrossRef]
- McArdle, M.A.; Finucane, O.M.; Connaughton, R.M.; McMorrow, A.M.; Roche, H.M. Mechanisms of obesity-induced inflammation and insulin resistance: Insights into the emerging role of nutritional strategies. Front. Endocrinol. 2013, 4, 52. [Google Scholar] [CrossRef]
- Chen, L.; Chen, R.; Wang, H.; Liang, F. Mechanisms Linking Inflammation to Insulin Resistance. Int. J. Endocrinol. 2015, 2015, 508409. [Google Scholar] [CrossRef] [Green Version]
- Okuda-Hanafusa, C.; Uchio, R.; Fuwa, A.; Kawasaki, K.; Muroyama, K.; Yamamoto, Y.; Murosaki, S. Turmeronol A and turmeronol B from Curcuma longa prevent inflammatory mediator production by lipopolysaccharide-stimulated RAW264. 7 macrophages, partially via reduced NF-κB signaling. Food Funct. 2019, 10, 5779–5788. [Google Scholar] [CrossRef]
- Sun, D.I.; Nizamutdinova, I.T.; Kim, Y.M.; Cai, X.F.; Lee, J.J.; Kang, S.S.; Kim, Y.S.; Kang, K.M.; Chai, G.Y.; Chang, K.C.; et al. Bisacurone inhibits adhesion of inflammatory monocytes or cancer cells to endothelial cells through down-regulation of VCAM-1 expression. Int. Immunopharmacol. 2008, 8, 1272–1281. [Google Scholar] [CrossRef]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr. Physiol. 2020, 10, 785–809. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. Mechanisms for insulin resistance: Common threads and missing links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef]
- Shalini, V.; Srinivas, L. Lipid peroxide induced DNA damage: Protection by turmeric (Curcuma longa). Mol. Cell Biochem. 1987, 77, 3–10. [Google Scholar] [CrossRef]
- Damame, H.; Rooge, S.; Patil, R.; Garad, C.; Arvindekar, A. Beta cell protective effect of Curcuma longa and Piper nigrum in cytokine cocktail induced apoptosis in Min6 pancreatic beta cells. Phytomed. Plus 2021, 1, 100072. [Google Scholar] [CrossRef]
- Mohankumar, S.; McFarlane, J.R. An aqueous extract of Curcuma longa (turmeric) rhizomes stimulates insulin release and mimics insulin action on tissues involved in glucose homeostasis in vitro. Phytother. Res. 2011, 25, 396–401. [Google Scholar] [CrossRef]
- Kasabri, V.; Flatt, P.R.; Abdel-Wahab, Y.H. In vitro modulation of pancreatic insulin secretion, extrapancreatic insulin action and peptide glycation by Curcuma longa aqueous extracts. J. Exp. Integr. Med. 2014, 4, 187. [Google Scholar] [CrossRef]
- Ashida, H.; Tian, X.; Kitakaze, T.; Yamashita, Y. Bisacurone suppresses hepatic lipid accumulation through inhibiting lipogenesis and promoting lipolysis. J. Clin. Biochem. Nutr. 2020, 67, 43–52. [Google Scholar] [CrossRef]
- Iglesias, M.A.; Ye, J.M.; Frangioudakis, G.; Saha, A.K.; Tomas, E.; Ruderman, N.B.; Cooney, G.J.; Kraegen, E.W. AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant high-fat-fed rats. Diabetes 2002, 51, 2886–2894. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.M.; Sun, R.Q.; Zeng, X.Y.; Choong, Z.H.; Wang, H.; Watt, M.J.; Ye, J.M. Activation of PPARα ameliorates hepatic insulin resistance and steatosis in high fructose-fed mice despite increased endoplasmic reticulum stress. Diabetes 2013, 62, 2095–2105. [Google Scholar] [CrossRef] [Green Version]
| Study 1 | Study 2 | |||
|---|---|---|---|---|
| Placebo (0.90 g/3 Tablets) | C. longa Extract (0.90 g/3 Tablets) | Placebo (0.97 g/2 Capsules) | C. longa Extract (0.97 g/2 Capsules) | |
| Energy, Kcal | 3.4 | 3.4 | 6.1 | 5.5 |
| Carbohydrate, g | 0.82 | 0.76 | 0.24 | 0.23 |
| Protein, g | 0 | 0.02 | 0.20 | 0.29 |
| Lipid, g | 0.01 | 0.03 | 0.47 | 0.38 |
| Sodium chloride, mg | 0.74 | 0.45 | 0.66 | 0.13 |
| Bisacurone, μg | 0 | 400 | 0 | 400 |
| Turmeronol A, μg | 0 | 80 | 0 | 100 |
| Turmeronol B, μg | 0 | 20 | 0 | 100 |
| Low-hsCRP Subgroup (<0.098 mg/dL) | High-hsCRP Subgroup (≥0.098 mg/dL) | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n = 26) | C. longa Extract (n = 29) | Placebo (n = 18) | C. longa Extract (n = 14) | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Sex, male/female, n | 16/10 | 17/12 | 6/12 | 6/8 | ||||
| Age, y | 57.8 | 5.4 | 59.0 | 5.5 | 59.6 | 5.6 | 58.3 | 5.1 |
| Physical measurements and tests | ||||||||
| Height, cm | 163.8 | 9.1 | 163.1 | 6.8 | 159.7 | 5.8 | 162.2 | 11.0 |
| Body weight, kg | 67.4 | 10.8 | 65.4 | 7.3 | 65.0 | 6.9 | 68.7 | 8.6 |
| BMI, kg/m2 | 25.1 | 2.9 | 24.5 | 1.5 | 25.4 | 1.9 | 26.1 | 2.0 |
| SBP, mmHg | 134.6 | 18.3 | 128.6 | 14.3 | 125.7 | 13.2 | 130.6 | 18.0 |
| DBP, mmHg | 84.1 | 12.4 | 80.5 | 10.4 | 80.1 | 10.3 | 83.0 | 10.5 |
| Serum inflammatory markers | ||||||||
| hsCRP, mg/dL | 0.055 | 0.019 | 0.059 | 0.026 | 0.182 | 0.079 | 0.165 | 0.068 |
| Metabolic markers | ||||||||
| Glucose, mg/dL | 83.2 | 5.8 | 85.8 | 4.7 | 85.7 | 6.2 | 86.6 | 10.4 |
| HbA1c, % | 5.39 | 0.18 | 5.50 | 0.27 | 5.60 | 0.26 | 5.52 | 0.30 |
| Triglyceride, mg/dL | 106.8 | 48.1 | 118.6 | 79.5 | 120.7 | 66.9 | 148.2 | 62.0 |
| Total cholesterol, mg/dL | 214.7 | 37.1 | 210.1 | 33.2 | 228.0 | 40.4 | 234.1 | 30.0 |
| LDL-cholesterol, mg/dL | 133.0 | 30.8 | 126.1 | 30.1 | 145.4 | 38.2 | 144.4 | 26.0 |
| HDL-cholesterol, mg/dL | 55.5 | 18.1 | 55.7 | 12.5 | 52.6 | 8.7 | 56.5 | 12.2 |
| Low-hsCRP Subgroup (<0.098 mg/dL) | High-hsCRP Subgroup (≥0.098 mg/dL) | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n = 19) | C. longa Extract (n = 26) | Placebo (n = 21) | C. longa Extract (n = 13) | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Sex, male/female, n | 8/11 | 11/15 | 8/13 | 4/9 | ||||
| Age, y | 56.5 | 4.4 | 56.1 | 4.6 | 56.7 | 4.4 | 57.9 | 5.7 |
| Physical measurements and tests | ||||||||
| Height, cm | 164.4 | 8.7 | 162.8 | 7.8 | 160.8 | 8.1 | 161.9 | 10.1 |
| Body weight, kg | 70.5 | 7.7 | 71.0 | 6.9 | 69.4 | 8.4 | 68.9 | 9.5 |
| BMI, kg/m2 | 26.1 | 1.8 | 26.8 | 1.5 | 26.8 | 1.7 | 26.2 | 1.6 |
| SBP, mmHg | 120.8 | 14.0 | 121.3 | 14.1 | 124.8 | 11.6 | 116.5 | 16.1 |
| DBP, mmHg | 76.9 | 11.6 | 78.3 | 9.4 | 78.2 | 9.0 | 76.2 | 10.1 |
| Serum inflammatory markers | ||||||||
| hsCRP, mg/dL | 0.042 | 0.025 | 0.049 | 0.031 | 0.126 | 0.016 | 0.142 | 0.078 |
| Metabolic markers | ||||||||
| Glucose, mg/dL | 93.5 | 10.8 | 88.0 | 6.9 | 91.1 | 6.3 | 89.5 | 5.5 |
| HbA1c, % | 5.63 | 0.22 | 5.58 | 0.26 | 5.63 | 0.19 | 5.48 | 0.26 |
| Triglyceride, mg/dL | 125.2 | 61.0 | 128.7 | 75.1 | 116.7 | 45.5 | 109.1 | 45.5 |
| Total cholesterol, mg/dL | 229.8 | 46.6 | 223.4 | 38.4 | 232.8 | 26.1 | 216.8 | 36.8 |
| LDL-cholesterol, mg/dL | 145.9 | 40.5 | 142.2 | 33.6 | 151.1 | 22.3 | 130.2 * | 27.5 |
| HDL-cholesterol, mg/dL | 59.7 | 11.2 | 55.7 | 12.0 | 56.7 | 12.7 | 63.3 | 23.0 |
| Change from Baseline | Repeated Measures Two-Way ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Week 4 | Week 8 | Week 12 | Group | Time | Interaction | ||||
| Mean | SD | Mean | SD | Mean | SD | ||||
| All study participants | |||||||||
| hsCRP, mg/dL | |||||||||
| Placebo | 0.016 | 0.072 | 0.036 | 0.092 | 0.057 | 0.138 | 0.077 | 0.098 | 0.243 |
| C. longa extract | 0.006 | 0.086 | −0.001 * | 0.071 | 0.015 ** | 0.068 | |||
| Glucose, mg/dL | |||||||||
| Placebo | 2.2 | 4.6 | 2.7 | 4.7 | 2.0 | 5.0 | 0.070 | 0.153 | 0.900 |
| C. longa extract | 0.1 ** | 6.5 | 1.0 * | 5.5 | −0.1 ** | 6.6 | |||
| Stratified analysis | |||||||||
| Low-hsCRP subgroup (<0.098 mg/dL) | |||||||||
| hsCRP, mg/dL | |||||||||
| Placebo | 0.042 | 0.064 | 0.055 | 0.080 | 0.083 | 0.150 | 0.013 | 0.281 | 0.387 |
| C. longa extract | 0.023 | 0.064 | 0.020 | 0.048 | 0.026 ** | 0.049 | |||
| Glucose, mg/dL | |||||||||
| Placebo | 1.2 | 4.6 | 2.4 | 4.7 | 1.4 | 5.3 | 0.576 | 0.396 | 0.829 |
| C. longa extract | 1.0 | 6.1 | 1.3 | 4.5 | 0.8 | 5.3 | |||
| High-hsCRP subgroup (≥0.098 mg/dL) | |||||||||
| hsCRP, mg/dL | |||||||||
| Placebo | −0.026 | 0.065 | 0.007 | 0.103 | 0.017 | 0.111 | 0.306 | 0.207 | 0.233 |
| C. longa extract | −0.041 | 0.124 | −0.056 ** | 0.091 | −0.017 | 0.101 | |||
| Glucose, mg/dL | |||||||||
| Placebo | 3.6 | 4.4 | 3.1 | 4.8 | 2.9 | 4.4 | 0.052 | 0.180 | 0.193 |
| C. longa extract | −1.4 ** | 7.3 | 0.4 ** | 7.6 | −2.0 ** | 8.8 | |||
| Change from Baseline | Repeated Measures Two-Way ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Week 4 | Week 8 | Week 12 | Group | Time | Interaction | ||||
| Mean | SD | Mean | SD | Mean | SD | ||||
| All study participants | |||||||||
| hsCRP, mg/dL | |||||||||
| Placebo | 0.019 | 0.044 | 0.036 | 0.091 | 0.016 | 0.047 | 0.057 | 0.363 | 0.302 |
| C. longa extract | 0.021 | 0.089 | 0.002 * | 0.032 | −0.007 | 0.035 | |||
| Glucose, mg/dL | |||||||||
| Placebo | −2.4 | 6.3 | −1.3 | 6.3 | −0.7 | 6.1 | 0.967 | 0.189 | 0.433 |
| C. longa extract | −1.4 | 5.5 | −1.8 | 7.2 | −0.9 | 6.3 | |||
| Stratified analysis | |||||||||
| Low-hsCRP subgroup (<0.098 mg/dL) | |||||||||
| hsCRP, mg/dL | |||||||||
| Placebo | 0.011 | 0.045 | 0.039 | 0.103 | 0.019 | 0.050 | 0.101 | 0.060 | 0.831 |
| C. longa extract | 0.033 | 0.095 | 0.010 | 0.028 | 0.008 | 0.022 | |||
| Glucose, mg/dL | |||||||||
| Placebo | −3.9 | 5.5 | −1.5 | 6.0 | −1.3 | 5.6 | 0.235 | 0.066 | 0.193 |
| C. longa extract | −0.6 * | 5.8 | −1.0 | 6.0 | 0.4 | 6.8 | |||
| High-hsCRP subgroup (≥0.098 mg/dL) | |||||||||
| hsCRP, mg/dL | |||||||||
| Placebo | 0.031 | 0.041 | 0.031 | 0.076 | 0.010 | 0.043 | 0.001 | 0.334 | 0.924 |
| C. longa extract | −0.024 | 0.039 | −0.019 * | 0.035 | −0.047 * | 0.034 | |||
| Glucose, mg/dL | |||||||||
| Placebo | −1.0 | 6.8 | −1.0 | 6.7 | −0.1 | 6.6 | 0.187 | 0.949 | 0.790 |
| C. longa extract | −3.2 | 4.8 | −3.4 | 9.2 | −3.6 * | 4.3 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchio, R.; Okuda-Hanafusa, C.; Saji, R.; Kawasaki, K.; Muroyama, K.; Murosaki, S.; Yamamoto, Y.; Hirose, Y. A Hot Water Extract of Curcuma longa L. Improves Fasting Serum Glucose Levels in Participants with Low-Grade Inflammation: Reanalysis of Data from Two Randomized, Double-Blind, Placebo-Controlled Trials. Nutrients 2022, 14, 3763. https://doi.org/10.3390/nu14183763
Uchio R, Okuda-Hanafusa C, Saji R, Kawasaki K, Muroyama K, Murosaki S, Yamamoto Y, Hirose Y. A Hot Water Extract of Curcuma longa L. Improves Fasting Serum Glucose Levels in Participants with Low-Grade Inflammation: Reanalysis of Data from Two Randomized, Double-Blind, Placebo-Controlled Trials. Nutrients. 2022; 14(18):3763. https://doi.org/10.3390/nu14183763
Chicago/Turabian StyleUchio, Ryusei, Chinatsu Okuda-Hanafusa, Ryosuke Saji, Kengo Kawasaki, Koutarou Muroyama, Shinji Murosaki, Yoshihiro Yamamoto, and Yoshitaka Hirose. 2022. "A Hot Water Extract of Curcuma longa L. Improves Fasting Serum Glucose Levels in Participants with Low-Grade Inflammation: Reanalysis of Data from Two Randomized, Double-Blind, Placebo-Controlled Trials" Nutrients 14, no. 18: 3763. https://doi.org/10.3390/nu14183763
APA StyleUchio, R., Okuda-Hanafusa, C., Saji, R., Kawasaki, K., Muroyama, K., Murosaki, S., Yamamoto, Y., & Hirose, Y. (2022). A Hot Water Extract of Curcuma longa L. Improves Fasting Serum Glucose Levels in Participants with Low-Grade Inflammation: Reanalysis of Data from Two Randomized, Double-Blind, Placebo-Controlled Trials. Nutrients, 14(18), 3763. https://doi.org/10.3390/nu14183763






