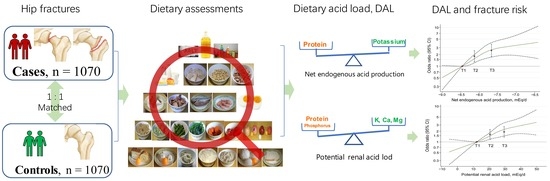
Dietary Acid Load Was Positively Associated with the Risk of Hip Fracture in Elderly Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dietary Assessments and Calculation of DAL
2.3. Covariates
2.4. Statistical Analyses
3. Results
3.1. Study Population Characteristics
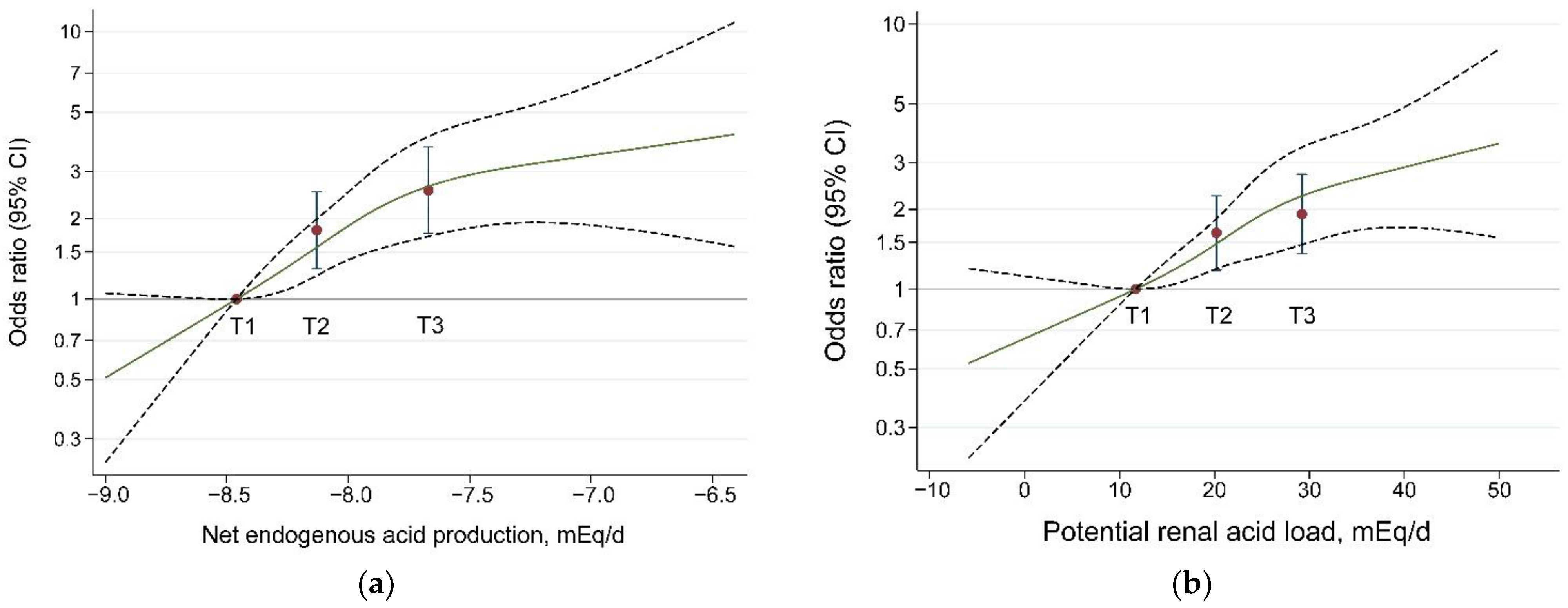
3.2. Dietary Acid Load and Hip Fracture Risk
3.3. Subgroup Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cummings, S.R.; Melton, L.J. Epidemiology and outcomes of osteoporotic fractures. Lancet 2002, 359, 1761–1767. [Google Scholar] [CrossRef]
- Tei, R.M.H.; Ramlau-Hansen, C.H.; Plana-Ripoll, O.; Brink, O.; Langdahl, B.L. OFELIA: Prevalence of Osteoporosis in Fragility Fracture Patients. Calcif. Tissue Int. 2019, 104, 102–114. [Google Scholar] [CrossRef]
- Mohd-Tahir, N.A.; Li, S.C. Economic burden of osteoporosis-related hip fracture in Asia: A systematic review. Osteoporos. Int. 2017, 28, 2035–2044. [Google Scholar] [CrossRef]
- Bliuc, D.; Alarkawi, D.; Nguyen, T.V.; Eisman, J.A.; Center, J.R. Risk of Subsequent Fractures and Mortality in Elderly Women and Men with Fragility Fractures with and without Osteoporotic Bone Density: The Dubbo Osteoporosis Epidemiology Study. J. Bone Miner. Res. 2015, 30, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Bliuc, D.; Nguyen, N.D.; Milch, V.E.; Nguyen, T.V.; Eisman, J.A.; Center, J.R. Mortality Risk Associated With Low-Trauma Osteoporotic Fracture and Subsequent Fracture in Men and Women. JAMA-J. Am. Med. Assoc. 2009, 301, 513–521. [Google Scholar] [CrossRef]
- Munoz-Garach, A.; Garcia-Fontana, B.; Munoz-Torres, M. Nutrients and Dietary Patterns Related to Osteoporosis. Nutrients 2020, 12, 1986. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R. Nutritional aspects of bone health. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Lichtenstein, A.H.; Dawson-Hughes, B.; Hannan, M.T.; Tucker, K.L. Adherence to the 2006 American Heart Association Diet and Lifestyle Recommendations for cardiovascular disease risk reduction is associated with bone health in older Puerto Ricans. Am. J. Clin. Nutr. 2013, 98, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Movassagh, E.Z.; Baxter-Jones, A.D.G.; Kontulainen, S.; Whiting, S.; Szafron, M.; Vatanparast, H. Vegetarian-style dietary pattern during adolescence has long-term positive impact on bone from adolescence to young adulthood: A longitudinal study. Nutr. J. 2018, 17, 36. [Google Scholar] [CrossRef]
- Jennings, A.; Cashman, K.D.; Gillings, R.; Cassidy, A.; Tang, J.; Fraser, W.; Dowling, K.G.; Hull, G.L.J.; Berendsen, A.A.M.; de Groot, L.C.P.G.M.; et al. A Mediterranean-like dietary pattern with vitamin D-3 (10 μg/d) supplements reduced the rate of bone loss in older Europeans with osteoporosis at baseline: Results of a 1-y randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 633–640. [Google Scholar] [CrossRef]
- Frassetto, L.A.; Todd, K.M.; Morris, R.C., Jr.; Sebastian, A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am. J. Clin. Nutr. 1998, 68, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Bullo, M.; Amigo-Correig, P.; Marquez-Sandoval, F.; Babio, N.; Martinez-Gonzalez, M.A.; Estruch, R.; Basora, J.; Sola, R.; Salas-Salvado, J. Mediterranean diet and high dietary acid load associated with mixed nuts: Effect on bone metabolism in elderly subjects. J. Am. Geriatr. Soc. 2009, 57, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Adeva, M.M.; Souto, G. Diet-induced metabolic acidosis. Clin. Nutr. 2011, 30, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Remer, T.; Dimitriou, T.; Manz, F. Dietary potential renal acid load and renal net acid excretion in healthy, free-living children and adolescents. Am. J. Clin. Nutr. 2003, 77, 1255–1260. [Google Scholar] [CrossRef]
- Remer, T.; Manz, F. Potential renal acid load of foods and its influence on urine pH. J. Am. Diet. Assoc. 1995, 95, 791–797. [Google Scholar] [CrossRef]
- Pedone, C.; Napoli, N.; Pozzilli, P.; Lauretani, F.; Bandinelli, S.; Ferrucci, L.; Antonelli-Incalzi, R. Quality of diet and potential renal acid load as risk factors for reduced bone density in elderly women. Bone 2010, 46, 1063–1067. [Google Scholar] [CrossRef][Green Version]
- Dargent-Molina, P.; Sabia, S.; Touvier, M.; Kesse, E.; Breart, G.; Clavel-Chapelon, F.; Boutron-Ruault, M.C. Proteins, dietary acid load, and calcium and risk of postmenopausal fractures in the E3N French women prospective study. J. Bone Miner. Res. 2008, 23, 1915–1922. [Google Scholar] [CrossRef]
- New, S.A.; MacDonald, H.M.; Campbell, M.K.; Martin, J.C.; Garton, M.J.; Robins, S.P.; Reid, D.M. Lower estimates of net endogenous non-carbonic acid production are positively associated with indexes of bone health in premenopausal and perimenopausal women. Am. J. Clin. Nutr. 2004, 79, 131–138. [Google Scholar] [CrossRef]
- Rahbar, A.; Larijani, B.; Nabipour, I.; Mohamadi, M.M.; Mirzaee, K.; Amiri, Z. Relationship among dietary estimates of net endogenous acid production, bone mineral density and biochemical markers of bone turnover in an Iranian general population. Bone 2009, 45, 876–881. [Google Scholar] [CrossRef]
- Tabatabai, L.S.; Cummings, S.R.; Tylavsky, F.A.; Bauer, D.C.; Cauley, J.A.; Kritchevsky, S.B.; Newman, A.; Simonsick, E.M.; Harris, T.B.; Sebastian, A.; et al. Arterialized venous bicarbonate is associated with lower bone mineral density and an increased rate of bone loss in older men and women. J. Clin. Endocrinol. Metab. 2015, 100, 1343–1349. [Google Scholar] [CrossRef]
- Remer, T.; Manz, F.; Alexy, U.; Schoenau, E.; Wudy, S.A.; Shi, L. Long-term high urinary potential renal acid load and low nitrogen excretion predict reduced diaphyseal bone mass and bone size in children. J. Clin. Endocrinol. Metab. 2011, 96, 2861–2868. [Google Scholar] [CrossRef] [PubMed]
- Wengreen, H.J.; Munger, R.G.; West, N.A.; Cutler, D.R.; Corcoran, C.D.; Zhang, J.; Sassano, N.E. Dietary protein intake and risk of osteoporotic hip fracture in elderly residents of Utah. J. Bone Miner. Res. 2004, 19, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Frassetto, L.; Banerjee, T.; Powe, N.; Sebastian, A. Acid Balance, Dietary Acid Load, and Bone Effects-A Controversial Subject. Nutrients 2018, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.J.; Johnson, L.K.; Hunt, J.R. A diet high in meat protein and potential renal acid load increases fractional calcium absorption and urinary calcium excretion without affecting markers of bone resorption or formation in postmenopausal women. J. Nutr. 2011, 141, 391–397. [Google Scholar] [CrossRef]
- McLean, R.R.; Qiao, N.; Broe, K.E.; Tucker, K.L.; Casey, V.; Cupples, L.A.; Kiel, D.P.; Hannan, M.T. Dietary acid load is not associated with lower bone mineral density except in older men. J. Nutr. 2011, 141, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Byberg, L.; Lindholm, B.; Larsson, T.E.; Lind, L.; Michaelsson, K.; Carrero, J.J. Dietary acid load, kidney function, osteoporosis, and risk of fractures in elderly men and women. Osteoporos. Int. 2015, 26, 563–570. [Google Scholar] [CrossRef]
- Zengin, A.; Pye, S.R.; Cook, M.J.; Adams, J.E.; Wu, F.C.; O’Neill, T.W.; Ward, K.A. Ethnic differences in bone geometry between White, Black and South Asian men in the UK. Bone 2016, 91, 180–185. [Google Scholar] [CrossRef]
- Putman, M.S.; Yu, E.W.; Lee, H.; Neer, R.M.; Schindler, E.; Taylor, A.P.; Cheston, E.; Bouxsein, M.L.; Finkelstein, J.S. Differences in skeletal microarchitecture and strength in African-American and white women. J. Bone Miner. Res. 2013, 28, 2177–2185. [Google Scholar] [CrossRef]
- Han, E.; Kim, G.; Hong, N.; Lee, Y.H.; Kim, D.W.; Shin, H.J.; Lee, B.W.; Kang, E.S.; Lee, I.K.; Cha, B.S. Association between dietary acid load and the risk of cardiovascular disease: Nationwide surveys (KNHANES 2008–2011). Cardiovasc. Diabetol. 2016, 15, 122. [Google Scholar] [CrossRef]
- Banerjee, T.; Crews, D.C.; Wesson, D.E.; Tilea, A.M.; Saran, R.; Rios-Burrows, N.; Williams, D.E.; Powe, N.R. High Dietary Acid Load Predicts ESRD among Adults with CKD. J. Am. Soc. Nephrol. 2015, 26, 1693–1700. [Google Scholar] [CrossRef]
- Gaede, J.; Nielsen, T.; Madsen, M.L.; Toft, U.; Jorgensen, T.; Overvad, K.; Tjonneland, A.; Hansen, T.; Allin, K.H.; Pedersen, O. Population-based studies of relationships between dietary acidity load, insulin resistance and incident diabetes in Danes. Nutr. J. 2018, 17, 91. [Google Scholar] [CrossRef]
- Zeng, F.F.; Wu, B.H.; Fan, F.; Xie, H.L.; Xue, W.Q.; Zhu, H.L.; Chen, Y.M. Dietary patterns and the risk of hip fractures in elderly Chinese: A matched case-control study. J. Clin. Endocrinol. Metab. 2013, 98, 2347–2355. [Google Scholar] [CrossRef]
- Fung, T.T.; McCullough, M.L.; Newby, P.K.; Manson, J.E.; Meigs, J.B.; Rifai, N.; Willett, W.C.; Hu, F.B. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2005, 82, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, E.; Iso, H.; Honjo, K.; Yatsuya, H.; Tamakoshi, A. No modifying effect of education level on the association between lifestyle behaviors and cardiovascular mortality: The Japan Collaborative Cohort Study. Sci. Rep. 2017, 7, 39820. [Google Scholar] [CrossRef]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef] [PubMed]
- Gholami, F.; Naghshi, S.; Samadi, M.; Rasaei, N.; Mirzaei, K. Dietary Acid Load and Bone Health: A Systematic Review and Meta-Analysis of Observational Studies. Front. Nutr. 2022, 9, 869132. [Google Scholar] [CrossRef] [PubMed]
- Zwart, S.R.; Rice, B.L.; Dlouhy, H.; Shackelford, L.C.; Heer, M.; Koslovsky, M.D.; Smith, S.M. Dietary acid load and bone turnover during long-duration spaceflight and bed rest. Am. J. Clin. Nutr. 2018, 107, 834–844. [Google Scholar] [CrossRef]
- Han, Y.; An, M.; Yang, L.; Li, L.; Rao, S.; Cheng, Y. Effect of Acid or Base Interventions on Bone Health: A Systematic Review, Meta-Analysis, and Meta-Regression. Adv. Nutr. 2021, 12, 1540–1557. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Butler, L.M.; van Dam, R.M.; Ang, L.W.; Yuan, J.M.; Koh, W.P. Adherence to a vegetable-fruit-soy dietary pattern or the Alternative Healthy Eating Index is associated with lower hip fracture risk among Singapore Chinese. J. Nutr. 2014, 144, 511–518. [Google Scholar] [CrossRef]
- Amodu, A.; Abramowitz, M.K. Dietary acid, age, and serum bicarbonate levels among adults in the United States. Clin. J. Am. Soc. Nephrol. 2013, 8, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, I.; Maher, T.; Hulter, H.N.; Schambelan, M.; Sebastian, A. Effect of diet on plasma acid-base composition in normal humans. Kidney Int. 1983, 24, 670–680. [Google Scholar] [CrossRef]
- Bushinsky, D.A. Stimulated osteoclastic and suppressed osteoblastic activity in metabolic but not respiratory acidosis. Am. J. Physiol. 1995, 268, C80–C88. [Google Scholar] [CrossRef] [PubMed]
- Frick, K.K.; Bushinsky, D.A. Metabolic acidosis stimulates RANKL RNA expression in bone through a cyclo-oxygenase-dependent mechanism. J. Bone Miner. Res. 2003, 18, 1317–1325. [Google Scholar] [CrossRef]
- Krieger, N.S.; Frick, K.K.; Bushinsky, D.A. Mechanism of acid-induced bone resorption. Curr. Opin. Nephrol. Hypertens. 2004, 13, 423–436. [Google Scholar] [CrossRef]
- Frick, K.K.; LaPlante, K.; Bushinsky, D.A. RANK ligand and TNF-alpha mediate acid-induced bone calcium efflux in vitro. Am. J. Physiol. Ren. Physiol. 2005, 289, F1005–F1011. [Google Scholar] [CrossRef] [PubMed]
- Menzel, J.; di Giuseppe, R.; Wientzek, A.; Kroke, A.; Boeing, H.; Weikert, C. Physical Activity, Bone Health, and Obesity in Peri-/Pre- and Postmenopausal Women: Results from the EPIC-Potsdam Study. Calcif. Tissue Int. 2015, 97, 376–384. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, G.W.; Compston, J.E.; Leslie, W.D.; Thabane, L.; Papaioannou, A.; Lau, A.; Wang, X.J.; Qin, C.H.; Chen, B.; Chen, M.S.; et al. Relationship Between Obesity and Risk of Major Osteoporotic Fracture in Postmenopausal Women: Taking Frailty Into Consideration. J. Bone Mineral. Res. 2020, 35, 2355–2362. [Google Scholar] [CrossRef]
- Xiang, B.Y.; Huang, W.; Zhou, G.Q.; Hu, N.; Chen, H.; Chen, C. Body mass index and the risk of low bone mass-related fractures in women compared with men A PRISMA-compliant meta-analysis of prospective cohort studies. Medicine 2017, 96, e5290. [Google Scholar] [CrossRef]
- Ahmed, T.; Haboubi, N. Assessment and management of nutrition in older people and its importance to health. Clin. Interv. Aging 2010, 5, 207–216. [Google Scholar] [CrossRef]
- Schulman, R.C.; Weiss, A.J.; Mechanick, J.I. Nutrition, bone, and aging: An integrative physiology approach. Curr. Osteoporos. Rep. 2011, 9, 184–195. [Google Scholar] [CrossRef]
- Parmenter, B.H.; Slater, G.J.; Frassetto, L.A. Accuracy and precision of estimation equations to predict net endogenous acid excretion using the Australian food database. Nutr. Diet. 2017, 74, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Parmenter, B.H.; Dymock, M.; Banerjee, T.; Sebastian, A.; Slater, G.J.; Frassetto, L.A. Performance of Predictive Equations and Biochemical Measures Quantifying Net Endogenous Acid Production and the Potential Renal Acid Load. Kidney Int. Rep. 2020, 5, 1738–1745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.; Ho, S.C. Validity and reproducibility of a food frequency Questionnaire among Chinese women in Guangdong province. Asia Pac. J. Clin. Nutr. 2009, 18, 240–250. [Google Scholar] [PubMed]
| Variable | Women (795 Pairs) | p-Value | Men (275 Pairs) | p-Value | ||
|---|---|---|---|---|---|---|
| Case | Control | Case | Control | |||
| Age, years | 71.1 ± 7.2 | 70.7 ± 6.9 | 0.233 | 69.7 ± 7.6 | 69.7 ± 7.2 | 0.982 |
| Body mass index, kg/m2 | 21.8 ± 3.4 | 23.3 ± 3.0 | <0.001 | 21.5 ± 3.0 | 23.4 ± 2.6 | <0.001 |
| Marital status, n (%) | <0.001 | <0.001 | ||||
| Married or cohabitation | 454 (57.3) | 536 (67.6) | 230 (83.0) | 256 (92.4) | ||
| Others | 339 (42.8) | 257 (32.4) | 47 (17.0) | 21 (7.6) | ||
| Education, n (%) | <0.001 | <0.001 | ||||
| Primary school or below | 457 (57.6) | 238 (30.0) | 101 (36.5) | 47 (17.0) | ||
| Junior high school | 119 (15.0) | 171 (21.56) | 57 (20.6) | 53 (19.1) | ||
| High school or above | 217 (27.4) | 384 (48.4) | 119 (43.0) | 177 (63.9) | ||
| Family monthly income (Yuan/person), n (%) | <0.001 | <0.001 | ||||
| ≤500 | 62 (7.8) | 14 (1.8) | 12 (4.3) | 1 (0.4) | ||
| 501–2000 | 212 (26.7) | 108 (13.6) | 48 (17.3) | 17 (6.1) | ||
| 2001–3000 | 172 (21.7) | 214 (27.0) | 50 (18.1) | 59 (21.3) | ||
| >3000 | 347 (43.8) | 457 (57.6) | 167 (60.3) | 200 (72.2) | ||
| Occupation, n (%) | <0.001 | <0.001 | ||||
| All mental work | 196 (24.7) | 253 (31.9) | 92 (33.2) | 88 (31.8) | ||
| Mainly mental work | 82 (10.3) | 173 (21.8) | 35 (12.6) | 59 (21.3) | ||
| Mainly physical labor | 184 (23.2) | 191 (24.1) | 70 (25.3) | 86 (31.) | ||
| All physical labor | 316 (39.9) | 158 (19.9) | 72 (26.0) | 39 (14.1) | ||
| Other | 15 (1.9) | 18 (2.3) | 8 (2.89) | 5 (1.8) | ||
| Saltiness of habitual diets, n (%) | 0.020 | <0.001 | ||||
| Light or very light | 339 (43.0) | 394 (50.0) | 73 (26.5) | 121 (44.0) | ||
| Moderate | 302 (38.3) | 271 (34.4) | 131 (47.6) | 102 (37.1) | ||
| Salty or very salty | 146 (18.5) | 123 (15.6) | 73 (26.5) | 52 (18.9) | ||
| Health lifestyle score, n (%) | <0.001 | 0.025 | ||||
| 0–1 | 148 (19.3) | 78 (10.0) | 49 (18.5) | 32 (11.9) | ||
| 2 | 288 (37.5) | 220 (28.2) | 87 (32.8) | 82 (30.4) | ||
| 3 | 263 (34.2) | 295 (37.8) | 102 (38.5) | 109 (40.4) | ||
| 4 | 69 (9.0) | 187 (24.0) | 27 (10.2) | 47 (17.4) | ||
| Alternate Mediterranean diet score, n (%) | <0.001 | <0.001 | ||||
| 0–2 | 244 (30.8) | 114 (14.4) | 81 (29.3) | 39 (14.2) | ||
| 3 | 182 (23.0) | 118 (14.9) | 56 (20.3) | 49 (17.8) | ||
| 4 | 175 (22.1) | 202 (25.5) | 59 (21.4) | 65 (23.6) | ||
| 5–8 | 191 (24.1) | 357 (45.1) | 80 (29.0) | 122 (44.4) | ||
| Physical activity, MET⋅h/day d | 66.7 ± 38.6 | 82.1 ± 53.1 | <0.001 | 70.6 ± 44.6 | 77.6 ± 49.1 | 0.081 |
| Estrogen user, n (%) | 11 (1.4) | 59 (7.5) | <0.001 | |||
| Years since menopause, years | 21.9 ± 8.3 | 20.7 ± 8.1 | 0.002 | |||
| Smoker, n (%) a | 37 (4.7) | 15 (1.9) | 0.002 | 143 (51.6) | 120 (43.3) | 0.060 |
| Passive smoker, n (%) b | 172 (21.7) | 142 (17.9) | <0.001 | 54 (19.5) | 30 (10.8) | 0.003 |
| Alcohol drinker, n (%) c | 20 (2.5) | 37 (4.7) | 0.022 | 53 (19.1) | 40 (14.4) | 0.150 |
| Tea drinker, n (%) | 252 (31.8) | 363 (45.8) | <0.001 | 119 (43.0) | 177 (63.9) | <0.001 |
| Calcium supplement user, n (%) | 268 (33.8) | 367 (46.3) | <0.001 | 45 (16.3) | 88 (31.8) | <0.001 |
| Multivitamin supplement user, n (%) | 80 (10.1) | 227 (28.6) | <0.001 | 22 (7.9) | 80 (28.9) | <0.001 |
| Dietary calcium intake (mg/d) | 441 (344, 555) | 493 (401, 607) | <0.001 | 534 (416, 659) | 572 (476, 689) | 0.004 |
| Dietary magnesium intake (mg/d) | 315(271,361) | 324(281,372) | 0.005 | 395 (351,440) | 407(363,458) | 0.013 |
| Dietary vitamin D intake (IU/d) | 66.9 (48.5, 92.3) | 68.6 (49.8, 96.2) | 0.265 | 82.2 (59.5, 115.3) | 85.0 (57.7, 116.9) | 0.678 |
| Net endogenous acid production, NEAP (mEq/d) | −8.02 (−8.25, −7.78) | −8.18 (−8.41, −7.90) | <0.001 | −7.82 (−8.03, −7.56) | −7.98 (−8.24, −7.70) | <0.001 |
| Potential renal acid load, PRAL (mEq/d) | 21.2 (16.7, 24.9) | 18.3 (12.9, 23.2) | <0.001 | 30.2 (27.1, 34.8) | 28.0 (22.3, 32.3) | 0.006 |
| Food Groups | Tertile 1 | Tertile 2 | Tertile 3 | p-Trend |
|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | ||
| Grain * | 167 (141, 203) | 185 (157, 221) | 192 (161, 230) | <0.001 |
| Soy foods * | 5.4 (2.4, 10.4) | 5.2 (2.6, 11.7) | 7.9 (2.8, 19.9) | 0.090 |
| Vegetables | 452 (357, 559) | 317.5 (249, 373) | 187 (121, 263) | <0.001 |
| Fruits | 155.3 (94.8, 204.7) | 94.2 (60.4, 133.2) | 36.6 (20.2, 70.0) | <0.001 |
| Meats | 58.1 (43.5, 79.5) | 67.3 (49.6, 93.0) | 84.1 (60.6, 126.9) | <0.001 |
| Poultry | 16.4 (9.5, 28.3) | 19.6 (11.3, 29.0) | 17.1 (10.7, 29.5) | 0.614 |
| Fish and shellfish | 34.9 (20.2, 54.2) | 35.2 (22.0, 55.7) | 27.0 (12.8, 49.4) | 0.196 |
| Eggs | 28.4 (19.3, 44.4) | 27.2 (18.6, 44.6) | 18.5 (11.4, 28.5) | <0.001 |
| Dairy foods * | 20.5 (9.2, 31.1) | 14.4 (5.7, 22.0) | 3.9 (0.6, 9.5) | <0.001 |
| NEAP/PRAL by Sex | Tertiles of Dietary Acid Load | p-Trend | ||
|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 (Highest) | ||
| Women and men | ||||
| NEAP, mEq/d | ||||
| n (case/control) | 176/356 | 371/357 | 523/357 | |
| Median (mEq/d) | −8.46 | −8.13 | −7.67 | |
| OR 1 c | 1.00 | 2.14 (1.68, 2.72) | 3.37 (2.62, 4.34) | <0.001 |
| OR 2 c | 1.00 | 1.82 (1.38, 2.40) | 2.73 (2.04, 3.65) | <0.001 |
| OR 3 c | 1.00 | 1.81 (1.30, 2.53) | 2.55 (1.76, 3.71) | <0.001 |
| PRAL, mEq/d | ||||
| n (case/control) | 185/356 | 371/357 | 514/357 | |
| Median (mEq/d) b | 11.7 | 20.2 | 29.2 | |
| OR 1 c | 2.00 (1.59, 2.53) | 3.02 (2.37,3.85) | <0.001 | |
| OR 2 c | 1.00 | 1.73 (1.32, 2.27) | 2.30 (1.74, 3.04) | <0.001 |
| OR 3 c | 1.00 | 1.63 (1.18, 2.25) | 1.92 (1.36, 2.71) | <0.001 |
| Women | ||||
| NEAP, mEq/d | ||||
| n (case/control) | 140/265 | 266/265 | 387/265 | |
| Median (mEq/d) | −8.48 | −8.17 | −7.75 | |
| OR 1 c | 1.00 | 1.93 (1.47, 2.54) | 3.07 (2.31, 4.08) | <0.001 |
| OR 2 c | 1.00 | 1.56 (1.14,2.15) | 2.27 (1.63,3.17) | <0.001 |
| OR 3 c | 1.00 | 1.60 (1.11,2.32) | 2.11 (1.42,3.15) | 0.002 |
| PRAL, mEq/d | ||||
| n (case/control) | 140/265 | 264/265 | 389/265 | |
| Median (mEq/d) b | 10.5 | 18.4 | 25.1 | |
| OR 1 c | 1.00 | 1.91 (1.45, 2.50) | 3.07 (2.31, 4.08) | <0.001 |
| OR 2 c | 1.00 | 1.84 (1.33,2.54) | 2.34 (1.67,3.27) | 0.001 |
| OR 3 c | 1.00 | 1.76 (1.21,2.56) | 1.97 (1.32,2.92) | 0.005 |
| Men | ||||
| NEAP, mEq/d | ||||
| n (case/control) | 36/91 | 105/92 | 136/92 | |
| Median (mEq/d) | −8.31 | −7.97 | −7.57 | |
| OR 1 c | 1.00 | 3.31 (1.93, 5.69) | 4.81 (2.74, 8.43) | <0.001 |
| OR 2 c | 1.00 | 2.22 (1.15,4.29) | 2.70 (1.39,5.23) | 0.034 |
| OR 3 c | 1.00 | 1.86 (0.76,4.54) | 1.77 (0.66,4.71) | 0.164 |
| PRAL, mEq/d | 20.1 | 28.3 | 34.9 | |
| n (case/control) | 45/91 | 107/92 | 125/92 | |
| Median (mEq/d) b | 11.7 | 20.2 | 29.2 | |
| OR 1 c | 1.00 | 2.39 (1.51, 3.79) | 2.94 (1.82, 4.75) | <0.001 |
| OR 2 c | 1.00 | 1.36 (0.74,2.50) | 2.03 (1.12,3.68) | 0.035 |
| OR 3 c | 1.00 | 1.12 (0.50,2.50) | 1.31 (0.58,2.95) | 0.459 |
| Subgroups | PRAL | p-Trend | p-Interaction | NEAP | p-Trend | p-Interaction | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 (Highest) | T1 | T2 | T3 (Highest) | |||||
| Age, year | 0.091 | 0.368 | ||||||||
| ≤65 | 1.00 | 1.02 (0.58, 1.78) | 0.94 (0.53, 1.66) | 0.807 | 1.00 | 1.62 (0.91, 2.88) | 1.38 (0.75, 2.54) | 0.036 | ||
| >65 | 1.00 | 1.84 (1.33, 2.56) | 2.32 (1.66, 3.25) | <0.001 | 1.00 | 1.71 (1.23, 2.34) | 2.54 (1.78, 3.63) | <0.001 | ||
| BMI, kg/m2 | 0.005 | <0.001 | ||||||||
| <23.0 | 1.00 | 1.65 (1.13, 2.56) | 2.33 (1.58, 3.46) | <0.001 | 1.00 | 2.06 (1.40, 3.12) | 3.48 (2.30, 5.27) | <0.001 | ||
| ≥23.0 | 1.00 | 1.91 (1.25, 2.91) | 1.47 (0.93, 2.31) | 0.152 | 1.00 | 1.95 (1.27, 2.98) | 1.57 (0.96, 2.56) | 0.221 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.-F.; Liu, Y.-P.; Liu, C.-Y.; Zhu, H.-L.; Wu, B.-H.; Li, B.-L.; Chen, Y.-M. Dietary Acid Load Was Positively Associated with the Risk of Hip Fracture in Elderly Adults. Nutrients 2022, 14, 3748. https://doi.org/10.3390/nu14183748
Li C-F, Liu Y-P, Liu C-Y, Zhu H-L, Wu B-H, Li B-L, Chen Y-M. Dietary Acid Load Was Positively Associated with the Risk of Hip Fracture in Elderly Adults. Nutrients. 2022; 14(18):3748. https://doi.org/10.3390/nu14183748
Chicago/Turabian StyleLi, Cheng-Feng, Yu-Ping Liu, Chun-Ying Liu, Hui-Lian Zhu, Bao-Hua Wu, Bao-Lin Li, and Yu-Ming Chen. 2022. "Dietary Acid Load Was Positively Associated with the Risk of Hip Fracture in Elderly Adults" Nutrients 14, no. 18: 3748. https://doi.org/10.3390/nu14183748
APA StyleLi, C.-F., Liu, Y.-P., Liu, C.-Y., Zhu, H.-L., Wu, B.-H., Li, B.-L., & Chen, Y.-M. (2022). Dietary Acid Load Was Positively Associated with the Risk of Hip Fracture in Elderly Adults. Nutrients, 14(18), 3748. https://doi.org/10.3390/nu14183748