Effect of the Intake of Lean Red-Meat from Beef-(Pirenaica Breed) versus Lean White-Meat on Body Composition, Fatty Acids Profile and Cardiovascular Risk Indicators: A Randomized Cross-Over Study in Healthy Young Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
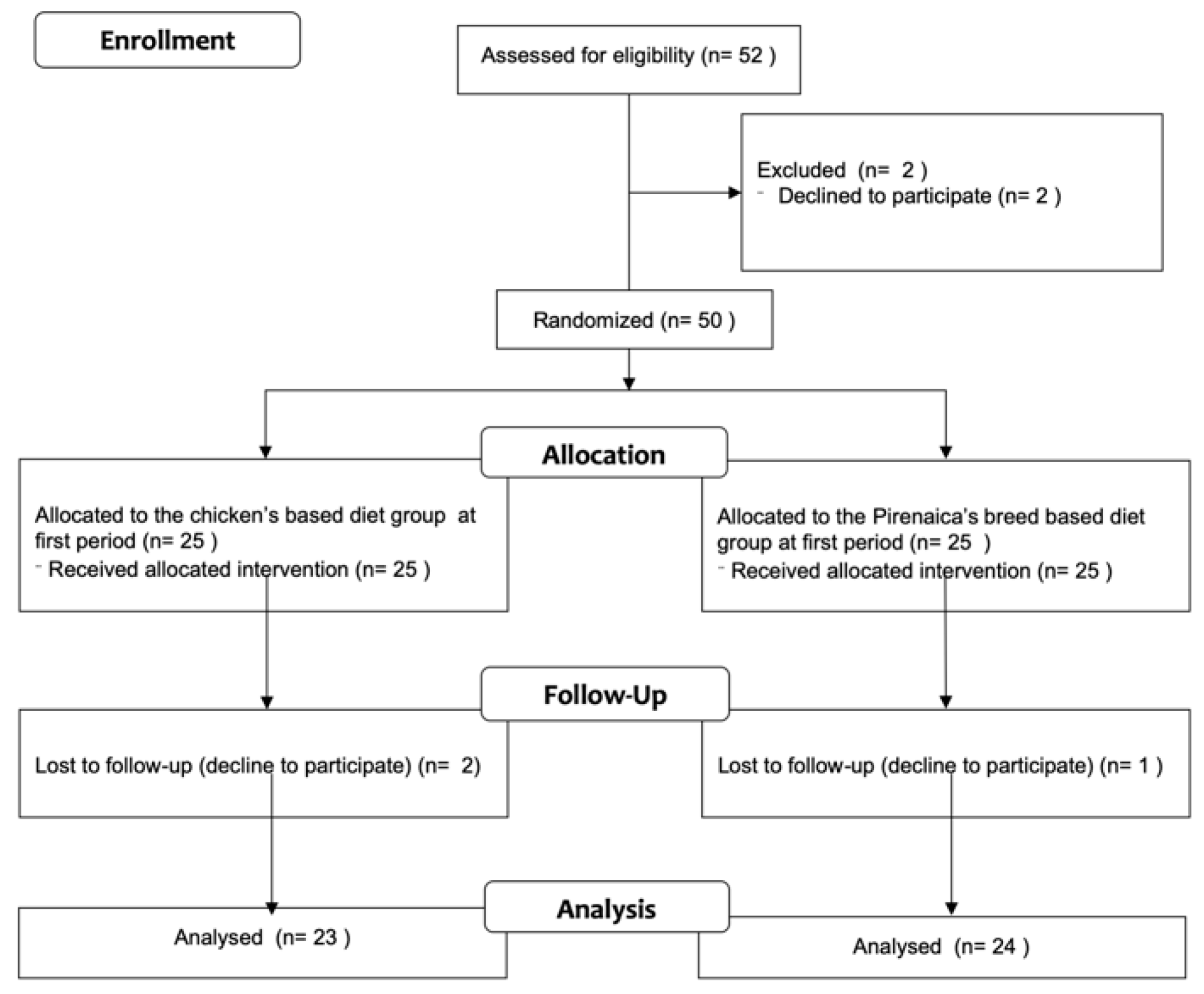
2.2. Participants
2.3. Socieconomic Indicators
2.4. Assessment of Anthropometrics and Lifestyle Behaviors
2.5. Intervention
2.6. Laboratory Analysis
2.7. Statistical Analysis
3. Results
4. Discussion
4.1. Meat Consumption and Dietary Guidelines
4.2. Meat Consumption and Body Composition and CVD Risk Factors
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Healthy Diet. Fact Sheet No. 394 [Updated August 2018]. Available online: https://www.who.int/publications/m/item/healthy-diet-factsheet394 (accessed on 6 August 2022).
- Herforth, A.; Arimond, M.; Álvarez-Sánchez, C.; Coates, J.; Christianson, K.; Muehlhoff, E. A Global Review of Food-Based Dietary Guidelines. Adv. Nutr. 2019, 10, 590–605. [Google Scholar] [CrossRef] [PubMed]
- McAllister, T.A.; Stanford, K.; Chaves, A.V.; Evans, P.R.; Eustaquio de Souza Figueiredo, E.; Ribeiro, G. Chapter 5—Nutrition, feeding and management of beef cattle in intensive and extensive production systems. In Animal Agriculture; Bazer, F.W., Lamb, G.C., Wu, G., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 75–98. [Google Scholar]
- Pistón, M.; Suárez, A.; Bühl, V.; Tissot, F.; Silva, J.; Panizzolo, L. Influence of cooking processes on Cu, Fe, Mn, Ni, and Zn levels in beef cuts. J. Food Compos. Anal. 2020, 94, 103624. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F.; Pintado, T.; Cofrades, S.; Ruiz-Capillas, C.; Bastida, S. Production variations of nutritional composition of commercial meat products. Food Res. Int. 2010, 43, 2378–2384. [Google Scholar] [CrossRef]
- Campo, M.M.; Muela, E.; Olleta, J.L.; Moreno, L.A.; Santaliestra-Pasias, A.M.; Mesana, M.I.; Sañudo, C. Influence of cooking method on the nutrient composition of Spanish light lamb. J. Food Compos. Anal. 2013, 31, 185–190. [Google Scholar] [CrossRef]
- Campo, M.M.; Muela, E.; Resconi, V.C.; Barahona, M.; Sañudo, C. Influence of commercial cut on proximate composition and fatty acid profile of Rasa Aragonesa light lamb. J. Food Compos. Anal. 2016, 53, 7–12. [Google Scholar] [CrossRef]
- Aune, D.; Ursin, G.; Veierød, M.B. Meat consumption and the risk of type 2 diabetes: A systematic review and meta-analysis of cohort studies. Diabetologia 2009, 52, 2277–2287. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wolk, A. Meat consumption and risk of colorectal cancer: A meta-analysis of prospective studies. Int. J. Cancer 2006, 119, 2657–2664. [Google Scholar] [CrossRef]
- Micha, R.; Mozaffarian, D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: A fresh look at the evidence. Lipids 2010, 45, 893–905. [Google Scholar] [CrossRef]
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Red Meat and Processed Meat; International Agency for Research on Cancer: Lyon, France, 2018.
- Xu, X.; Yu, E.; Gao, X.; Song, N.; Liu, L.; Wei, X.; Zhang, W.; Fu, C. Red and processed meat intake and risk of colorectal adenomas: A meta-analysis of observational studies. Int. J. Cancer 2013, 132, 437–448. [Google Scholar] [CrossRef]
- Klurfeld, D.M. Research gaps in evaluating the relationship of meat and health. Meat Sci. 2015, 109, 86–95. [Google Scholar] [CrossRef]
- Johnston, B.C.; Zeraatkar, D.; Han, M.A.; Vernooij, R.W.M.; Valli, C.; El Dib, R.; Marshall, C.; Stover, P.J.; Fairweather-Taitt, S.; Wójcik, G.; et al. Unprocessed Red Meat and Processed Meat Consumption: Dietary Guideline Recommendations from the Nutritional Recommendations (NutriRECS) Consortium. Ann. Intern. Med. 2019, 171, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Ekmekcioglu, C.; Wallner, P.; Kundi, M.; Weisz, U.; Haas, W.; Hutter, H.P. Red meat, diseases, and healthy alternatives: A critical review. Crit. Rev. Food Sci. Nutr. 2018, 58, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Campo, M.M.; Sañudo, C.; Panea, B.; Alberti, P.; Santolaria, P. Breed type and ageing time effects on sensory characteristics of beef strip loin steaks. Meat Sci. 1999, 51, 383–390. [Google Scholar] [CrossRef]
- Christensen, M.; Ertbjerg, P.; Failla, S.; Sañudo, C.; Richardson, R.I.; Nute, G.R.; Olleta, J.L.; Panea, B.; Albertí, P.; Juárez, M.; et al. Relationship between collagen characteristics, lipid content and raw and cooked texture of meat from young bulls of fifteen European breeds. Meat Sci. 2011, 87, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Resano, H.; Olaizola, A.M.; Dominguez-Torreiro, M. Exploring the influence of consumer characteristics on veal credence and experience guarantee purchasing motivators. Meat Sci. 2018, 141, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aranceta Bartrina, J.; Arija Val, V.; Maíz Aldalur, E.; Martínez de la Victoria Muñoz, E.; Ortega Anta, R.M.; Pérez-Rodrigo, C.; Quiles Izquierdo, J.; Rodríguez Martín, A.; Román Viñas, B.; Salvador Castell, G.; et al. Dietary guidelines for the Spanish population (SENC, December 2016); the new graphic icon of healthy nutrition. Nutr. Hosp. 2016, 33 (Suppl. 8), 1–48. [Google Scholar] [CrossRef]
- Martin-Moreno, J.M.; Boyle, P.; Gorgojo, L.; Maisonneuve, P.; Fernandez-Rodriguez, J.C.; Salvini, S.; Willett, W.C. Development and validation of a food frequency questionnaire in Spain. Int. J. Epidemiol. 1993, 22, 512–519. [Google Scholar] [CrossRef]
- de la Fuente-Arrillaga, C.; Ruiz, Z.V.; Bes-Rastrollo, M.; Sampson, L.; Martinez-González, M.A. Reproducibility of an FFQ validated in Spain. Public Health Nutr. 2010, 13, 1364–1372. [Google Scholar] [CrossRef]
- Ottevaere, C.; Huybrechts, I.; De Bourdeaudhuij, I.; Sjöström, M.; Ruiz, J.R.; Ortega, F.B.; Hagströmer, M.; Widhalm, K.; Molnár, D.; Moreno, L.A.; et al. Comparison of the IPAQ-A and actigraph in relation to VO2max among European adolescents: The HELENA study. J. Sci. Med. Sport 2011, 14, 317–324. [Google Scholar] [CrossRef]
- Rey-Lopez, J.P.; Ruiz, J.R.; Ortega, F.B.; Verloigne, M.; Vicente-Rodriguez, G.; Gracia-Marco, L.; Gottrand, F.; Molnar, D.; Widhalm, K.; Zaccaria, M.; et al. Reliability and validity of a screen time-based sedentary behaviour questionnaire for adolescents: The HELENA study. Eur. J. Public Health 2012, 22, 373–377. [Google Scholar] [CrossRef] [Green Version]
- Lepage, G.; Roy, C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986, 27, 114–120. [Google Scholar] [CrossRef]
- Mesana Graffe, M.I.; Santaliestra Pasias, A.M.; Fleta Zaragozano, J.; Campo Arribas, M.d.M.; Sanudo Astiz, C.; Valbuena Turienzo, I.; Martínez, P.; Horno Delgado, J.; Moreno Aznar, L.A. Changes in body composition and cardiovascular risk indicators in healthy Spanish adolescents after lamb- (Ternasco de Aragon) or chicken-basic diets. Nutr. Hosp. 2013, 28, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Gallego, R.; Perez-Calahorra, S.; Cenarro, A.; Bea, A.M.; Andres, E.; Horno, J.; Ros, E.; Civeira, F. Effect of lean red meat from lamb v. lean white meat from chicken on the serum lipid profile: A randomised, cross-over study in women. Br. J. Nutr. 2012, 107, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Rouhani, M.H.; Salehi-Abargouei, A.; Surkan, P.J.; Azadbakht, L. Is there a relationship between red or processed meat intake and obesity? A systematic review and meta-analysis of observational studies. Obes. Rev. 2014, 15, 740–748. [Google Scholar] [CrossRef]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Boffetta, P. Health risk factors associated with meat, fruit and vegetable consumption in cohort studies: A comprehensive meta-analysis. PLoS ONE 2017, 12, e0183787. [Google Scholar] [CrossRef]
- Bergeron, N.; Chiu, S.; Williams, P.T.; MKing, S.; Krauss, R.M. Effects of red meat, white meat, and nonmeat protein sources on atherogenic lipoprotein measures in the context of low compared with high saturated fat intake: A randomized controlled trial. Am. J. Clin. Nutr. 2019, 110, 24–33. [Google Scholar] [CrossRef]
- Kim, K.; Hyeon, J.; Lee, S.A.; Kwon, S.O.; Lee, H.; Keum, N.; Lee, J.; Park, S.M. Role of Total, Red, Processed, and White Meat Consumption in Stroke Incidence and Mortality: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. J. Am. Heart Assoc. 2017, 6, e005983. [Google Scholar] [CrossRef]
- Haskins, C.P.; Henderson, G.; Champ, C.E. Meat, eggs, full-fat dairy, and nutritional boogeymen: Does the way in which animals are raised affect health differently in humans? Crit. Rev. Food Sci. Nutr. 2019, 59, 2709–2719. [Google Scholar] [CrossRef]
- Średnicka-Tober, D.; Barański, M.; Seal, C.; Sanderson, R.; Benbrook, C.; Steinshamn, H.; Gromadzka-Ostrowska, J.; Rembiałkowska, E.; Skwarło-Sońta, K.; Eyre, M.; et al. Composition differences between organic and conventional meat: A systematic literature review and meta-analysis. Br. J. Nutr. 2016, 115, 994–1011. [Google Scholar] [CrossRef]
- Wood, J.D.; Richardson, R.I.; Nute, G.R.; Fisher, A.V.; Campo, M.M.; Kasapidou, E.; Sheard, P.R.; Enser, M. Effects of fatty acids on meat quality: A review. Meat Sci. 2004, 66, 21–32. [Google Scholar] [CrossRef]
- De la Fuente, J.; Díaz, M.T.; Alvarez, I.; Oliver, M.A.; Font I Furnols, M.; Sañudo, C.; Campo, M.; Montossi, F.; Nute, G.; Cañeque, V. Fatty acid and vitamin E composition of intramuscular fat in cattle reared in different production systems. Meat Sci. 2009, 82, 331–337. [Google Scholar] [CrossRef] [PubMed]

| Grilled | Breaded and Deep Fried | Stewed | |
|---|---|---|---|
| Olive oil | 10 mL virgin olive oil | 30 mL virgin olive oil | 10 mL virgin olive oil |
| Cooking method | Simple grill | Fried | Stew |
| Internal temperature | 75 °C | 75 °C | 75 °C |
| Cooking temperature | 200 °C | 200 °C | 200 °C |
| Time of cooking | 60 min | 90 min | 120 min |
| Additional foods Both recipes | Onions/Salt/black pepper/Garlic | Eggs/potatoes/bread crumb/black pepper/salt/herbs de Provence | Onions/water/tomatoes/white wine/mushrooms/carrots/salt/pepper/almonds/parsley/garlic/salt |
| Beef recipes | Meat broth/brandy/cooking cream/butter | Beef broth (beef, onions, carrots, leeks and water) | |
| Chicken recipes | Tomatoes/red and green peppers/white wine/vinegar | Chicken broth (chicken, onions, carrots, leeks and water) | |
| Beef cut Chicken cut | Loin Thigh and drumstick | Silverside Breast | Brisket Thigh and drumstick |
| Skin on chicken | Yes | No | Yes |
| Total Sample n = 47 | Participants Who Started with the Pyrenees’ Beef Diet n = 24 | Participants Who Started with the Chicken’s Diet n = 23 | p | ||
|---|---|---|---|---|---|
| Gender n (%) | Female | 23 (48.9) | 7 (29.2) | 16 (69.6) | 0.006 |
| Male | 24 (51.1) | 17 (70.8) | 7 (30.4) | ||
| Age (years ± SD) | 19.9 ± 1.75 | 20.5 ± 2.09 | 19.3 ± 1.02 | 0.022 | |
| Maternal education, n (%) | Low | 10 (21.3) | 4 (16.7) | 6 (26.1) | 0.691 |
| Medium | 25 (53.2) | 14 (58.3) | 11 (47.8) | ||
| High | 12 (25.5) | 6 (25.0) | 6 (26.1) | ||
| Paternal education, n (%) | Low | 13 (28.9) | 6 (25.0) | 7 (33.3) | 0.814 |
| Medium | 20 (44.4) | 11 (45.8) | 9 (42.9) | ||
| High | 12 (26.7) | 7 (29.2) | 5 (23.8) | ||
| BLOOD SAMPLES at baseline | |||||
| Hematology | Hemoglobin (106/μdL) | 15.18 (1.12) | 14.7 (0.94) | 1.000 | |
| Hematocrit (%) | 44.61 (2.93) | 43.9 (2.77) | 0.062 | ||
| Biochemistry | Glucose (mg/dL) | 80.5 (5.87) | 80.1 (4.10) | 0.965 | |
| GOT (U/L) | 27.96 (10.58) | 22.96 (5.10) | 0.217 | ||
| GPT (U/L) | 25.71 (10.98) | 19.57 (4.99) | 0.684 | ||
| GGT (U/L) | 17.33 (8.64) | 13.65 (2.85) | 0.193 | ||
| Total cholesterol (mg/dL) | 151.08 (22.85) | 148.04 (41.57) | 0.946 | ||
| Triglycerides (mg/dL) | 72.79 (30.63) | 62.30 (18.38) | 0.286 | ||
| HDL cholesterol (mg/dL) | 53.13 (13.02) | 58.04 (16.44) | 0.976 | ||
| LDL cholesterol (mg/dL) | 83.33 (21.32) | 77.66 (33.94) | 0.726 | ||
| Iron metabolism | Iron (μg/dL) (n = 46) | 112.42 (46.33) | 95.73 (35.87) | 0.167 | |
| Ferritin (ng/mL) | 88.56 (58.03) | 55.75 (30.53) | 0.472 | ||
| Transferrin (mg/dL) | 242.38 (25.37) | 261.43 (33.69) | 0.491 | ||
| Transferrin Saturation Index (%) | 36.71 (14.37) | 29.02 (11.47) | 0.049 | ||
| Other minerals | Magnesium (mmol/L) | 0.87 (0.06) | 0.89 (0.08) | 0.074 | |
| Calcium (mg/dL) | 10.22 (0.29) | 10.10 (0.45) | 0.971 | ||
| Zinc (μg/dL) | 88.93 (9.32) | 88.17 (13.97) | 0.327 | ||
| Other variables | Apolipoprotein A1 (mg/dL) | 156.96 (21.22) | 166.70 (29.42) | 0.562 | |
| Apolipoprotein B (mg/dL) | 78.5 (17.00) | 73.43 (24.87) | 1.000 | ||
| C Reactive Protein (mg/dL) | 0.35 (0.56) | 0.22 (0.07) | 0.837 | ||
| Chicken-Based Diet Group n = 47 | Pirenaica Breed-Based Diet Group n = 47 | Mean Differences between Beginning and End between Both Groups n = 47 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | F | p1 | Before | After | F | p1 | Mean Differences | 95% IC | F | p2 | |
| Body Composition | ||||||||||||
| Body Mass Index (kg/m2) | 23.3 ± 3.45 | 23.35 ± 3.27 | 0.278 | 0.600 | 23.4 ± 3.40 | 23.3 ± 3.32 | 2.011 | 0.163 | 0.19 | −0.16; 0.55 | 1.170 | 0.285 |
| Fat mass index (kg/m2) | 15.1 ± 6.46 | 14.9 ± 6.34 | 0.073 | 0.788 | 15.2 ± 6.51 | 14.8 ± 6.20 | 2.924 | 0.094 | 0.22 | −0.19; 0.63 | 1.143 | 0.291 |
| Waist Circumference (cm) | 76.4 ± 8.47 | 81.5 ± 36.28 | 0.998 | 0.323 | 76.9 ± 8.98 | 76.4 ± 8.27 | 3.492 | 0.068 | 5.71 | −4.58; 16.00 | 1.249 | 0.270 |
| Blood Samples | ||||||||||||
| Biochemistry | ||||||||||||
| GOT (U/L) | 25.3 ± 7.82 | 26.0 ± 7.23 | 0.333 | 0.567 | 27.2 ± 9.1 | 25.7 ± 6.6 | 0.979 | 0.328 | 2.23 | −1.65; 6.12 | 1.341 | 0.253 |
| Triglycerides (mg/dL) | 75.1 ± 38.77 | 79.7 ± 32.08 | 1.833 | 0.182 | 70.3 ± 26.28 | 81.3 ± 37.01 | 7.915 | 0.007 | −6.09 | −17.39; 5.22 | 1.176 | 0.284 |
| HDL cholesterol (mg/dL) | 56.9 ± 14.66 | 56.5 ± 13.83 | 0.110 | 0.741 | 56.9 ± 15.19 | 57.1 ± 14.51 | 0.016 | 0.901 | −0.57 | −4.38; 3.24 | 0.090 | 0.765 |
| Total cholesterol/HDL cholesterol | 2.7 ± 0.70 | 2.8 ± 0.74 | 2.640 | 0.111 | 2.8 ± 0.79 | 2.8 ± 0.77 | 0.633 | 0.430 | 0.04 | 0.14; 0.21 | 0.177 | 0.676 |
| Iron metabolism | ||||||||||||
| Iron (μg/dL) (n = 46) | 101.1 ± 39.6 | 97.3 ± 34.02 | 0.363 | 0.550 | 112.6 ± 44.09 | 99.0 ± 33.78 | 3.363 | 0.073 | −4.81 | −18.16; 8.53 | 0.970 | 0.330 |
| Transferrin Saturation Index (%) | 30.6 ± 11.33 | 28.87 ± 9.93 | 0.945 | 0.336 | 35.5 ± 14.39 | 29.6 ± 11.2 | 6.826 | 0.012 | 4.15 | −1.18; 9.47 | 2.460 | 0.124 |
| Other minerals | ||||||||||||
| Zinc (μg/dL) | 89.4 ± 12.58 | 91.3 ± 12.57 | 1.994 | 0.165 | 91.1 ± 12.71 | 96.1 ± 22.31 | 3.017 | 0.089 | −3.17 | −8.93; 2.59 | 1.229 | 0.274 |
| Other variables | ||||||||||||
| Apolipoprotein A1 (mg/dL) | 161.6 ± 25.86 | 157.5 ± 21.31 | 2.427 | 0.126 | 160.1 ± 23.86 | 158.8 ± 25.42 | 0.239 | 0.627 | −2.68 | −9.75; 4.38 | 0.585 | 0.448 |
| Fatty Acids profile | ||||||||||||
| Palmitic Acid (%) | 21.9 ± 2.80 | 22.3 ± 2.59 | 1.105 | 0.299 | 22.3 ± 2.34 | 22.2 ± 2.91 | 0.115 | 0.736 | 0.51 | −0.72; 1.73 | 0.692 | 0.410 |
| Stearic Acid (%) | 7.2 ± 1.00 | 7.4 ± 0.82 | 1.774 | 0.190 | 7.1 ± 1.02 | 7.2 ± 0.85 | 0.008 | 0.927 | 0.17 | −0.35; 0.69 | 0.437 | 0.512 |
| Oleic Acid (%) | 20.0 ± 4.15 | 19.8 ± 3.4 | 0.139 | 0.711 | 19.2 ± 3.04 | 20.3 ± 3.91 | 4.376 | 0.042 | −0.82 | −2.12; 0.49 | 1.603 | 0.213 |
| AA_W6 (%) | 7.3 ± 1.99 | 6.7 ± 1.83 | 11.198 | 0.002 | 7.3 ± 1.53 | 6.9 ± 1.49 | 4.142 | 0.048 | −0.20 | −0.76; 0.37 | 0.493 | 0.486 |
| EPA (%) | 0.0 ± 0.0 | 0.03 ± 0.19 | 1.704 | 0.199 | 0.0 ± 0.0 | 0.04 ± 0.19 | 1.703 | 0.199 | 0.03 | −0.04; 0.09 | 0.789 | 0.379 |
| DHA (%) | 1.4 ± 0.83 | 1.7 ± 1.78 | 1.085 | 0.303 | 1.3 ± 0.96 | 1.3 ± 0.80 | 0.021 | 0.885 | 0.27 | −0.39; 0.93 | 0.681 | 0.414 |
| SFA (%) | 30.9 ± 2.91 | 31.6 ± 2.69 | 2.813 | 0.101 | 31.1 ± 2.17 | 30.9 ± 2.70 | 0.060 | 0.807 | 0.76 | −0.52; 2.03 | 1.434 | 0.238 |
| UFA (%) | 69.1 ± 2.91 | 68.4 ± 2.69 | 2.813 | 0.101 | 69.0 ± 2.17 | 69.1 ± 2.70 | 0.060 | 0.807 | −0.76 | −2.03; 0.52 | 1.434 | 0.238 |
| MUFA (%) | 21.0 ± 4.31 | 20.8 ± 3.55 | 0.212 | 0.647 | 20.0 ± 3.20 | 21.1 ± 3.85 | 4.131 | 0.048 | −0.84 | −2.13; 0.46 | 1.704 | 0.199 |
| DUFA (%) | 39.4 ± 5.13 | 39.1 ± 4.04 | 0.171 | 0.681 | 40.3 ± 4.43 | 39.8 ± 3.91 | 0.604 | 0.441 | −0.01 | −1.54; 1.51 | 0.000 | 0.985 |
| MUFA/DUFA (%) | 0.55 ± 0.17 | 0.54 ± 0.13 | 0.306 | 0.583 | 0.51 ± 0.13 | 0.54 ± 0.14 | 2.227 | 0.143 | −0.03 | −0.08; 0.02 | 1.113 | 0.298 |
| PUFA (%) | 48.0 ± 4.73 | 47.5 ± 3.68 | 0.743 | 0.394 | 48.9 ± 4.38 | 48.0 ± 4.00 | 1.676 | 0.202 | −0.02 | −0.06; 0.02 | 0.965 | 0.332 |
| MUFA/PUFA (%) | 0.45 ± 0.13 | 0.44 ± 0.11 | 0.118 | 0.733 | 0.42 ± 0.10 | 045 ± 0.12 | 2.906 | 0.095 | −0.02 | −0.06; 0.02 | 0.965 | 0.332 |
| PUFA n6 (%) | 45.7 ± 7.61 | 45.0 ± 7.12 | 1.533 | 0.223 | 47.6 ± 4.37 | 45.9 ± 7.23 | 2.641 | 0.111 | 0.83 | −1.80; 3.46 | 0.409 | 0.526 |
| PUFA n3 (%) | 1.37 ± 0.83 | 1.7 ± 1.79 | 1.043 | 0.243 | 1.3 ± 0.96 | 1.3 ± 0.82 | 0.003 | 0.959 | 0.30 | −0.36; 0.95 | 0.836 | 0.366 |
| PUFA > 18C n6 (%) | 7.3 ± 1.99 | 6.7 ± 1.83 | 11.198 | 0.002 | 7.3 ± 1.53 | 6.9 ± 1.49 | 4.142 | 0.048 | −0.20 | −0.76; 0.37 | 0.493 | 0.486 |
| PUFA > 18C n3 (%) | 1.4 ± 0.83 | 1.7 ± 1.79 | 1.403 | 0.243 | 1.3 ± 0.96 | 1.3 ± 0.82 | 0.003 | 0.959 | 0.30 | −0.36; 0.95 | 0.836 | 0.366 |
| SFA/MUFA (%) | 1.5 ± 0.43 | 1.6 ± 0.32 | 0.224 | 0.638 | 1.6 ± 0.29 | 1.5 ± 0.33 | 2.957 | 0.092 | 0.07 | −0.08; 0.21 | 0.982 | 0.328 |
| Chicken-Based Diet Group n = 23 | Pirenaica Breed-Based Diet Group n = 24 | Mean Differences between Beginning and End between Control and Intervention in the First Period n = 47 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | F | p1 | Before | After | F | p1 | Mean Differences | 95% IC | F | p2 | |
| Blood Pressure | ||||||||||||
| Systolic Pressure (mmHg) | 109.99 ± 10.15 | 113.23 ± 9.82 | 5.94 | 0.023 | 116.04 ± 15.28 | 117 ± 13.95 | 0.728 | 0.402 | 1.927 | −2.20; 6.05 | 0.885 | 0.352 |
| Diastolic Pressure (mmHg) | 66.81 ± 6.92 | 67.03 ± 6.80 | 0.032 | 0.860 | 67.19 ± 5.33 | 68.43 ± 6.18 | 0.866 | 0.362 | −1.019 | −4.66; 2.62 | 0.318 | 0.576 |
| Blood Samples | ||||||||||||
| Hematology | ||||||||||||
| Hemoglobin (106/μdL) | 109.99 ± 10.15 | 113.23 ± 9.82 | 1.694 | 0.002 | 116.04 ± 15.28 | 117 ± 13.95 | 3.473 | 0.075 | −0.169 | −0.47; 0.13 | 1.319 | 0.257 |
| Hematocrit (%) | 66.81 ± 6.92 | 67.03 ± 6.80 | 1.611 | 0.218 | 67.19 ± 5.33 | 68.43 ± 6.18 | 2.435 | 0.132 | −1.025 | −2.06; 0.01 | 4.000 | 0.052 |
| Biochemistry | ||||||||||||
| Glucose (mg/dL) | 80.13 ± 4.10 | 77.13 ± 5.83 | 8.496 | 0.008 | 80.46 ± 5.87 | 77.50 ± 6.90 | 6.573 | 0.017 | −0.042 | −3.17; 3.08 | 0.001 | 0.979 |
| GPT (U/L) | 19.57 ± 5.00 | 23.09 ± 10.3 | 2.673 | 0.116 | 25.11 ± 10.98 | 28.96 ± 11.91 | 1.741 | 0.200 | 0.272 | −6.34; 6.88 | 0.007 | 0.934 |
| GGT (U/L) | 19.57 ± 5.00 | 23.09 ± 10.3 | 30.646 | <0.001 | 25.11 ± 10.98 | 28.96 ± 11.91 | 20.748 | <0.001 | −1.697 | −3.66; 0.26 | 3.037 | 0.088 |
| Total cholesterol (mg/dL) | 148.04 ± 41.57 | 149.22 ± 36.28 | 0.182 | 0.674 | 151.08 ± 22.85 | 152.71 ± 27.07 | 0.290 | 0.596 | −0.451 | −8.70; 7.80 | 0.012 | 0.913 |
| LDL cholesterol (mg/dL) | 77.65 ± 33.94 | 74.48 ± 31.34 | 2.073 | 0.164 | 83.33 ± 21.32 | 80.00 ± 21.35 | 2.249 | 0.147 | 0.159 | −6.15; 6.47 | 0.003 | 0.960 |
| LDL cholesterol/HDL cholesterol | 1.40 ± 0.71 | 1.31 ± 0.61 | 1.842 | 0.188 | 1.70 ± 0.68 | 1.56 ± 0.57 | 4.903 | 0.037 | 0.05 | −0.13; 0.24 | 0.367 | 0.548 |
| Iron metabolism | ||||||||||||
| Ferritin (ng/mL) | 55.75 ± 30.53 | 44.11 ± 45.70 | 1.296 | 0.267 | 88.56 ± 58.03 | 66.92 ± 47.01 | 24.707 | <0.001 | 10.000 | −12.05; 32.07 | 0.835 | 0.366 |
| Transferrin (mg/dL) | 261.43 ± 33.69 | 276.33 ± 30.01 | 12.811 | 0.002 | 242.38 ± 25.38 | 266.42 ± 33.26 | 31.398 | <0.001 | −9.146 | −21.20; 2.91 | 2.336 | 0.133 |
| Other minerals | ||||||||||||
| Magnesium (mmol/L) | 0.89 ± 0.08 | 0.83 ± 0.05 | 9.625 | 0.005 | 0.87 ± 0.06 | 0.84 ± 0.05 | 3.119 | 0.091 | −0.032 | −0.08; 0.02 | 1.599 | 0.213 |
| Calcium (mg/dL) | 10.10 ± 0.45 | 9.52 ± 0.40 | 30.985 | <0.001 | 10.22 ± 0.29 | 9.46 ± 0.26 | 123.626 | <0.001 | 0.167 | −0.08; 0.42 | 1.810 | 0.185 |
| Other variables | ||||||||||||
| Apolipoprotein B (mg/dL) | 73.43 ± 24.87 | 69.61 ± 19.91 | 4.738 | 0.041 | 78.50 ± 17.00 | 76.21 ± 16.06 | 2.510 | 0.127 | −1.534 | −6.10; 3.03 | 0.458 | 0.502 |
| C Reactive Protein (mg/dL) | 0.22 ± 0.08 | 0.51 ± 0.09 | 134.096 | <0.001 | 0.34 ± 0.56 | 0.52 ± 0.14 | 2.244 | 0.148 | 0.117 | −0.13; 0.36 | 0.929 | 0.340 |
| Fatty Acids | ||||||||||||
| Linoleic Acid (%) | 38.01 ± 3.34 | 39.36 ± 4.72 | 2.005 | 0.171 | 39.74 ± 4.72 | 39.78 ± 3.87 | 0.002 | 0.961 | 1.308 | 1.20; 3.81 | 1.11 | 0.298 |
| Blood Samples Iron Metabolism | Chicken-Based Diet Group | Pyrenees Beef-Based Diet Group | Mean Differences between Beginning and End between Both Groups | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | F | p1 | Before | After | F | p1 | Mean Diff | 95% IC | F | p2 | ||
| Overall sample (n = 47) | |||||||||||||
| Iron (μg/dL) (n = 46) | Male | 98.8 ± 35.30 | 102.5 ± 37.41 | 0.279 | 0.602 | 116.91 ± 43.55 | 104.96 ± 30.97 | 1.065 | 0.313 | 12.21 | −18.78; 43.20 | 0.671 | 0.422 |
| Female | 103.50 ± 44.37 | 91.78 ± 29.92 | 1.175 | 0.290 | 108.96 ± 30.97 | 94.13 ± 36.50 | 2.741 | 0.112 | 0.000 | −24; 44; 24.44 | 0.000 | 1.000 | |
| Transferrin Saturation Index (%) | Male | 30.53 ± 10.63 | 30.91 ± 10.25 | 0.035 | 0.852 | 39.47 ± 15.10 | 32.50 ± 10.63 | 3.239 | 0.085 | 7.86 | 1.99; 17.72 | 2.734 | 0.112 |
| Female | 30.76 ± 12.25 | 26.73 ± 9.32 | 1.799 | 0.194 | 31.36 ± 12.62 | 26.50 ± 11.15 | 4.211 | 0.052 | 1.06 | −5.79; 7.91 | 0.104 | 0.750 | |
| 1st period of the study (Control: n = 23. Intervention. n = 24) | |||||||||||||
| Ferritin (ng/mL) | Male | 65.13 ± 28.34 | 76.00 ± 72.69 | 0.125 | 0.736 | 111.42 ± 51.75 | 86.00 ± 41.86 | 20.218 | <0.001 | 36.29 | −7.44; 80.02 | 2.962 | 0.099 |
| Female | 51.65 ± 31.41 | 30.16 ± 16.35 | 13.828 | 0.002 | 33.05 ± 26.31 | 20.57 ± 16.11 | 6.921 | 0.039 | −9.00 | −28.46; 10.46 | 0.925 | 0.347 | |
| Transferrin (mg/dL) | Male | 257.14 ± 31.27 | 279.55 ± 34.72 | 8.216 | 0.029 | 236.12 ± 23.22 | 252.32 ± 25.26 | 21.192 | <0.001 | 1.05 | −12.22; 14.32 | 0.027 | 0.871 |
| Female | 263.31 ± 35.52 | 274.91 ± 28.84 | 5.746 | 0.030 | 257.57 ± 25.51 | 300.66 ± 24.63 | 24.308 | <0.001 | 6.74 | −5.16; 18.63 | 1.387 | 0.252 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santaliestra-Pasías, A.M.; Miguel-Berges, M.L.; Campo, M.M.; Guerrero, A.; Olleta, J.L.; Santolaria, P.; Moreno, L.A. Effect of the Intake of Lean Red-Meat from Beef-(Pirenaica Breed) versus Lean White-Meat on Body Composition, Fatty Acids Profile and Cardiovascular Risk Indicators: A Randomized Cross-Over Study in Healthy Young Adults. Nutrients 2022, 14, 3724. https://doi.org/10.3390/nu14183724
Santaliestra-Pasías AM, Miguel-Berges ML, Campo MM, Guerrero A, Olleta JL, Santolaria P, Moreno LA. Effect of the Intake of Lean Red-Meat from Beef-(Pirenaica Breed) versus Lean White-Meat on Body Composition, Fatty Acids Profile and Cardiovascular Risk Indicators: A Randomized Cross-Over Study in Healthy Young Adults. Nutrients. 2022; 14(18):3724. https://doi.org/10.3390/nu14183724
Chicago/Turabian StyleSantaliestra-Pasías, Alba M., María L. Miguel-Berges, María M. Campo, Ana Guerrero, José Luis Olleta, Pilar Santolaria, and Luis A. Moreno. 2022. "Effect of the Intake of Lean Red-Meat from Beef-(Pirenaica Breed) versus Lean White-Meat on Body Composition, Fatty Acids Profile and Cardiovascular Risk Indicators: A Randomized Cross-Over Study in Healthy Young Adults" Nutrients 14, no. 18: 3724. https://doi.org/10.3390/nu14183724
APA StyleSantaliestra-Pasías, A. M., Miguel-Berges, M. L., Campo, M. M., Guerrero, A., Olleta, J. L., Santolaria, P., & Moreno, L. A. (2022). Effect of the Intake of Lean Red-Meat from Beef-(Pirenaica Breed) versus Lean White-Meat on Body Composition, Fatty Acids Profile and Cardiovascular Risk Indicators: A Randomized Cross-Over Study in Healthy Young Adults. Nutrients, 14(18), 3724. https://doi.org/10.3390/nu14183724









