Higher Intake of Fat, Vitamin E-(β+γ), Magnesium, Sodium, and Copper Increases the Susceptibility to Prostatitis-like Symptoms: Evidence from a Chinese Adult Cohort
Abstract
:1. Introduction
2. Materials and Methods
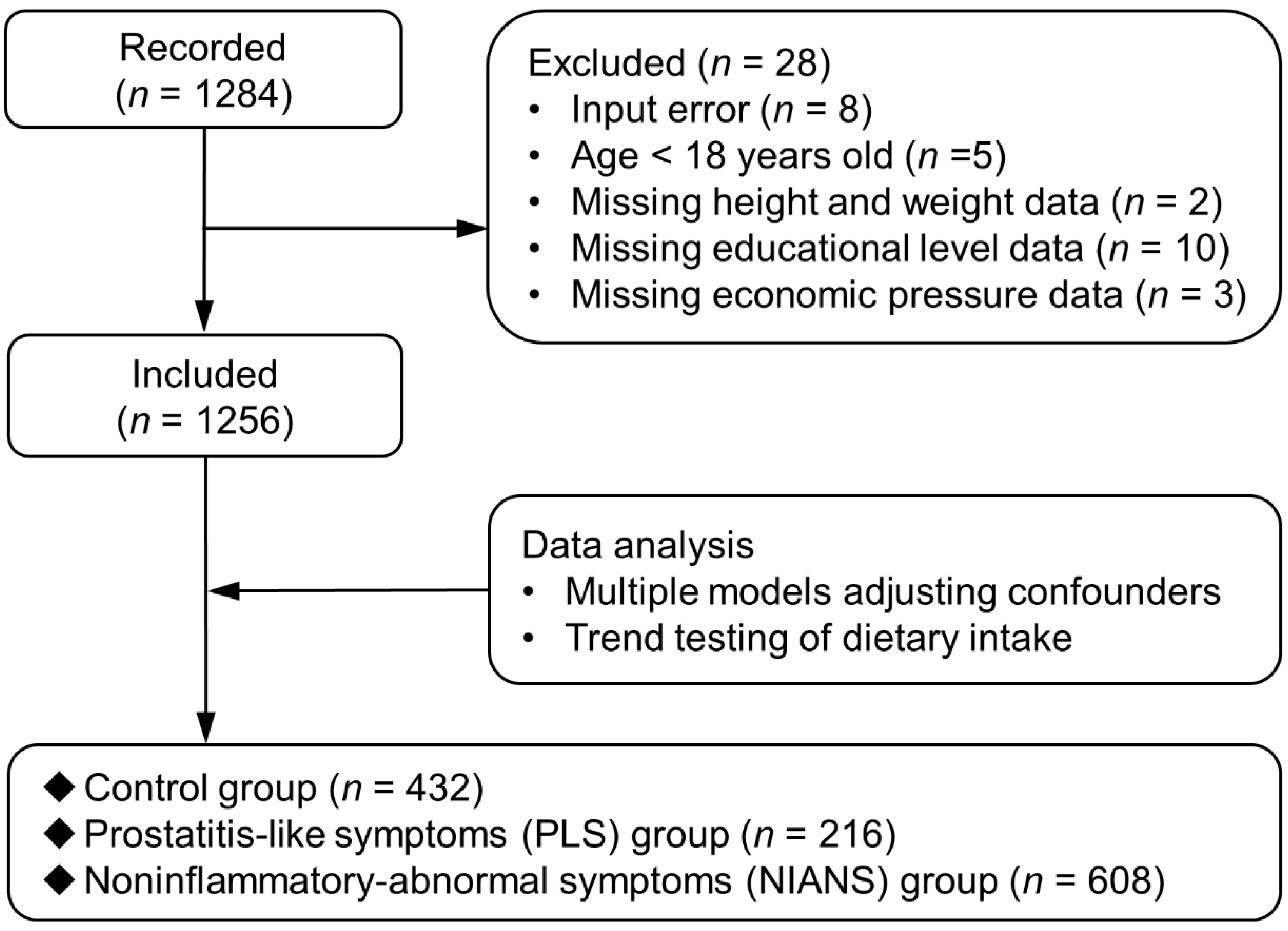
2.1. Participants Summary
2.2. Inclusion and Exclusion Criteria
2.3. Variables Definition and Data Collection
2.4. Statistical Analyses
3. Results
3.1. Basic Information of Enrolled Participants
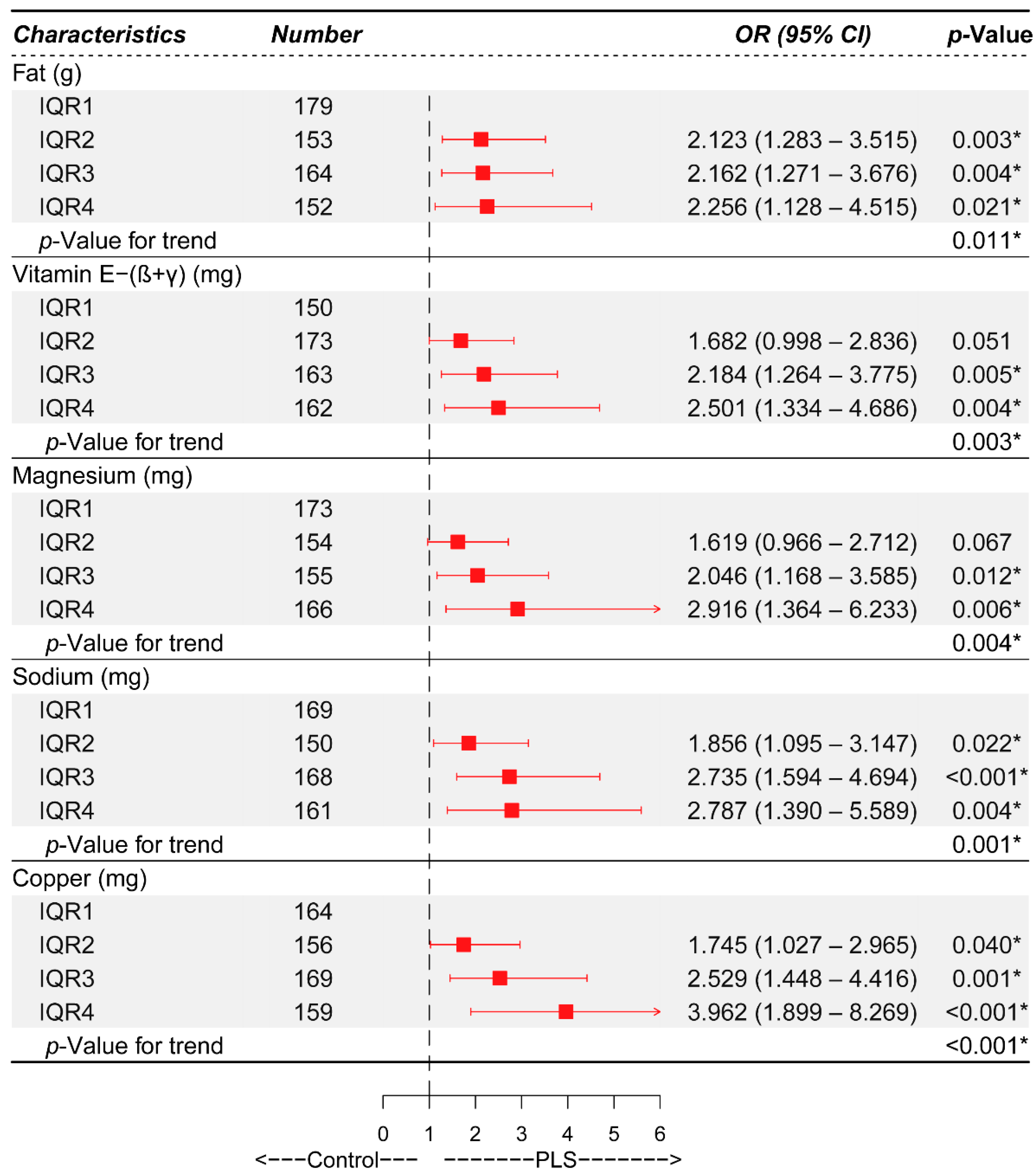
3.2. Identification of Independent Risk Factors of PLS via Different Logistic Regression Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehik, A.; Hellström, P.; Lukkarinen, O.; Sarpola, A.; Jarvelin, M.-R. Epidemiology of prostatitis in Finnish men: A population-based cross-sectional study. Br. J. Urol. 2000, 86, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.N.; Lee, S.W.H.; Jeon, J.; Cheah, P.Y.; Liong, M.L.; Riley, D.E. Epidemiology of prostatitis. Int. J. Antimicrob. Agents 2008, 31, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.A.; Pitts, M.K.; Richters, J.; Simpson, J.M.; Shelley, J.M.; Smith, A.M. National prevalence of urogenital pain and prostatitis-like symptoms in Australian men using the National Institutes of Health Chronic Prostatitis Symptoms Index. Br. J. Urol. 2010, 105, 373–379. [Google Scholar] [CrossRef]
- Liang, C.-Z.; Li, H.-J.; Wang, Z.-P.; Xing, J.-P.; Hu, W.-L.; Zhang, T.-F.; Ge, W.-W.; Hao, Z.-Y.; Zhang, X.-S.; Zhou, J.; et al. The Prevalence of Prostatitis-Like Symptoms in China. J. Urol. 2009, 182, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, C.; Peng, Y.; Lu, J.; Yang, N.Q.; Chen, L.; Zhang, G.Q.; Tang, L.K.; Dai, J.C. Association of diet and lifestyle with chronic prostatitis/chronic pelvic pain syndrome and pain severity: A case–control study. Prostate Cancer Prostatic Dis. 2015, 19, 92–99. [Google Scholar] [CrossRef]
- Bartoletti, R.; Cai, T.; Mondaini, N.; Dinelli, N.; Pinzi, N.; Pavone, C.; Gontero, P.; Gavazzi, A.; Giubilei, G.; Prezioso, D.; et al. Prevalence, Incidence Estimation, Risk Factors and Characterization of Chronic Prostatitis/Chronic Pelvic Pain Syndrome in Urological Hospital Outpatients in Italy: Results of a Multicenter Case-Control Observational Study. J. Urol. 2007, 178, 2411–2415. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, C.; Kong, X.; Meng, J.; Fan, S.; Ding, Y.; Fang, Q.; Dong, T.; Mm, H.Z.; Ni Mm, J.; et al. Identification of novel susceptibility factors related to CP/CPPS-like symptoms: Evidence from a multicenter case-control study. Prostate 2022, 82, 772–782. [Google Scholar] [CrossRef]
- Breser, M.L.; Salazar, F.C.; Rivero, V.E.; Motrich, R.D. Immunological Mechanisms Underlying Chronic Pelvic Pain and Prostate Inflammation in Chronic Pelvic Pain Syndrome. Front. Immunol. 2017, 8, 898. [Google Scholar] [CrossRef]
- Vykhovanets, E.V.; Shukla, S.; MacLennan, G.T.; Vykhovanets, O.V.; Bodner, D.R.; Gupta, S. Il-1β-induced post-transition effect of NF-kappaB provides time-dependent wave of signals for initial phase of intrapostatic inflammation. Prostate 2009, 69, 633–643. [Google Scholar] [CrossRef]
- Breser, M.L.; Motrich, R.; Sanchez, L.R.; Mackern-Oberti, J.P.; Rivero, V.E. Expression of CXCR3 on Specific T Cells Is Essential for Homing to the Prostate Gland in an Experimental Model of Chronic Prostatitis/Chronic Pelvic Pain Syndrome. J. Immunol. 2013, 190, 3121–3133. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Lewis, E.D.; Pae, M.; Meydani, S.N. Nutritional Modulation of Immune Function: Analysis of Evidence, Mechanisms, and Clinical Relevance. Front. Immunol. 2019, 9, 3160. [Google Scholar] [CrossRef] [PubMed]
- Dolan, L.C.; Matulka, R.A.; Burdock, G.A. Naturally Occurring Food Toxins. Toxins 2010, 2, 2289–2332. [Google Scholar] [CrossRef]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Meydani, S.N. Age-Associated Changes in Immune Function: Impact of Vitamin E Intervention and the Underlying Mechanisms. Endocr. Metab. Immune Disord. Drug Targets 2014, 14, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Laires, M.J.; Monteiro, C. Exercise, magnesium and immune function. Magnes Res. 2008, 21, 92–96. [Google Scholar]
- Petrović, J.; Stanić, D.; Dmitrašinović, G.; Plećaš-Solarović, B.; Ignjatović, S.; Batinić, B.; Popović, D.; Pešić, V. Magnesium Supplementation Diminishes Peripheral Blood Lymphocyte DNA Oxidative Damage in Athletes and Sedentary Young Man. Oxidative Med. Cell. Longev. 2016, 2016, 2019643. [Google Scholar] [CrossRef]
- Saeed, F.; Nadeem, M.; Ahmed, R.S.; Tahir Nadeem, M.; Arshad, M.S.; Ullah, A. Studying the impact of nutritional immunology underlying the modulation of immune responses by nutritional compounds—A review. Food Agric. Immunol. 2016, 27, 205–229. [Google Scholar] [CrossRef]
- Medina, K.L. Overview of the Immune System. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 133, pp. 61–76. [Google Scholar]
- Altobelli, G.G.; Van Noorden, S.; Balato, A.; Cimini, V. Copper/Zinc Superoxide Dismutase in Human Skin: Current Knowledge. Front. Med. 2020, 7, 183. [Google Scholar] [CrossRef]
- Nickel, J.C.; Downey, J.; Hunter, D.; Clark, J. Prevalence of prostatitis-like symptoms in a population based study using the Na-tional Institutes of Health chronic prostatitis symptom index. J. Urol. 2001, 165, 842–845. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, Y.; Li, F.; Shao, Y.; Sun, Z.; Zhong, C.; Fan, P.; Li, Z.; Zhang, M.; Li, X.; et al. Development and validation of a photographic atlas of food portions for accurate quantification of dietary intakes in China. J. Hum. Nutr. Diet. 2021, 34, 604–615. [Google Scholar] [CrossRef]
- Yang, Y.W.Z.; He, M.; Pan, X. Chinese Food Composition Table Standard Edition, 6th ed.; Peking University Medical Press: Beijing, China, 2019. [Google Scholar]
- Casas, R.; Sacanella, E.; Estruch, R. The Immune Protective Effect of the Mediterranean Diet against Chronic Low-grade Inflammatory Diseases. Endocr. Metab. Immune Disord. Drug Targets 2014, 14, 245–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, K.; Giugliano, D. Diet and inflammation: A link to metabolic and cardiovascular diseases. Eur. Hear. J. 2005, 27, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Ceriello, A.; Esposito, K. The Effects of Diet on Inflammation: Emphasis on the Metabolic Syndrome. J. Am. Coll. Cardiol. 2006, 48, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Hersoug, L.-G.; Linneberg, A. The link between the epidemics of obesity and allergic diseases: Does obesity induce decreased immune tolerance? Allergy 2007, 62, 1205–1213. [Google Scholar] [CrossRef]
- Jeffery, N.M.; Sanderson, P.; Newsholme, A.E.; Calder, P. Effects of varying the type of saturated fatty acid in the rat diet upon serum lipid levels and spleen lymphocyte functions. Biochim. Biophys. Acta BBA Lipids Lipid Metab. 1997, 1345, 223–236. [Google Scholar] [CrossRef]
- Jeffery, N.M.; Cortina, M.; Newsholme, E.A.; Calder, P.C. Effects of variations in the proportions of saturated, monounsaturated and polyunsaturated fatty acids in the rat diet on spleen lymphocyte functions. Br. J. Nutr. 1997, 77, 805–823. [Google Scholar] [CrossRef]
- Wallace, F.A.; Miles, E.A.; Evans, C.; Stock, T.E.; Yaqoob, P.; Calder, P.C. Dietary fatty acids influence the production of Th1- but not Th2-type cytokines. J. Leukoc. Biol. 2001, 69, 449–457. [Google Scholar]
- Usoro, O.B.; Mousa, S.A. Vitamin E Forms in Alzheimer’s Disease: A Review of Controversial and Clinical Experiences. Crit. Rev. Food Sci. Nutr. 2010, 50, 414–419. [Google Scholar] [CrossRef]
- Wells, S.R.; Jennings, M.H.; Rome, C.; Hadjivassiliou, V.; Papas, K.A.; Alexander, J.S. α-, γ- and δ-tocopherols reduce inflammatory angiogenesis in human microvascular endothelial cells. J. Nutr. Biochem. 2010, 21, 589–597. [Google Scholar] [CrossRef]
- Lee, G.Y.; Han, S.N. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef]
- Subramaniam, S.; Rao, J.S.A.; Ramdas, P.; Ng, M.H.; Kutty, M.K.; Selvaduray, K.R.; Radhakrishnan, A.K. Reduced infiltration of regulatory T cells in tumours from mice fed daily with gamma-tocotrienol supplementation. Clin. Exp. Immunol. 2021, 206, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Schwalfenberg, G.K.; Genuis, S.J. The Importance of Magnesium in Clinical Healthcare. Scientifica 2017, 2017, 4179326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shechter, M. Magnesium and cardiovascular system. Magnes. Res. 2010, 23, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.R.; Appel, L.J.; Whelton, P.K. Sodium Intake and All-Cause Mortality Over 20 Years in the Trials of Hypertension Prevention. J. Am. Coll. Cardiol. 2016, 68, 1609–1617. [Google Scholar] [CrossRef]
- Hucke, S.; Eschborn, M.; Liebmann, M.; Herold, M.; Freise, N.; Engbers, A.; Ehling, P.; Meuth, S.G.; Roth, J.; Kuhlmann, T.; et al. Sodium chloride promotes pro-inflammatory macrophage polarization thereby aggravating CNS autoimmunity. J. Autoimmun. 2016, 67, 90–101. [Google Scholar] [CrossRef]
- Kleinewietfeld, M.; Manzel, A.; Titze, J.; Kvakan, H.; Yosef, N.; Linker, R.A.; Muller, D.N.; Hafler, D.A. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013, 496, 518–522. [Google Scholar] [CrossRef]
- Wu, C.; Yosef, N.; Thalhamer, T.; Zhu, C.; Xiao, S.; Kishi, Y.; Regev, A.; Kuchroo, V.K. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 2013, 496, 513–517. [Google Scholar] [CrossRef]
- Knezović, A.; Kolobarić, N.; Drenjančević, I.; Mihaljević, Z.; Šušnjara, P.; Jukić, I.; Stupin, M.; Kibel, A.; Marczi, S.; Mihalj, M.; et al. Role of Oxidative Stress in Vascular Low-Grade Inflammation Initiation Due to Acute Salt Loading in Young Healthy Individuals. Antioxidants 2022, 11, 444. [Google Scholar] [CrossRef]
- Hernandez, A.L.; Kitz, A.; Wu, C.; Lowther, D.E.; Rodriguez, D.M.; Vudattu, N.; Deng, S.; Herold, K.C.; Kuchroo, V.K.; Kleinewietfeld, M.; et al. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J. Clin. Investig. 2015, 125, 4212–4222. [Google Scholar] [CrossRef]
- Besold, A.N.; Culbertson, E.M.; Culotta, V.C. The Yin and Yang of copper during infection. JBIC J. Biol. Inorg. Chem. 2016, 21, 137–144. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, R.; Shen, J.; Jin, Y.; Chang, C.; Hong, M.; Guo, S.; He, D. Circulating Level of Blood Iron and Copper Associated with Inflammation and Disease Activity of Rheumatoid Arthritis. Biol. Trace Element Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Dong, J.; Cheng, N.; Yang, R.; Han, Y.; Han, Y. Inflammatory cytokines expression in Wilson’s disease. Neurol. Sci. 2019, 40, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Zhao, J.; Bulek, K.; Tang, F.; Chen, X.; Cai, G.; Jia, S.; Fox, P.L.; Huang, E.; Pizarro, T.T.; et al. Inflammation mobilizes copper metabolism to promote colon tumorigenesis via an IL-17-STEAP4-XIAP axis. Nat. Commun. 2020, 11, 900. [Google Scholar] [CrossRef] [Green Version]
| Variables | Total (n = 1256) | Control Group (n = 432) | PLS Group (n = 216) | NIANS Group (n = 608) | p-Value 1 | p-Value 2 | p-Value 3 |
|---|---|---|---|---|---|---|---|
| Age (y) * | 21.0 (20.0, 26.0) | 21.0 (20.0, 25.0) | 26.0 (23.0, 38.0) | 21.0 (20.0, 24.0) | <0.001 | <0.001 | 0.612 |
| BMI (kg/m2) * | 22.6 (20.5, 25.0) | 22.5 (20.5, 24.6) | 22.9 (20.9, 25.5) | 22.6 (20.4, 25.0) | 0.316 | 0.129 | 0.646 |
| Educational level # | <0.001 | <0.001 | 0.277 | ||||
| Senior high school or bachelor’s degrees | 1093 (87.0%) | 387 (89.6%) | 165 (76.4%) | 541 (89.0%) | |||
| Junior high school or below | 99 (7.9%) | 27 (6.2%) | 42 (19.4%) | 30 (5.0%) | |||
| Master’s degrees or above | 64 (5.1%) | 18 (4.2%) | 9 (4.2%) | 37 (6.0%) | |||
| Economic pressure # | 0.091 | 0.404 | 0.023 | ||||
| None | 404 (32.2%) | 161 (37.3%) | 66 (30.6%) | 177 (29.1%) | |||
| Mild | 484 (38.5%) | 148 (34.3%) | 80 (37.0%) | 256 (42.1%) | |||
| Moderate | 274 (21.8%) | 90 (20.8%) | 51 (23.6%) | 133 (21.9%) | |||
| Severe | 94 (7.5%) | 33 (7.6%) | 19 (8.8%) | 42 (6.9%) | |||
| Total food intake per day (g) * | 1107.0 (760.0, 1542.5) | 1045.7 (720.0, 1502.5) | 1150.6 (814.6, 1613.1) | 1121.6 (789.7, 1545.1) | 0.091 | 0.044 | 0.098 |
| Total energy intake per day (KJ) * | 5157.4 (3545.2, 7656.7) | 4779.0 (3290.0, 7607.9) | 5675.9 (3808.2, 7716.0) | 5328.4 (3610.9, 7619.8) | 0.039 | 0.019 | 0.055 |
| Protein (g) * | 64.7 (42.1, 99.7) | 59.9 (38.5, 96.1) | 68.8(48.7, 99.7) | 65.4 (43.7, 105.0) | 0.024 | 0.017 | 0.023 |
| Fat (g) * | 33.8 (21.4, 56.0) | 32.4 (18.0, 54.5) | 37.6 (24.4, 54.4) | 35.1 (22.8, 57.0) | 0.022 | 0.031 | 0.013 |
| Carbohydrate (g) * | 162.9(108.5, 240.9) | 151.6 (103.4, 228.8) | 173.6 (125.0, 244.8) | 163.0 (108.7, 240.0) | 0.065 | 0.019 | 0.312 |
| Dietary fiber (g) * | 7.24 (4.4, 12.2) | 6.7 (3.8, 11.7) | 8.5 (5.3, 13.2) | 7.2 (4.3, 12.4) | 0.002 | <0.001 | 0.124 |
| Cholesterol (mg) * | 346.2 (156.9, 519.8) | 311.0 (137.7, 482.1) | 385.5(180.6, 545.3) | 356.3 (162.9, 539.3) | 0.016 | 0.006 | 0.044 |
| Ash (g) * | 8.0 (5.2, 12.1) | 7.3 (4.8, 11.5) | 8.7 (5.9, 12.6) | 8.1 (5.3, 12.0) | 0.007 | 0.003 | 0.025 |
| Vitamin A (µg) * | 494.9(287.6, 802.9) | 481.5 (261.0, 770.5) | 495.5 (323.7, 847.4) | 501.0 (296.8, 804.7) | 0.120 | 0.080 | 0.082 |
| Carotene (µg) * | 1465.3 (539.3, 2868.0) | 1312.8 (459.3, 2897.9) | 1668.1 (873.0, 3068.7) | 1440.1 (541.7, 2775.6) | 0.046 | 0.022 | 0.691 |
| Retinol (µg) * | 173.2 (98.1, 272.4) | 158.9 (89.8, 258.8) | 159.3 (88.9, 248.9) | 189.4 (108.0, 292.0) | 0.003 | 0.841 | 0.002 |
| Thiamin (mg) * | 0.6 (0.44, 0.9) | 0.5 (0.3, 0.9) | 0.6 (0.4, 0.9) | 0.6 (0.4, 0.9) | 0.088 | 0.095 | 0.042 |
| Riboflavin (mg) * | 0.8 (0.5, 1.2) | 0.8 (0.5, 1.2) | 0.85 (0.6, 1.2) | 0.8 (0.6, 1.3) | 0.022 | 0.060 | 0.008 |
| Vitamin C (mg) * | 53.9 (28.2, 94.4) | 51.9 (24.5, 91.3) | 62.8 (33.5, 107.9) | 52.5 (28.2, 92.2) | 0.014 | 0.005 | 0.527 |
| Vitamin E [total (mg)] * | 10.4 (5.9, 18.7) | 9.9(5.3, 17.3) | 13.0 (7.0, 20.6) | 10.4 (6.0, 18.5) | 0.005 | 0.001 | 0.154 |
| Vitamin E-α (mg) * | 4.6 (2.7, 7.7) | 4.2 (2.4, 7.2) | 4.9 (3.2, 8.0) | 4.7 (2.7, 7.7) | 0.008 | 0.002 | 0.091 |
| Vitamin E-(β+γ) (mg) * | 3.4 (1.8, 7.0) | 3.1 (1.6, 6.4) | 4.1 (2.4, 7.9) | 3.4 (1.7, 7.0) | 0.006 | 0.001 | 0.286 |
| Vitamin E-δ (mg) * | 1.8 (0.9, 3.5) | 1.8 (0.9, 3.2) | 1.9 (1.1, 3.9) | 1.8 (0.9, 3.5) | 0.174 | 0.062 | 0.631 |
| Calcium (mg) * | 522.6 (323.2, 782.3) | 492.6 (296.7, 760.3) | 519.3 (332.7, 747.9) | 557.5 (341.0, 798.6) | 0.119 | 0.335 | 0.042 |
| Zinc (mg) * | 9.3 (6.2, 14.5) | 8.6(5.7, 13.7) | 10.1 (6.9, 14.3) | 9.6 (6.4, 15.1) | 0.060 | 0.038 | 0.049 |
| Magnesium (mg) * | 219.1 (143.6, 331.7) | 209.0 (131.3, 319.8) | 242.8 (162.3, 368.2) | 221.0 (146.9, 326.4) | 0.005 | 0.001 | 0.072 |
| Selenium (mg) * | 34.4 (21.4, 55.5) | 31.3(19.5, 51.9) | 37.6 (25.4, 58.8) | 35.3 (21.5, 55.5) | 0.017 | 0.006 | 0.047 |
| Phosphorus (mg) * | 948.4 (626.2, 1377.8) | 868.98 (604.2, 1326.3) | 996.73 (669.9, 1308.7) | 973.64 (647.4, 1409.3) | 0.052 | 0.078 | 0.022 |
| Potassium (mg) * | 1556.9 (1013.5, 2456.7) | 1438.6 (894.0, 2382.2) | 1688.3 (1108.7, 2475.3) | 1589.4 (1046.1, 2467.0) | 0.010 | 0.007 | 0.016 |
| Sodium (mg) * | 565.6 (342.4, 958.0) | 521.1 (298.9, 882.7) | 659.3 (437.4, 1013.1) | 558.4 (356.3, 959.4) | <0.001 | <0.001 | 0.038 |
| Iron (mg) * | 16.1 (10.2, 24.7) | 14.9 (9.6, 23.8) | 17.3 (11.6, 24.8) | 16.3(10.5, 25.1) | 0.036 | 0.019 | 0.045 |
| Copper (mg) * | 1.3 (0.8, 2.1) | 1.2 (0.7, 1.9) | 1.5 (1.0, 2.3) | 1.3 (0.8, 2.2) | <0.001 | <0.001 | 0.070 |
| Manganese (mg) * | 3.7 (2.3, 5.6) | 3.4 (2.2, 5.4) | 4.1 (2.7, 5.7) | 3.8 (2.3, 5.6) | 0.017 | 0.005 | 0.107 |
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | No. | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| Fat (g) * | |||||||||||
| IQR1 | 179 | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| IQR2 | 153 | 2.181 (1.351, 3.522) | 0.001 | 2.053 (1.256, 3.355) | 0.004 | 2.037 (1.244, 3.336) | 0.005 | 2.102 (1.271, 3.476) | 0.004 | 2.123 (1.283, 3.515) | 0.003 |
| IQR3 | 164 | 2.282 (1.425, 3.653) | 0.001 | 1.928 (1.187, 3.133) | 0.008 | 1.933 (1.186, 3.150) | 0.008 | 2.054 (1.218, 3.466) | 0.007 | 2.162 (1.271, 3.676) | 0.004 |
| IQR4 | 152 | 1.807 (1.112, 2.937) | 0.017 | 1.681 (1.023, 2.762) | 0.041 | 1.690 (1.026, 2.786) | 0.039 | 1.927 (1.015, 3.655) | 0.045 | 2.256 (1.128, 4.515) | 0.021 |
| Carotene (µg) * | |||||||||||
| IQR1 | 163 | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| IQR2 | 157 | 1.812 (1.116, 2.944) | 0.016 | 1.630 (0.993, 2.674) | 0.053 | 1.587 (0.964, 2.612) | 0.069 | 1.582 (0.959, 2.607) | 0.072 | 1.571 (0.953, 2.591) | 0.077 |
| IQR3 | 159 | 1.979 (1.223, 3.202) | 0.005 | 1.603 (0.974, 2.638) | 0.064 | 1.621 (0.983, 2.673) | 0.059 | 1.606 (0.963, 2.679) | 0.069 | 1.580 (0.945, 2.641) | 0.081 |
| IQR4 | 169 | 1.705 (1.056, 2.753) | 0.029 | 1.371 (0.835, 2.252) | 0.212 | 1.408 (0.855, 2.318) | 0.178 | 1.375 (0.775, 2.439) | 0.276 | 1.331 (0.744, 2.383) | 0.335 |
| Vitamin E-α (mg) * | |||||||||||
| IQR1 | 165 | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| IQR2 | 165 | 1.960 (1.212, 3.171) | 0.006 | 1.738 (1.061, 2.844) | 0.028 | 1.780 (1.084, 2.921) | 0.023 | 1.789 (1.083, 2.955) | 0.023 | 1.803 (1.091, 2.980) | 0.022 |
| IQR3 | 157 | 2.012 (1.238, 3.269) | 0.005 | 1.766 (1.074, 2.904) | 0.025 | 1.805 (1.093, 2.980) | 0.021 | 1.825 (1.074, 3.101) | 0.026 | 1.844 (1.084, 3.135) | 0.024 |
| IQR4 | 161 | 1.882 (1.159, 3.056) | 0.011 | 1.436 (0.866, 2.381) | 0.161 | 1.486 (0.893, 2.471) | 0.127 | 1.519 (0.825, 2.794) | 0.179 | 1.617 (0.862, 3.034) | 0.134 |
| Vitamin E-(β+γ) (mg) * | |||||||||||
| IQR1 | 150 | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| IQR2 | 173 | 1.719 (1.037, 2.848) | 0.036 | 1.591 (0.952, 2.657) | 0.076 | 1.583 (0.946, 2.650) | 0.080 | 1.640 (0.974, 2.761) | 0.063 | 1.682 (0.998, 2.836) | 0.051 |
| IQR3 | 163 | 2.384 (1.444, 3.936) | 0.001 | 1.958 (1.170, 3.276) | 0.011 | 1.946 (1.159, 3.268) | 0.012 | 2.095 (1.217, 3.609) | 0.008 | 2.184 (1.264, 3.775) | 0.005 |
| IQR4 | 162 | 2.471 (1.497, 4.079) | <0.001 | 1.892 (1.124, 3.182) | 0.016 | 1.927 (1.143, 3.251) | 0.014 | 2.215 (1.210, 4.055) | 0.010 | 2.501 (1.334, 4.686) | 0.004 |
| Magnesium (mg) * | |||||||||||
| IQR1 | 173 | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| IQR2 | 154 | 1.695 (1.042, 2.757) | 0.033 | 1.464 (0.890, 2.408) | 0.134 | 1.457 (0.885, 2.401) | 0.139 | 1.568 (0.938, 2.619) | 0.086 | 1.619 (0.966, 2.712) | 0.067 |
| IQR3 | 155 | 1.934 (1.195, 3.129) | 0.007 | 1.635 (0.999, 2.676) | 0.051 | 1.619 (0.986, 2.657) | 0.057 | 1.858 (1.079, 3.200) | 0.025 | 2.046 (1.168, 3.585) | 0.012 |
| IQR4 | 166 | 2.250 (1.406, 3.601) | 0.001 | 1.699 (1.041, 2.774) | 0.034 | 1.693 (1.034, 2.770) | 0.036 | 2.295 (1.148, 4.587) | 0.019 | 2.916 (1.364, 6.233) | 0.006 |
| Potassium (mg) * | |||||||||||
| IQR1 | 174 | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| IQR2 | 157 | 1.909 (1.181, 3.086) | 0.008 | 1.616 (0.987, 2.647) | 0.057 | 1.617 (0.985, 2.656) | 0.057 | 1.652 (0.994, 2.746) | 0.053 | 1.670 (1.004, 2.779) | 0.048 |
| IQR3 | 159 | 2.257 (1.404, 3.627) | 0.001 | 1.925 (1.185, 3.130) | 0.008 | 1.929 (1.183, 3.147) | 0.008 | 2.018 (1.174, 3.471) | 0.011 | 2.064 (1.196, 3.560) | 0.009 |
| IQR4 | 158 | 1.789 (1.105, 2.895) | 0.018 | 1.400 (0.850, 2.306) | 0.186 | 1.442 (0.873, 2.381) | 0.153 | 1.595 (0.773, 3.290) | 0.206 | 1.714 (0.815, 3.603) | 0.155 |
| Sodium (mg) * | |||||||||||
| IQR1 | 169 | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| IQR2 | 150 | 2.045 (1.234, 3.391) | 0.006 | 1.751 (1.043, 2.940) | 0.034 | 1.743 (1.037, 2.929) | 0.036 | 1.823 (1.077, 3.086) | 0.025 | 1.856 (1.095, 3.147) | 0.022 |
| IQR3 | 168 | 2.906 (1.790, 4.720) | <0.001 | 2.418 (1.470, 3.975) | 0.001 | 2.346 (1.421, 3.871) | 0.001 | 2.568 (1.509, 4.370) | 0.001 | 2.735 (1.594, 4.694) | <0.001 |
| IQR4 | 161 | 2.359 (1.440, 3.864) | 0.001 | 1.878 (1.129, 3.124) | 0.015 | 1.868 (1.120, 3.113) | 0.017 | 2.279 (1.200, 4.328) | 0.012 | 2.787 (1.390, 5.589) | 0.004 |
| Copper (mg) * | |||||||||||
| IQR1 | 164 | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| IQR2 | 156 | 1.688 (1.020, 2.793) | 0.042 | 1.510 (0.904, 2.524) | 0.116 | 1.527 (0.913, 2.555) | 0.107 | 1.701 (1.002, 2.886) | 0.049 | 1.745 (1.027, 2.965) | 0.040 |
| IQR3 | 169 | 2.191 (1.347, 3.563) | 0.002 | 1.912 (1.164, 3.141) | 0.010 | 1.867 (1.134, 3.075) | 0.014 | 2.261 (1.315, 3.888) | 0.003 | 2.529 (1.448, 4.416) | 0.001 |
| IQR4 | 159 | 2.826 (1.735, 4.602) | <0.001 | 2.073 (1.245, 3.453) | 0.005 | 2.091 (1.253, 3.488) | 0.005 | 3.212 (1.604, 6.431) | 0.001 | 3.962 (1.899, 8.269) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Jin, C.; Ding, Y.; Tao, Y.; Zhang, Y.; Fu, Z.; Zhou, T.; Zhang, L.; Song, Z.; Hao, Z.; et al. Higher Intake of Fat, Vitamin E-(β+γ), Magnesium, Sodium, and Copper Increases the Susceptibility to Prostatitis-like Symptoms: Evidence from a Chinese Adult Cohort. Nutrients 2022, 14, 3675. https://doi.org/10.3390/nu14183675
Zhang M, Jin C, Ding Y, Tao Y, Zhang Y, Fu Z, Zhou T, Zhang L, Song Z, Hao Z, et al. Higher Intake of Fat, Vitamin E-(β+γ), Magnesium, Sodium, and Copper Increases the Susceptibility to Prostatitis-like Symptoms: Evidence from a Chinese Adult Cohort. Nutrients. 2022; 14(18):3675. https://doi.org/10.3390/nu14183675
Chicago/Turabian StyleZhang, Meng, Chen Jin, Yang Ding, Yuqing Tao, Yulin Zhang, Ziyue Fu, Tao Zhou, Li Zhang, Zhengyao Song, Zongyao Hao, and et al. 2022. "Higher Intake of Fat, Vitamin E-(β+γ), Magnesium, Sodium, and Copper Increases the Susceptibility to Prostatitis-like Symptoms: Evidence from a Chinese Adult Cohort" Nutrients 14, no. 18: 3675. https://doi.org/10.3390/nu14183675
APA StyleZhang, M., Jin, C., Ding, Y., Tao, Y., Zhang, Y., Fu, Z., Zhou, T., Zhang, L., Song, Z., Hao, Z., Meng, J., & Liang, C. (2022). Higher Intake of Fat, Vitamin E-(β+γ), Magnesium, Sodium, and Copper Increases the Susceptibility to Prostatitis-like Symptoms: Evidence from a Chinese Adult Cohort. Nutrients, 14(18), 3675. https://doi.org/10.3390/nu14183675







