Gastric Emptying of Maltodextrin versus Phytoglycogen Carbohydrate Solutions in Healthy Volunteers: A Quasi-Experimental Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Beverages
2.3. Test Day
2.4. Gamma Scintigraphy
2.5. Blood Samples
2.6. Statistical Analysis
3. Results
3.1. Participants
3.2. Gastric Emptying
3.3. Blood Measures
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feldheiser, A.; Aziz, O.; Baldini, G.; Cox, B.P.B.W.; Fearon, K.C.H.; Feldman, L.S.; Gan, T.J.; Kennedy, R.H.; Ljungqvist, O.; Lobo, D.N.; et al. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 2: Consensus statement for anaesthesia practice. Acta Anaesthesiol. Scand. 2016, 60, 289–334. [Google Scholar] [CrossRef] [PubMed]
- Melloul, E.; Lassen, K.; Roulin, D.; Grass, F.; Perinel, J.; Adham, M.; Wellge, E.B.; Kunzler, F.; Besselink, M.G.; Asbun, H.; et al. Guidelines for Perioperative Care for Pancreatoduodenectomy: Enhanced Recovery After Surgery (ERAS) Recommendations. World J. Surg. 2020, 44, 2056–2084. [Google Scholar]
- Pogatschnik, C.; Steiger, E. Review of Preoperative Carbohydrate Loading. Nutr. Clin. Pract. 2015, 30, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Bilku, D.K.; Dennison, A.R.; Hall, T.C.; Metcalfe, M.S.; Garcea, G. Role of preoperative carbohydrate loading: A systematic review. Ann. R. Coll. Surg. Engl. 2014, 96, 15–22. [Google Scholar] [CrossRef]
- Smith, I.; Kranke, P.; Murat, I.; Smith, A.; O’Sullivan, G.; Søreide, E.; Spies, C. Perioperative fasting in adults and children: Guidelines from the European Society of Anaesthesiology. Eur. J. Anaesthesiol. 2011, 28, 556–569. [Google Scholar] [CrossRef]
- Nygren, J. The metabolic effects of fasting and surgery. Best Pract. Res. Clin. Anaesthesiol. 2006, 20, 429–438. [Google Scholar] [CrossRef]
- Piehl Aulin, K.; Söderlund, K.; Hultman, E. Muscle glycogen resynthesis rate in humans after supplementation of drinks containing carbohydrates with low and high molecular masses. Eur. J. Appl. Physiol. 2000, 81, 346–351. [Google Scholar] [CrossRef]
- Stephens, F.B.; Roig, M.; Armstrong, G.; Greenhaff, P.L. Post-exercise ingestion of a unique, high molecular weight glucose polymer solution improves performance during a subsequent bout of cycling exercise. Randomized. Control. Trial 2008, 26, 149–154. [Google Scholar] [CrossRef]
- Oliver, J.M.; Almada, A.L.; Van Eck, L.E.; Shah, M.; Mitchell, J.B.; Jones, M.T.; Jagim, A.R.; Rowlands, D.S. Ingestion of High Molecular Weight Carbohydrate Enhances Subsequent Repeated Maximal Power: A Randomized Controlled Trial. PLoS ONE 2016, 11, e0163009. [Google Scholar] [CrossRef]
- Almada, A.L.; Van Eck, L.E.; Shah, M.; Jones, M.T.; Jagim, A.; Dalton, R.; Mitchell, J.; Oliver, J.M. Effect of post-exercise ingestion of different molecular weight carbohydrate solutions. Part 1: The glucose and insulin response. J. Int. Soc. Sport Nutr. 2015, 12, P30. [Google Scholar] [CrossRef]
- Bandegan, A.; Huang, L.; Longstaffe, F.J.; Lemon, P.W.R. Dose–Response Oxidation of Ingested Phytoglycogen during Exercise in Endurance-Trained Men. J. Nutr. 2021, 151, 2942–2948. [Google Scholar] [CrossRef] [PubMed]
- Nickels, J.D.; Atkinson, J.; Papp-Szabo, E.; Stanley, C.; Diallo, S.O.; Perticaroli, S.; Baylis, B.; Mahon, P.; Ehlers, G.; Katsaras, J.; et al. Structure and Hydration of Highly-Branched, Monodisperse Phytoglycogen Nanoparticles. Biomacromolecules 2016, 17, 735–743. [Google Scholar] [CrossRef] [PubMed]
- American Society of Anesthesiologists. Practice Guidelines for Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration: Application to Healthy Patients Undergoing Elective Procedures. Anesthesiology 2017, 126, 376–393. [Google Scholar] [CrossRef] [PubMed]
- Dobson, G.; Chow, L.; Filteau, L.; Hurdle, H.; McIntyre, I.; Milne, A.; Milkovich, R.; Perrault, M.-A.; Sparrow, K.; Swart, P.A.; et al. Guidelines to the Practice of Anesthesia–Revised Edition 2021. Can. J. Anesth. 2021, 68, 92–129. [Google Scholar] [CrossRef] [PubMed]
- Leiper, J.B.; Piehl Aulin, K.; Soderlund, K. Improved Gastric Emptying Rate in Humans of a Unique Glucose Polymer with Gel-forming Properties. Scand. J. Gastroenterol. 2009, 35, 1143–1149. [Google Scholar] [CrossRef]
- Maltodextrin-American Chemical Society. Available online: https://www.acs.org/content/acs/en/molecule-of-the-week/archive/m/maltodextrin.html (accessed on 26 March 2022).
- Vist, G.E.; Maughan, R.J. The effect of osmolality and carbohydrate content on the rate of gastric emptying of liquids in man. J. Physiol. 1995, 486, 523. [Google Scholar] [CrossRef]
- Hellström, P.M.; Grybäck, P.; Jacobsson, H. The physiology of gastric emptying. Best Pract. Res. Clin. Anaesthesiol. 2006, 20, 397–407. [Google Scholar] [CrossRef]
- Sharma, S.; Deo, A.S.; Raman, P. Effectiveness of standard fasting guidelines as assessed by gastric ultrasound examination: A clinical audit. Indian J. Anaesth. 2018, 62, 747. [Google Scholar] [CrossRef]
- Van de Putte, P.; Perlas, A. The link between gastric volume and aspiration risk. In search of the Holy Grail? Anaesthesia 2018, 73, 274–279. [Google Scholar] [CrossRef]
- Doctor, J.; Chandan, P.; Shetty, N.; Gala, K.; Ranganathan, P. Ultrasound-guided assessment of gastric residual volume in patients receiving three types of clear fluids: A randomised blinded study. Indian J. Anaesth. 2021, 65, 289–294. [Google Scholar]
- Morrison, C.E.; Ritchie-Mclean, S.; Jha, A.; Mythen, M. Two hours too long: Time to review fasting guidelines for clear fluids. Br. J. Anaesth. 2020, 124, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Kaška, M.; Grosmanová, T.; Havel, E.; Hyšpler, R.; Petrová, Z.; Brtko, M.; Bareš, P.; Bareš, D.; Schusterová, B.; Pyszková, L.; et al. The impact and safety of preoperative oral or intravenous carbohydrate administration versus fasting in colorectal surgery—A randomized controlled trial. Wien. Klin. Wochenschr. 2010, 122, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Itou, K.; Fukuyama, T.; Suzuki, N.; Taniguchi, H.; Iwao, Y.; Hinenoya, H.; Kim, C.; Sanui, M.; Taniguchi, H.; Miyao, H.; et al. Safety and efficacy of oral rehydration therapy until 2 h before surgery: A multicenter randomized controlled trial. J. Anesth. 2012, 26, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Chari, P.; Singh, H. Fluid deprivation before operation. The effect of a small drink. Anaesthesia 1989, 44, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Dalal, K.S.; Rajwade, D.; Suchak, R. “Nil per oral after midnight”: Is it necessary for clear fluids? Indian J. Anaesth. 2010, 54, 445. [Google Scholar] [CrossRef]
- Knight, L.C. Update on GI Radiopharmaceuticals and Dosimetry Estimates. Semin. Nucl. Med. 2012, 42, 138. [Google Scholar] [CrossRef]
- Mudie, D.M.; Murray, K.; Hoad, C.L.; Pritchard, S.E.; Garnett, M.C.; Amidon, G.L.; Gowland, P.A.; Spiller, R.C.; Amidon, G.E.; Marcian, L.; et al. Quantification of Gastrointestinal Liquid Volumes and Distribution Following a 240 mL Dose of Water in the Fasted State. Mol. Pharm. 2014, 11, 3039–3047. [Google Scholar] [CrossRef]
- de Waal, T.; Rubbens, J.; Grimm, M.; Vandecaveye, V.; Tack, J.; Weitschies, W.; Brouwers, J.; Augustijns, P. Exploring the Effect of Esomeprazole on Gastric and Duodenal Fluid Volumes and Absorption of Ritonavir. Pharmaceutics 2020, 12, 670. [Google Scholar] [CrossRef]
- Goyal, R.K.; Guo, Y.; Mashimo, H. Advances in the physiology of gastric emptying. Neurogastroenterol. Motil. 2019, 31, e13546. [Google Scholar] [CrossRef]
- Woerle, H.J.; Albrecht, M.; Linke, R.; Zschau, S.; Neumann, C.; Nicolaus, M.; Gerich, J.; Göke, B.; Schirra, J. Importance of changes in gastric emptying for postprandial plasma glucose fluxes in healthy humans. Am. J. Physiol.-Endocrinol. Metab. 2008, 294, E103–E109. [Google Scholar] [CrossRef]
- Rowlands, D.S.; Wallis, G.A.; Shaw, C.; Jentjens, R.L.P.G.; Jeukendrup, A.E. Glucose polymer molecular weight does not affect exogenous carbohydrate oxidation. Med. Sci. Sports Exerc. 2005, 37, 1510–1516. [Google Scholar] [CrossRef] [PubMed]
- Mesbah, A.; Thomas, M. Preoperative fasting in children. BJA Educ. 2017, 17, 346–350. [Google Scholar] [CrossRef] [Green Version]
| Characteristic | Intervention Group | p-Value | |
|---|---|---|---|
| Maltodextrin | Phytoglycogen | ||
| n | 5 | 5 | |
| Age (years) | 27.1 ± 1.66 | 24.8 ± 0.86 | 0.40 |
| BMI (kg/m2) | 24.9 ± 2.32 | 25.6 ± 1.80 | 1 |
| Sex (male/female) | 3/2 | 3/2 | |
| Time Point | %Gastric Emptying | p-Value | |
|---|---|---|---|
| Intervention Group | |||
| Maltodextrin | Phytoglycogen | ||
| 45 min | 38.6 ± 6.06 | 68.0 ± 3.24 | 0.01 |
| 90 min | 66.8 ± 5.57 | 87.4 ± 0.96 | 0.01 |
| 120 min | 83.8 ± 6.07 | 95.6 ± 0.88 | 0.17 |
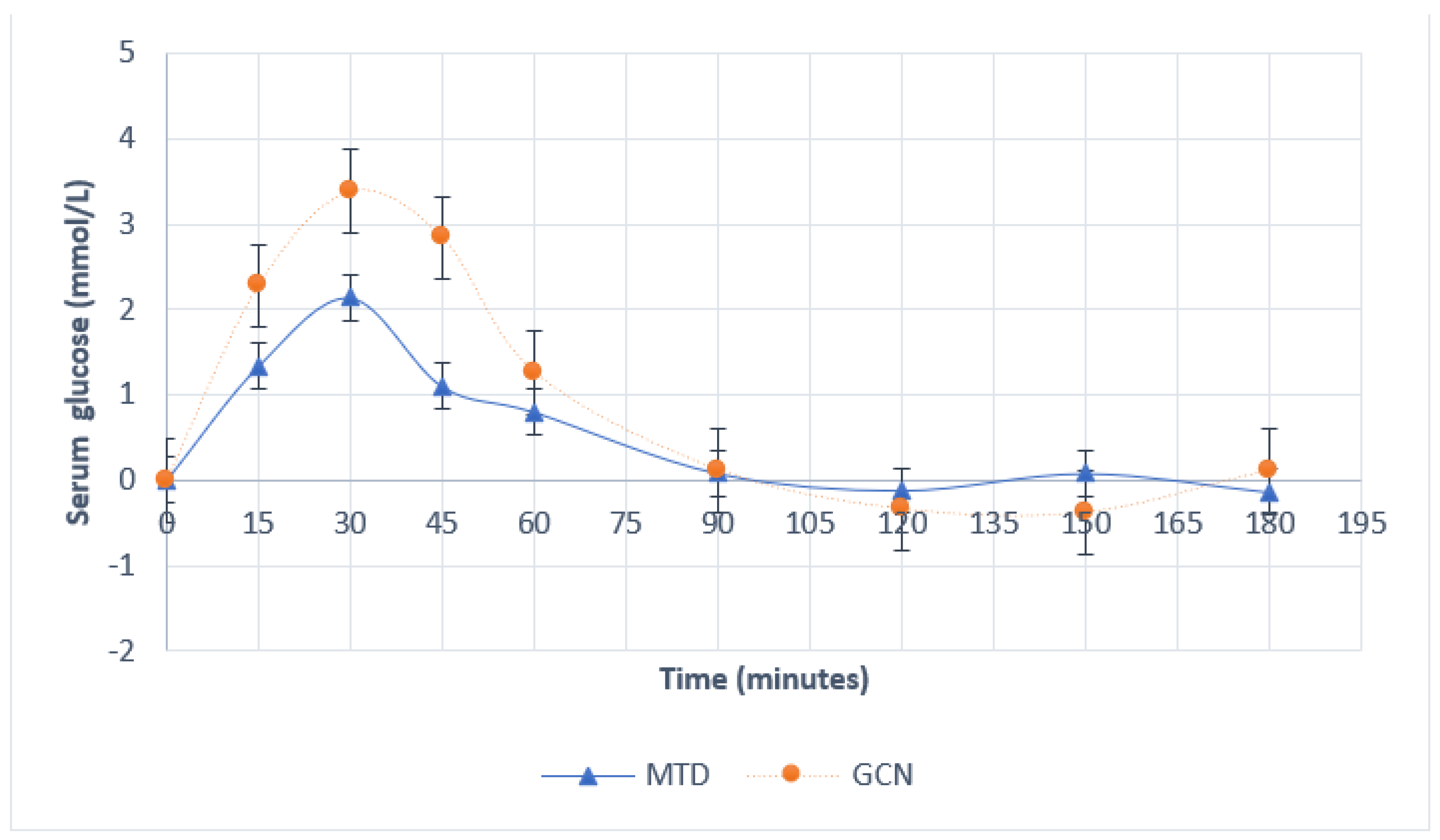
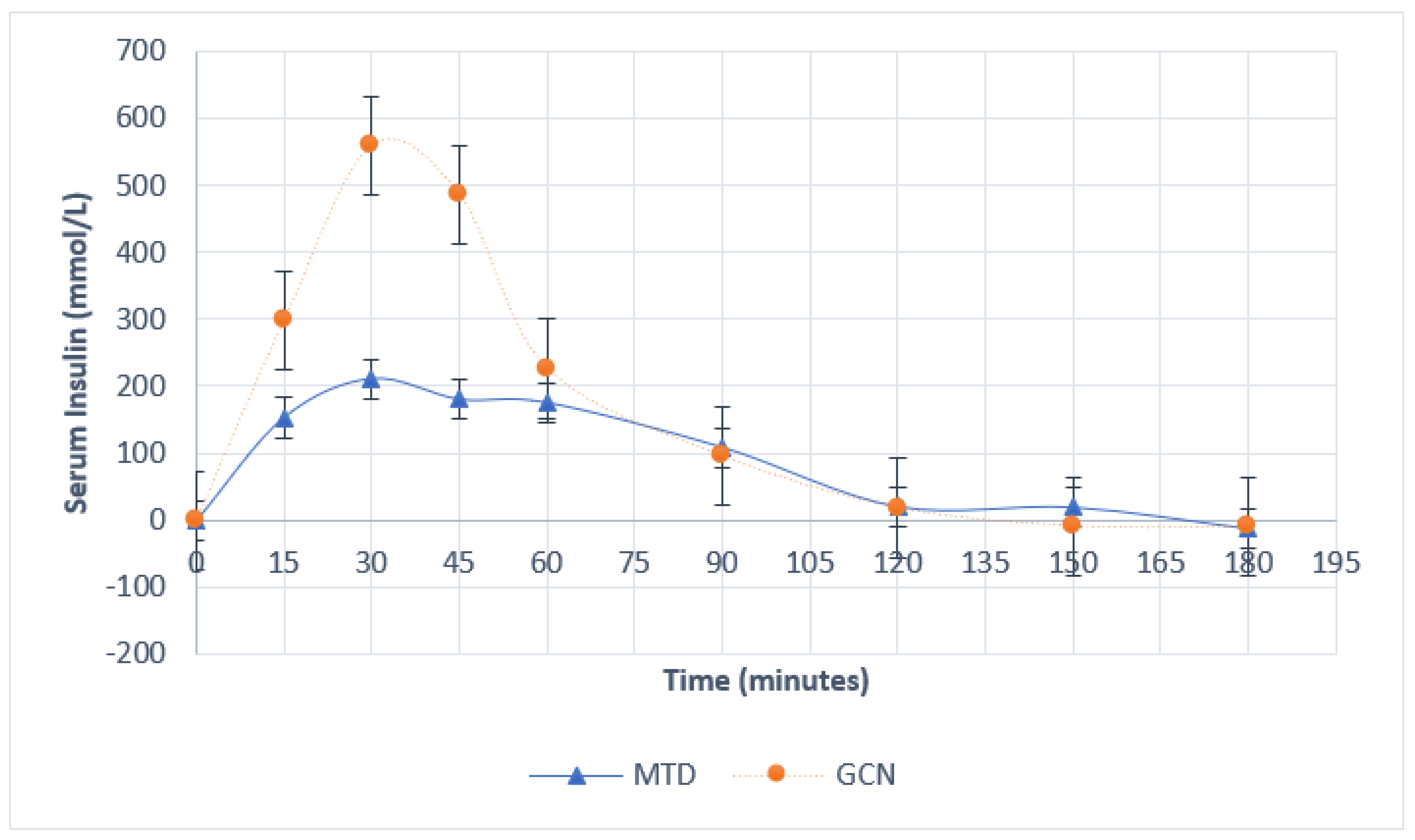
| Time Point (Minutes) | Serum Glucose (mmol/L) | Serum Insulin (mmol/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention Group | p-Value | Intervention Group | p-Value | |||||||
| Maltodextrin | Phytoglycogen | Maltodextrin | Phytoglycogen | |||||||
| 15 | 1.34 | ±0.48 | 2.28 | ±0.22 | 0.29 | 153.0 | ±50.53 | 298.6 | ±114.91 | 0.83 |
| 30 | 2.14 | ±0.38 | 3.38 | ±0.25 | 0.06 | 210.6 | ±34.38 | 559.6 | ±207.53 | 0.06 |
| 45 | 1.10 | ±0.54 | 2.84 | ±0.43 | 0.09 | 180.0 | ±25.43 | 486.8 | ±217.50 | 0.21 |
| 60 | 0.80 | ±0.55 | 1.26 | ±0.33 | 0.67 | 175.0 | ±33.20 | 226.6 | ±78.86 | 0.83 |
| 90 | 0.08 | ±0.46 | 0.12 | ±0.29 | 1 | 108.6 | ±33.02 | 95.8 | ±20.43 | 0.83 |
| 120 | −0.12 | ±0.45 | −0.34 | ±0.31 | 0.91 | 20.6 | ±27.8 | 18.2 | ±11.18 | 0.83 |
| 150 | 0.08 | ±0.45 | −0.38 | ±0.20 | 0.67 | 19.6 | ±25.69 | −8.8 | ±8.48 | 1 |
| 180 | −0.14 | ±0.33 | 0.12 | ±0.10 | 0.24 | −12.0 | ±12.95 | −10.4 | ±6.84 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammond, L.R.D.; Barfett, J.; Baker, A.; McGlynn, N.D. Gastric Emptying of Maltodextrin versus Phytoglycogen Carbohydrate Solutions in Healthy Volunteers: A Quasi-Experimental Study. Nutrients 2022, 14, 3676. https://doi.org/10.3390/nu14183676
Hammond LRD, Barfett J, Baker A, McGlynn ND. Gastric Emptying of Maltodextrin versus Phytoglycogen Carbohydrate Solutions in Healthy Volunteers: A Quasi-Experimental Study. Nutrients. 2022; 14(18):3676. https://doi.org/10.3390/nu14183676
Chicago/Turabian StyleHammond, Leila R. D., Joseph Barfett, Andrew Baker, and Néma D. McGlynn. 2022. "Gastric Emptying of Maltodextrin versus Phytoglycogen Carbohydrate Solutions in Healthy Volunteers: A Quasi-Experimental Study" Nutrients 14, no. 18: 3676. https://doi.org/10.3390/nu14183676
APA StyleHammond, L. R. D., Barfett, J., Baker, A., & McGlynn, N. D. (2022). Gastric Emptying of Maltodextrin versus Phytoglycogen Carbohydrate Solutions in Healthy Volunteers: A Quasi-Experimental Study. Nutrients, 14(18), 3676. https://doi.org/10.3390/nu14183676







