The Potential of Pectins to Modulate the Human Gut Microbiota Evaluated by In Vitro Fermentation: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
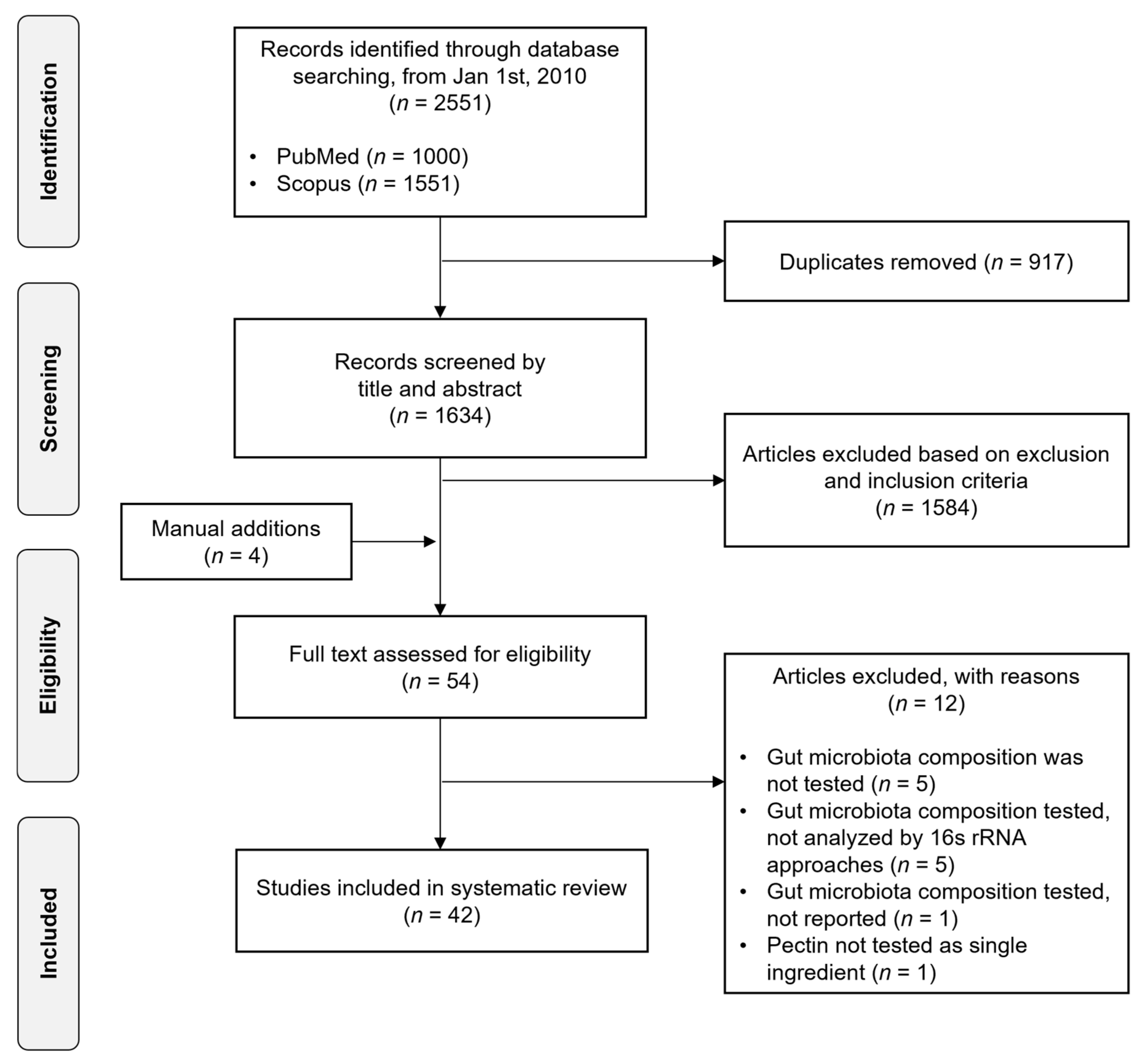
2.1. Search Strategy and Eligibility Criteria
2.2. Study Screening
3. Results
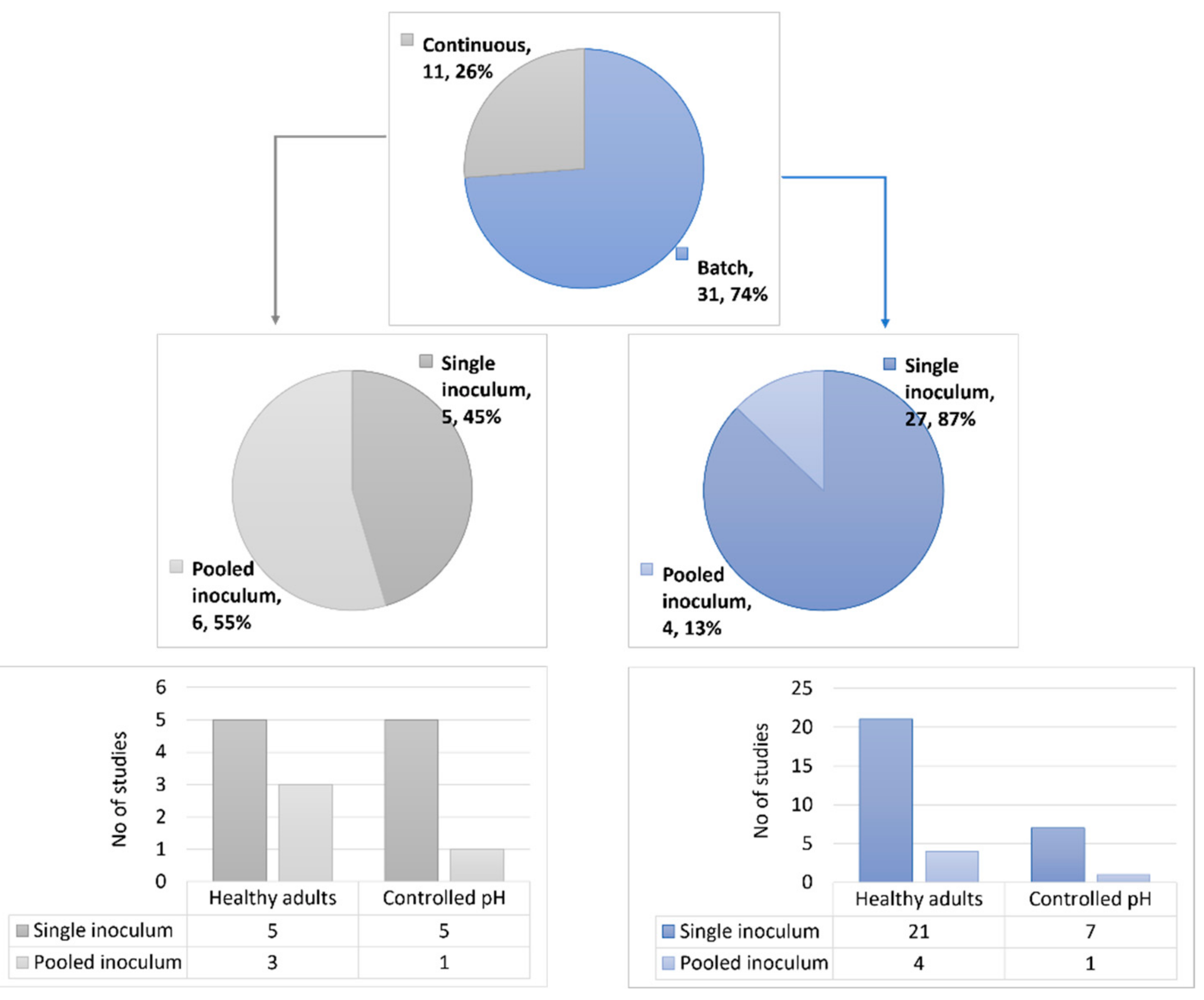
3.1. Study Selection and Characteristics
3.2. Tested Products
3.3. Effects on Gut Microbiota Composition and SCFA Production
| Ref. | Subjects (Age, Years) | Test Products 1 | Comparators | Methods 2 | Main Outcomes, Including Changes in Gut Microbiota Composition and SCFA Production Linked to Pectic Substrates |
|---|---|---|---|---|---|
| SINGLE DONOR (n = 27) | |||||
| HEALTHY ADULTS (n = 21) | |||||
| Non-controlled pH (n = 15) | |||||
| Cantu-Jungles, 2021 [24] | 10 (26–42 y) | Citrus pectin (GalA 3 74%, >6.7% methoxy group, Sigma, St. Louis, MO, USA) | Blank, FOS from chicory (>95%, Sigma, USA), RS2 from potato (Bob’s Red Mill, Clackamas, OR, USA), and insoluble β-glucan | 50 mg/50 mL; 0 and 24 h |
|
| Wu, 2021 [25] | 4 (18–30 y) | RGI-enriched fraction (MW 1.93 × 105 Da, polydispersity 1.63, Rha:GalA:Gal = 1:0.8:18) from Okra fruit (Abelmoschus esculentus, harvested from Chengdu, Sichuan, China) | Basal medium, FOS (Sigma, St. Louis, MO, USA) | 1% w/v; 0, 6, 12, 24, and 48 h |
|
| Yu, 2020 [26] | 9 (25–40 y) | Pectin (ND) | No fiber, inulin (ND), andcellulose (ND) | 5 g/L pectin, 10 g/L inulin, 20 g/L cellulose; 0 and 24 h |
|
| Cui, 2020 [27] | 4 (age ND) | Orange or grapefruit pectin: P2 (acidic, pH 2, DE 71%), P10 (alkali, pH 10, DE 2%), C (cellulase, DE 69%), P2 + C (acid +cellulase, DE 65%), and P10 + C (alkali + cellulase, more RG1, DE 15%) | Baseline | 1% w/v; 0, 4, 8, 12, 24, 48, and 72 h |
|
| Bang, 2018 [28] | 3 (29–30 y) | Citrus pectin (GalA > 74%, Sigma, St. Louis, MO, USA) | Baseline | 1%; 0, 6, 12, 18, 24, 36, and 48 h |
|
| Tuncil, 2017 [29] | 3 donors (age ND) | PGalA from citrus pectin (Megazyme, Wicklow, Ireland) | FOS (Sigma, St. Louis, MO, USA), galactomannan (Carob) and Xyloglucan (Tamarind) (Megazyme, Wicklow, Ireland), and Arabinoxylan | 50 mg/5 mL; 0, 2, 4, 6, 8, 10, 12, and 24 h |
|
| Min, 2015 [30] | 4 (23–28 y) | High methoxy pectin (HMP, DM 76%, DP492, Tic Gums, Belcamp, MD, USA), SBP (DM 21%, DP3729, Herbstreith & Fox (Elmsford, N.Y., USA), pectin from soy (DM 23%, DP1510) | FOS (95% purity, DP 3–5, Ingredion, USA) | Unclear concentration; 0, 6, 12, 24, and 30 h |
|
| Van den Abbeele, 2020 [31] | 1 (26 y) | RGI from carrot (min. 80% purity; Nutrileads, Wageningen, The Netherlands) | Blank and inulin (average DP > 23, Beneo, Mannheim, Germany) | 5 g/L; 0, 6, 24, and 48 h; targeted bacterial groups. |
|
| Gómez, 2016 [32] | 3 (age ND) | SBP, SBPOS (DM 50%, DA 37%, mostly AOS, and pH 1.8), Lemon pectin (LP), lemon POS (LPOS, DM 62%, DA 4.6%, more oligogalacturonides) | FOS from chicory (Sigma, St. Louis, MO, USA) | 10 g/L; 0, 5, 10, and 24 h; targeted bacterial groups. |
|
| Sulek, 2014 [33] | 6 (41 ± 9 y) | Sugar beet AOS (Danisco A/S, Nakskov, Denmark), base solution (BA), LA fraction (<1 kDa), and HA fraction (>1 kDa) | No CHO in media; FOS from chicory (>95%, Beneo, Tienen, Belgium) | 5 g/L; 0 and 24 h; targeted bacterial groups. |
|
| Gómez, 2014 [34] | 3 donors(age ND) | Orange pectin and orange POS (≈90% oligomers, 53.4% OGalA, 25.3% AOS, and 16.5% GOS) | No fiber in media; FOS (>95% purity, Sigma, St. Louis, MO, USA) | 10 g/L; 0, 5, 10, and 24 h; targeted bacterial groups. |
|
| Gullón, 2011 [35] | 1 (age ND) | Apple-derived oligosaccharides: GLOS, AOS, GOS, OGalA, and XOS; total oligomers (OS) | No CHO in culture media | 10 g/L; 0, 7, 10, 12, 24, 32, and 48 h; Targeted bacterial groups. |
|
| Holck, 2011 [36] | 6 (41 ± 9 y) | Sugar beet AOS (Danisco A/S, Nakskov, Denmark): small (mostly DP 2–5), small and feruloylated; long (mostly DP 5–10), and long and feruloylated | FOS from chicory (>95%, DP 2–8, Beneo, Tienen, Belgium) | 5 g/L; 0 and 24 h; targeted phyla (2) and genera (2) |
|
| Thomassen 2011 [37] | 3 (43 ± 10 y) | Destarched potato pulp (DNE, no enzyme), destarched potato pulp (DPP, enzyme treated), crude potato pulp (CNE, no enzyme), crude potato pulp (CPP, enzyme treated), CCP fractions: CPP < 10 kDa, CPP 10–100 kDa, and CPP > 100 kDa. | FOS from chicory (DP 2–8, Beneo, Tienen, Belgium) | 5 g/L; 0 and 24 h. |
|
| Adamberg, 2018 [9] | 5 (28–48 y) | Arabino-galactan from larch tree (AG, DP > 23, Sigma, USA), and citrus pectin (GalA >74%, Sigma, St. Louis, MO, USA); | Culture medium without CHO, mucin from porcine stomach (type III, Sigma, St. Louis, MO, USA), GOS (DP 2–10, Friesland Campina, Wolvega, The Netherlands), inulin (HSI, DP 2–8), and lnulin (HP, DP > 23% (Beneo, Oreye, Belgium), Levan (DP > 100), RS (Cargill, Malchin, Germany) xylan and chitin from shrimp cells (Sigma, St. Louis, MO, USA) | 5 g/L; 0, 24 and 48 h |
|
| Specific pH range (n =6) | |||||
| Johnson, 2015 [38] | 3 (age ND) | Pectin (ND) | Control medium (low fibers and inulin (ND) | 1.5 g; pH 6.7–6.9; 0, 5, 10, 24, 30, and 48 h. |
|
| Reichardt, 2018 [39] | 3 (age ND) | RGI from potato (Megazyme, Bray Ireland), and apple pectin (Sigma, UK) | FOS (95%, DP 2–8) and Inulin (99%, DP > 23) (Beneo, Tienen, Belgium), arabinoxylan (Megazyme, Bray, Ireland), barley β-glucan (PolyCell Technologies, Crookston, USA), RS2 and RS3 (National Starch and Chemical Comp., Bridgewater, USA), FiberSol (Matsutani, Itami-City, Japan) | 0.2% w/v; pH 5.5 and 6.5; 0, 6, and 24 h |
|
| Di, 2017 [40] | 5 (30 ± 7 y) | POS1 (MW 72.8 × 103, DM 40%, Gal:Rha 3.14), POS2 (MW 811 × 103, DM 42%, Gal:Rha 1.97), MCP1 (MW 9.2 × 103, DM 5%, Gal:Rha 2.92), and MCP2 (MW 17.7 × 103, Gal:Rha 4.47, DM 3%) from orange peels (EcoNugenics Inc., Santa Rosa, CA, USA) | Inulin (99%, Beneo, Tienen, Belgium) | 1% w/v; pH 6.7–6.9;0, 10, 24, 36, and 48 h; targeted bacterial groups. |
|
| Moon, 2015 [41] | 3 (age ND) | Debranched sugar beet arabinan (LAR, average MW 18 kDa, Megazyme, Wicklow, Ireland) and sugar beet linear AOS (LAOS, 50% DP3, 29% DP2, 20% DP4, and 1% DP5). | FOS (DP 3–5, Wako, Osaka, Japan) | 1% w/v; pH 6.8; 0, 12, and 24 h; targeted bacterial groups. |
|
| Onumpai, 2011 [42] | 4 (30 ± 4 y) | PGalA (Sigma, St. Louis, MO, USA); OGalA DP5 (DP 1–10), OGalA DP 9 (DP 4–23), methylated citrus pectin (MPec, DM 34.5%, Danisco A/S, Copenhagen, Denmark), methylated OGalA (MOGalA, DP 1–10), RGI (A. thaliana seed mucilage), oligorhamnogalacturonides (Orham, DP 2–19), potato galactan and beet arabinan (British Sugar, Peterborough, UK), oligogalactosides (PGOS, DP 1–10), oligoarabinosides (OAr, DP 1–11) | Inulin (>97%, Beneo ST, Orafti, Tienen, Belgium) | 1% w/v; pH 6.7–6.9;0, 12, 24, and 36 h; targeted bacterial groups. |
|
| Ferreira-Lazarte, 2018 [43] | 5 (31± 4 y) | Sunflower pectin (DM 45.7%, 800–100 kDa), sunflower MP (DM 17%, 12.5 kDa), Artichoke pectin (DM 8.9%, >500 kDa), artichoke MP (DM 8.5%), citrus pectin (Ceamsa, Pontevedra, Spain, DM 70.7%), and citrus MP (DM 14.2%) | Negative: no CHO. Positive: FOS (ND) and inulin (ND). | 1% w/v; pH 6.7–6.9;0, 10, 24, 36, and 48 h; targeted bacterial groups. |
|
| SPECIFIC POPULATIONS (n = 6) | |||||
| Non-controlled pH (n = 5) | |||||
| Van Trijp, 2020 [44] | 5 ileostomy subjects (30–75 y) | Lemon pectin (DM 67%, CP Kelco, Lille Skensved, Denmark) | Inulin and FOS (DP 2–60, Sensus, Roosendaal, the Netherlands), GOS (69%, DP 2–6, Friesland Campina, Wageningen, the Netherlands), and potato IMMP (92% α-1-6, average DP 50, Avebe, Veendam, Belgium) | 10 g/L; 0, 5 ,7 ,9, and 24 h |
|
| Yang, 2013 [45] | 15 adult patients (age ND) | Pectin (TIC gums, White Marsh, MD, USA): 35% polymeric uronic acid residues, DM 72%, MW peak at 9.4 × 105, and 38% free glucose; botanical origin ND | Guar gum (TIC gums, White Marsh, MD, USA), agave inulin (Ciranda, Hudson, WI, USA), corn RS2 (70% high amylose, Cargill, Cedar Rapids, IA, USA), oat β-glucan (Quaker, Chicago, IL, USA), corn arabinoxylan (AX, Bunge Milling, Danville, IL, USA) | 1% w/v; 0 and 12 h. |
|
| Vigsnæs, 2011 [46] | 12 UC patients with 6 healthy adults (41 ± 9 y) | Sugar beet AOS (DP 2–10, Danisco A/S, Nakskov, Denmark) and arabinose moiety (85 mol%, 125 mg/g free sugars, ferulic acid 36 µg/g) | No substrate; FOS (95%, Beneo, DP 2–8, Tienen, Belgium) | 5 g/L; 0 and 24 h; targeted bacterial groups. |
|
| Holck, 2011 [47] | 3 UC remission (36 ± 5 y); 3 UC relapse (44 ± 6 y); 3 healthy (43 ± 10 y) | HG oligosaccharides (DP4 and DP5) from SBP (Danisco A/S, Nakskov, Denmark) | Baseline | 5 g/L; 0 and 24 h; targeted phyla (n = 2). |
|
| Jin, 2019 [48] | 17 patients with cirrhosis and 17 healthy (18–80 y) | Citrus pectin (Unipectine™, Cargill Inc., Wayzata, MN, USA) | Baseline, RS type 4 (Fibersym® RW, MGP Ingredients, Atchison, KS, USA), lactulose (LL, Sigma, St. Louis, MO, USA), arabinoxylan (AX, Corn Biofiber Gum Agrifiber Holdings LLC, (Mundelein, IL, USA) | 2%; 0 and 14 h |
|
| Specific pH range (n = 1) | |||||
| Adamberg, 2018 [49] | 7 OW (7–14 y) with 6 healthy NW (4–15 y) | Apple pectin (AP, Sigma, St. Louis, MO, USA) | Arabinogalactan (AG) | From 0.2 L/h to 0.06 L/h; pH 7; 0 and 10 h |
|
| POOLED (ALL HEALTHY DONORS) (n = 4) | |||||
| Non-controlled pH (n = 3) | |||||
| Perez-Burillo, 2019 [50] | 3 (mean BMI 21.3, age ND) | Citrus fiber (42% pectin and 25% cellulose and hemicellulose; Fiberstars, USA) | Control salami (no fiber), inulin (99.5%, Beneo, Belgium), acacia gum (Arabinogalactan, Nexira, France) | 2% in salami; 0 and 24 h | ↑ Dorea and Clostridium cluster XIVb with citrus and acacia fiber. ↓ Escherichia/Shigella with citrus and acacia fiber.↑ SCFA (total and individuals) vs. control salami for all fiber-salami. |
| Cantu-Jungles, 2019 [51] | 3 (age ND) | Isolated highly-branched RGI (AGI), HG, and AGI (uronic acid/(Ara + Gal): 1.3, HG − DM 79%). Xyloglucan (XYG, tucumã pulp). | FOS (95%, Sigma, St. Louis, MO, USA) | 1% w/v; 0, 4, 8, 12, and 24 h |
|
| Leijdekkers, 2014 [52] | 10 (44 ± 7 y) | SBPOS (90%, 15% average DP5, GalA 43%; Cosun, Breda, the Netherlands) | FOS (95%, Sensus, Roosendaal, the Netherlands) | 1% w/v; 0, 3, 6, 9, 12, and 24 h |
|
| Specific pH range (n = 1) | |||||
| Ramasamy, 2014 [53] | 8 (25–45 y) | Chicory root pulp (62% pectin and 38% uronic acid; Sensus, Roosendaal, the Netherlands) | Baseline | 1% w/v; pH 5.8–6.3;0, 2, 6, 8, 12, and 24 h. |
|
| Ref | Subjects (Age, Years) | Product Tested 1 | Comparator | Methods 2 | Main Outcomes, Including Changes in Gut Microbiota Composition and SCFA Production Linked to Pectic Substrates |
|---|---|---|---|---|---|
| SINGLE DONOR (ALL HEALTHY DONORS) (n = 5) | |||||
| Chung, 2019 [54] | 2 (53–64 y) | Apple pectin (Unipectin, Cargill, Belgium) | Inulin, AXOS 3, mixture 1 (all), and mixture 2 (all and RS, galactomannan, and β-glucan) | 4.2 g/L (for single substrate); pH 6.1 ± 0.1; 20 days; single-stage anaerobic |
|
| Chung, 2016 [55] | 3 (age ND) | Apple pectin (Sigma, St. Louis, MO, USA) | Inulin DP < 10 (Oligo-Fiber DS2, Cargill) | 0.5% w/v; pH 5.5–6.9; 12 days; single-stage anaerobic |
|
| Ferreira, 2019 [4] | 1 (age ND) | Citrus pectin, (DM 70%, average MW 350 kDa, GalA 66%, Ceamsa, Pontevedra, Spain) | Baseline | 3% w/v; pH 5.6 (AC), 6.3 (TC), and 6.8 (DC); 14 days; SIMGI; targeted bacterial groups |
|
| Van den Abbeele, 2021 [56] | 4 (29–33 y) | RGI (80%, carrot, Nutrileads, Wageningen, the Netherlands) | Baseline | 3 g/d;pH 5.7–5.9 (PC) and 6.6–6.9 (DC);21 days;SHIME® |
|
| Khodaei, 2016 [57] | 1 (age ND) | RGI from potato (90.8% polysaccharides, 6.5% DP 2–70, and 2.7% DP1; Megazyme, Wicklow, Ireland); oligo-RGI (51% DP 2–12 and 6.3% DP1; 73% Gal), Oligo-RGI (GOS, no polysaccharides, 51% DP 13–70, and 6.1% DP1; 70% Gal). | FOS (>95%, DP 2–8, Beneo, Belgium); 3.2 g/L CHO as negative control | 9.7 g/L; pH 6.2; 4 days; BIOSTAT®; targeted bacterial groups |
|
| POOLED (n = 6) | |||||
| HEALTHY ADULTS (n = 3) | |||||
| Larsen, 2019 [58] | 8 (25–42 y) | Potato fiber (FiberBind, KMC, Brande, Denmark, 65% dietary fiber, containing pectin, cellulose, and hemicellulose). Pectin fraction consisted of GalA (13.1%) and rhamnose (0.5%) | Baseline, native potato starch (NS), and potato cross-linked resistant starch (RS) | 7.5 g/d; pH 5.8; 0, 24, 48, 56, and 72 h TIM-2 |
|
| Larsen, 2019 [59] | 8 (25–42 y) | Citrus pectins with various DM and extraction processes (P1–P3, P5–P8; CP Kelco, Lille Skensved, Denmark), SBP (P4), and RGI (P10). | Baseline | 7.5 g/d; pH 5.8; 0, 24, 48, 56, and 72 h; TIM-2 |
|
| Bianchi, 2019 [60] | 3 (age ND) | Lemon pectin (harshly extracted, LM, CP Kelco, Lille Skensved, Denmark) and probiotic strain B. longum BB-46 (Chr. Hansen, Hørsholm, Denmark) | Probiotic strain only | 2% w/v (8 g/d); pH 5.6–5.9 (AC), 6.1–6.9 (TC), and 6.6–6.9 (DC); 7 days for each treatment; SHIME®. |
|
| SPECIFIC POPULATIONS (n = 3) | |||||
| Aguirre, 2014 [17] | 4 lean healthy adults (BMI 23) and 4 obese adults (BMI 33) (age ND) | Apple fiber (23% uronic acid; CSM, Bingen, Germany) and SBP (GENU pectin, DE 53%, and 58% uronic acid; CP Kelco, CPKelco, Nijmegen, the Netherlands). | SIEM (control), GOS (97%, DP 2–6; Friesland Campina, Beilen, the Netherlands), lactulose (98%, Sigma, Zwijndrecht, the Netherlands) | 7.5 g/d; pH 5.8; 0 and 72 h; TIM-2 (PC conditions) |
|
| Bianchi, 2018 [61] | 3 obese adults (BMI > 30 Kg/m2, age ND) | Lemon pectin (harshly extracted, DM 36%, CP Kelco, Lille Skensved, Denmark) | Baseline | 2% (w/v); pH 5.6–5.9 (AC), 6.1–6.4 (TC), 6.6–6.9 (DC); 7 days; SHIME® |
|
| Míguez, 2020 [62] | 6 elderly subjects (60–83 y) | POS mixtures (OGs 44.4%, AOS 16.9%, and GOS 11.6%) | Baseline and FOS from chicory (Sigma, Madrid, Spain) | 6.5 g/d; pH 5.8; 0, 24, 48, and 72 h; TIM-2 |
|
4. Discussion
4.1. Influence of the Methodology on the Fermentation of Pectic Substrates
4.2. Common Features for Pectic Substrates in Terms of Fermentation Rate, Gut Microbiota Composition, and SCFA Production
4.2.1. The Effects of Pectic Substrates on the Gut Microbiota Composition
4.2.2. The Effects of Pectic Substrates on the Production of SCFA
4.2.3. The Impact on Fermentative Activities Based on Donor Health Status
4.3. Structure-Function Relationship of Pectic Substrates
4.3.1. Degree of Methyl-Esterification
4.3.2. Composition of Neutral Sugars
4.3.3. Distribution of HG and RG Fractions
4.3.4. Degree of Branching
4.3.5. Molecular Weight
4.3.6. Other Structural Characteristics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ndeh, D.; Gilbert, H.J. Biochemistry of complex glycan depolymerisation by the human gut microbiota. FEMS Microbiol. Rev. 2018, 42, 146–164. [Google Scholar] [CrossRef]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-related gelling of pectins and linking with other natural compounds: A review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef] [PubMed]
- Beukema, M.; Faas, M.M.; de Vos, P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: Impact via gut microbiota and direct effects on immune cells. Exp. Mol. Med. 2020, 52, 1364–1376. [Google Scholar] [CrossRef]
- Ferreira-Lazarte, A.; Moreno, F.J.; Cueva, C.; Gil-Sánchez, I.; Villamiel, M. Behaviour of citrus pectin during its gastrointestinal digestion and fermentation in a dynamic simulator (simgi®). Carbohydr. Polym. 2019, 207, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Holloway, W.D.; Tasman-Jones, C.; Maher, K. Pectin digestion in humans. Am. J. Clin. Nutr. 1983, 37, 253–255. [Google Scholar] [CrossRef]
- Yeo, S.-K.; Liong, M.-T. Angiotensin I-converting enzyme inhibitory activity and bioconversion of isoflavones by probiotics in soymilk supplemented with prebiotics. Int. J. Food Sci. Nutr. 2010, 61, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Panel, E.N. Scientific opinion on the substantiation of health claims related to pectins and reduction of post-prandial glycaemic responses (ID 786), maintenance of normal blood cholesterol concentrations (ID 818) and increase in satiety leading to a reduction in energy intake (ID 4692) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1747. [Google Scholar]
- FDA. Review of the Scientific Evidence on the Physiological Effects of Certain Non-Digestible Carbohydrates. Available online: www.fda.gov (accessed on 29 January 2021).
- Adamberg, K.; Kolk, K.; Jaagura, M.; Vilu, R.; Adamberg, S. The composition and metabolism of faecal microbiota is specifically modulated by different dietary polysaccharides and mucin: An isothermal microcalorimetry study. Benef. Microbes 2018, 9, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Dupont, C.; Kalach, N.; Soulaines, P.; Bradatan, E.; Lachaux, A.; Payot, F.; De Blay, F.; Guénard-Bilbault, L.; Hatahet, R.; Mulier, S.; et al. Safety of a New Amino Acid Formula in Infants Allergic to Cow’s Milk and Intolerant to Hydrolysates. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, G.H.; Teka, T.; Zaman, B.; Majid, N.; Khatun, M.; Fuchs, G.J. Clinical studies in persistent diarrhea: Dietary management with green banana or pectin in Bangladeshi children. Gastroenterology 2001, 121, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Inokuchi, R.; Fukushima, K.; Naraba, H.; Takahashi, Y.; Sonoo, T.; Hashimoto, H.; Doi, K.; Morimura, N. Pectin-containing liquid enteral nutrition for critical care: A historical control and propensity score matched study. Asia Pac. J. Clin. Nutr. 2019, 28, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Tabei, I.; Tsuchida, S.; Akashi, T.; Ookubo, K.; Hosoda, S.; Furukawa, Y.; Tanabe, Y.; Tamura, Y. Effects of a novel method for enteral nutrition infusion involving a viscosity-regulating pectin solution: A multicenter randomized controlled trial. Clin. Nutr. ESPEN 2018, 23, 34–40. [Google Scholar] [CrossRef]
- An, R.; Wilms, E.; Smolinska, A.; Hermes, G.D.A.; Masclee, A.A.M.; de Vos, P.; Schols, H.A.; van Schooten, F.J.; Smidt, H.; Jonkers, D.; et al. Sugar Beet Pectin Supplementation Did Not Alter Profiles of Fecal Microbiota and Exhaled Breath in Healthy Young Adults and Healthy Elderly. Nutrients 2019, 11, 2193. [Google Scholar] [CrossRef] [PubMed]
- So, D.; Whelan, K.; Rossi, M.; Morrison, M.; Holtmann, G.; Kelly, J.T.; Shanahan, E.R.; Staudacher, H.M.; Campbell, K.L. Dietary fiber intervention on gut microbiota composition in healthy adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 107, 965–983. [Google Scholar] [CrossRef]
- Aguirre, M.; Jonkers, D.M.; Troost, F.J.; Roeselers, G.; Venema, K. In vitro characterization of the impact of different substrates on metabolite production, energy extraction and composition of gut microbiota from lean and obese subjects. PLoS ONE 2014, 9, e113864. [Google Scholar] [CrossRef]
- Venema, K.; van den Abbeele, P. Experimental models of the gut microbiome. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 115–126. [Google Scholar] [CrossRef]
- Swanson, K.S.; de Vos, W.M.; Martens, E.C.; Gilbert, J.A.; Menon, R.S.; Soto-Vaca, A.; Hautvast, J.; Meyer, P.D.; Borewicz, K.; Vaughan, E.E.; et al. Effect of fructans, prebiotics and fibres on the human gut microbiome assessed by 16S rRNA-based approaches: A review. Benef. Microbes 2020, 11, 101–129. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Ashaolu, J.O.; Adeyeye, S.A.O. Fermentation of prebiotics by human colonic microbiota in vitro and short-chain fatty acids production: A critical review. J. Appl. Microbiol. 2021, 130, 677–687. [Google Scholar] [CrossRef]
- Elshahed, M.S.; Miron, A.; Aprotosoaie, A.C.; Farag, M.A. Pectin in diet: Interactions with the human microbiome, role in gut homeostasis, and nutrient-drug interactions. Carbohydr. Polym. 2021, 255, 117388. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oren, A.; Garrity, G. List of new names and new combinations previously effectively, but not validly, published. Int. J. Syst. Evol. Microbiol. 2020, 70, 4043–4049. [Google Scholar] [CrossRef] [PubMed]
- Cantu-Jungles, T.M.; Bulut, N.; Chambry, E.; Ruthes, A.; Iacomini, M.; Keshavarzian, A.; Johnson, T.A.; Hamaker, B.R. Dietary Fiber Hierarchical Specificity: The Missing Link for Predictable and Strong Shifts in Gut Bacterial Communities. mBio 2021, 12, e0102821. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-T.; Nie, X.; Gan, R.-Y.; Guo, H.; Fu, Y.; Yuan, Q.; Zhang, Q.; Qin, W. In vitro digestion and fecal fermentation behaviors of a pectic polysaccharide from okra (Abelmoschus esculentus) and its impacts on human gut microbiota. Food Hydrocoll. 2021, 114, 106577. [Google Scholar] [CrossRef]
- Yu, X.; Gurry, T.; Nguyen, L.T.T.; Richardson, H.S.; Alm, E.J. Prebiotics and Community Composition Influence Gas Production of the Human Gut Microbiota. mBio 2020, 11, e00217-20. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, C.; Zhao, S.; Tian, G.; Wang, F.; Li, C.; Wang, F.; Zheng, J. Alkali+ cellulase-extracted citrus pectins exhibit compact conformation and good fermentation properties. Food Hydrocoll. 2020, 108, 106079. [Google Scholar] [CrossRef]
- Bang, S.J.; Kim, G.; Lim, M.Y.; Song, E.J.; Jung, D.H.; Kum, J.S.; Nam, Y.D.; Park, C.S.; Seo, D.H. The influence of in vitro pectin fermentation on the human fecal microbiome. AMB Express 2018, 8, 98. [Google Scholar] [CrossRef]
- Tuncil, Y.E.; Nakatsu, C.H.; Kazem, A.E.; Arioglu-Tuncil, S.; Reuhs, B.; Martens, E.; Hamaker, B.R. Delayed utilization of some fast-fermenting soluble dietary fibers by human gut microbiota when presented in a mixture. J. Funct. Foods 2017, 32, 347–357. [Google Scholar] [CrossRef]
- Min, B.; Koo, O.K.; Park, S.H.; Jarvis, N.; Ricke, S.C.; Crandall, P.G.; Lee, S.-O. Fermentation patterns of various pectin sources by human fecal microbiota. Food Nutr. Sci. 2015, 6, 1103–1114. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Verstrepen, L.; Ghyselinck, J.; Albers, R.; Marzorati, M.; Mercenier, A. A Novel Non-Digestible, Carrot-Derived Polysaccharide (cRG-I) Selectively Modulates the Human Gut Microbiota while Promoting Gut Barrier Integrity: An Integrated in vitro Approach. Nutrients 2020, 12, 1917. [Google Scholar] [CrossRef]
- Gomez, B.; Gullón, B.; Yanez, R.; Schols, H.A.; Alonso, J.L. Prebiotic potential of pectins and pecti oligosaccharides derived from lemon peel wastes and sugar beet pulp: A comparative evaluation. J. Funct. Foods 2016, 20, 108–121. [Google Scholar] [CrossRef]
- Sulek, K.; Vigsnaes, L.K.; Schmidt, L.R.; Holck, J.; Frandsen, H.L.; Smedsgaard, J.; Skov, T.H.; Meyer, A.S.; Licht, T.R. A combined metabolomic and phylogenetic study reveals putatively prebiotic effects of high molecular weight arabino-oligosaccharides when assessed by in vitro fermentation in bacterial communities derived from humans. Anaerobe 2014, 28, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Gómez, B.; Gullón, B.; Remoroza, C.; Schols, H.A.; Parajó, J.C.; Alonso, J.L. Purification, characterization, and prebiotic properties of pectic oligosaccharides from orange peel wastes. J. Agric. Food Chem. 2014, 62, 9769–9782. [Google Scholar] [CrossRef] [PubMed]
- Gullon, B.; Gullon, P.; Sanz, Y.; Alonso, J.L.; Parajó, J.C. Prebiotic potential of a refined product containing pectic oligosaccharides. LWT Food Sci. Technol. 2011, 44, 1687–1696. [Google Scholar] [CrossRef]
- Holck, J.; Lorentzen, A.; Vigsnæs, L.K.; Licht, T.R.; Mikkelsen, J.D.; Meyer, A.S. Feruloylated and nonferuloylated arabino-oligosaccharides from sugar beet pectin selectively stimulate the growth of Bifidobacterium spp. in human fecal in vitro fermentations. J. Agric. Food Chem. 2011, 59, 6511–6519. [Google Scholar] [CrossRef]
- Thomassen, L.V.; Vigsnæs, L.K.; Licht, T.R.; Mikkelsen, J.D.; Meyer, A.S. Maximal release of highly bifidogenic soluble dietary fibers from industrial potato pulp by minimal enzymatic treatment. Appl. Microbiol. Biotechnol. 2011, 90, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.P.; Walton, G.E.; Psichas, A.; Frost, G.S.; Gibson, G.R.; Barraclough, T.G. Prebiotics Modulate the Effects of Antibiotics on Gut Microbial Diversity and Functioning in vitro. Nutrients 2015, 7, 4480–4497. [Google Scholar] [CrossRef]
- Reichardt, N.; Vollmer, M.; Holtrop, G.; Farquharson, F.M.; Wefers, D.; Bunzel, M.; Duncan, S.H.; Drew, J.E.; Williams, L.M.; Milligan, G.; et al. Specific substrate-driven changes in human faecal microbiota composition contrast with functional redundancy in short-chain fatty acid production. ISME J. 2018, 12, 610–622. [Google Scholar] [CrossRef]
- Di, R.; Vakkalanka, M.S.; Onumpai, C.; Chau, H.K.; White, A.; Rastall, R.A.; Yam, K.; Hotchkiss, A.T., Jr. Pectic oligosaccharide structure-function relationships: Prebiotics, inhibitors of Escherichia coli O157:H7 adhesion and reduction of Shiga toxin cytotoxicity in HT29 cells. Food Chem. 2017, 227, 245–254. [Google Scholar] [CrossRef]
- Moon, J.S.; Shin, S.Y.; Choi, H.S.; Joo, W.; Cho, S.K.; Li, L.; Kang, J.H.; Kim, T.J.; Han, N.S. In vitro digestion and fermentation properties of linear sugar-beet arabinan and its oligosaccharides. Carbohydr. Polym. 2015, 131, 50–56. [Google Scholar] [CrossRef]
- Onumpai, C.; Kolida, S.; Bonnin, E.; Rastall, R.A. Microbial utilization and selectivity of pectin fractions with various structures. Appl. Environ. Microbiol. 2011, 77, 5747–5754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira-Lazarte, A.; Kachrimanidou, V.; Villamiel, M.; Rastall, R.A.; Moreno, F.J. In vitro fermentation properties of pectins and enzymatic-modified pectins obtained from different renewable bioresources. Carbohydr. Polym. 2018, 199, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Van Trijp, M.P.H.; Rösch, C.; An, R.; Keshtkar, S.; Logtenberg, M.J.; Hermes, G.D.A.; Zoetendal, E.G.; Schols, H.A.; Hooiveld, G. Fermentation Kinetics of Selected Dietary Fibers by Human Small Intestinal Microbiota Depend on the Type of Fiber and Subject. Mol. Nutr. Food Res. 2020, 64, e2000455. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Martínez, I.; Walter, J.; Keshavarzian, A.; Rose, D.J. In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe 2013, 23, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Vigsnæs, L.K.; Holck, J.; Meyer, A.S.; Licht, T.R. In vitro fermentation of sugar beet arabino-oligosaccharides by fecal microbiota obtained from patients with ulcerative colitis to selectively stimulate the growth of Bifidobacterium spp. and Lactobacillus spp. Appl. Environ. Microbiol. 2011, 77, 8336–8344. [Google Scholar] [CrossRef]
- Holck, J.; Hjerno, K.; Lorentzen, A.; Vigsnæs, L.K.; Hemmingsen, L.; Licht, T.R.; Mikkelsen, J.D.; Meyer, A.S. Tailored enzymatic production of oligosaccharides from sugar beet pectin and evidence of differential effects of a single DP chain length difference on human faecal microbiota composition after in vitro fermentation. Process. Biochem. 2011, 46, 1039–1049. [Google Scholar] [CrossRef]
- Jin, M.; Kalainy, S.; Baskota, N.; Chiang, D.; Deehan, E.C.; McDougall, C.; Tandon, P.; Martínez, I.; Cervera, C.; Walter, J.; et al. Faecal microbiota from patients with cirrhosis has a low capacity to ferment non-digestible carbohydrates into short-chain fatty acids. Liver Int. 2019, 39, 1437–1447. [Google Scholar] [CrossRef]
- Adamberg, K.; Adamberg, S. Selection of fast and slow growing bacteria from fecal microbiota using continuous culture with changing dilution rate. Microb. Ecol. Health Dis. 2018, 29, 1549922. [Google Scholar] [CrossRef]
- Perez-Burillo, S.; Mehta, T.; Pastoriza, S.; Kramer, D.; Paliy, O.; Rufian-Henares, J. Potential probiotic salami with dietary fiber modulates antioxidant capacity, short chain fatty acid production and gut microbiota community structure. LWT Food Sci. Technol. 2019, 105, 355–362. [Google Scholar] [CrossRef]
- Cantu-Jungles, T.M.; do Nascimento, G.E.; Zhang, X.; Iacomini, M.; Cordeiro, L.M.C.; Hamaker, B.R. Soluble xyloglucan generates bigger bacterial community shifts than pectic polymers during in vitro fecal fermentation. Carbohydr. Polym. 2019, 206, 389–395. [Google Scholar] [CrossRef]
- Leijdekkers, A.G.; Aguirre, M.; Venema, K.; Bosch, G.; Gruppen, H.; Schols, H.A. In vitro fermentability of sugar beet pulp derived oligosaccharides using human and pig fecal inocula. J. Agric. Food Chem. 2014, 62, 1079–1087. [Google Scholar] [CrossRef]
- Ramasamy, U.S.; Venema, K.; Schols, H.A.; Gruppen, H. Effect of soluble and insoluble fibers within the in vitro fermentation of chicory root pulp by human gut bacteria. J. Agric. Food Chem. 2014, 62, 6794–6802. [Google Scholar] [CrossRef]
- Chung, W.S.F.; Walker, A.W.; Vermeiren, J.; Sheridan, P.O.; Bosscher, D.; Garcia-Campayo, V.; Parkhill, J.; Flint, H.J.; Duncan, S.H. Impact of carbohydrate substrate complexity on the diversity of the human colonic microbiota. FEMS Microbiol. Ecol. 2019, 95, fiy201. [Google Scholar] [CrossRef]
- Chung, W.S.; Walker, A.W.; Louis, P.; Parkhill, J.; Vermeiren, J.; Bosscher, D.; Duncan, S.H.; Flint, H.J. Modulation of the human gut microbiota by dietary fibres occurs at the species level. BMC Biol. 2016, 14, 3. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Duysburgh, C.; Cleenwerck, I.; Albers, R.; Marzorati, M.; Mercenier, A. Consistent Prebiotic Effects of Carrot RG-I on the Gut Microbiota of Four Human Adult Donors in the SHIME(®) Model despite Baseline Individual Variability. Microorganisms 2021, 9, 2142. [Google Scholar] [CrossRef]
- Khodaei, N.; Fernandez, B.; Fliss, I.; Karboune, S. Digestibility and prebiotic properties of potato rhamnogalacturonan I polysaccharide and its galactose-rich oligosaccharides/oligomers. Carbohydr. Polym. 2016, 136, 1074–1084. [Google Scholar] [CrossRef]
- Larsen, N.; de Souza, C.B.; Krych, L.; Kot, W.; Leser, T.D.; Sørensen, O.B.; Blennow, A.; Venema, K.; Jespersen, L. Effect of potato fiber on survival of Lactobacillus species at simulated gastric conditions and composition of the gut microbiota in vitro. Food Res. Int. 2019, 125, 108644. [Google Scholar] [CrossRef]
- Larsen, N.; Bussolo de Souza, C.; Krych, L.; Barbosa Cahú, T.; Wiese, M.; Kot, W.; Hansen, K.M.; Blennow, A.; Venema, K.; Jespersen, L. Potential of Pectins to Beneficially Modulate the Gut Microbiota Depends on Their Structural Properties. Front. Microbiol. 2019, 10, 223. [Google Scholar] [CrossRef]
- Bianchi, F.; Larsen, N.; Tieghi, T.M.; Adorno, M.A.T.; Saad, S.M.I.; Jespersen, L.; Sivieri, K. In vitro modulation of human gut microbiota composition and metabolites by Bifidobacterium longum BB-46 and a citric pectin. Food Res. Int. 2019, 120, 595–602. [Google Scholar] [CrossRef]
- Bianchi, F.; Larsen, N.; de Mello Tieghi, T.; Adorno, M.A.T.; Kot, W.; Saad, S.M.I.; Jespersen, L.; Sivieri, K. Modulation of gut microbiota from obese individuals by in vitro fermentation of citrus pectin in combination with Bifidobacterium longum BB-46. Appl. Microbiol. Biotechnol. 2018, 102, 8827–8840. [Google Scholar] [CrossRef]
- Míguez, B.; Vila, C.; Venema, K.; Parajó, J.C.; Alonso, J.L. Prebiotic effects of pectooligosaccharides obtained from lemon peel on the microbiota from elderly donors using an in vitro continuous colon model (TIM-2). Food Funct. 2020, 11, 9984–9999. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Khan, T.M.; Duncan, S.H.; Harmsen, H.J.; Garcia-Gil, L.J.; Flint, H.J. Cultured representatives of two major phylogroups of human colonic Faecalibacterium prausnitzii can utilize pectin, uronic acids, and host-derived substrates for growth. Appl. Environ. Microbiol. 2012, 78, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Ladirat, S.E.; Schols, H.A.; Nauta, A.; Schoterman, M.H.; Keijser, B.J.; Montijn, R.C.; Gruppen, H.; Schuren, F.H. High-throughput analysis of the impact of antibiotics on the human intestinal microbiota composition. J. Microbiol. Methods 2013, 92, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Southgate, D.A.; Branch, W.J.; Wiggins, H.S.; Houston, H.; Jenkins, D.J.; Jivraj, T.; Hill, M.J. The digestion of pectin in the human gut and its effect on calcium absorption and large bowel function. Br. J. Nutr. 1979, 41, 477–485. [Google Scholar] [CrossRef]
- Hamberg, O.; Rumessen, J.J.; Gudmand-Høyer, E. Inhibition of starch absorption by dietary fibre. A comparative study of wheat bran, sugar-beet fibre, and pea fibre. Scand. J Gastroenterol. 1989, 24, 103–109. [Google Scholar] [CrossRef]
- Christl, S.U.; Murgatroyd, P.R.; Gibson, G.R.; Cummings, J.H. Production, metabolism, and excretion of hydrogen in the large intestine. Gastroenterology 1992, 102, 1269–1277. [Google Scholar] [CrossRef]
- Grabitske, H.A.; Slavin, J.L. Gastrointestinal effects of low-digestible carbohydrates. Crit. Rev. Food Sci. Nutr. 2009, 49, 327–360. [Google Scholar] [CrossRef]
- Müller, M.; Hermes, G.D.A.; Canfora, E.E.; Smidt, H.; Masclee, A.A.M.; Zoetendal, E.G.; Blaak, E.E. Distal colonic transit is linked to gut microbiota diversity and microbial fermentation in humans with slow colonic transit. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G361–G369. [Google Scholar] [CrossRef]
- El Kaoutari, A.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef]
- Chung, W.S.F.; Meijerink, M.; Zeuner, B.; Holck, J.; Louis, P.; Meyer, A.S.; Wells, J.M.; Flint, H.J.; Duncan, S.H. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol. Ecol. 2017, 93, fix127. [Google Scholar] [CrossRef]
- Martens, E.C.; Lowe, E.C.; Chiang, H.; Pudlo, N.A.; Wu, M.; McNulty, N.P.; Abbott, D.W.; Henrissat, B.; Gilbert, H.J.; Bolam, D.N.; et al. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol. 2011, 9, e1001221. [Google Scholar] [CrossRef]
- Pryde, S.E.; Duncan, S.H.; Hold, G.L.; Stewart, C.S.; Flint, H.J. The microbiology of butyrate formation in the human colon. FEMS Microbiol. Lett. 2002, 217, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Asnicar, F.; Berry, S.E.; Valdes, A.M.; Nguyen, L.H.; Piccinno, G.; Drew, D.A.; Leeming, E.; Gibson, R.; Le Roy, C.; Khatib, H.A.; et al. Microbiome connections with host metabolism and habitual diet from 1098 deeply phenotyped individuals. Nat. Med. 2021, 27, 321–332. [Google Scholar] [CrossRef]
- Sokol, H.; Seksik, P.; Furet, J.P.; Firmesse, O.; Nion-Larmurier, I.; Beaugerie, L.; Cosnes, J.; Corthier, G.; Marteau, P.; Doré, J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm. Bowel Dis. 2009, 15, 1183–1189. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, H.; Chen, S.; He, J.; Zhou, Y.; Nie, Y. Systematic review and meta-analysis of the role of Faecalibacterium prausnitzii alteration in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2021, 36, 320–328. [Google Scholar] [CrossRef]
- Lecronier, M.; Tashk, P.; Tamzali, Y.; Tenaillon, O.; Denamur, E.; Barrou, B.; Aron-Wisnewsky, J.; Tourret, J. Gut microbiota composition alterations are associated with the onset of diabetes in kidney transplant recipients. PLoS ONE 2020, 15, e0227373. [Google Scholar] [CrossRef] [PubMed]
- Ruengsomwong, S.; La-Ongkham, O.; Jiang, J.; Wannissorn, B.; Nakayama, J.; Nitisinprasert, S. Microbial Community of Healthy Thai Vegetarians and Non-Vegetarians, Their Core Gut Microbiota, and Pathogen Risk. J. Microbiol. Biotechnol. 2016, 26, 1723–1735. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Fechner, A.; Kiehntopf, M.; Jahreis, G. The formation of short-chain fatty acids is positively associated with the blood lipid-lowering effect of lupin kernel fiber in moderately hypercholesterolemic adults. J. Nutr. 2014, 144, 599–607. [Google Scholar] [CrossRef] [Green Version]
- Pomare, E.W.; Branch, W.J.; Cummings, J.H. Carbohydrate fermentation in the human colon and its relation to acetate concentrations in venous blood. J. Clin. Investig. 1985, 75, 1448–1454. [Google Scholar] [CrossRef]
- Dusková, D.; Marounek, M. Fermentation of pectin and glucose, and activity of pectin-degrading enzymes in the rumen bacterium Lachnospira multiparus. Lett. Appl. Microbiol. 2001, 33, 159–163. [Google Scholar] [CrossRef]
- Olano-Martin, E.; Gibson, G.R.; Rastell, R.A. Comparison of the in vitro bifidogenic properties of pectins and pectic-oligosaccharides. J. Appl. Microbiol. 2002, 93, 505–511. [Google Scholar] [CrossRef]
- Thorning, T.K.; Bertolt, C.J.; Nielsen, M.S.; Ritz, C.; Astrup, A.; Raben, A. Potato Fibers Have Positive Effects on Subjective Appetite Sensations in Healthy Men, but Not on Fecal Fat Excretion: A Randomized Controlled Single-Blind Crossover Trial. Nutrients 2020, 12, 3496. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Hachelaf, W.; Suau, A.; Boudraa, G.; Bouziane-Nedjadi, K.; Rigottier-Gois, L.; Touhami, M.; Desjeux, J.F.; Pochart, P. Effects on faecal microbiota of dietary and acidic oligosaccharides in children during partial formula feeding. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 580–588. [Google Scholar] [CrossRef]
- Heinken, A.; Khan, M.T.; Paglia, G.; Rodionov, D.A.; Harmsen, H.J.; Thiele, I. Functional metabolic map of Faecalibacterium prausnitzii, a beneficial human gut microbe. J. Bacteriol. 2014, 196, 3289–3302. [Google Scholar] [CrossRef]
- Zdunek, A.; Pieczywek, P.M.; Cybulska, J. The primary, secondary, and structures of higher levels of pectin polysaccharides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1101–1117. [Google Scholar] [CrossRef]
- Rastall, R.A.; Diez-Municio, M.; Forssten, S.; Hamaker, B.R.; Meyer, A.S.; Moreno, F.J.; Respondek, F.; Stahl, B.; Venema, K.; Wiese, M. Structure and function of non-digestible carbohydrates in the gut microbiome. Beneficial. Microbes 2022, 13, 95–108. [Google Scholar] [CrossRef]
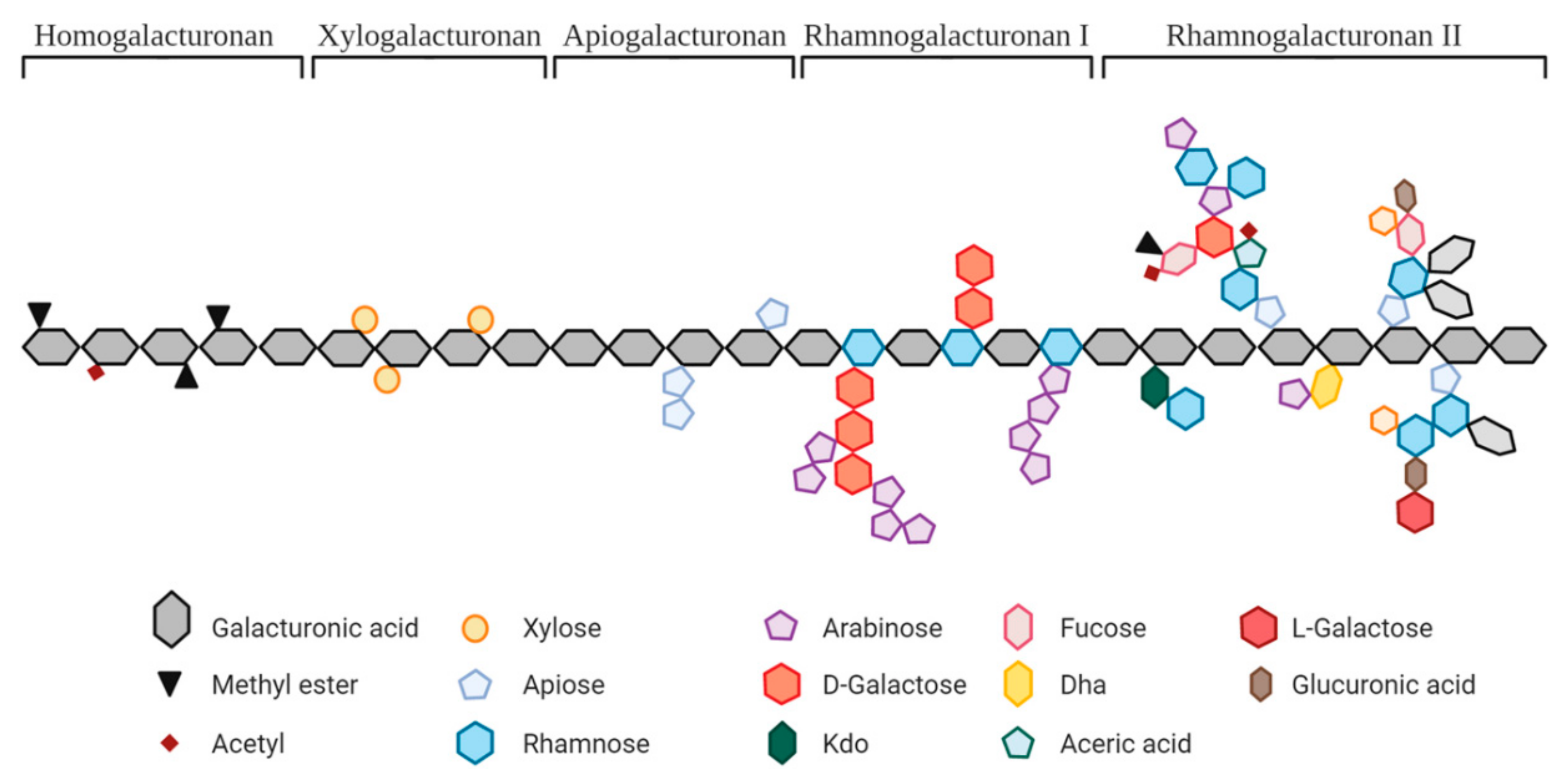

| Pectic Substrates | Origin | Molecular Structure and Main Linkages | Other Parameters Described in the Studies |
|---|---|---|---|
| Pectin | Citrus, apple, sugar beet, soy, sunflower, artichoke, and prune | (GalA)n and/or (GalA-Rha)n and/or (GalA-Gal)n; α(1,4); α(1,2) | GalA: 32–88% DE: 2–79% |
| Hydrolyzed pectin | Citrus, sunflower, and artichoke | GalA: 56–79% DE: 5–17% MW: 9.2–300 kDa | |
| OS from pectin | Methylated citrus pectin, orange or lemon peel, and apple | GalA: 42–96%; DE: 29–62%; DP 1–10 or MW > 23 kDa | |
| Sugar beet | GalA: <2–78%; Ara: 10–85%; DP 2–10 or MW: <1–12 kDa | ||
| Polygalacturonic acid | Citrus pectin | α(1,4)GalAn | GalA: >90% |
| OS from PolyGalA | Polygalacturonic acid | GalA: 91–98% DP 1–23 | |
| RG1- enriched | Okra, carrot, A. thaliana seed mucilage, prune, lime, and potato | α-(1,2)(Rha)n and α-(1,4)(GalA)n and β-(1,4)(Gal)n (potato only), and α-Ara and β-D-Gal residues of different sizes | GalA: 10–25%; Ara > 48%; Potato: Gal 61%, 34 kDa |
| OS from RG1 | A. thaliana seed mucilage; Potato | Potato: >70% Gal; DP 2–70 | |
| Arabinan | Sugar beet | α-(1,5)(Ara)n and possible Ara residues or short side chains | MW: 18 kDa, debranched, Ara:Gal:Rha = 71:26:3 |
| OS from Arabinan | DP 1–11, Ara: 93.4% | ||
| Arabinogalactan | Acacia fiber and larch tree | AGI: β-(1,4)-D-(Gal)n and occasional β-(1,3), and α-Ara/Fuc/GlucA AGII: β-(1,3)-D-(Gal)n and β-(1,6)-D-Gal/Ara | MW: 300–800 kDa |
| Galactan | Potato | β-(1,4)(Gal)n and may contain Ara/Rha/GalA | MW: ~100 kDa |
| OS from Galactan | Gal: 95%, DP 1–10 | ||
| Galactomannan | Carob tree and guar plant | Man(β-1,4)[Gal(α-1,6)]β-Man | MW: 1.07 × 105–0.67 × 106 kDa |
| Fibers rich in pectin | Potato | α-(1,2)(Rha)n and α-(1,4)(GalA)n and β-(1,4)(Gal)n side chains | 65% fiber; GalA: 13% |
| Chicory root pulp | Pectin fraction: (GalA)n and/or (GalA-Rha)n; α(1,4); α(1,2). Inulin fraction: β-(2-1)(Fru)n | 62% pectin, uronic acids 38% | |
| Apple | α-(1,2)(Rha)n and α-(1,4)(GalA)n and α-(1,4)(Ara)n, β-(1,4)(Gal)n | GalA: 23%, 60% total sugars (45% glucose) | |
| Citrus fiber | (GalA)n and/or (GalA-Rha)n; α(1,4); α(1,2) | 42% pectin, 25% cellulose and hemicellulose |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascale, N.; Gu, F.; Larsen, N.; Jespersen, L.; Respondek, F. The Potential of Pectins to Modulate the Human Gut Microbiota Evaluated by In Vitro Fermentation: A Systematic Review. Nutrients 2022, 14, 3629. https://doi.org/10.3390/nu14173629
Pascale N, Gu F, Larsen N, Jespersen L, Respondek F. The Potential of Pectins to Modulate the Human Gut Microbiota Evaluated by In Vitro Fermentation: A Systematic Review. Nutrients. 2022; 14(17):3629. https://doi.org/10.3390/nu14173629
Chicago/Turabian StylePascale, Nélida, Fangjie Gu, Nadja Larsen, Lene Jespersen, and Frederique Respondek. 2022. "The Potential of Pectins to Modulate the Human Gut Microbiota Evaluated by In Vitro Fermentation: A Systematic Review" Nutrients 14, no. 17: 3629. https://doi.org/10.3390/nu14173629
APA StylePascale, N., Gu, F., Larsen, N., Jespersen, L., & Respondek, F. (2022). The Potential of Pectins to Modulate the Human Gut Microbiota Evaluated by In Vitro Fermentation: A Systematic Review. Nutrients, 14(17), 3629. https://doi.org/10.3390/nu14173629





