A Mediterranean-Diet-Based Nutritional Intervention for Children with Prediabetes in a Rural Town: A Pilot Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
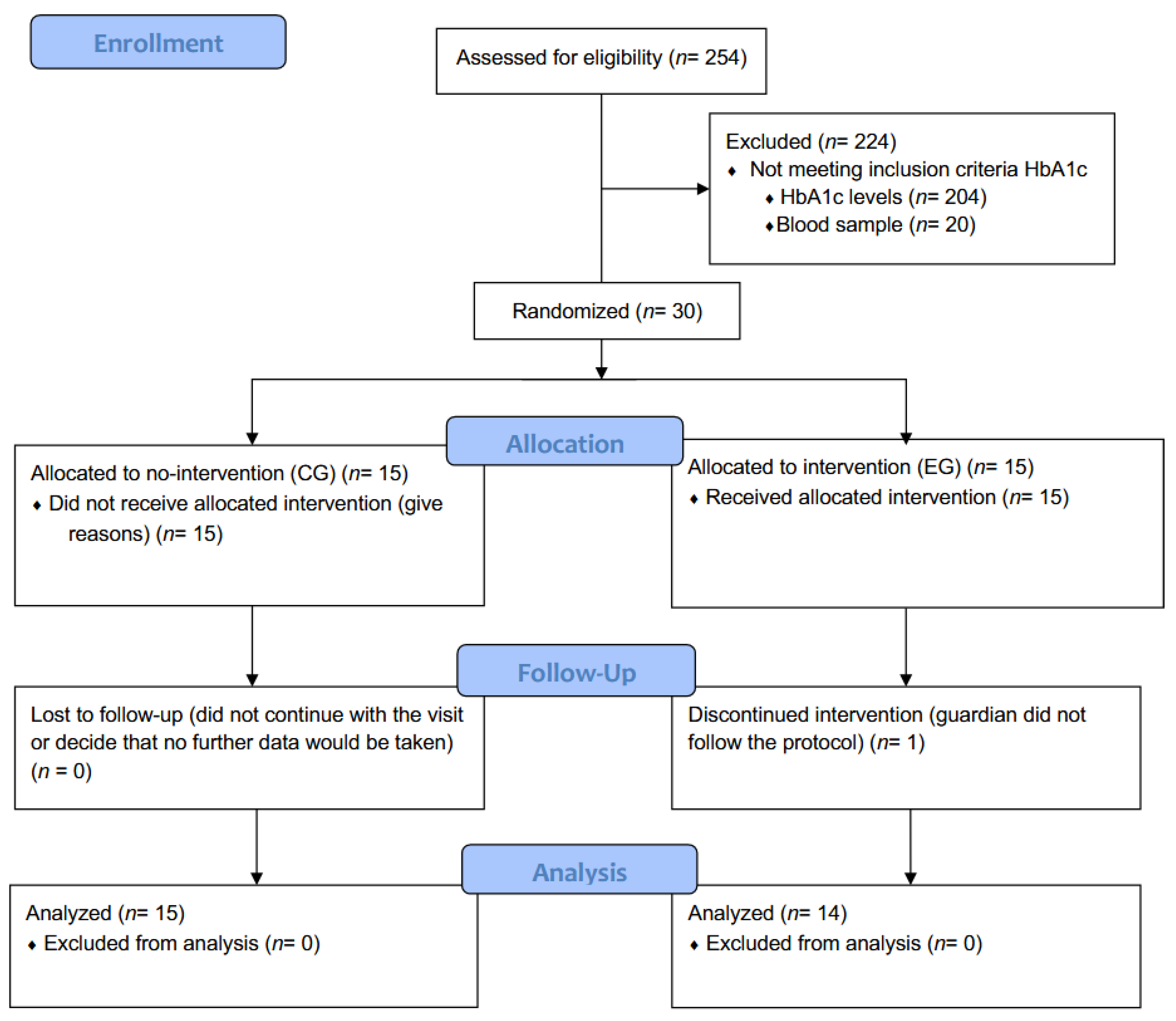
2.2. Study Population, Sampling, and Data Collection
2.3. Nutritional Intervention and Evaluation
2.4. Parameters and Measuring Instruments
2.5. Statistical Analysis
2.6. Ethical Aspects
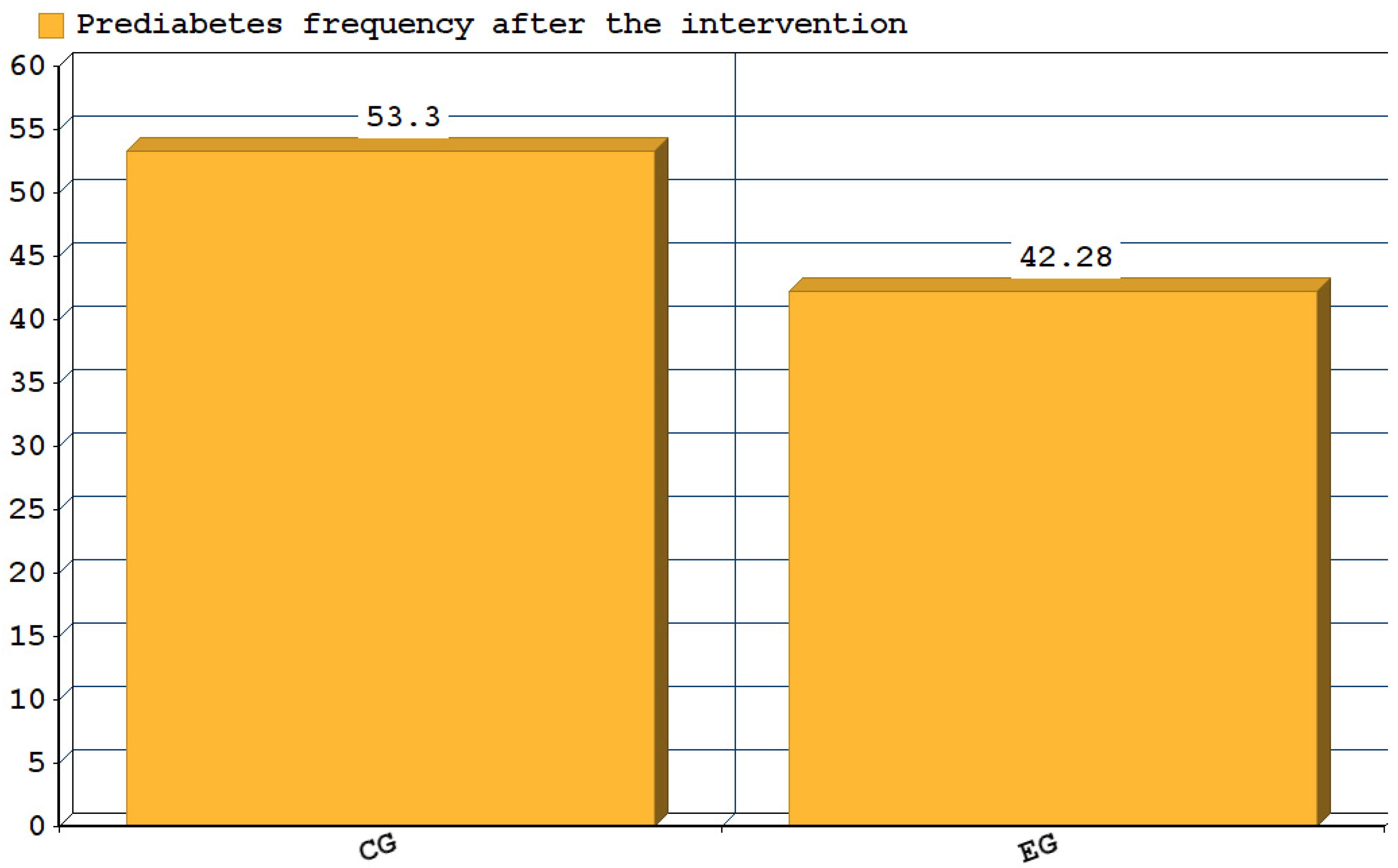
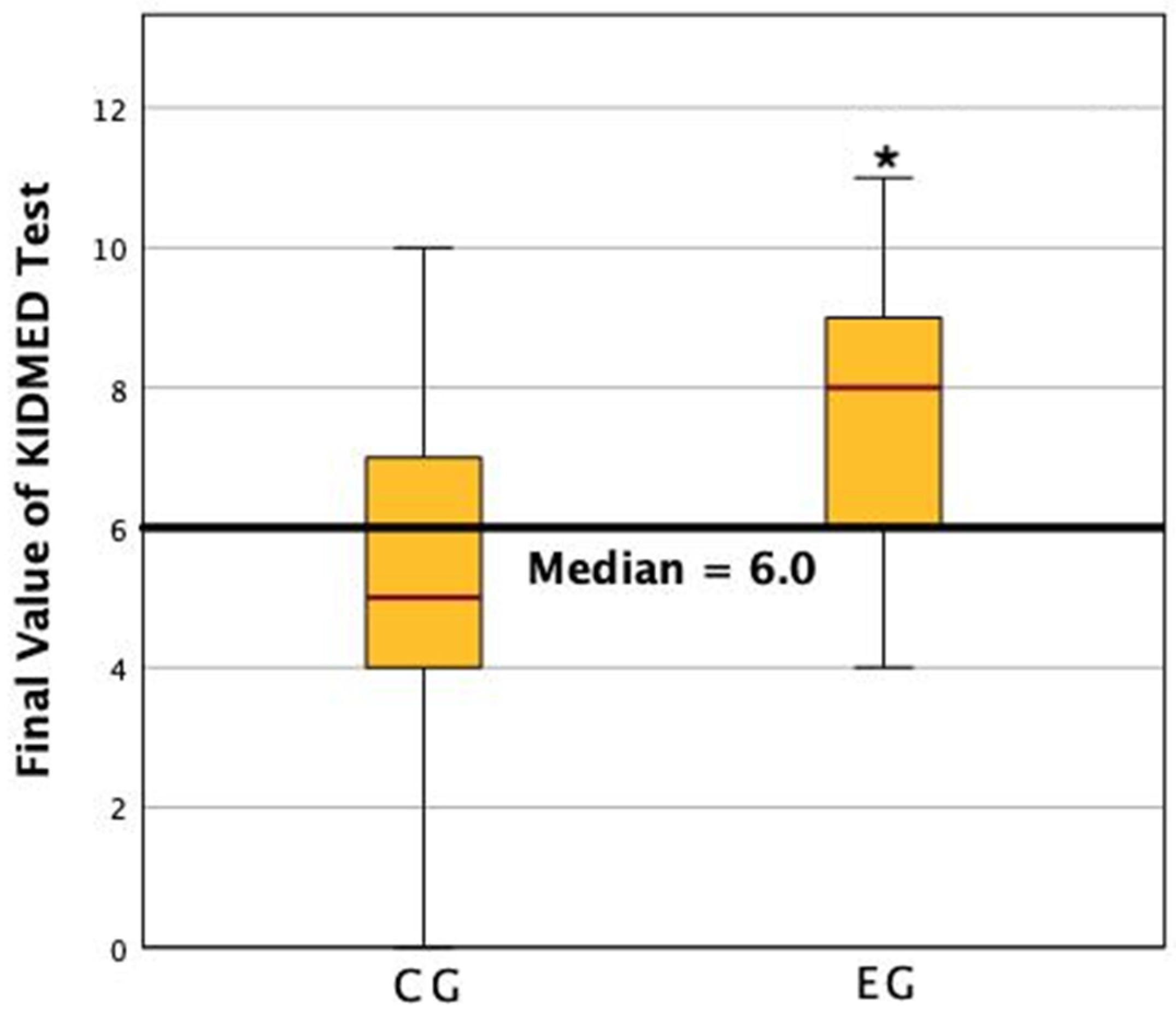
3. Results
4. Discussion
Limitations, Implications for the Field, and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stephenson, J.; Heslehurst, N.; Hall, J.; Schoenaker, D.A.J.M.; Hutchinson, J.; Cade, J.E.; Poston, L.; Barrett, G.; Crozier, S.R.; Barker, M.; et al. Before the Beginning: Nutrition and Lifestyle in the Preconception Period and Its Importance for Future Health. Lancet 2018, 391, 1830–1841. [Google Scholar] [CrossRef]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef] [PubMed]
- Valerio, G.; Baroni, M.G.; Brufani, C.; Forziato, C.; Grugni, G.; Licenziati, M.R.; Maffeis, C.; Miraglia Del Giudice, E.; Morandi, A.; Pacifico, L.; et al. Childhood Obesity Classification Systems and Cardiometabolic Risk Factors: A Comparison of the Italian, World Health Organization and International Obesity Task Force References. Ital. J. Pediatr. 2017, 43, 19. [Google Scholar] [CrossRef] [PubMed]
- Atzendorf, J.; Apfelbacher, C.; Gomes de Matos, E.; Kraus, L.; Piontek, D. Patterns of Multiple Lifestyle Risk Factors and Their Link to Mental Health in the German Adult Population: A Cross-Sectional Study. BMJ Open 2018, 8, e022184. [Google Scholar] [CrossRef] [PubMed]
- Morales-Suárez-Varela, M.; Rubio-López, N.; Ruso, C.; Llopis-Gonzalez, A.; Ruiz-Rojo, E.; Redondo, M.; Pico, Y. Anthropometric Status and Nutritional Intake in Children (6–9 Years) in Valencia (Spain): The ANIVA Study. Int. J. Environ. Res. Public Health 2015, 12, 16082–16095. [Google Scholar] [CrossRef]
- Brambilla, P.; Pozzobon, G.; Pietrobelli, A. Physical Activity as the Main Therapeutic Tool for Metabolic Syndrome in Childhood. Int. J. Obes. 2011, 35, 16–28. [Google Scholar] [CrossRef]
- Kosti, R.I.; Panagiotakos, D.B. The Epidemic of Obesity in Children and Adolescents in the World. Cent. Eur. J. Public Health 2006, 14, 151–159. [Google Scholar] [CrossRef]
- Aranceta-Bartrina, J.; Gianzo-Citores, M.; Pérez-Rodrigo, C. Prevalence of Overweight, Obesity and Abdominal Obesity in the Spanish Population Aged 3 to 24 Years. The ENPE Study. Rev. Esp. Cardiol. 2020, 73, 290–299. [Google Scholar] [CrossRef]
- World Health Organization. Taking Action on Childhood Obesity Report; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Echeverría, G.; McGee, E.; Urquiaga, I.; Jiménez, P.; D’Acuña, S.; Villarroel, L.; Velasco, N.; Leighton, F.; Rigotti, A. Inverse Associations between a Locally Validated Mediterranean Diet Index, Overweight/Obesity, and Metabolic Syndrome in Chilean Adults. Nutrients 2017, 9, 862. [Google Scholar] [CrossRef]
- Peng, W.; Goldsmith, R.; Berry, E.M. Demographic and Lifestyle Factors Associated with Adherence to the Mediterranean Diet in Relation to Overweight/Obesity among Israeli Adolescents: Findings from the Mabat Israeli National Youth Health and Nutrition Survey. Public Health Nutr. 2017, 20, 883–892. [Google Scholar] [CrossRef]
- Gracia-Arnaiz, M. Taking Measures in Times of Crisis: The Political Economy of Obesity Prevention in Spain. Food Policy 2017, 68, 65–76. [Google Scholar] [CrossRef]
- Aguilar-Morales, I.; Colin-Ramirez, E.; Rivera-Mancía, S.; Vallejo, M.; Vázquez-Antona, C. Performance of Waist-to-Height Ratio, Waist Circumference, and Body Mass Index in Discriminating Cardio-Metabolic Risk Factors in a Sample of School-Aged Mexican Children. Nutrients 2018, 10, 1850. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Ragab, S.H.; Ismail, N.A.; Awad, M.A.; Kandil, M.E. Echocardiographic Assessment of Epicardial Adipose Tissue in Obese Children and Its Relation to Clinical Parameters of Metabolic Syndrome. J. Saudi Heart Assoc. 2013, 25, 108. [Google Scholar] [CrossRef][Green Version]
- Castillo, E.H.; Borges, G.; Talavera, J.O.; Orozco, R.; Vargas-Alemán, C.; Huitrón-Bravo, G.; Diaz-Montiel, J.C.; Castañón, S.; Salmerón, J. Body Mass Index and the Prevalence of Metabolic Syndrome among Children and Adolescents in Two Mexican Populations. J. Adolesc. Health 2007, 40, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Hahad, O.; Prochaska, J.H.; Daiber, A.; Münzel, T. Environmental Noise-Induced Effects on Stress Hormones, Oxidative Stress, and Vascular Dysfunction: Key Factors in the Relationship between Cerebrocardiovascular and Psychological Disorders. Oxid. Med. Cell. Longev. 2019, 2019, 4623109. [Google Scholar] [CrossRef]
- Markopoulou, P.; Papanikolaou, E.; Analytis, A.; Zoumakis, E.; Siahanidou, T. Preterm Birth as a Risk Factor for Metabolic Syndrome and Cardiovascular Disease in Adult Life: A Systematic Review and Meta-Analysis. J. Pediatr. 2019, 210, 69–80.e5. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Pomeranz, J.; Wilde, P. Adoption and Design of Emerging Dietary Policies to Improve Cardiometabolic Health in the US. Curr. Atheroscler. Rep. 2018, 20, 25. [Google Scholar] [CrossRef]
- Alp, H.; Eklioğlu, B.S.; Atabek, M.E.; Karaarslan, S.; Baysal, T.; Altın, H.; Karataş, Z.; Şap, F. Evaluation of Epicardial Adipose Tissue, Carotid Intima-Media Thickness and Ventricular Functions in Obese Children and Adolescents. J. Pediatr. Endocrinol. Metabol. 2014, 27, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S. Profiles of Body Mass Index and Blood Pressure among Young Adults Categorised by Waist-to-Height Ratio Cut-Offs in Shandong, China. Ann. Hum. Biol. 2019, 46, 409–414. [Google Scholar] [CrossRef]
- Eklioğlu, B.S.; Atabek, M.E.; Akyürek, N.; Alp, H. Prediabetes and Cardiovascular Parameters in Obese Children and Adolescents. J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 80–85. [Google Scholar] [CrossRef]
- Ighbariya, A.; Weiss, R. Insulin Resistance, Prediabetes, Metabolic Syndrome: What Should Every Pediatrician Know? J. Clin. Res. Pediatri. Endocrinol. 2018, 9 (Suppl. S2), 49–57. [Google Scholar] [CrossRef] [PubMed]
- Andes, L.J.; Cheng, Y.J.; Rolka, D.B.; Gregg, E.W.; Imperatore, G. Prevalence of Prediabetes Among Adolescents and Young Adults in the United States, 2005–2016. JAMA Pediatr. 2020, 174, e194498. [Google Scholar] [CrossRef] [PubMed]
- Cosson, E.; Hamo-Tchatchouang, E.; Banu, I.; Nguyen, M.-T.; Chiheb, S.; Ba, H.; Valensi, P. A Large Proportion of Prediabetes and Diabetes Goes Undiagnosed When Only Fasting Plasma Glucose and/or HbA1c Are Measured in Overweight or Obese Patients. Diabetes Metab. 2010, 36, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Song, Q.; Ren, Y.; Chen, X.; Jiang, X.; Hu, D. Global Prevalence of Prediabetes in Children and Adolescents: A Systematic Review and Meta-Analysis. J. Diabetes 2022, 14, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Al Amiri, E.; Abdullatif, M.; Abdulle, A.; Al Bitar, N.; Afandi, E.Z.; Parish, M.; Darwiche, G. The Prevalence, Risk Factors, and Screening Measure for Prediabetes and Diabetes among Emirati Overweight/Obese Children and Adolescents. BMC Public Health 2015, 15, 1298. [Google Scholar] [CrossRef]
- Pandey, U.; Midha, T.; Rao, Y.K.; Katiyar, P.; Wal, P.; Kaur, S.; Martolia, D.S. Anthropometric Indicators as Predictor of Prediabetes in Indian Adolescents. Indian Heart J. 2017, 69, 474–479. [Google Scholar] [CrossRef]
- Pandey, M.; Chalk, W. Lockdown: ‘Noisy Neighbours Are Ruining My Life’. BBC News, 12 May 2020; 3. [Google Scholar]
- Cabana, F.; Jasmi, R.; Maguire, R. Great Ape Nutrition: Low-Sugar and High-Fibre Diets Can Lead to Increased Natural Behaviours, Decreased Regurgitation and Reingestion, and Reversal of Prediabetes. Int. Zoo Yearb. 2018, 52, 48–61. [Google Scholar] [CrossRef]
- Mazahery, H.; Gammon, C.; Lawgun, D.; Conlon, C.; Beck, K.; Von Hurst, P. Pre-Diabetes Prevalence and Associated Factors in New Zealand School Children: A Cross-Sectional Study. N. Z. Med. J. 2021, 134, 76–90. [Google Scholar]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef]
- Crespo-Blanco, M.C.; Varillas-Delgado, D.; Blanco-Abril, S.; Cid-Exposito, M.G.; Robledo-Martín, J. Effectiveness of an Intervention Programme on Adherence to the Mediterranean Diet in a Preschool Child: A Randomised Controlled Trial. Nutrients 2022, 14, 1536. [Google Scholar] [CrossRef]
- Finicelli, M.; Di Salle, A.; Galderisi, U.; Peluso, G. The Mediterranean Diet: An Update of the Clinical Trials. Nutrients 2022, 14, 2956. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Dinu, M.; Pagliai, G.; Cesari, F.; Gori, A.M.; Sereni, A.; Becatti, M.; Fiorillo, C.; Marcucci, R.; Casini, A. Low-Calorie Vegetarian versus Mediterranean Diets for Reducing Body Weight and Improving Cardiovascular Risk Profile. Circulation 2018, 137, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Díaz-López, A.; Ruiz-Canela, M.; Basora, J.; Fitó, M.; Corella, D.; Serra-Majem, L.; Wärnberg, J.; Romaguera, D.; Estruch, R.; et al. Effect of a Lifestyle Intervention Program with Energy-Restricted Mediterranean Diet and Exercise on Weight Loss and Cardiovascular Risk Factors: One-Year Results of the PREDIMED-Plus Trial. Diabetes Care 2019, 42, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Pascual Fuster, V.; Pérez Pérez, A.; Carretero Gómez, J.; Caixàs Pedragós, A.; Gómez-Huelgas, R.; Pérez-Martínez, P. Executive Summary: Updates to the Dietary Treatment of Prediabetes and Type 2 Diabetes Mellitus. Endocrinol. Diabetes Nutr. 2021, 68, 277–287. [Google Scholar] [CrossRef]
- van Zuuren, E.J.; Fedorowicz, Z.; Kuijpers, T.; Pijl, H. Effects of Low-Carbohydrate- Compared with Low-Fat-Diet Interventions on Metabolic Control in People with Type 2 Diabetes: A Systematic Review including GRADE Assessments. Am. J. Clin. Nutr. 2018, 108, 300–331. [Google Scholar] [CrossRef]
- Rojo-Martínez, G.; Valdés, S.; Soriguer, F.; Vendrell, J.; Urrutia, I.; Pérez, V.; Ortega, E.; Ocón, P.; Montanya, E.; Menéndez, E.; et al. Incidence of Diabetes Mellitus in Spain as Results of the Nation-Wide Cohort Diabetes Study. Sci. Rep. 2020, 10, 2765. [Google Scholar] [CrossRef]
- Azzi, J.L.; Azzi, S.; Lavigne-Robichaud, M.; Vermeer, A.; Barresi, T.; Blaine, S.; Giroux, I. Participant Evaluation of a Prediabetes Intervention Program Designed for Rural Adults. Can. J. Diet. Pract. Res. 2020, 81, 80–85. [Google Scholar] [CrossRef]
- Arenaza, L.; Medrano, M.; Amasene, M.; Rodríguez-Vigil, B.; Díez, I.; Graña, M.; Tobalina, I.; Maiz, E.; Arteche, E.; Larrarte, E.; et al. Prevention of Diabetes in Overweight/Obese Children through a Family Based Intervention Program including Supervised Exercise (PREDIKID Project): Study Protocol for a Randomized Controlled Trial. Trials 2017, 18, 372. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; the CONSORT Group. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. BMC Med. 2010, 8, 18. [Google Scholar] [CrossRef]
- Albert Pérez, E.; Mateu Olivares, V.; Martínez-Espinosa, R.; Molina Vila, M.; Reig García-Galbis, M. New Insights about How to Make an Intervention in Children and Adolescents with Metabolic Syndrome: Diet, Exercise vs. Changes in Body Composition. A Systematic Review of RCT. Nutrients 2018, 10, 878. [Google Scholar] [CrossRef]
- Dominguez-Riscart, J.; Buero-Fernandez, N.; Garcia-Zarzuela, A.; Morales-Perez, C.; Garcia-Ojanguren, A.; Lechuga-Sancho, A.M. Adherence to Mediterranean Diet Is Associated with Better Glycemic Control in Children with Type 1 Diabetes: A Cross-Sectional Study. Front. Nutr. 2022, 9, 813989. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, M.; Chiefari, E.; Arcidiacono, B.; Corigliano, D.M.; Brunetti, F.S.; Maggisano, V.; Russo, D.; Foti, D.P.; Brunetti, A. Mediterranean Diet Nutrients to Turn the Tide against Insulin Resistance and Related Diseases. Nutrients 2020, 12, 1066. [Google Scholar] [CrossRef] [PubMed]
- Bučan Nenadić, D.; Kolak, E.; Selak, M.; Smoljo, M.; Radić, J.; Vučković, M.; Dropuljić, B.; Pijerov, T.; Babić Cikoš, D. Anthropometric Parameters and Mediterranean Diet Adherence in Preschool Children in Split-Dalmatia County, Croatia—Are They Related? Nutrients 2021, 13, 4252. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E.; Ávila, J.M.; Castillo, A.; Valero, T.; Del Pozo, S.; Rodriguez, P.; Bartrina, J.A.; Gil, Á.; González-Gross, M.; Ortega, R.M.; et al. The ANIBES Study on Energy Balance in Spain: Design, Protocol and Methodology. Nutrients 2015, 7, 970. [Google Scholar] [CrossRef]
- Ministerio de Sanidad, Servicios Sociales e Igualdad; Agencia Española de Consumo, Seguridad Alimentaria y Nutrición (AECOSAN). Programa de Alimentación, Nutrición y Gastronomía Para Educación Infantil (PANGEI). El Gusto Es Mío; Secretaría General Técnica, Centro de Publicaciones, Ministerio de Educación, Cultura y Deporte: Madrid, Spain, 2016; ISBN 030-16-552-0.
- Costarelli, V.; Koretsi, E.; Georgitsogianni, E. Health-Related Quality of Life of Greek Adolescents: The Role of the Mediterranean Diet. Qual. Life Res. 2013, 22, 951–956. [Google Scholar] [CrossRef]
- Costarelli, V.; Michou, M.; Svoronou, E.; Koutava, N.; Symvoulidou, M.; Anastasiou, K.; Abeliotis, K. ‘Healthy Children, Healthy Planet’: A Pilot School-Based Educational Intervention. Health Educ. J. 2022, 81, 183–195. [Google Scholar] [CrossRef]


| Variables | Control Group (CG) (n = 15) | Experimental Group (EG) (n = 14) | Differences between GC vs. GE (p-Value) |
|---|---|---|---|
| Gender (boys) | 7 (46.7%) | 8 (57.1%) | 0.6 |
| Weight (kg) | 41.12 ± 12.04 | 44.18 ± 16 | 0.36 |
| Height (m) | 140.95 ± 9.29 | 143.2 ± 27.4 | 0.25 |
| BMI 1 (kg/m2) | 20.2 ± 4.1 | 20.9 ± 4.3 | 0.42 |
| Waist circumference (cm) | 68.9 ± 12.8 | 74.8 ± 15.3 | 0.4 |
| Arm circumference (cm) | 21.3 ± 3.2 | 21.9 ± 4.7 | 0.13 |
| Hip circumference (cm) | 88.9 ± 20.3 | 85.8 ± 12.5 | 0.51 |
| BF% 1 | 29.9 ± 2.2 | 31.5 ± 3.9 | 0.58 |
| FF% 1 | 24.3 ± 7.9 | 25.3 ± 7.9 | 0.41 |
| Hb1Ac | 5.86 ± 0.2 | 5.91 ± 0.1 | 0.47 |
| Insulin level | 11.72 ± 7.16 | 12 ± 10.01 | 0.68 |
| Variables | Control Group (CG) (n = 15) | Differences between V0 and V4 (p-Value) a | Experimental Group (EG) (n = 14) | Differences between V0 and V4 (p-Value) | Differences GC vs. GE (p-Value) b |
|---|---|---|---|---|---|
| Weight (kg) | 40.27 ± 11.64 | −0.85 (0.23) | 42.97 ± 15.87 | −1.83 (0.13) | 0.46 |
| Height (m) | 142.45 ± 10.17 | 1.5 (0.85) | 144.73 ± 13.39 | 1.5 (0.75) | 0.91 |
| BMI 1 (kg/m2) | 19.58 ± 4.07 | −0.62 (0.19) | 20.61 ± 4.4 | −0.69 (0.13) | 0.62 |
| Waist circumference (cm) | 66.48 ± 12.74 | −2.32 (0.02) | 71.61 ± 13.65 | −3.19 (0.001) | 0.58 |
| Arm circumference (cm) | 18.94 ± 3.06 | −2.36 (0.018) | 19.39 ± 4.7 | −2.51 (0.012) | 0.45 |
| Hip circumference (cm) | 88.77 ± 23.32 | −0.13 (p < 0.001) | 85.42 ± 18.25 | −0.38 (<0.001) | 0.17 |
| BF% 1 | 29.59 ± 3.11 | −0.31 (0.39) | 31 ± 3.71 | −0.5 (0.22) | 0.26 |
| FF% 1 | 27.28 ± 8.9 | 2.98 (0.003) | 27.77 ± 10.87 | 2.47 (0.016) | 0.82 |
| Hb1Ac | 5.56 ± 0.2 | −0.3 (<0.001) | 5.51 ± 0.1 | −0.27 (0.001) | 0.51 |
| Insulin (mIU/L) | 10.8 ± 5.89 | −0.8 (0.41) | 7.3 ± 4.59 | −4.8 (0.006) | 0.046 |
| Items | CG | Differences between Visits | GE | Differences between Visits | Differences between GC and GE per Items | ||
|---|---|---|---|---|---|---|---|
| Initial Visit | Last Visit | Initial Visit | Last Visit | ||||
| Consume ≥ 2 servings of vegetables per day, one of them raw | 8.9% | 67.7% | <0.001 | 7.1% | 80% | <0.001 | 0.41 |
| Use 3–4 tablespoons of extra virgin olive oil raw and for cooking | 68% | 80% | 0.25 | 78.6% | 100% | 0.014 | 0.02 |
| Eat ≥ 3 whole servings of fruit per day | 4.3% | 21.12% | 0.02 | 7.1% | 30% | 0.016 | 0.158 |
| Consume natural juices or smoothies | 6.7% | 33.3% | 0.02 | 0% | 80% | <0.001 | <0.001 |
| Preferably consume more whole grains (bread, pasta, and rice) than refined ones | 0% | 57.85 | <0.001 | 0% | 70% | <0.001 | 0.16 |
| Eat ≥ 3 servings of legumes per week | 45% | 68% | 0.04 | 50% | 90% | 0.004 | 0.014 |
| Eat ≥ 3–4 handfuls per week of natural nuts | 16.3% | 36.7% | 0.016 | 14.3% | 50% | <0.001 | 0.045 |
| Consume ≥ 3 servings of oily and/or white fish per week | 48% | 80% | <0.001 | 42.9% | 80% | <0.001 | 0.45 |
| Preferably consume lean meats without skin (chicken, turkey, rabbit, and pork tenderloin) | 65% | 73.3% | 0.16 | 64.3% | 100% | <0.001 | 0.012 |
| Do not consume red and/or processed meats weekly (ribs, chops, hamburger, commercial sausages, etc.) | 23.3% | 66.7% | 0.002 | 0% | 70% | <0.001 | 0.16 |
| Do not consume carbonated and/or sugary drinks weekly | 13.3% | 43.3% | <0.001 | 7.1% | 50% | <0.001 | 0.84 |
| Do not consume industrial pastries. treats and snacks weekly | 0% | 43.3% | <0.001 | 0% | 50% | <0.001 | 0.049 |
| Consume quality and minimally processed dairy products (natural yogurt, fresh cheese, etc.) | 0% | 13.3% | 0.04 | 0% | 20% | 0.02 | 0.05 |
| Water is the main daily drink | 60% | 100% | 0.004 | 71.4% | 100% | 0.01 | 0.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blancas-Sánchez, I.M.; Del Rosal Jurado, M.; Aparicio-Martínez, P.; Quintana Navarro, G.; Vaquero-Abellan, M.; Castro Jiménez, R.A.; Fonseca Pozo, F.J. A Mediterranean-Diet-Based Nutritional Intervention for Children with Prediabetes in a Rural Town: A Pilot Randomized Controlled Trial. Nutrients 2022, 14, 3614. https://doi.org/10.3390/nu14173614
Blancas-Sánchez IM, Del Rosal Jurado M, Aparicio-Martínez P, Quintana Navarro G, Vaquero-Abellan M, Castro Jiménez RA, Fonseca Pozo FJ. A Mediterranean-Diet-Based Nutritional Intervention for Children with Prediabetes in a Rural Town: A Pilot Randomized Controlled Trial. Nutrients. 2022; 14(17):3614. https://doi.org/10.3390/nu14173614
Chicago/Turabian StyleBlancas-Sánchez, Isabel María, María Del Rosal Jurado, Pilar Aparicio-Martínez, Gracia Quintana Navarro, Manuel Vaquero-Abellan, Rafael A. Castro Jiménez, and Francisco Javier Fonseca Pozo. 2022. "A Mediterranean-Diet-Based Nutritional Intervention for Children with Prediabetes in a Rural Town: A Pilot Randomized Controlled Trial" Nutrients 14, no. 17: 3614. https://doi.org/10.3390/nu14173614
APA StyleBlancas-Sánchez, I. M., Del Rosal Jurado, M., Aparicio-Martínez, P., Quintana Navarro, G., Vaquero-Abellan, M., Castro Jiménez, R. A., & Fonseca Pozo, F. J. (2022). A Mediterranean-Diet-Based Nutritional Intervention for Children with Prediabetes in a Rural Town: A Pilot Randomized Controlled Trial. Nutrients, 14(17), 3614. https://doi.org/10.3390/nu14173614







