Do Lifestyle Interventions before Gastric Bypass Prevent Weight Regain after Surgery? A Five-Year Longitudinal Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Times of Assessment of Follow-Up
2.3. Baseline Measures
2.4. Bariatric Surgery Procedure
2.5. Follow-Up Measures
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Apovian, C. Obesity: Definition, comorbidities, causes, and burden. Am. J. Manag. Care 2016, 22, S176–S185. [Google Scholar] [PubMed]
- Haththotuwa, R.N.; Wijeyaratne, C.N.; Senarath, U. Worldwide epidemic of obesity. In Obesity and Obstetrics, 2nd ed.; Mahmood, T.A., Arulkumaran, S., Chervenak, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–8. [Google Scholar]
- El Ghoch, M.; Fakhoury, R. Challenges and new directions in obesity management: Lifestyle modification programmes, pharmacotherapy and bariatric surgery. J. Popul. Ther. Clin. Pharmacol. 2019, 26, e1–e4. [Google Scholar] [CrossRef]
- Busetto, L.; Bettini, S.; Makaronidis, J.; Roberts, C.A.; Halford, J.C.G.; Batterham, R. Mechanisms of weight regain. Eur. J. Intern. Med. 2021, 93, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Martins, C. Do we really know what drives relapse in obesity management? Eur. J. Intern. Med. 2022, 95, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Montesi, L.; El Ghoch, M.; Brodosi, L.; Calugi, S.; Marchesini, G.; Dalle Grave, R. Long-term weight loss maintenance for obesity: A multidisciplinary approach. Diabetes. Metab. Syndr. Obes. 2016, 9, 37–46. [Google Scholar] [PubMed]
- Kreidieh, D.; Fakhoury, R.; El Ghoch, M. Exploring the effectiveness of a 1.5-Year weight management intervention for adults with obesity. Clin. Nutr. ESPEN. 2021, 42, 2015–2220. [Google Scholar] [CrossRef] [PubMed]
- Maggard, M.A.; Shugarman, L.R.; Suttorp, M.; Maglione, M.; Sugerman, H.J.; Livingston, E.H.; Nguyen, N.T.; Li, Z.; Mojica, W.A.; Hilton, L.; et al. Meta-analysis: Surgical treatment of obesity. Ann. Intern. Med. 2005, 142, 547–559. [Google Scholar] [CrossRef]
- Oliveira Magro, D.; Geloneze, B.; Delfini, R.; Contini Pareja, B.; Callejas, F.; Pareja, J. Long-term weight regain after gastric bypass: A 5-year prospective study. Obes. Surg. 2008, 18, 648–651. [Google Scholar] [CrossRef]
- Rudolph, A.; Hilbert, A. Post-operative behavioural management in bariatric surgery: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2013, 14, 292–302. [Google Scholar] [CrossRef]
- Brazil, J.; Finucane, F. Structured Lifestyle Modification Prior to Bariatric Surgery: How Much is Enough? Obes. Surg. 2021, 31, 4585–4591. [Google Scholar] [CrossRef]
- Esquivel, M.M.; Azagury, D. Preoperative Weight Loss Before Bariatric Surgery-The Debate Continues. JAMA Netw. Open 2020, 3, e204994. [Google Scholar] [CrossRef]
- Chinaka, U.; Fultang, J.; Ali, A., Jr.; Bakhshi, A. Pre-specified Weight Loss Before Bariatric Surgery and Postoperative Outcomes. Cureus 2020, 12, e12406. [Google Scholar] [CrossRef]
- Dalle Grave, R.; Centis, E.; Marzocchi, R.; El Ghoch, M.; Marchesini, G. Major factors for facilitating change in behavioral strategies to reduce obesity. Psychol. Res. Behav. Manag. 2013, 6, 101–110. [Google Scholar]
- Alami, R.S.; Morton, J.M.R.S.; Lie, J.; Sanchez, B.R.; Peters, A.; Curet, M. Is there a benefit to preoperative weight loss in gastric bypass patients? A prospective randomized trial. Surg. Obes. Relat. Dis. 2007, 3, 141–146. [Google Scholar] [CrossRef]
- Vines, L.; Schiesser, M. Gastric bypass: Current results and different techniques. Dig. Surg. 2014, 31, 33–39. [Google Scholar] [CrossRef]
- Verma, J. Repeated Measures Design for Empirical Researchers; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Williamson, D.A.; Bray, G.A.; Ryan, D. Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obesity 2015, 23, 2319–2320. [Google Scholar] [CrossRef]
- Kushner, B.S.; Eagon, J. Systematic Review and Meta-Analysis of the Effectiveness of Insurance Requirements for Supervised Weight Loss Prior to Bariatric Surgery. Obes. Surg. 2021, 31, 5396–5408. [Google Scholar] [CrossRef]
- Bettini, S.; Belligoli, A.; Fabris, R.; Busetto, L. Diet approach before and after bariatric surgery. Rev. Endocr. Metab. Disord. 2020, 21, 297–306. [Google Scholar] [CrossRef]
- Gerber, P.; Anderin, C.; Thorell, A. Weight loss prior to bariatric surgery: An updated review of the literature. Scand. J. Surg. 2015, 104, 33–39. [Google Scholar] [CrossRef]
- Van Nieuwenhove, Y.; Dambrauskas, Z.; Campillo-Soto, A.; van Dielen, F.; Wiezer, R.; Janssen, I.; Kramer, M.; Thorell, A. Preoperative very low-calorie diet and operative outcome after laparoscopic gastric bypass: A randomized multicenter study. Arch. Surg. 2011, 146, 1300–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, L.F.; Tan, T.L.; Holmes, P.A.; Becker, D.A.; Horn, J.; Bixler, E. Can morbidly obese patients safely lose weight preoperatively? Am. J. Surg. 1995, 169, 245–253. [Google Scholar] [CrossRef]
- Coffin, B.; Maunoury, V.; Pattou, F.; Hébuterne, X.; Schneider, S.; Coupaye, M.; Ledoux, S.; Iglicki, F.; Mion, F.; Robert, M.; et al. Impact of Intragastric Balloon Before Laparoscopic Gastric Bypass on Patients with Super Obesity: A Randomized Multicenter Study. Obes. Surg. 2017, 27, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Kalarchian, M.A.; Marcus, M.D.; Courcoulas, A.P.; Cheng, Y.; Levine, M. Preoperative lifestyle intervention in bariatric surgery: A randomized clinical trial. Surg. Obes. Relat. Dis. 2016, 12, 180–187. [Google Scholar] [CrossRef]
- Solomon, H.; Liu, G.Y.; Alami, R.; Morton, J.; Curet, M. Benefits to patients choosing preoperative weight loss in gastric bypass surgery: New results of a randomized trial. J. Am. Coll. Surg. 2009, 208, 241–245. [Google Scholar] [CrossRef]
- Gade, H.; Friborg, O.; Rosenvinge, J.H.; Cvancarova Småstuen, M.; Hjelmesæth, J. The Impact of a Preoperative Cognitive Behavioural Therapy (CBT) on Dysfunctional Eating Behaviours, Affective Symptoms and Body Weight 1 Year after Bariatric Surgery: A Randomised Controlled Trial. Obes. Surg. 2015, 25, 2112–2119. [Google Scholar] [CrossRef]
- Courcoulas, A.P.; Christian, N.J.; Belle, S.H.; Berk, P.D.; Flum, D.R.; Garcia, L.; Horlick, M.; Kalarchian, M.A.; King, W.C.; Mitchell, J.E.; et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA 2013, 310, 2416–2425. [Google Scholar] [CrossRef]
- Teckler, A.; McLeroy, K. The importance of external validity. Am. J. Public Health 2008, 98, 9–10. [Google Scholar] [CrossRef]
- Little, R.; Yau, L. Intent-to-treat analysis for longitudinal studies with drop-outs. Biometrics 1996, 52, 1324–1333. [Google Scholar] [CrossRef]
- Correa, P. Limitations of retrospective research. Am. J. Psychiatry 2011, 168, 436–437. [Google Scholar] [CrossRef]
| LSI n = 91 | Non-LSI n = 123 | Total n = 214 | Significance | |
|---|---|---|---|---|
| Age (years) | 43.59 ± 10.36 | 44.22 ± 11.21 | 43.95 ± 10.83 | p = 0.670 |
| Sex | X2 = 1.72, p = 0.190 | |||
| Male | 23(25.3) | 22(17.9) | 45(21.0) | |
| Female | 68(74.7) | 101(82.1) | 169(79.0) | |
| Marital Status | X2 = 0.677, p = 0.411 | |||
| No | 13(14.3) | 13(10.6) | 26(12.1) | |
| Yes | 78(85.7) | 110(89.4) | 188(87.9) | |
| Employment | X2 = 0.113, p = 0.736 | |||
| No | 7(7.7) | 8(6.5) | 15(7.0) | |
| Yes | 84(92.3) | 115(93.5) | 199(93.0) | |
| Height (cm) | 1.65 ± 0.08 | 1.64 ± 0.09 | 1.64 ± 0.09 | p = 0.317 |
| Weight (Kg) | 122.98 ± 22.04 | 120.72 ± 20.30 | 121.70 ± 21.04 | p = 0.444 |
| BMI (Kg/m2) | 45.16 ± 6.36 | 45.02 ± 5.90 | 45.08 ± 6.08 | p = 0.862 |
| Hypertension | X2 = 1.90, p = 0.168 | |||
| No | 57(62.6) | 88(71.5) | 145(67.8) | |
| Yes | 34(37.4) | 35(28.5) | 69(32.2) | |
| Type 2 Diabetes | X2 = 0.103, p = 0.748 | |||
| No | 177(84.6) | 106(86.2) | 183(85.5) | |
| Yes | 14(15.4) | 17(13.8) | 31(14.5) | |
| Dyslipidemia | X2 = 2.12, p = 0.146 | |||
| No | 84(92.3) | 119(96.7) | 203(94.9) | |
| Yes | 7(7.7) | 4(3.3) | 11(5.1) |
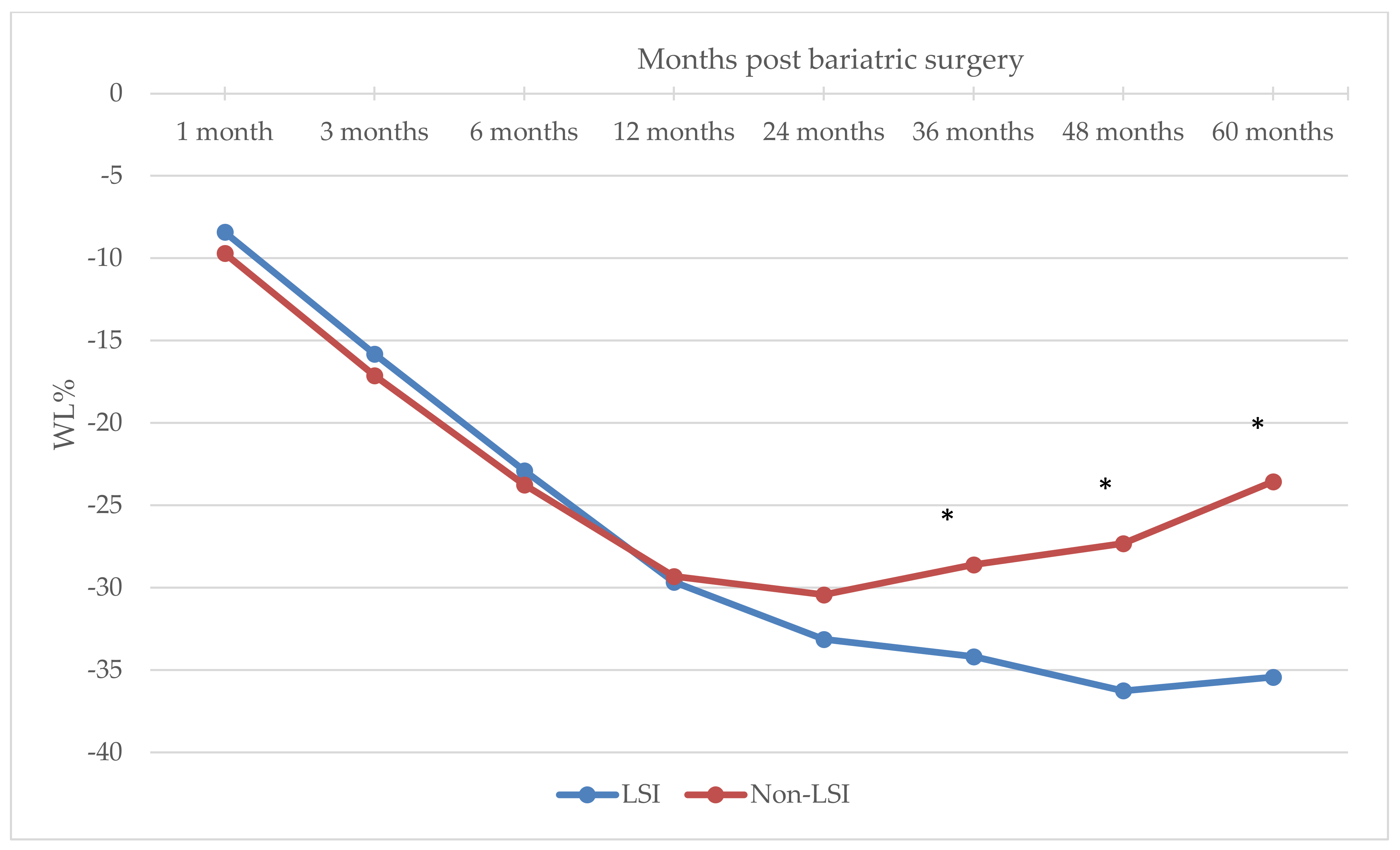
| Months Post-Bariatric | LSI n = 91 | Non-LSI n = 123 | Significance ¥ |
|---|---|---|---|
| % Weight loss | |||
| 1 month | −8.41 ± 3.06 | −9.70 ± 4.74 | 0.025 |
| 3 months | −15.81 ± 4.80 | −17.13 ± 5.76 | 0.077 |
| 6 months | −22.89 ± 6.02 | −23.76 ± 7.20 a | 0.348 |
| 12 months | −29.66 ± 7.75 | −29.31 ± 8.79 b | 0.758 |
| 24 months | −33.13 ± 7.71 | −30.43 ± 8.88 | 0.021 |
| 36 months | −34.18 ± 7.91 | −28.61 ± 9.22 b | <0.0001 |
| 48 months | −36.25 ± 7.50 | −27.32 ± 9.62 | <0.0001 |
| 60 months | −35.42 ± 7.37 | −23.55 ± 9.87 a | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaccaro, S.; Itani, L.; Scazzina, F.; Bonilauri, S.; Cartelli, C.M.; El Ghoch, M.; Pellegrini, M. Do Lifestyle Interventions before Gastric Bypass Prevent Weight Regain after Surgery? A Five-Year Longitudinal Study. Nutrients 2022, 14, 3609. https://doi.org/10.3390/nu14173609
Vaccaro S, Itani L, Scazzina F, Bonilauri S, Cartelli CM, El Ghoch M, Pellegrini M. Do Lifestyle Interventions before Gastric Bypass Prevent Weight Regain after Surgery? A Five-Year Longitudinal Study. Nutrients. 2022; 14(17):3609. https://doi.org/10.3390/nu14173609
Chicago/Turabian StyleVaccaro, Salvatore, Leila Itani, Francesca Scazzina, Stefano Bonilauri, Concetto Maria Cartelli, Marwan El Ghoch, and Massimo Pellegrini. 2022. "Do Lifestyle Interventions before Gastric Bypass Prevent Weight Regain after Surgery? A Five-Year Longitudinal Study" Nutrients 14, no. 17: 3609. https://doi.org/10.3390/nu14173609
APA StyleVaccaro, S., Itani, L., Scazzina, F., Bonilauri, S., Cartelli, C. M., El Ghoch, M., & Pellegrini, M. (2022). Do Lifestyle Interventions before Gastric Bypass Prevent Weight Regain after Surgery? A Five-Year Longitudinal Study. Nutrients, 14(17), 3609. https://doi.org/10.3390/nu14173609










