The Gut Microbiome among Postmenopausal Latvian Women in Relation to Dietary Habits
Abstract
:1. Introduction
2. Materials and Methods
- 45 to 69 years old;
- Postmenopausal (no menstrual bleeding for at least 12 months).
- 3.
- Women receiving glucocorticoid therapy;
- 4.
- Women diagnosed with osteoporosis prior to the study;
- 5.
- Women with severe chronic diseases;
- 6.
- Women with chronic digestive diseases and, therefore, following a specific diet.
3. Results
3.1. Characteristics of the Participants Involved in the Study
3.2. Dietary Habits among Study Participants
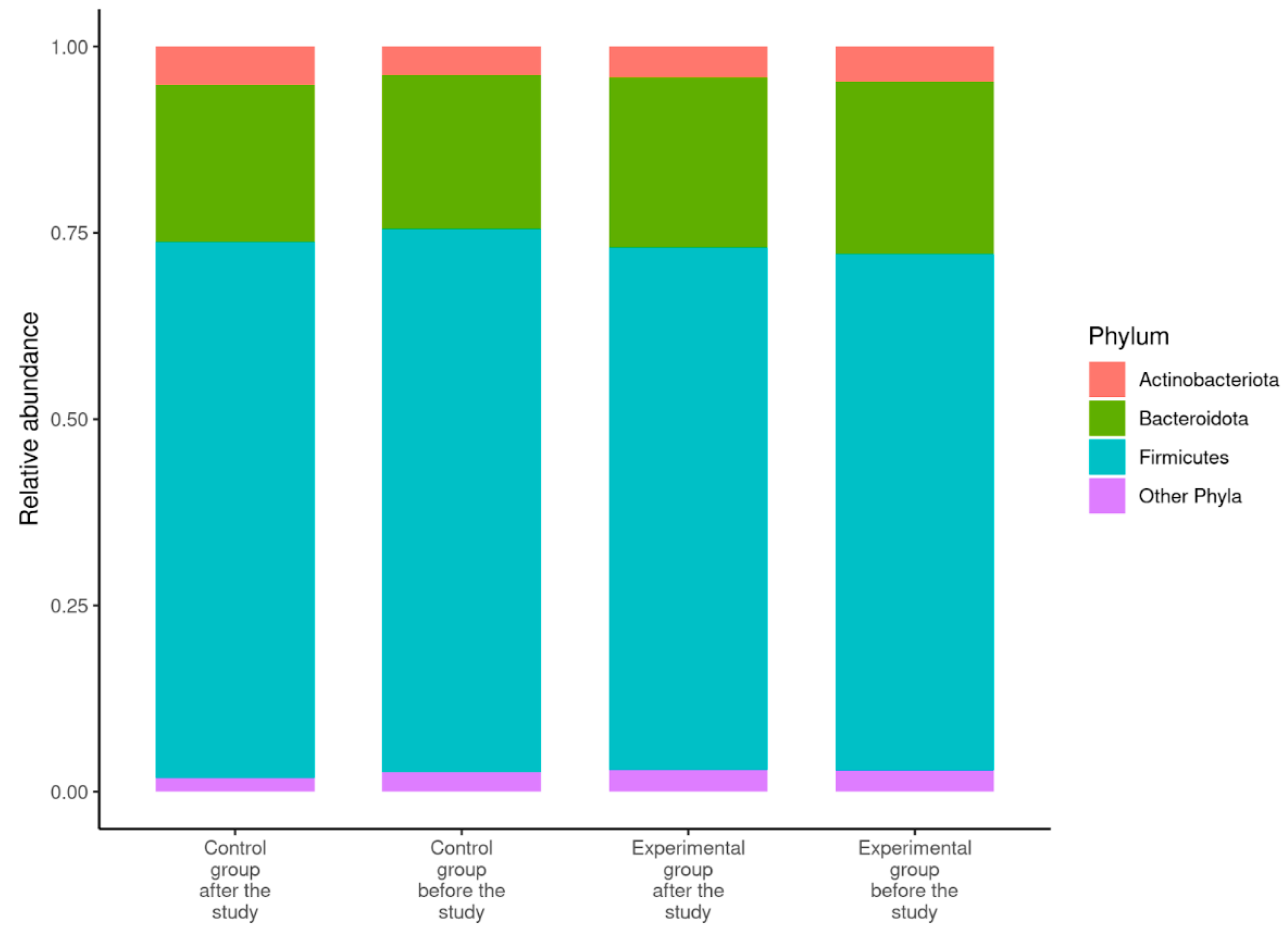
3.3. Description of the Gut Microbiome
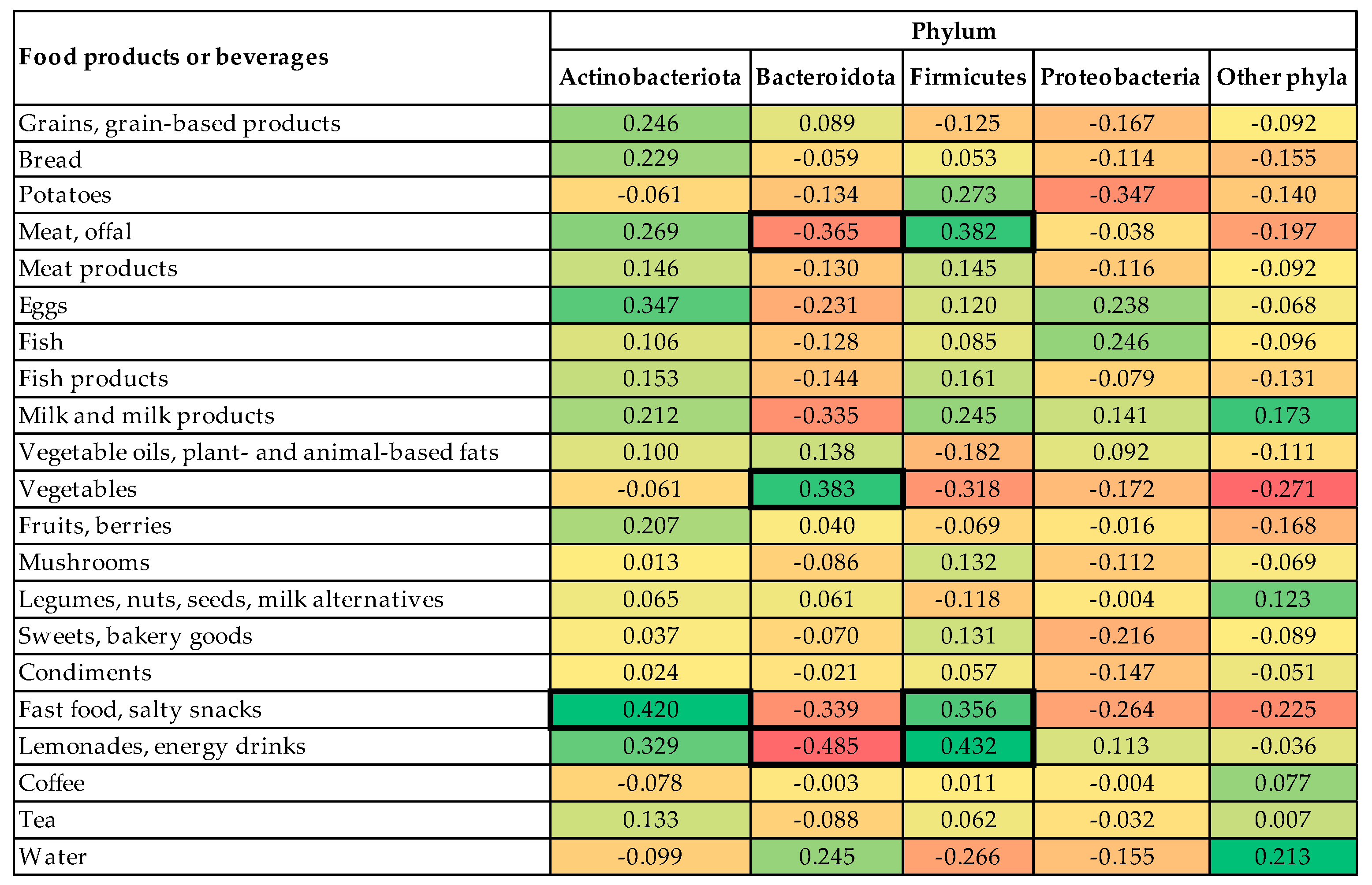
- the first profile was associated with a high abundance of phylum Actinobacteriota, low abundance of phylum Bacteroidota, and high intake of fast food and salty snacks, sweets, and bakery goods (explaining 11.71% of total variance);
- the second profile was associated with a high abundance of phylum Bacteroidota, low abundance of phylum Firmicutes, and high intake of vegetables, potatoes but low intake of legumes, nuts, seeds, and milk alternatives (explaining 8.81% of total variance);
- the third profile was associated with low abundance of phylum Proteobacteria and a low intake of lemonades and energy drinks (explaining 5.49% of total variance).
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stiemsma, L.T.; Nakamura, R.E.; Nguyen, J.G.; Michels, K.B. Does Consumption of Fermented Foods Modify the Human Gut Microbiota? J. Nutr. 2020, 150, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Hua, F.; Ding, W. Gut Microbiome and Osteoporosis. Aging Dis. 2020, 11, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Salazar, N.; González, S.; Nogacka, A.M.; Rios-Covián, D.; Arboleya, S.; Gueimonde, M.; de los Reyes-Gavilán, C.G. Microbiome: Effects of Ageing and Diet. Curr. Issues Mol. Biol. 2020, 36, 33–62. [Google Scholar] [CrossRef]
- Cheng, M.; Ning, K. Stereotypes About Enterotype: The Old and New Ideas. Genom. Proteom. Bioinform. 2019, 17, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Lisko, D.J.; Johnston, G.P.; Johnston, C.G. Effects of Dietary Yogurt on the Healthy Human Gastrointestinal (GI) Microbiome. Microorganisms 2017, 5, 6. [Google Scholar] [CrossRef]
- Bibbò, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The role of diet on gut microbiota composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749. [Google Scholar] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.; Dold, S.; Rehman, A.; Bird, J.K.; Steinert, R.E. Vitamins, the gut microbiome and gastrointestinal health in humans. Nutr. Res. 2021, 95, 35–53. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, J.; Li, X.; Sun, Q.; Qin, P.; Wang, Q. Compositional and functional features of the female premenopausal and postmenopausal gut microbiota. FEBS Lett. 2019, 593, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Ong, A.M.; Kang, K.; Weiler, H.A.; Morin, S.N. Fermented Milk Products and Bone Health in Postmenopausal Women: A Systematic Review of Randomized Controlled Trials, Prospective Cohorts, and Case-Control Studies. Adv. Nutr. 2020, 11, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Rettedal, E.A.; Ilesanmi-Oyelere, B.L.; Roy, N.C.; Coad, J.; Kruger, M.C. The Gut Microbiome Is Altered in Postmenopausal Women With Osteoporosis and Osteopenia. JBMR Plus 2021, 5, e10452. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xu, S.; Zhang, B.; Xiao, C.; Chen, Z.; Si, F.; Fu, J.; Lin, X.; Zheng, G.; Yu, G.; et al. Gut microbiota and metabolite alterations associated with reduced bone mineral density or bone metabolic indexes in postmenopausal osteoporosis. Aging 2020, 12, 8583–8604. [Google Scholar] [CrossRef]
- Brahe, L.K.; Le Chatelier, E.; Prifti, E.; Pons, N.; Kennedy, S.; Hansen, T.; Pedersen, O.; Astrup, A.; Ehrlich, S.D.; Larsen, L.H. Specific gut microbiota features and metabolic markers in postmenopausal women with obesity. Nutr. Diabetes. 2015, 5, e159. [Google Scholar] [CrossRef] [PubMed]
- Frankenfeld, C.L.; Hullar, M.A.J.; Maskarinec, G.; Monroe, K.R.; Shepherd, J.A.; Franke, A.A.; Randolph, T.W.; Wilkens, L.R.; Boushey, C.J.; Le Marchand, L.; et al. The Gut Microbiome Is Associated with Circulating Dietary Biomarkers of Fruit and Vegetable Intake in a Multiethnic Cohort. J. Acad. Nutr. Diet. 2022, 122, 78–98. [Google Scholar] [CrossRef] [PubMed]
- Fisberg, M.; Machado, R. History of yogurt and current patterns of consumption. Nutr. Rev. 2015, 73 (Suppl. 1), 4–7. [Google Scholar] [CrossRef]
- El-Abbadi, N.H.; Dao, M.C.; Meydani, S.N. Yogurt: Role in healthy and active aging. Am. J. Clin. Nutr. 2014, 99 (Suppl. 5), 1263S–1270S. [Google Scholar] [CrossRef]
- Republic of Latvia. Cabinet Regulation No. 97. Regulations Regarding Requirements for the Classification, Quality and Labelling of Milk Products and Composite Milk Products. 2011. Available online: https://likumi.lv/ta/en/en/id/225278-regulations-regarding-requirements-for-the-classification-quality-and-labelling-of-milk-products-and-composite-milk-products (accessed on 24 August 2022).
- Le Roy, C.I.; Kurilshikov, A.; Leeming, E.R.; Visconti, A.; Bowyer, R.C.E.; Menni, C.; Fachi, M.; Koutnikova, H.; Veiga, P.; Zhernakova, A.; et al. Yoghurt consumption is associated with changes in the composition of the human gut microbiome and metabolome. BMC Microbiol. 2022, 22, 39. [Google Scholar] [CrossRef]
- Filteau, M.; Matamoros, S.; Savard, P.; Roy, D. Molecular monitoring of fecal microbiota in healthy adults following probiotic yogurt intake. PharmaNutrition 2013, 1, 123–129. [Google Scholar] [CrossRef]
- Institute of Food Safety; Animal Health and Environment. Gut Microbiome Composition and Diversity among Health and Lifestyle Induced Dietary Regimen. 2021. Available online: https://bior.lv/en/about-bior/news/gut-microbiome-composition-and-diversity-among-health-and-lifestyle-induced-dietary-regimen (accessed on 24 August 2022).
- Latvian Biomedical Research and Study Centre. Latvian Microbiome Project. 2022. Available online: https://latvijasmikrobioms.lv/eng/ (accessed on 24 August 2022).
- World Health Organization. The WHO STEPwise Approach to Noncommunicable Disease Risk Factor Surveillance. 2017. Available online: https://www.who.int/teams/noncommunicable-diseases/surveillance/systems-tools/steps (accessed on 24 August 2022).
- Comeau, A.M.; Douglas, G.M.; Langille, M.G.I. Microbiome Helper: A Custom and Streamlined Workflow for Microbiome Research. mSystems 2017, 2, e00127-16. [Google Scholar] [CrossRef]
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every base matters: Assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef]
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.A.; et al. Improved Bacterial 16S rRNA Gene (V4 and V4–5) and Fungal Internal Transcribed Spacer Marker Gene Primers for Microbial Community Surveys. mSystems 2015, 1, e00009-15. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Marcel, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.; McMurdie, P.; Rosen, M.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Quast, Q.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Beule, L.; Karlovsky, P. Improved normalization of species count data in ecology by scaling with ranked subsampling (SRS): Application to microbial communities. PeerJ 2020, 8, e9593. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E. A mathematical theory of communication. Bell. Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Sørensen, T.J. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content and its application to analyses of the vegetation on Danish commons. K. Dan Vidensk Selsk. Bil. Skr. 1984, 5, 1–34. [Google Scholar]
- Veselības Ministrija. Veselīga Uztura Ieteikumi Pieaugušajiem. Gads. 2020. Available online: https://www.vm.gov.lv/lv/media/3017/download (accessed on 24 June 2022).
- Nordic Nutrition Recommendations. 2014. Available online: https://norden.diva-portal.org/smash/get/diva2:704251/FULLTEXT01.pdf (accessed on 24 August 2022).
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarăes, V.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J.P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts of Gut Microbiota Associated with Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front. Microbiol. 2018, 9, 890. [Google Scholar] [CrossRef] [PubMed]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef]
- Gui, X.; Yang, Z.; Li, M.D. Effect of Cigarette Smoke on Gut Microbiota: State of Knowledge. Front. Physiol. 2021, 12, 673341. [Google Scholar] [CrossRef]
- Lee, S.H.; Yun, Y.; Kim, S.J.; Lee, E.J.; Chang, Y.; Ryu, S.; Shin, H.; Kim, H.L.; Kim, H.N.; Lee, J.H. Association between cigarette smoking status and composition of gut microbiota: Population-based cross-sectional study. J. Clin. Med. 2018, 7, 282. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, K.; Mokkala, K. Overall Dietary Quality Relates to Gut Microbiota Diversity and Abundance. Int. J. Mol. Sci. 2019, 20, 1835. [Google Scholar] [CrossRef]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef]
- Rose, D.J. Impact of whole grains on the gut microbiota: The next frontier for oats? Br. J. Nutr. 2014, 112 (Suppl. 2), S44–S49. [Google Scholar] [CrossRef]
- Seal, C.L.; Courtin, C.M.; Venema, K.; de Vries, J. Health benefits of whole grain: Effects on dietary carbohydrate quality, the gut microbiome, and consequences of processing. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2742–2768. [Google Scholar] [CrossRef]
- Veselības Ministrija. Pārtikas Drošības, Dzīvnieku Veselības un Vides Zinātniskais Institūts “BIOR”. Pētījums Par Sāls un Joda Patēriņu Latvijas Pieaugušo Iedzīvotāju Populācijā. Gads. 2020. Available online: https://esparveselibu.lv/sites/default/files/2020-09/P%C4%93t%C4%ABjums%20par%20s%C4%81ls%20un%20joda%20pat%C4%93ri%C5%86u%20Latvijas%20pieaugu%C5%A1o%20iedz%C4%ABvot%C4%81ju%20popul%C4%81cij%C4%81_0.pdf (accessed on 24 August 2022).
- Van Hul, M.; Le Roy, T.; Prifti, E.; Dao, M.C.; Paquot, A.; Zucker, J.D.; Delzenne, N.M.; Muccioli, G.; Clément, K.; Cani, P.D. From correlation to causality: The case of Subdoligranulum. Gut Microbes. 2020, 12, 1849998. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on the Substantiation of Health Claims Related to Live Yoghurt Cultures and Improved Lactose Digestion (ID 1143, 2976) Pursuant to Article 13 of Regulation (EC) No. 1924/2006. Available online: https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2010.1763 (accessed on 24 August 2022).
- Savaiano, D.A.; Hutkins, R.W. Yogurt, cultured fermented milk, and health: A systematic review. Nutr. Rev. 2021, 79, 599–614. [Google Scholar] [CrossRef] [PubMed]
| Packaging: Cup Weight/Capacity: 350 g List of Ingredients: Milk, Milk Protein, Lactic Acid Bacteria 1.4 × 109 CFU g−1 | |
|---|---|
| Nutrition Declaration | Values Per 100 g |
| Energy value | 289–327 kJ/69–78 kcal |
| Fat, of which | 3.5–4.5 g |
| –saturates | 2.4–3.0 g |
| Carbohydrates, of which | 4.5 g |
| –sugars | 4.5 g |
| Protein | 4.8 g |
| Salt 1 | 0.10 g |
| Calcium | 132 mg |
| Vitamin D | <0.10 μg |
| Characteristics | Control Group (n = 26) | Experimental Groups (n = 26) |
|---|---|---|
| Age (years) | 58 ± 5 (49–69) | 57 ± 4 (51–69) |
| Education level | Secondary education (n = 2) Higher education (n = 24) | Secondary education (n = 2) Higher education (n = 24) |
| Body mass index (kg m−2) | 27.82 ± 4.62 (20.87–38.93) | 28.35 ± 6.26 (19.20–44.47) |
| Waist circumference (cm) 1 | 93 ± 12 (72–112) | 93 ± 14 (73–124) |
| Waist-to-hip ratio 1 | 0.86 ± 0.08 (0.70–1.01) | 0.85 ± 0.07 (0.76–1.02) |
| Last menstrual period 1 | 12 months (n = 4) Before 13–24 months (n = 1) More than 24 months ago (n = 20) | 12 months (n = 2) Before 13–24 months (n = 7) More than 24 months ago (n = 17) |
| Smoking | Never (n = 15) Used to smoke (n = 8) Occasionally (n = 2) | Never (n = 20) Used to smoke (n = 4) Occasionally (n = 2) |
| Daily (n = 1) | Daily (n = 0) | |
| Use of alcohol | Never (n = 2) Once a month or less often (n = 6) 2 to 4 times a month (n = 14) 2 to 3 times a week (n = 2) 4 and more times a week (n = 2) | Never (n = 2) Once a month or less often (n = 11) 2 to 4 times a month (n = 10) 2 to 3 times a week (n = 3) 4 and more times a week (n = 0) |
| Bone mineral density level | Normal (n = 10) Osteopenia (n = 8) Osteoporosis (n = 8) | Normal (n = 13) Osteopenia (n = 11) Osteoporosis (n = 2) |
| Use of antibiotics in the last year | Yes (n = 6) No (n = 20) | Yes (n = 4) No (n = 22) |
| Characteristics | Control Group (n = 26) | Experimental Group (n = 26) | ||
|---|---|---|---|---|
| Before the Study | After the Study | Before the Study | After the Study | |
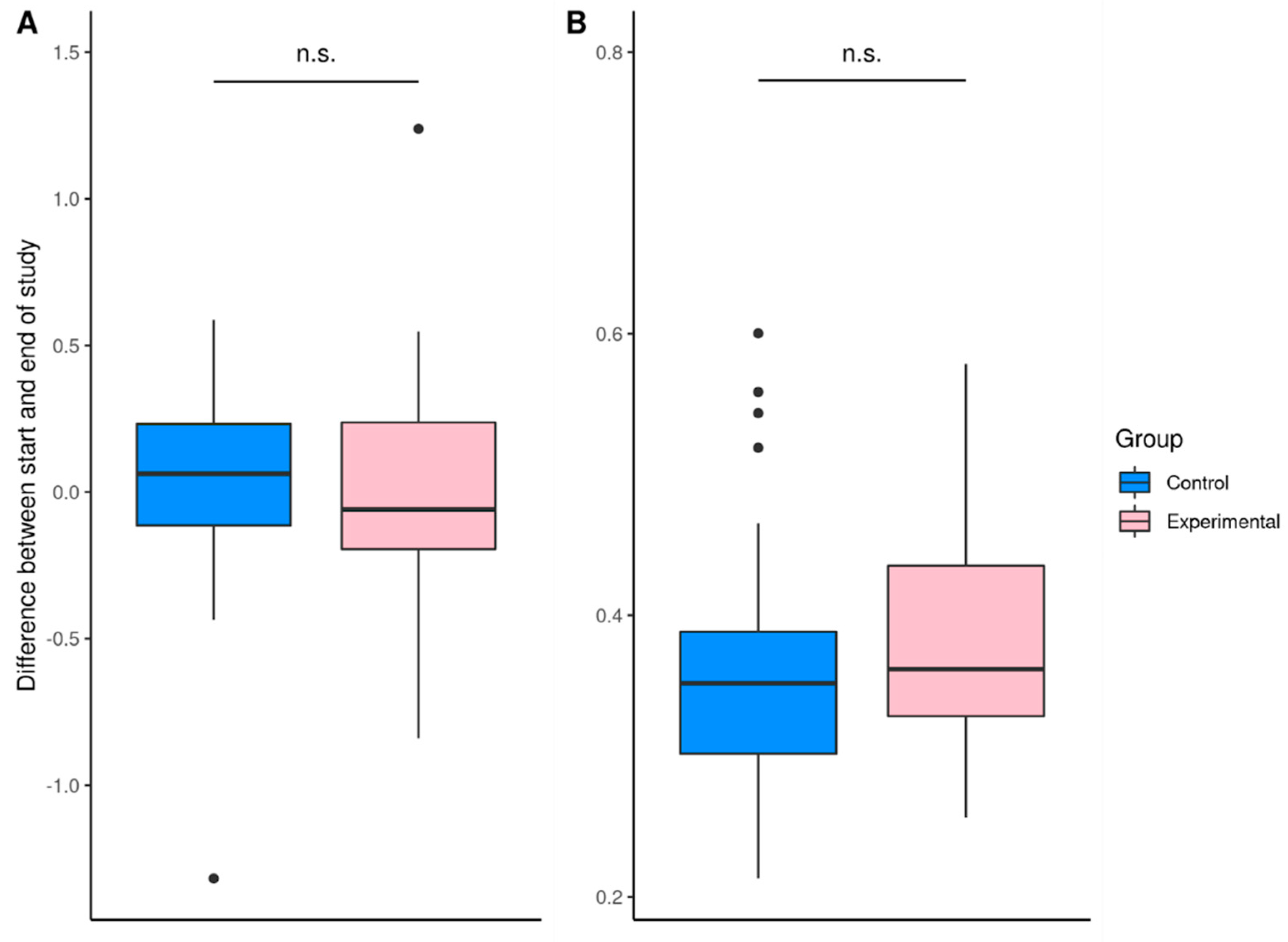
| Ratio of Firmicutes:Bacteroidota | 4.06 ± 1.71 1 (1.78–7.87) | 4.06 ± 2.12 (1.41–10.11) | 3.65 ± 2.01 (1.27–8.58) | 4.07 ± 3.03 (1.03–14.43) |
| Ratio of Proteobacteria:Actinobacteria | 0.29 ± 0.29 (0.02–1.19) | 0.24 ± 0.28 (0.00–1.11) | 0.37 ± 0.48 (0.02–2.39) | 0.41 ± 0.69 (0.01–2.77) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aumeistere, L.; Ķibilds, J.; Siksna, I.; Neimane, L.V.; Kampara, M.; Ļubina, O.; Ciproviča, I. The Gut Microbiome among Postmenopausal Latvian Women in Relation to Dietary Habits. Nutrients 2022, 14, 3568. https://doi.org/10.3390/nu14173568
Aumeistere L, Ķibilds J, Siksna I, Neimane LV, Kampara M, Ļubina O, Ciproviča I. The Gut Microbiome among Postmenopausal Latvian Women in Relation to Dietary Habits. Nutrients. 2022; 14(17):3568. https://doi.org/10.3390/nu14173568
Chicago/Turabian StyleAumeistere, Līva, Juris Ķibilds, Inese Siksna, Lolita Vija Neimane, Māra Kampara, Olga Ļubina, and Inga Ciproviča. 2022. "The Gut Microbiome among Postmenopausal Latvian Women in Relation to Dietary Habits" Nutrients 14, no. 17: 3568. https://doi.org/10.3390/nu14173568
APA StyleAumeistere, L., Ķibilds, J., Siksna, I., Neimane, L. V., Kampara, M., Ļubina, O., & Ciproviča, I. (2022). The Gut Microbiome among Postmenopausal Latvian Women in Relation to Dietary Habits. Nutrients, 14(17), 3568. https://doi.org/10.3390/nu14173568






