Breast Milk Retinol Levels after Vitamin A Supplementation at Different Postpartum Amounts and Intervals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Type and Population
2.2. Sample Size Calculation
2.3. Participant Recruitment
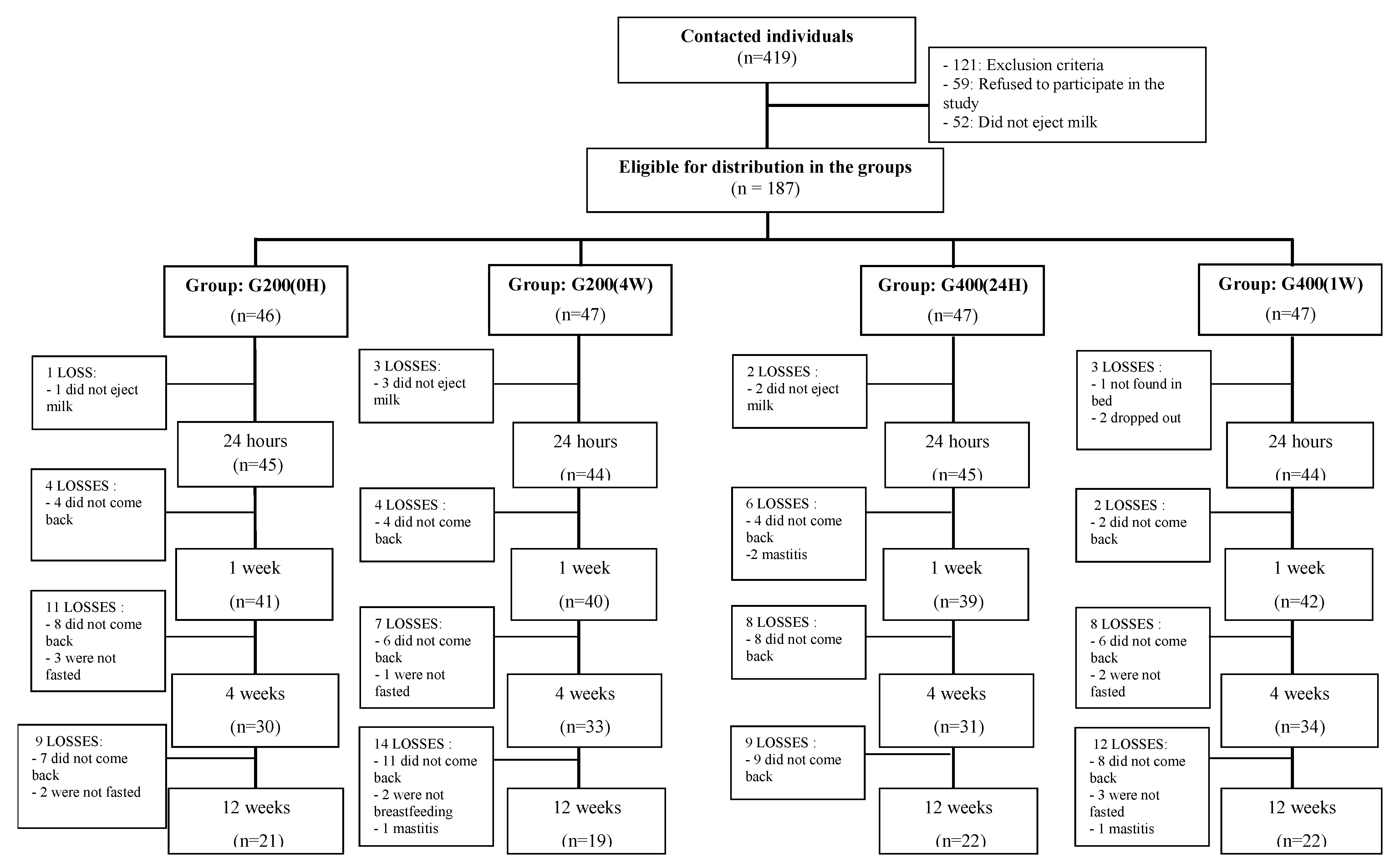
2.4. Study Design (Groups and Follow-Up)
- G200 (0H) = Single VA supplementation: 200,000 IU given orally immediately after delivery;
- G200 (4W) = Single VA supplementation: 200,000 IU given orally four weeks after delivery;
- G400 (24H) = Double VA supplementation: 200,000 IU given orally immediately after delivery + 200,000 IU given orally 24 h after the first supplement (total of 400,000 IU of VA).
- G400 (1W) = Double VA supplementation: 200,000 IU given orally immediately after delivery + 200,000 IU given orally one week after the first supplement (total of 400,000 IU of VA).
2.5. Intervention and Breast Milk Collection
2.6. Anthropometric Parameters
2.7. Other Variables of Interest
2.8. Biochemical Retinol Assessment
2.9. Statistical Analyses
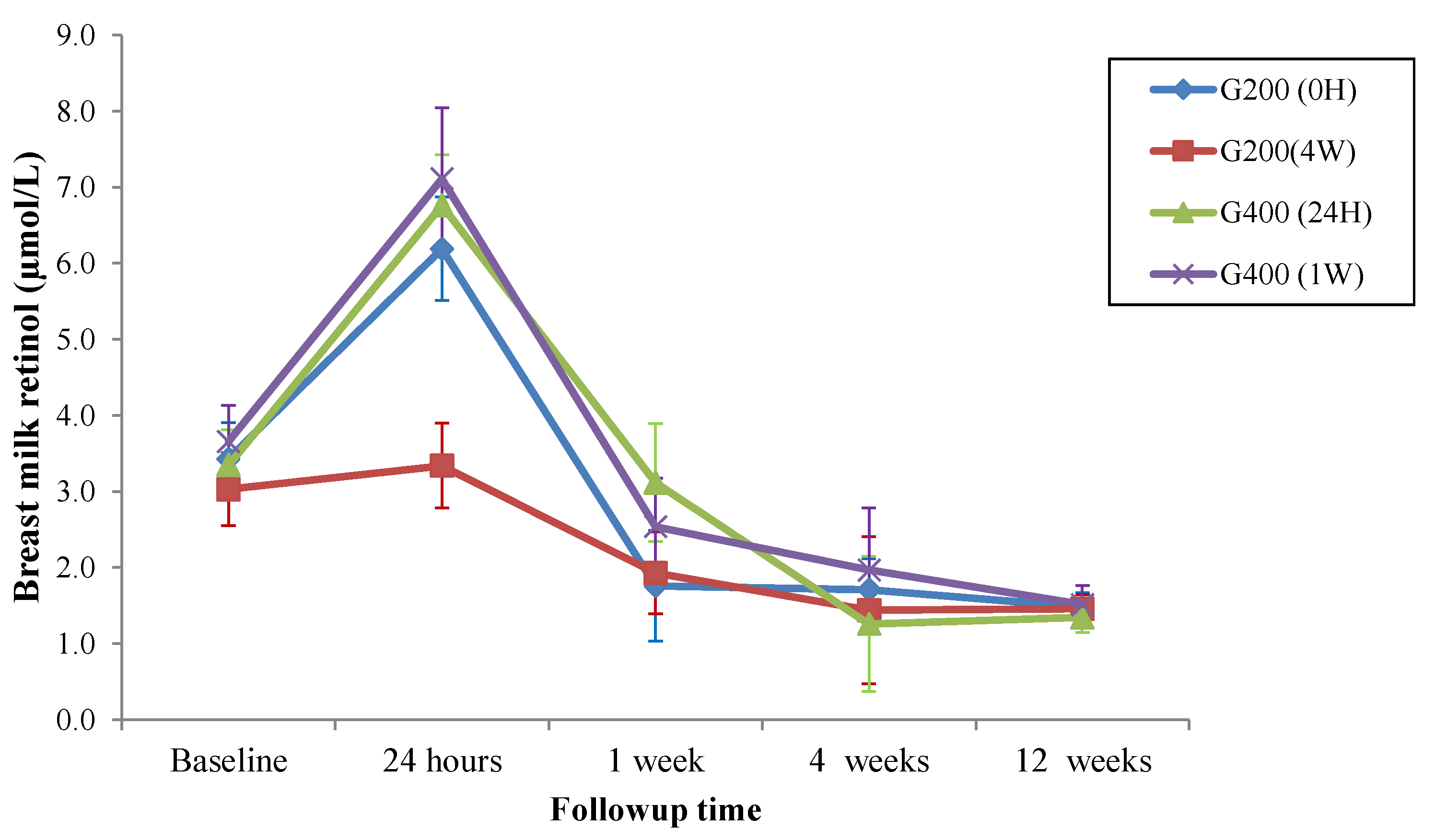
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005: WHO Global DATABASE on Vitamin A Deficiency; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Allen, L.H.; Haskell, M. Vitamin A requirements of infants under six months of age. Food Nutr. Bull. 2001, 22, 214–234. [Google Scholar] [CrossRef]
- Miller, M.; Humphre, J.; Johnson, E.; Marinda, E.; Brookmeyer, R.; Katz, J. Why Do Children Become Vitamin A Deficient? J. Nutr. 2002, 132, 2867S–2880S. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization (PAHO). Providing Vitamin A Supplements though Immunization and Other Health Contacts for Children 6–59 Months and Women up to 6 Weeks Postpartum, 2nd ed.; A Guide for Health Workers; PAHO: Washington, DC, USA, 2001. [Google Scholar]
- World Health Organization; WHO/UNICEF/IVACG Task Force; UNICEF; International Vitamin A Consultative Group. Vitamin A Supplements: A Guide to Their Use in the Treatment of Vitamin A Deficiency and Xerophthalmia, 2nd ed.; WHO: Geneva, Switzerland, 1997. [Google Scholar]
- Bahl, R.; Bhandari, N.; Wahed, M.A.; Kumar, G.T.; Bhan, M.K.; WHO/CHD Immunization-Linked Vitamin A Group. Vitamin A supplementation of women postpartum and of their infants at immunization alters breast milk retinol and infant Vitamin A status. J. Nutr. 2002, 132, 3243–3248. [Google Scholar]
- International Vitamin A Consultative Group (IVACG). Os Acordos de Annecy para Avaliação e Controlo da Deficiência de Vitamina A: Sumário das Recomendações e Clarificações; IVACG: Washington, DC, USA, 2002. [Google Scholar]
- Ahmad, S.M.; Hossain, M.I.; Bergman, P.; Kabir, Y.; Raqib, R. The effect of postpartum vitamin A supplementation on breast milk immune regulators and infant immune functions: Study protocol of a randomized, controlled trial. Trials 2015, 16, 129. [Google Scholar] [CrossRef]
- Macias, C.; Schweigert, F.J. Changes in the concentration of carotenoids, vitamin A, alpha tocopherol and total lipids in human milk throughout early lactation. Ann. Nutr. Metab. 2001, 45, 82–85. [Google Scholar] [CrossRef]
- Darboe, M.K.; Thurnham, D.I.; Morgan, G.; Adegbola, R.; Secka, O.; Solon, J.A.; Jackson, S.J.; Northrop-Clewes, C.; Fulford, T.J.; Doherty, C.P.; et al. Effectiveness of an early supplementation scheme of high-dose vitamin A versus standard WHO protocol in Gambian mothers and infants: A randomised controlled trial. Lancet 2007, 369, 2088–2096. [Google Scholar] [CrossRef]
- Bezerra, D.S.; Araújo, K.F.; Azevêdo, G.M.M.; Dimenstein, R. A Randomized Trial Evaluating the Effect of 2 Regimens of Maternal Vitamin A Supplementation on Breast Milk Retinol Levels. J. Hum. Lact. 2010, 26, 148–156. [Google Scholar]
- Santos, C.S.; Kruze, I.; Fernandes, T.; Andreto, L.M.; Figueiroa, J.N.; Diniz, A.S. The Effect of a Maternal Double Megadose of Vitamin A Supplement on Serum Levels of Retinol in Children Aged under Six Months. J. Nutr. Metab. 2013, 2013, 1–8. [Google Scholar]
- World Health Organization (WHO). Guideline: Vitamin A Supplementation in Postpartum Women; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Manual de Condutas Gerais do Programa Nacional de Suplementação de Vitamina A; Brasil, Ministério da Saúde. Secretaria de Atenção à Saúde. Departamento de Atenção Básica; Ministério da Saúde: Brasília, Spain, 2013.
- Oficio Circular nº 17-2016—Encerramento da Suplementação de Puérperas com Megadoses de Vitamina A no Programa Nacional de Suplementação de Vitamina A; Brasil, Ministério da Saúde, Secretaria de Atenção à Saúde, Departamento de Atenção Básica; Ministério da Saúde: Brasília, Spain, 2016.
- Dror, D.K.; Allen, L.H. Retinol-to-fat ratio and retinol concentration in human milk show similar time trends and associations with maternal factors at the population level: A systematic review and meta-analysis. Adv. Nutr. 2018, 9, 332S–346S. [Google Scholar]
- Dimenstein, R.; Dantas, J.C.O.; Medeiros, A.C.P.; Cunha, L.R.F. Influência da idade gestacional e da paridade sobre a concentração de retinol no colostro humano. Arch. Latinoam. De Nutr. 2010, 60, 235–239. [Google Scholar]
- Garza, C.; Johnson, C.A.; Smith, E.O.B.; Nichols, B.L. Changes in the nutrient composition of human milk during gradual weaning. Am. J. Clin. Nutr. 1983, 37, 61–65. [Google Scholar] [CrossRef]
- Atalah, E.; Castillo, C.; Castro, R.; Aldea, A. Propuesta de um nuevo estandar de evaluación nutricional em embarazadas/Proposal of a new standard for the nutritional assessment of pregnant women. Rev. Med. Chile 1997, 125, 1429–1436. [Google Scholar] [PubMed]
- Orientações Básicas para a Coleta, Processamento, Análise de Dados e Informação em Serviços de Saúde: Norma Técnica do Sistema de Vigilância Alimentar e Nutricional—SISVAN; Brasil, Ministério da Saúde, Secretaria de Atenção à Saúde, Departamento de Atenção Básica; Ministério da Saúde: Brasília, Spain, 2004.
- World Health Organization (WHO). Anthro Para Computadoraspersonales, Versión 3.01, 2009: Software Para Evaluarelcrecimiento y Desarrollo de Losniñosdel Mundo; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Giuliano, A.R.; Neilson, E.M.; Kelly, B.E.; Canfield, L.M. Simultaneous quantitation and separation of carotenoids and retinol in human milk by high-performance liquid chromatography. Method Enzym. 1992, 213, 391–399. [Google Scholar]
- World Health Organization (WHO). Indicators for Assessing Vitamin A Deficiency and Their Application in Monitoring and Evaluation Intervention Programmes; WHO: Geneva, Switzerland, 1996. [Google Scholar]
- Gannon, B.M.; Jones, C.; Mehta, S. Requisitos de vitamina A na gravidez e na lactação. Curr. Dev. Nutr. 2020, 4, nzaa142. [Google Scholar] [CrossRef] [PubMed]
- Dunchek, B.; Nussenblatt, B.; Ricks, M.O.; Kumwenda, N.; Neville, M.C.; Moncrief, D.T.; Taha, T.E.; Semba, R.D. Breast milk retinol concentrations are not associated with systemic inflammation among breat-feeding women in Malawi. J. Nutr. 2005, 135, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Keikha, M.; Shayan-Moghadam, R.; Bahreynian, M.; Kelishadi, R. Nutritional supplements and mother’s milk composition: A systematic review of interventional studies. Int. Breastfeed. J. 2021, 16, 1–30. [Google Scholar] [CrossRef]
- Rajwar, E.; Shradha, S.P.; Bhumika, T.V.; Zinnia, S. Effect of vitamin A, calcium and vitamin D fortification and supplementation on nutritional status of women: An overview of systematic reviews. Syst. Rev. 2020, 9, 248. [Google Scholar] [CrossRef]
- Soares, M.M.; Silva, M.A.; Garcia, P.; Silva, L.; Costa, G.; Araújo, R.; Cotta, R. Effect of vitamin A supplementation: A systematic review. Ciênc. Saúde Colet. 2019, 24, 827–838. [Google Scholar] [CrossRef]
- Tomiya, M.T.; de Arruda, I.K.; da Silva, D.A.; Santana, R.A.; da Silveira, K.C.; Andreto, L.M. The effect of vitamin a supplementation with 400,000 IU vs 200,000 IU on retinol concentrations in the breast milk: A randomized clinical trial. Clin. Nutr. 2017, 36, 100–106. [Google Scholar] [CrossRef]
- Neves, P.A.; Saunders, C.; Barros, D.C.; Ramalho, A. Vitamin A supplementation in Brazilian pregnant and postpartum women: A systematic review. Rev. Bras. Epidemiol. 2015, 18, 824–836. [Google Scholar] [CrossRef]
- Grilo, E.C.; Medeiros, W.F.; Silva, A.G.; Gurgel, C.S.; Ramalho, H.M.; Dimenstein, R. Maternal supplementation with a megadose of vitamin A reduces colostrum level of α-tocopherol: A randomised controlled trial. J. Hum. Nutr. Diet. 2016, 29, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Hu, P.; Yang, Y.; Xu, F.; Li, F.; Luxo Xie, Z.; Wang, Z. Impacto da Suplementação Oral Diária de Vitamina A de Baixa Dose Materna no Par Mãe-Bebê: Um Ensaio Randomizado Controlado por Placebo na China. Nutrients 2021, 13, 2370. [Google Scholar] [CrossRef] [PubMed]
- Koroma, A.S.; Conteh, S.G.; Bah, M.; Kamara, H.I.; Turay, M.; Kandeh, A.; Macauley, A.; Allieu, H.; A Kargbo, A.; Sonnie, M.; et al. Routine vitamin A supplementation and other high impact interventions in Sierra Leone. Matern. Child Nutr. 2020, 16, e13041. [Google Scholar] [CrossRef] [PubMed]
| Variables | G200 (0H) (n = 21) | G200 (4W) (n = 19) | G400 (24H) (n = 22) | G400 (1W) (n = 22) | p-Value | Total (n = 84) |
|---|---|---|---|---|---|---|
| MATERNAL | ||||||
| Age (years) | 24.6 ± 3.9 a | 26.8 ± 5.6 a | 24.0 ± 4.5 a | 26.7 ± 5.1 a | 0.15 c | 25.5 ± 4.9 |
| Parity | 1.1 ± 1.7 a | 0.9 ± 1.1 a | 0.9 ± 0.8 a | 1.3 ± 1.1 a | 0.66 c | 1.0 ± 1.2 |
| Marital status | 0.84 d | |||||
| Single | 4 (4.9) b | 2 (25) b | 3 (3.7) b | 2 (2.5) b | 11 (13.6) | |
| Married | 17 (21.0) b | 16 (19.8) b | 19 (23.5) b | 18 (22.2) b | 70 (86.4) | |
| People per household | 0.47 d | |||||
| Up to three | 14 (17.6) b | 11 (13.8) b | 13 (16.3) b | 13 (16.4) b | 51 (64.1) | |
| Four or more | 7 (8.8) b | 7 (8.8) b | 8 (10) b | 7 (8.8) b | 29 (36.3) | |
| Education level | 0.48 d | |||||
| High school and higher education | 6 (7.4) b | 5 (6.1) b | 8 (9.9) b | 9 (11.0) b | 28 (34.6) | |
| Up to elementary school | 15 (18.5) b | 13 (16.1) b | 14 (17.3) b | 11 (13.6) b | 53 (65.3) | |
| Family income (minimum salaries) | 0.98 d | |||||
| ≤1 | 13 (16.0) b | 11 (13.6) b | 12 (14.8) b | 14 (17.3) b | 50 (61.7) | |
| >1 | 8 (9.9) b | 7 (8.7) b | 10 (12.3) b | 6 (7.4) b | 31 (38.3) | |
| Residence location | 0.57 d | |||||
| Rural | 5 (6.2) b | 3 (3.7) b | 3 (3.7) b | 6 (7.4) b | 17 (21.0) | |
| Urban | 16 (19.8) b | 15 (18.5) b | 19 (23.5) b | 14 (17.3) b | 64 (79.0) | |
| Gestational nutritional status | 0.57 d | |||||
| Low weight | 5 (6.3) b | 2 (2.5) b | 5 (6.3) b | 1 (1.3) b | 13 (16.3) | |
| Normal weight | 9 (11.3) b | 7 (8.8) b | 6 (7.5) b | 12 (15.0) b | 34 (42.4) | |
| Excessive weight | 7 (8.8) b | 8 (10.0) b | 10 (12.5) b | 8 (10.1) b | 33 (41.3) | |
| Delivery route | 0.44 d | |||||
| Vaginal | 16 (19.0) b | 12 (14.3) b | 16 (19.0) b | 19 (22.6) b | 63 (75.0) | |
| Caesarian | 5 (6.0) b | 7 (8.3) b | 6 (7.2) b | 3 (3.6) b | 21(25.0) | |
| INFANTS | ||||||
| Gestational age (weeks) | 39.8 ± 1.1 a | 39.8 ± 1.2 a | 40.1 ± 1.2 a | 38.8 ± 1.4 a | 0.89 c | 37.3 ± 2.3 |
| Sex | 0.05 d | |||||
| Female | 8 (9.5) b | 14 (16.7) b | 8 (9.5) b | 8 (9.5) b | 38 (45.2) | |
| Male | 13 (15.5) b | 5 (6.0) b | 14 (16.7) b | 14 (16.7) b | 46 (54.8) | |
| Weight (kg) | 3357.9 ± 368.3 a | 3430.3 ± 454.6 a | 3283.6 ± 435.4 a | 3410.7 ± 301.9 a | 0.62 c | 3368.6 ± 389.6 |
| Length (cm) | 49.0 ± 1.5 a | 49.3 ± 1.8 a | 48.2 ± 1.7 a | 49.0 ± 1.6 a | 0.24 c | 48.9 ± 1.7 |
| Source | Model | ||
|---|---|---|---|
| Wald χ2 | df | p | |
| Group | 3.20 | 3 | 0.36 |
| Follow-up time | 92.71 | 4 | <0.001 * |
| Group * follow-up time | 65.53 | 12 | <0.001 * |
| Living location | 0.50 | 1 | 0.47 |
| Family income | 1.35 | 1 | 0.24 |
| Education level | 1.30 | 1 | 0.25 |
| Anthropometric nutritional status | 1.70 | 3 | 0.63 |
| Follow-Up | Groups | |||
|---|---|---|---|---|
| G200 (0H) (n = 21) | G200 (4W) (n = 19) | G400 (24H) (n = 22) | G400 (1W) (n = 22) | |
| Baseline (IC95%) | 3.43 (2.50–4.36) a | 3.03 (2.09–3.97) d | 3.35 (2.44–4.25) f | 3.66 (2.72–4.60) i |
| 24 h (IC95%) | 6.19 (4.89–7.50) b | 3.34 (2.20–4.47) d | 6.76 (5.38–8.14) g | 7.11 (5.18–9.05) j |
| 1 week (IC95%) | 1.76 (1.04–2.29) c | 1.93 (1.24–2.61) d,e | 3.12 (1.87–4.37) f | 2.54 (1.87–3.20) i,l |
| 4 weeks (IC95%) | 1.71 (1.15–2.28) c | 1.44 (1.01–1.88) e | 1.26 (0.89–1.63) h | 1.97 (1.53–2.41) l |
| 12 weeks (IC95%) | 1.48 (1.11–1.85) c | 1.46 (1.09–1.82) e | 1.35 (0.96–1.74) h | 1.51 (1.02–2.01) l |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezerra, D.S.; de Melo, A.T.A.; de Oliveira, K.C.d.A.N.; de Araújo, K.Q.M.A.; Medeiros, M.S.M.d.F.; Santos, F.A.P.S.d.; Medeiros, J.F.P.; Lima, M.S.R.; da Silva, A.G.C.L.; Ribeiro, K.D.d.S.; et al. Breast Milk Retinol Levels after Vitamin A Supplementation at Different Postpartum Amounts and Intervals. Nutrients 2022, 14, 3570. https://doi.org/10.3390/nu14173570
Bezerra DS, de Melo ATA, de Oliveira KCdAN, de Araújo KQMA, Medeiros MSMdF, Santos FAPSd, Medeiros JFP, Lima MSR, da Silva AGCL, Ribeiro KDdS, et al. Breast Milk Retinol Levels after Vitamin A Supplementation at Different Postpartum Amounts and Intervals. Nutrients. 2022; 14(17):3570. https://doi.org/10.3390/nu14173570
Chicago/Turabian StyleBezerra, Danielle S., Andressa T. A. de Melo, Kátia C. de A. N. de Oliveira, Karoline Q. M. A. de Araújo, Monalisa S. M. de F. Medeiros, Flávia A. P. S. dos Santos, Jeane F. P. Medeiros, Mayara S. R. Lima, Ana Gabriella C. L. da Silva, Karla Danielly da S. Ribeiro, and et al. 2022. "Breast Milk Retinol Levels after Vitamin A Supplementation at Different Postpartum Amounts and Intervals" Nutrients 14, no. 17: 3570. https://doi.org/10.3390/nu14173570
APA StyleBezerra, D. S., de Melo, A. T. A., de Oliveira, K. C. d. A. N., de Araújo, K. Q. M. A., Medeiros, M. S. M. d. F., Santos, F. A. P. S. d., Medeiros, J. F. P., Lima, M. S. R., da Silva, A. G. C. L., Ribeiro, K. D. d. S., Dimenstein, R., & Osório, M. M. (2022). Breast Milk Retinol Levels after Vitamin A Supplementation at Different Postpartum Amounts and Intervals. Nutrients, 14(17), 3570. https://doi.org/10.3390/nu14173570






