Updated Information of the Effects of (Poly)phenols against Type-2 Diabetes Mellitus in Humans: Reinforcing the Recommendations for Future Research
Abstract
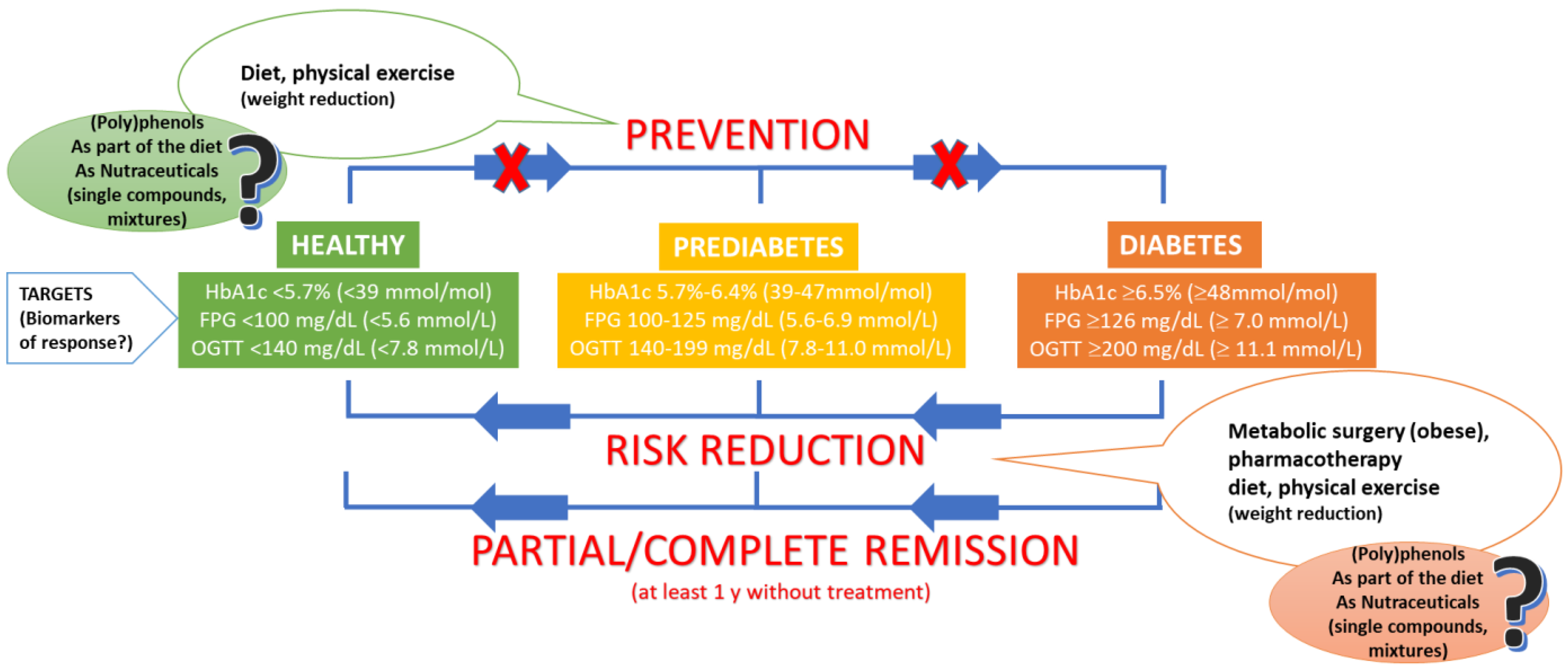
:1. Type 2 Diabetes Mellitus: Current Scenario
2. (Poly)phenols: A Brief Overview
3. Updated Status of the Evidence of the Benefits of (Poly)phenols against T2DM in Humans
3.1. Results from Recent Systematic Reviews and Meta-Analyses: What Are the Main Messages?
3.2. Results from Very Recent RCTs: Are We Improving RCTs Design to Achieve Better Evidence for the Effects (Poly)phenol against T2DM?
4. How Can Future Pre-Clinical Studies Be Improved to Further Support the Evidence of (Poly)phenols against T2DM?
4.1. In Vitro Testing Using Cell Cultures and Cell-Free Assays
4.2. Animal Models
5. General Discussion and Recommendations to Advance the Understanding of the Effects of (Poly)phenols on T2DM
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation (IDF). Key Global Findings 2021 Diabetes Atlas. Available online: https://diabetesatlas.org/ (accessed on 9 August 2022).
- Matafome, P.; Seiça, R. Function and Dysfunction of Adipose Tissue. Obes. Brain Funct. 2017, 19, 3–31. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Medical Care in Diabetes. Abridged for Primary Care Providers. Clin. Diabetes 2022, 40, 10–38. [Google Scholar] [CrossRef] [PubMed]
- Riddle, M.C.; Cefalu, W.T.; Evans, P.H.; Gerstein, H.C.; Nauck, M.A.; Oh, W.K.; Rothberg, A.E.; le Roux, C.W.; Rubino, F.; Schauer, P.; et al. Consensus Report: Definition and Interpretation of Remission in Type 2 Diabetes. Diabetes Care 2021, 44, 2438–2444. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; Oliveira, J.; Pinho, A.; Carvalho, E. The Role of Nutraceutical Containing Polyphenols in Diabetes Prevention. Metabolites 2022, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Hjorth, M.F.; Astrup, A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; McArdle, P.; Taplin, J.; Unwin, D.; Unwin, J.; Deakin, T.; Wheatley, S.; Murdoch, C.; Malhotra, A.; Mellor, D. Dietary strategies for remission of type 2 diabetes: A narrative review. J. Hum. Nutr. Diet. 2022, 35, 165–178. [Google Scholar] [CrossRef]
- Taylor, S.I.; Yazdi, Z.S.; Beitelshees, A.L. Pharmacological treatment of hyperglycemia in type 2 diabetes. J. Clin. Investig. 2021, 131, e142243. [Google Scholar] [CrossRef]
- Lacroix, S.; Klicic Badoux, J.; Scott-Boyer, M.P.; Parolo, S.; Matone, A.; Priami, C.; Morine, M.J.; Kaput, J.; Moco, S. A computationally driven analysis of the polyphenol-protein interactome. Sci. Rep. 2018, 8, 2232. [Google Scholar] [CrossRef]
- Truzzi, F.; Tibaldi, C.; Zhang, Y.; Dinelli, G.; D′Amen, E. An Overview on Dietary Polyphenols and Their Biopharmaceutical Classification System (BCS). Int. J. Mol. Sci. 2021, 22, 5514. [Google Scholar] [CrossRef]
- Wisnuwardani, R.W.; De Henauw, S.; Androutsos, O.; Forsner, M.; Gottrand, F.; Huybrechts, I.; Knaze, V.; Kersting, M.; Le Donne, C.; Marcos, A.; et al. Estimated dietary intake of polyphenols in European adolescents: The HELENA study. Eur. J. Nutr. 2019, 58, 2345–2363. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Amawi, H.; Ashby, C.R., Jr.; Tiwari, A.K. Cancer chemoprevention through dietary flavonoids: What’s limiting? Chin. J. Cancer 2017, 36, 50. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Castro-Barquero, S.; Becerra-Tomás, N.; Babio, N.; Martínez-González, M.Á.; Corella, D.; Fitó, M.; Romaguera, D.; Vioque, J.; Alonso-Gomez, A.M.; et al. Adopting a High-Polyphenolic Diet Is Associated with an Improved Glucose Profile: Prospective Analysis within the PREDIMED-Plus Trial. Antioxidants 2022, 11, 316. [Google Scholar] [CrossRef]
- Raimundo, A.F.; Félix, F.; Andrade, R.; García-Conesa, M.T.; González-Sarrías, A.; Gilsa-Lopes, J.; Dulce do, Ó.; Raimundo, A.; Ribeiro, R.; Rodriguez-Mateos, A.; et al. Combined effect of interventions with pure or enriched mixtures of (poly)phenols and anti-diabetic medication in type 2 diabetes management: A meta-analysis of randomized controlled human trials. Eur. J. Nutr. 2020, 59, 1329–1343. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martínez, L.; Periago, M.J.; García-Alonso, J.; García-Conesa, M.T.; González-Barrio, R. A Systematic Review of the Cardiometabolic Benefits of Plant Products Containing Mixed Phenolics and Polyphenols in Postmenopausal Women: Insufficient Evidence for Recommendations to This Specific Population. Nutrients 2021, 13, 4276. [Google Scholar] [CrossRef]
- Macena, M.L.; Nunes, L.F.D.S.; da Silva, A.F.; Pureza, I.R.O.M.; Praxedes, D.R.S.; Santos, J.C.F.; Bueno, N.B. Effects of dietary polyphenols in the glycemic, renal, inflammatory, and oxidative stress biomarkers in diabetic nephropathy: A systematic review with meta-analysis of randomized controlled trials. Nutr. Rev. 2022, 20, nuac035. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Bai, Y.; Yang, K.; Chen, G. Effects of green tea consumption on glycemic control: A systematic review and meta-analysis of randomized controlled trials. Nutr. Metab. 2020, 17, 56. [Google Scholar] [CrossRef] [PubMed]
- Bag, S.; Mondal, A.; Majumder, A.; Banik, A. Tea and its phytochemicals: Hidden health benefits & modulation of signaling cascade by phytochemicals. Food Chem. 2022, 371, 131098. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Hu, T.; Zhao, H. Metabolic fate of tea polyphenols and their crosstalk with gut microbiota. Food Sci. Hum. Wellness 2022, 11, 455–466. [Google Scholar] [CrossRef]
- Zhao, T.; Li, C.; Wang, S.; Song, X. Green Tea (Camellia sinensis): A Review of Its Phytochemistry, Pharmacology, and Toxicology. Molecules 2022, 27, 3909. [Google Scholar] [CrossRef]
- Jeyaraman, M.M.; Al-Yousif, N.S.H.; Singh Mann, A.; Dolinsky, V.W.; Rabbani, R.; Zarychanski, R.; Abou-Setta, A.M. Resveratrol for adults with type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2020, 1, CD011919. [Google Scholar] [CrossRef] [PubMed]
- Nyambuya, T.M.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Mxinwa, V.; Mokgalaboni, K.; Orlando, P.; Silvestri, S.; Louw, J.; Tiano, L.; Dludla, P.V. A Meta-Analysis of the Impact of Resveratrol Supplementation on Markers of Renal Function and Blood Pressure in Type 2 Diabetic Patients on Hypoglycemic Therapy. Molecules 2020, 25, 5645. [Google Scholar] [CrossRef] [PubMed]
- Delpino, F.M.; Figueiredo, L.M. Resveratrol supplementation and type 2 diabetes: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 4465–4480. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Geng, J.; Zhao, H.; Li, X.; Song, G. Effects of Resveratrol on Metabolic Indicators in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Int. J. Clin. Pract. 2022, 31, 9734738. [Google Scholar] [CrossRef] [PubMed]
- Rajha, H.N.; Paule, A.; Aragonès, G.; Barbosa, M.; Caddeo, C.; Debs, E.; Dinkova, R.; Eckert, G.P.; Fontana, A.; Gebrayel, P.; et al. Recent Advances in Research on Polyphenols: Effects on Microbiota, Metabolism, and Health. Mol. Nutr. Food Res. 2022, 66, e2100670. [Google Scholar] [CrossRef]
- Jianbo, X. Recent advances in dietary flavonoids for management of type 2 diabetes. Curr. Opin. Food Sci. 2022, 44, 100806. [Google Scholar] [CrossRef]
- Gómez-Martínez, S.; Díaz-Prieto, L.E.; Vicente Castro, I.; Jurado, C.; Iturmendi, N.; Martín-Ridaura, M.C.; Calle, N.; Dueñas, M.; Picón, M.J.; Marcos, A.; et al. Moringa oleifera Leaf Supplementation as a Glycemic Control Strategy in Subjects with Prediabetes. Nutrients 2021, 14, 57. [Google Scholar] [CrossRef]
- Grabež, M.; Škrbić, R.; Stojiljković, M.P.; Vučić, V.; Rudić Grujić, V.; Jakovljević, V.; Djuric, D.M.; Suručić, R.; Šavikin, K.; Bigović, D.; et al. A prospective, randomized, double-blind, placebo-controlled trial of polyphenols on the outcomes of inflammatory factors and oxidative stress in patients with type 2 diabetes mellitus. Rev. Cardiovasc. Med. 2022, 23, 57. [Google Scholar] [CrossRef]
- Vodouhè, M.; Marois, J.; Guay, V.; Leblanc, N.; Weisnagel, S.J.; Bilodeau, J.F.; Jacques, H. Marginal Impact of Brown Seaweed Ascophyllum nodosum and Fucus vesiculosus Extract on Metabolic and Inflammatory Response in Overweight and Obese Prediabetic Subjects. Mar. Drugs 2022, 20, 174. [Google Scholar] [CrossRef]
- Mahjabeen, W.; Khan, D.A.; Mirza, S.A. Role of resveratrol supplementation in regulation of glucose hemostasis, inflammation and oxidative stress in patients with diabetes mellitus type 2: A randomized, placebo-controlled trial. Complementary Ther. Med. 2022, 66, 102819. [Google Scholar] [CrossRef]
- Moreno Uclés, R.; González-Sarrías, A.; Espín, J.C.; Tomás-Barberán, F.A.; Janes, M.; Cheng, H.; Finley, J.; Greenway, F.; Losso, J.N. Effects of red raspberry polyphenols and metabolites on the biomarkers of inflammation and insulin resistance in type 2 diabetes: A pilot study. Food Funct. 2022, 13, 5166–5176. [Google Scholar] [CrossRef]
- Duarte, I.; de Souza, M.C.M.; Curinga, R.M.; Mendonça, H.M.; de Lacerda de Oliveira, L.; Milenkovic, D.; Hassimotto, N.M.A.; Costa, A.M.; Malaquias, J.V.; Dos Santos Borges, T.K. Effect of Passiflora setacea juice and its phenolic metabolites on insulin resistance markers in overweight individuals and on microglial cell activity. Food Funct. 2022, 13, 6498–6509. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Pu, Y.; Xu, Y.; He, X.; Cao, J.; Ma, Y.; Jiang, W. Anti-diabetic and anti-obesity: Efficacy evaluation and exploitation of polyphenols in fruits and vegetables. Food Res. Int. 2022, 157, 111202. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Sánchez, M.A.; González-Sarrías, A.; Romo-Vaquero, M.; García-Villalba, R.; Selma, M.V.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Dietary phenolics against colorectal cancer--From promising preclinical results to poor translation into clinical trials: Pitfalls and future needs. Mol. Nutr. Food Res. 2015, 59, 1274–1291. [Google Scholar] [CrossRef] [PubMed]
- García-Conesa, M.T.; Larrosa, M. Polyphenol-Rich Foods for Human Health and Disease. Nutrients 2020, 12, 400. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.B.; Styring, A.K.; McCullagh, J.S.O. Polyphenols: Bioavailability, Microbiome Interactions and Cellular Effects on Health in Humans and Animals. Pathogens 2022, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; Selma, M.V.; Tomás-Barberán, F.A.; González-Sarrías, A.; Espín, J.C. Where to Look into the Puzzle of Polyphenols and Health? The Postbiotics and Gut Microbiota Associated with Human Metabotypes. Mol. Nutr. Food Res. 2020, 64, e1900952. [Google Scholar] [CrossRef]
- Zhang, Y.; Aisker, G.; Dong, H.; Halemahebai, G.; Zhang, Y.; Tian, L. Urolithin A suppresses glucolipotoxicity-induced ER stress and TXNIP/NLRP3/IL-1β inflammation signal in pancreatic β cells by regulating AMPK and autophagy. Phytomedicine 2021, 93, 153741. [Google Scholar] [CrossRef]
- García-Díez, E.; López-Oliva, M.E.; Pérez-Jiménez, J.; Martín, M.A.; Ramos, S. Metabolic regulation of (-)-epicatechin and the colonic metabolite 2.;3-dihydroxybenzoic acid on the glucose uptake.; lipid accumulation and insulin signalling in cardiac H9c2 cells. Food Funct. 2022, 13, 5602–5615. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, P.; Liu, H.; Sun, X.; Liang, J.; Sun, L.; Chen, Y. Hypoglycemic activity of Origanum vulgare L. and its main chemical constituents identified with HPLC-ESI-QTOF-MS. Food Funct. 2021, 12, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Liu, Y.; Huang, Q.; Farag, M.A.; Li, X.; Wan, X.; Zhao, C. Nutritional assessment models for diabetes and aging. Food Front. 2022, 1–17. [Google Scholar] [CrossRef]
- Rocha, S.; Lucas, M.; Araújo, A.N.; Corvo, M.L.; Fernandes, E.; Freitas, M. Optimization and Validation of an In Vitro Standardized Glycogen Phosphorylase Activity Assay. Molecules 2021, 26, 4635. [Google Scholar] [CrossRef] [PubMed]
- Kottaisamy, C.P.D.; Raj, D.S.; Prasanth Kumar, V.; Sankaran, U. Experimental animal models for diabetes and its related complications-a review. Lab. Anim. Res. 2021, 37, 23. [Google Scholar] [CrossRef] [PubMed]
- Niewiadomska, J.; Gajek-Marecka, A.; Gajek, J.; Noszczyk-Nowak, A. Biological Potential of Polyphenols in the Context of Metabolic Syndrome: An Analysis of Studies on Animal Models. Biology 2022, 11, 559. [Google Scholar] [CrossRef]
- Boonphang, O.; Ontawong, A.; Pasachan, T.; Phatsara, M.; Duangjai, A.; Amornlerdpison, D.; Jinakote, M.; Srimaroeng, C. Antidiabetic and Renoprotective Effects of Coffea arabica Pulp Aqueous Extract through Preserving Organic Cation Transport System Mediated Oxidative Stress Pathway in Experimental Type 2 Diabetic Rats. Molecules 2021, 26, 1907. [Google Scholar] [CrossRef]
- Taïlé, J.; Bringart, M.; Planesse, C.; Patché, J.; Rondeau, P.; Veeren, B.; Clerc, P.; Gauvin-Bialecki, A.; Bourane, S.; Meilhac, O.; et al. Antioxidant Polyphenols of Antirhea borbonica Medicinal Plant and Caffeic Acid Reduce Cerebrovascular.; Inflammatory and Metabolic Disorders Aggravated by High-Fat Diet-Induced Obesity in a Mouse Model of Stroke. Antioxidants 2022, 11, 858. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Castro-Barquero, S.; Lamuela-Raventós, R.M.; Doménech, M.; Estruch, R. Relationship between Mediterranean Dietary Polyphenol Intake and Obesity. Nutrients 2018, 10, 1523. [Google Scholar] [CrossRef]
- Ofosu, F.K.; Daliri, E.B.-M.; Elahi, F.; Chelliah, R.; Lee, B.-H.; Oh, D.-H. New Insights on the Use of Polyphenols as Natural Preservatives and Their Emerging Safety Concerns. Front. Sustain. Food Syst. 2020, 4, 525810. [Google Scholar] [CrossRef]
- Trindade, L.R.; da Silva, D.V.T.; Baião, D.D.S.; Paschoalin, V.M.F. Increasing the Power of Polyphenols through Nanoencapsulation for Adjuvant Therapy against Cardiovascular Diseases. Molecules 2021, 26, 4621. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Dong, Y.; Wang, F.; Zhang, Y. Nanoformulations to Enhance the Bioavailability and Physiological Functions of Polyphenols. Molecules 2020, 25, 4613. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Le, Y.; Tang, S.Q.; He, W.Y.; He, J.; Wang, Y.H.; Wang, H.B. Painful Diabetic Neuropathy Is Associated with Compromised Microglial IGF-1 Signaling Which Can Be Rescued by Green Tea Polyphenol EGCG in Mice. Oxidative Med. Cell. Longev. 2022, 6773662. [Google Scholar] [CrossRef]
- Morand, C.; De Roos, B.; Garcia-Conesa, M.T.; Gibney, E.R.; Landberg, R.; Manach, C.; Milenkovic, D.; Rodriguez-Mateos, A.; Van de Wiele, T.; Tomas-Barberan, F. Why interindividual variation in response to consumption of plant food bioactives matters for future personalised nutrition. Proc. Nutr. Soc. 2020, 79, 225–235. [Google Scholar] [CrossRef] [PubMed]
- COST Action POSITIVe. 2014–2018. Available online: https://www.cost.eu/actions/FA1403. (accessed on 8 August 2022).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menezes, R.; Matafome, P.; Freitas, M.; García-Conesa, M.-T. Updated Information of the Effects of (Poly)phenols against Type-2 Diabetes Mellitus in Humans: Reinforcing the Recommendations for Future Research. Nutrients 2022, 14, 3563. https://doi.org/10.3390/nu14173563
Menezes R, Matafome P, Freitas M, García-Conesa M-T. Updated Information of the Effects of (Poly)phenols against Type-2 Diabetes Mellitus in Humans: Reinforcing the Recommendations for Future Research. Nutrients. 2022; 14(17):3563. https://doi.org/10.3390/nu14173563
Chicago/Turabian StyleMenezes, Regina, Paulo Matafome, Marisa Freitas, and María-Teresa García-Conesa. 2022. "Updated Information of the Effects of (Poly)phenols against Type-2 Diabetes Mellitus in Humans: Reinforcing the Recommendations for Future Research" Nutrients 14, no. 17: 3563. https://doi.org/10.3390/nu14173563
APA StyleMenezes, R., Matafome, P., Freitas, M., & García-Conesa, M.-T. (2022). Updated Information of the Effects of (Poly)phenols against Type-2 Diabetes Mellitus in Humans: Reinforcing the Recommendations for Future Research. Nutrients, 14(17), 3563. https://doi.org/10.3390/nu14173563









