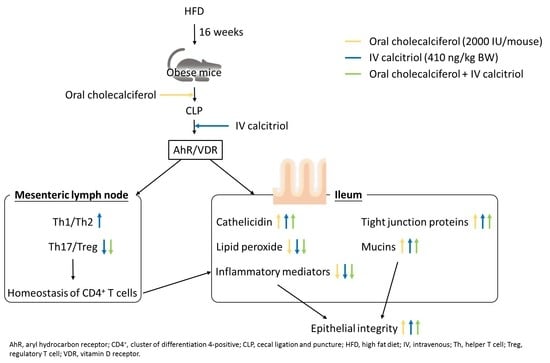
Effects of Different Routes and Forms of Vitamin D Administration on Mesenteric Lymph Node CD4+ T Cell Polarization and Intestinal Injury in Obese Mice Complicated with Polymicrobial Sepsis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Preparations
2.2. Experimental Procedures
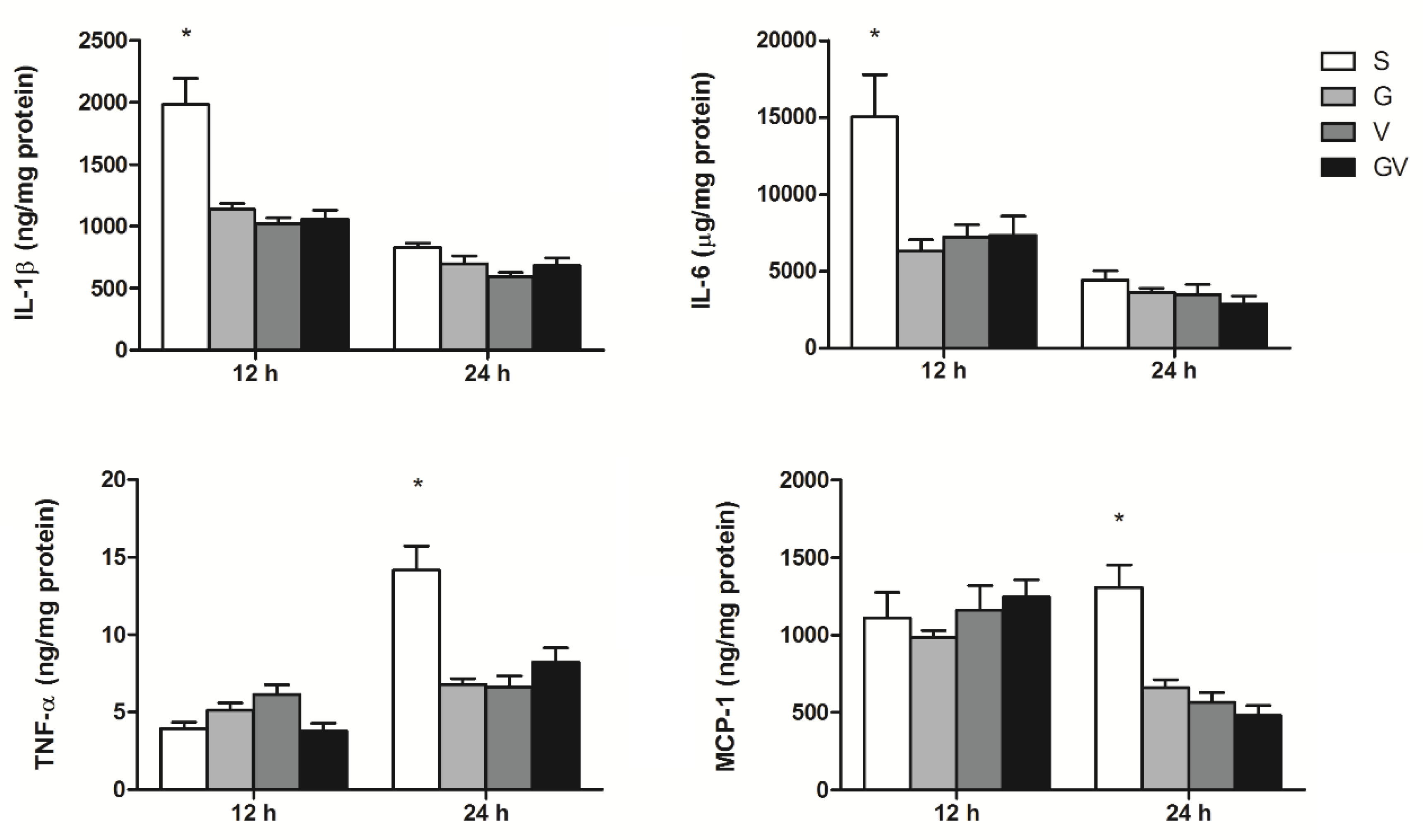
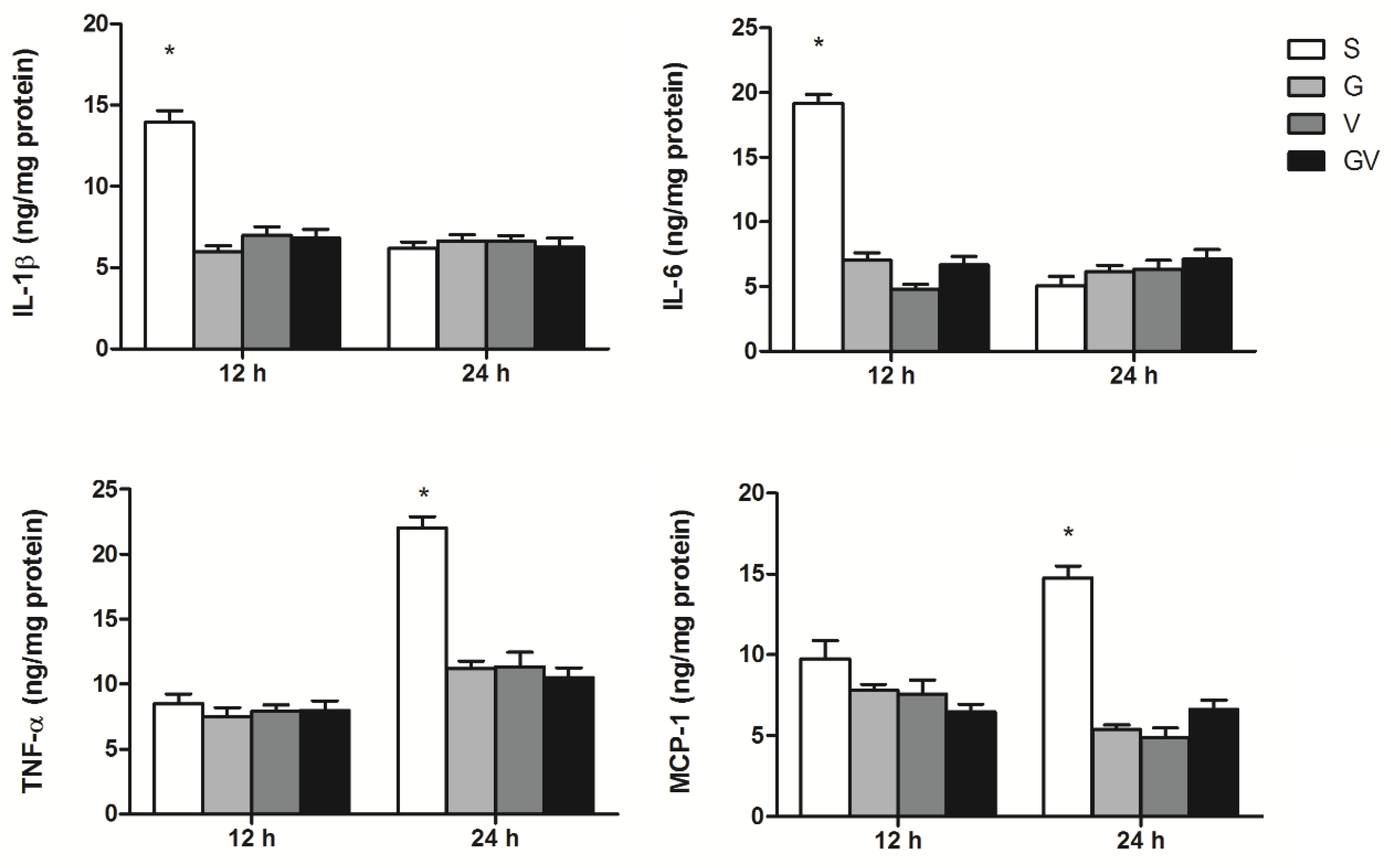
2.3. Inflammatory Mediator Concentrations in PLF
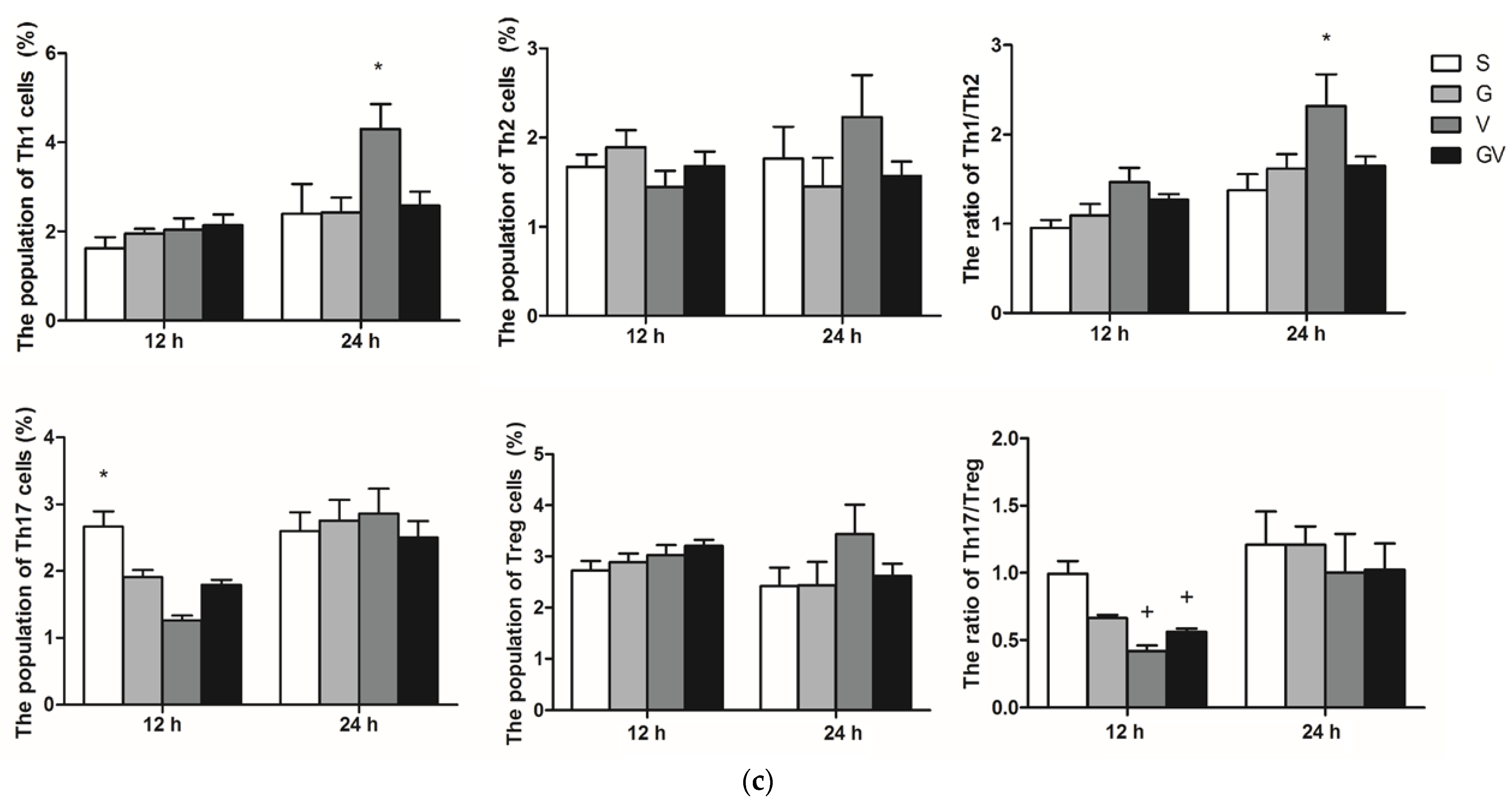
2.4. Distribution of CD4+ T Cell Subpopulations in MLNs
2.5. Tight Junction (TJ) Protein Levels in Ileum Tissues
2.6. Concentrations of Cathelicidin in Ileum Tissues
2.7. Messenger (m)RNA Extraction and Analysis of a Real-Time Reverse-Transcription (RT) Quantitative Polymerase Chain Reaction (qPCR)
2.8. Analysis of Thiobarbituric-Acid-Reactive Substances (TBARSs) in the Ileum
2.9. Statistical Analysis
3. Results
3.1. PLF Inflammatory Mediator Concentrations
3.2. Distribution of CD4 T Cell Subpopulations in MLNs
3.3. Inflammatory Mediator Concentrations in Intestinal Tissues
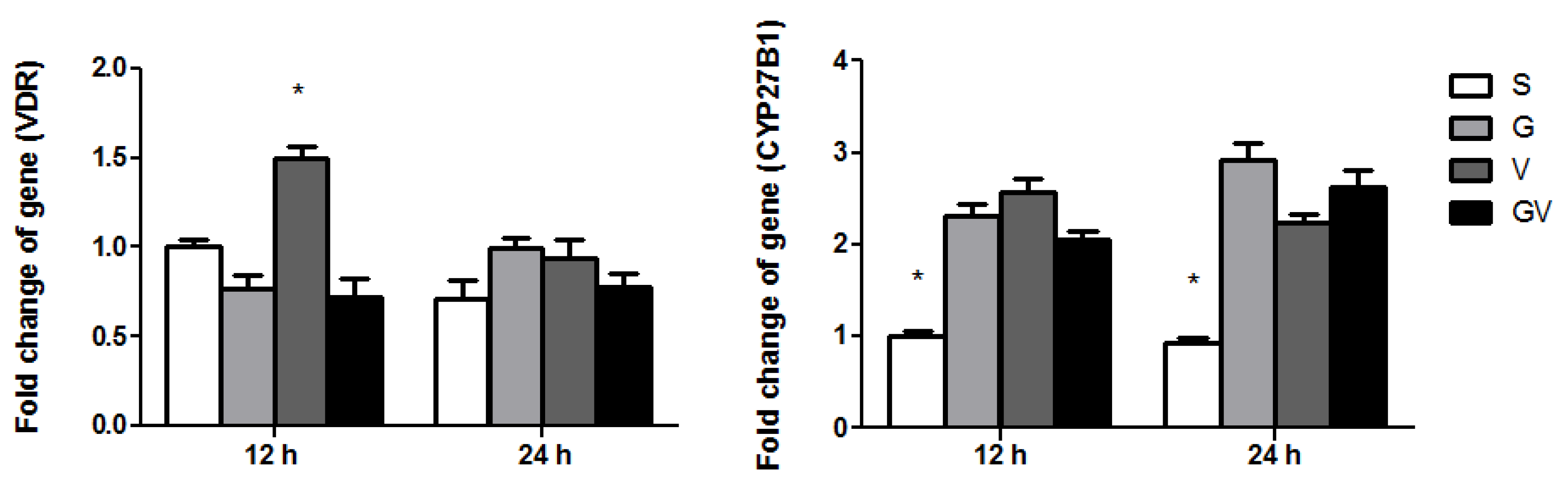
3.4. VDR and CYP27B1 Gene Expressions in the Ileum
3.5. Cathelicidin Levels in the Ileum
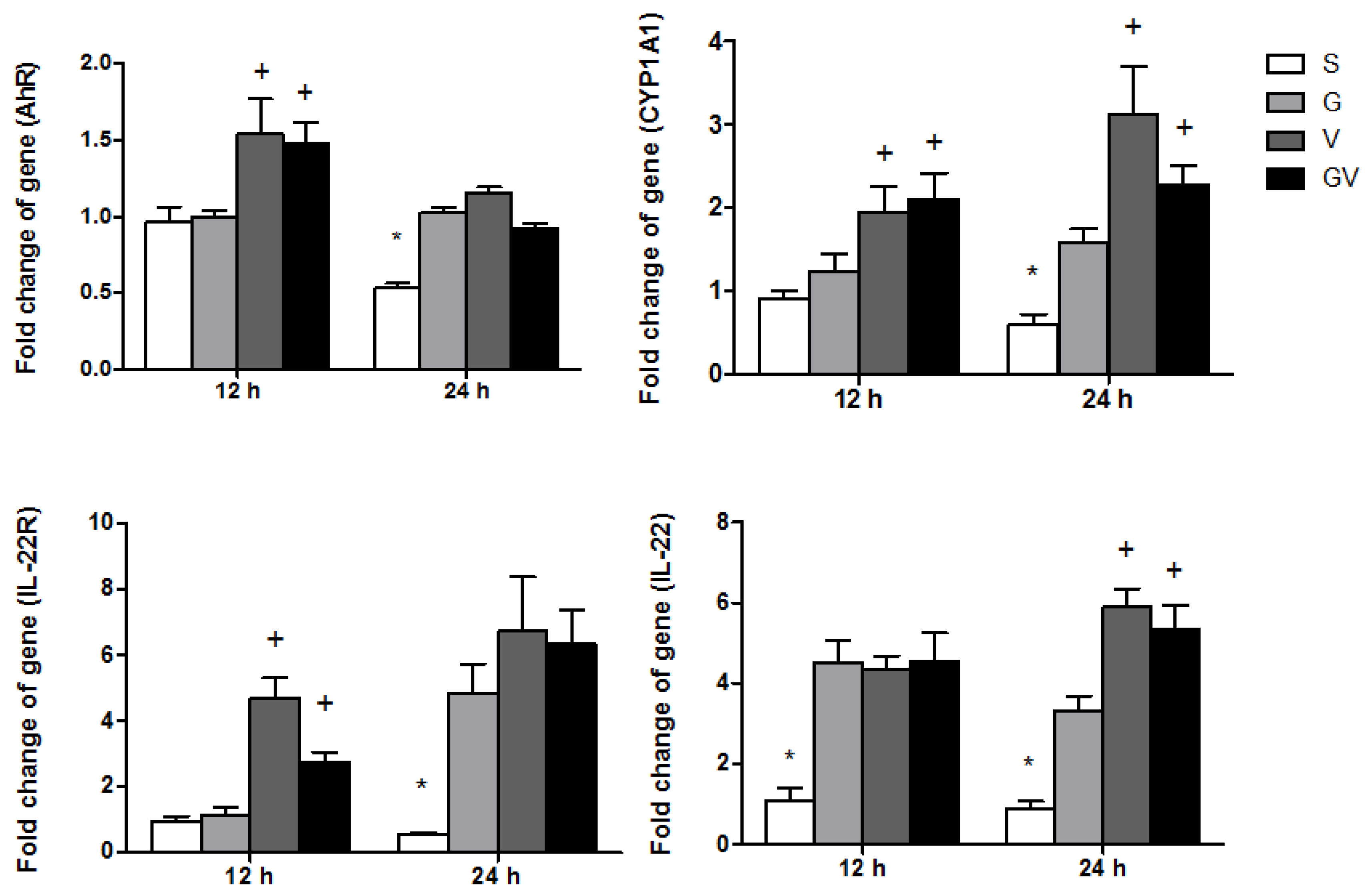
3.6. AhR-Associated Gene Expression in Ileum Tissues
3.7. Expressions of TJ Protein Levels and Mucosal Repair Genes in the Ileum
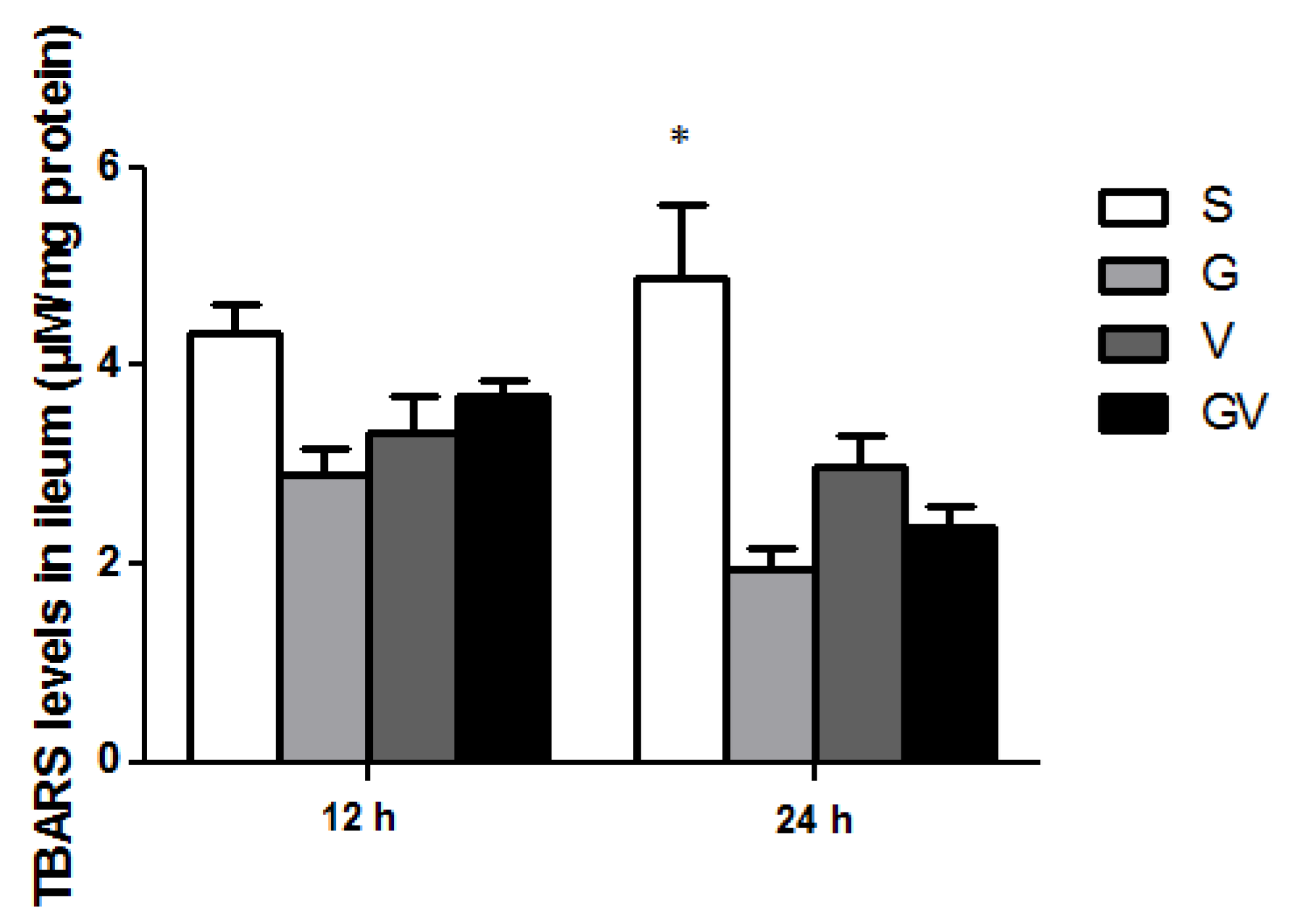
3.8. Lipid Peroxide Concentrations in the Ileum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Andersen, C.J.; Murphy, K.E.; Fernandez, M.L. Impact of obesity and metabolic syndrome on immunity. Adv. Nutr. 2016, 7, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Schetz, M.; De Jong, A.; Deane, A.M.; Druml, W.; Hemelaar, P.; Pelosi, P.; Pickkers, P.; Reintam-Blaser, A.; Roberts, J.; Sakr, Y. Obesity in the critically ill: A narrative review. Intensive Care Med. 2019, 45, 757–769. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kompoti, M. Obesity and infection. Lancet Infect Dis 2006, 6, 438–446. [Google Scholar] [CrossRef]
- Papadimitriou-Olivgeris, M.; Aretha, D.; Zotou, A.; Koutsileou, K.; Zbouki, A.; Lefkaditi, A.; Sklavou, C.; Marangos, M.; Fligou, F. The role of obesity in sepsis outcome among critically ill patients: A retrospective cohort analysis. Biomed. Res. Int. 2016, 2016, 5941279. [Google Scholar] [CrossRef] [PubMed]
- Border, J.R.; Hassett, J.; LaDuca, J.; Seibel, R.; Steinberg, S.; Mills, B.; Losi, P.; Border, D. The gut origin septic states in blunt multiple trauma (ISS = 40) in the ICU. Ann. Surg. 1987, 206, 427–448. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, C.J.; MacFie, J.; Mitchell, C.J.; Johnstone, D.; Sagar, P.M.; Sedman, P.C. Microbiology of bacterial translocation in humans. Gut 1998, 42, 29–35. [Google Scholar] [CrossRef]
- Fakhoury, H.M.A.; Kvietys, P.R.; AlKattan, W.; Anouti, F.A.; Elahi, M.A.; Karras, S.N.; Grant, W.B. Vitamin D and intestinal homeostasis: Barrier, microbiota, and immune modulation. J. Steroid. Biochem. Mol. Biol. 2020, 200, 105663. [Google Scholar] [CrossRef]
- Yoseph, B.P.; Klingensmith, N.J.; Liang, Z.; Breed, E.R.; Burd, E.M.; Mittal, R.; Dominguez, J.A.; Petrie, B.; Ford, M.L.; Coopersmith, C.M. Mechanisms of intestinal barrier dysfunction in sepsis. Shock 2016, 46, 52–59. [Google Scholar] [CrossRef]
- Yue, C.; Ma, B.; Zhao, Y.; Li, Q.; Li, J. Lipopolysaccharide-induced bacterial translocation is intestine site-specific and associates with intestinal mucosal inflammation. Inflammation 2012, 35, 1880–1888. [Google Scholar] [CrossRef]
- MacFie, J. Current status of bacterial translocation as a cause of surgical sepsis. Br. Med. Bull. 2004, 71, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Owens, W.E.; Berg, R.D. Bacterial translocation from the gastrointestinal tract of athymic (nu/nu) mice. Infect. Immun. 1980, 27, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Pepper, M.; Jenkins, M.K. Origins of CD4(+) effector and central memory T cells. Nat. Immunol. 2011, 12, 467–471. [Google Scholar]
- Battault, S.; Whiting, S.J.; Peltier, S.L.; Sadrin, S.; Gerber, G.; Maixent, J.M. Vitamin D metabolism, functions and needs: From science to health claims. Eur. J. Nutr. 2013, 52, 429–441. [Google Scholar] [CrossRef]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, hormone, and immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Snyder, L.; Lin, Y.D.; Yang, L. Vitamin D and 1,25(oH)2D regulation of T cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic effects of vitamin D on human health and disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N. Editorial: Vitamin D and IBD: Can we get over the “Causation” Hump? Am. J. Gastroenterol. 2016, 111, 720–722. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Hansen, T.I.; Gubatan, J.M.; Jensen, K.B.; Rejnmark, L. Managing vitamin D deficiency in inflammatory bowel disease. Frontline Gastroenterol. 2019, 10, 394–400. [Google Scholar] [CrossRef]
- de Haan, K.; Groeneveld, A.B.; de Geus, H.R.; Egal, M.; Struijs, A. Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: Systematic review and meta-analysis. Crit. Care 2014, 18, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upala, S.; Sanguankeo, A.; Permpalung, N. Significant association between vitamin D deficiency and sepsis: A systematic review and meta-analysis. BMC Anesth. 2015, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W.; Meyer, M.B.; Lee, S.M.; Onal, M.; Benkusky, N.A. The vitamin D receptor: Contemporary genomic approaches reveal new basic and translational insights. J. Clin. Investig. 2017, 127, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Brożyna, A.A.; Zmijewski, M.A.; Janjetovic, Z.; Kim, T.K.; Slominski, R.M.; Tuckey, R.C.; Mason, R.S.; Jetten, A.M.; Guroji, P.; et al. The role of classical and novel forms of vitamin D in the pathogenesis and progression of nonmelanoma skin cancers. Adv. Exp. Med. Biol. 2020, 1268, 257–283. [Google Scholar] [PubMed]
- Song, Y.; Slominski, R.M.; Qayyum, S.; Kim, T.K.; Janjetovic, Z.; Raman, C.; Tuckey, R.C.; Song, Y.; Slominski, A.T. Molecular and structural basis of interactions of vitamin D3 hydroxyderivatives with aryl hydrocarbon receptor (AhR): An integrated experimental and computational study. Int. J. Biol. Macromol. 2022, 209, 1111–1123. [Google Scholar] [CrossRef]
- Maëva, M.; Elodie, B.; Nathalie, R.; Manon, D.; Marjolène, S.; Valentine, D.; Morgane, M.; Ivan, W.; Frédéric, D.; Romain, V.; et al. AhR/IL-22 pathway as new target for the treatment of post-infectious irritable bowel syndrome symptoms. Gut Microbes 2022, 14, 2022997. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Q.; Yu, K.; Fan, X.; Xiao, W.; Cai, Y.; Xu, P.; Yu, M.; Yang, H. 6-Formylindolo(3,2-b)carbazole induced aryl hydrocarbon receptor activation prevents intestinal barrier dysfunction through regulation of claudin-2 expression. Chem. Biol. Interact. 2018, 288, 83–90. [Google Scholar] [CrossRef]
- Amrein, K.; Sourij, H.; Wagner, G.; Holl, A.; Pieber, T.R.; Smolle, K.H.; Stojakovic, T.; Schnedl, C.; Dobnig, H. Short-term effects of high-dose oral vitamin D3 in critically ill vitamin D deficient patients: A randomized, double-blind, placebo-controlled pilot study. Crit. Care 2011, 15, R104. [Google Scholar] [CrossRef]
- Quraishi, S.A.; De Pascale, G.; Needleman, J.S.; Nakazawa, H.; Kaneki, M.; Bajwa, E.K.; Camargo, C.A., Jr.; Bhan, I. Effect of cholecalciferol supplementation on vitamin D status and cathelicidin levels in sepsis: A randomized, placebo-controlled trial. Crit. Care Med. 2015, 43, 1928–1937. [Google Scholar] [CrossRef]
- Han, J.E.; Jones, J.L.; Tangpricha, V.; Brown, M.A.; Brown, L.A.S.; Hao, L.; Hebbar, G.; Lee, M.J.; Liu, S.; Ziegler, T.R.; et al. High dose vitamin D administration in ventilated intensive care unit patients: A pilot double blind randomized controlled trial. J. Clin. Transl. Endocrinol. 2016, 4, 59–65. [Google Scholar] [CrossRef]
- Zand, L.; Kumar, R. The use of vitamin D metabolites and analogues in the treatment of chronic kidney disease. Endocrinol. Metab. Clin. N. Am. 2017, 46, 983–1007. [Google Scholar] [CrossRef] [PubMed]
- Leaf, D.E.; Raed, A.; Donnino, M.W.; Ginde, A.A.; Waikar, S.S. Randomized controlled trial of calcitriol in severe sepsis. Am. J. Respir. Crit. Care Med. 2014, 190, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.F.; Yao, D.H.; Hu, Y.Y.; Li, Y. Vitamin D improves intestinal barrier function in cirrhosis rats by upregulating heme oxygenase-1 expression. Biomolecules 2019, 27, 222–230. [Google Scholar] [CrossRef]
- Meeker, S.; Seamons, A.; Maggio-Price, L.; Paik, J. Protective links between vitamin D, inflammatory bowel disease and colon cancer. World J. Gastroenterol. 2016, 22, 933–948. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.L.; Wu, J.M.; Su, L.H.; Yang, P.J.; Lee, P.C.; Chen, K.Y.; Yeh, S.L.; Lin, M.T. Intravenous calcitriol administration regulates the renin-angiotensin system and attenuates acute lung injury in obese mice complicated with polymicrobial sepsis. Biomed. Pharm. 2021, 141, 111856. [Google Scholar] [CrossRef]
- Langlois, P.L.; Szwec, C.; D’Aragon, F.; Heyland, D.K.; Manzanares, W. Vitamin D supplementation in the critically ill: A systematic review and meta-analysis. Clin. Nutr. 2018, 37, 1238–1246. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Yeh, C.L.; Wu, J.M.; Yang, P.J.; Lee, P.C.; Chen, K.Y.; Huang, C.C.; Yeh, S.L.; Lin, M.T. Intravenous calcitriol administration modulates mesenteric lymph node CD4(+) T-cell polarization and attenuates intestinal inflammation in obese mice complicated with polymicrobial sepsis. JPEN J. Parenter. Enter. Nutr. 2022, 46, 1371–1383. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Wu, S.; Sun, J. Vitamin D, Vitamin D receptor, and tissue barriers. Tissue Barriers 2013, 1, e23118. [Google Scholar] [CrossRef]
- Sosa Henríquez, M.; Gómez de Tejada Romero, M.J. Cholecalciferol or calcifediol in the management of vitamin D deficiency. Nutrients 2020, 12, 1617. [Google Scholar] [CrossRef] [PubMed]
- Asrani, V.M.; Brown, A.; Huang, W.; Bissett, I.; Windsor, J.A. Gastrointestinal dysfunction in critical illness: A review of scoring tools. JPEN J. Parenter. Enter. Nutr. 2020, 44, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, H.; Vargas, J.G.; Mokhtari, Z.; Jarick, K.J.; Ulbrich, M.; Mosca, J.P.; Viera, E.A.; Graf, C.; Le, D.D.; Heinze, K.G.; et al. Mesenteric lymph node transplantation in mice to study immune responses of the gastrointestinal tract. Front. Immunol. 2021, 12, 689896. [Google Scholar] [CrossRef] [PubMed]
- DiPiro, J.T. Cytokine networks with infection: Mycobacterial infections, leishmaniasis, human immunodeficiency virus infection, and sepsis. Pharmacotherapy 1997, 17, 205–223. [Google Scholar]
- Bettelli, E.; Korn, T.; Oukka, M.; Kuchroo, V.K. Induction and effector functions of T(H)17 cells. Nature 2008, 453, 1051–1057. [Google Scholar] [CrossRef]
- Zhang, N.; Pan, H.F.; Ye, D.Q. Th22 in inflammatory and autoimmune disease: Prospects for therapeutic intervention. Mol. Cell Biochem. 2011, 353, 41–46. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef]
- Cabrera-Perez, J.; Condotta, S.A.; Badovinac, V.P.; Griffith, T.S. Impact of sepsis on CD4 T cell immunity. J. Leukoc. Biol. 2014, 96, 767–777. [Google Scholar] [CrossRef]
- Ferguson, N.R.; Galley, H.F.; Webster, N.R. T helper cell subset ratios in patients with severe sepsis. Intensive Care Med. 1999, 25, 106–109. [Google Scholar] [CrossRef]
- Gupta, D.L.; Bhoi, S.; Mohan, T.; Galwnkar, S.; Rao, D.N. Coexistence of Th1/Th2 and Th17/Treg imbalances in patients with post traumatic sepsis. Cytokine 2016, 88, 214–221. [Google Scholar] [CrossRef]
- Song, G.Y.; Chung, C.S.; Chaudry, I.H.; Ayala, A. Immune suppression in polymicrobial sepsis: Differential regulation of Th1 and Th2 responses by p38 MAPK. J. Surg. Res. 2000, 91, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Kasten, K.R.; Tschöp, J.; Adediran, S.G.; Hildeman, D.A.; Caldwell, C.C. T cells are potent early mediators of the host response to sepsis. Shock 2010, 34, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Gregori, S.; Giarratana, N.; Smiroldo, S.; Uskokovic, M.; Adorini, L. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes 2002, 51, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Gan, X.; Li, X.; Sun, H.; Chen, Z.; Lu, H. Vitamin D alleviates acute graft-versus-host disease through promoting the generation of foxp3(+) T cells. Ann. Transl. Med. 2019, 7, 748. [Google Scholar] [CrossRef]
- Aslam, M.M.; John, P.; Bhatti, A.; Jahangir, S.; Kamboh, M.I. Vitamin D as a principal factor in mediating rheumatoid arthritis-derived immune response. Biomed. Res. Int. 2019, 2019, 3494937. [Google Scholar] [CrossRef]
- Bouillon, R.; Haussler, M.; Bikle, D.; Christakos, S.; Welsh, J. Introduction: Special issue on vitamin D dedicated to the memory of Anthony W Norman. JBMR Plus 2021, 5, e10445. [Google Scholar] [CrossRef]
- Hu, Z.; Murakami, T.; Suzuki, K.; Tamura, H.; Reich, J.; Kuwahara-Arai, K.; Iba, T.; Nagaoka, I. Antimicrobial cathelicidin peptide LL-37 inhibits the pyroptosis of macrophages and improves the survival of polybacterial septic mice. Int. Immunol. 2016, 28, 245–253. [Google Scholar] [CrossRef]
- Wang, T.T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar] [CrossRef]
- Ho, J.; Chan, H.; Liang, Y.; Liu, X.; Zhang, L.; Li, Q.; Zhang, Y.; Zeng, J.; Ugwu, F.N.; Ho, I.H.T.; et al. Cathelicidin preserves intestinal barrier function in polymicrobial sepsis. Crit. Care 2020, 24, 47. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon. Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Meeker, S.; Seamons, A.; Paik, J.; Treuting, P.M.; Brabb, T.; Grady, W.M.; Maggio-Price, L. Increased dietary vitamin D suppresses MAPK signaling, colitis, and colon cancer. Cancer Res. 2014, 74, 4398–4408. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Munsick, C.; Bemiss, C.; Mahon, B.D. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J. Nutr. 2000, 130, 2648–2652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Liu, W.; Sun, T.; Huang, Y.; Wang, Y.; Deb, D.K.; Yoon, D.; Kong, J.; Thadhani, R.; Li, Y.C. 1,25-Dihydroxyvitamin D promotes negative feedback regulation of TLR signaling via targeting microRNA-155-SOCS1 in macrophages. J. Immunol. 2013, 190, 3687–3695. [Google Scholar] [CrossRef]
- Afaq, F.; Zaid, M.A.; Pelle, E.; Khan, N.; Syed, D.N.; Matsui, M.S.; Maes, D.; Mukhtar, H. Aryl hydrocarbon receptor is an ozone sensor in human skin. J. Invest. Derm. 2009, 129, 2396–2403. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Cella, M.; McDonald, K.G.; Garlanda, C.; Kennedy, G.D.; Nukaya, M.; Mantovani, A.; Kopan, R.; Bradfield, C.A.; Newberry, R.D.; et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat. Immunol 2011, 13, 144–151. [Google Scholar] [CrossRef]
- Eyerich, K.; Dimartino, V.; Cavani, A. IL-17 and IL-22 in immunity: Driving protection and pathology. Eur. J. Immunol. 2017, 47, 607–614. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Q.; Wang, C.; Liu, X.; Li, N.; Li, J. Disruption of tight junctions during polymicrobial sepsis in vivo. J. Pathol. 2009, 218, 210–221. [Google Scholar] [CrossRef]
- Turner, J.R. Molecular basis of epithelial barrier regulation: From basic mechanisms to clinical application. Am. J. Pathol. 2006, 169, 1901–1909. [Google Scholar] [CrossRef]
- Beeman, N.; Webb, P.G.; Baumgartner, H.K. Occludin is required for apoptosis when claudin-claudin interactions are disrupted. Cell Death Dis. 2012, 3, e273. [Google Scholar] [CrossRef]
- Shirazi, T.; Longman, R.J.; Corfield, A.P.; Probert, C.S. Mucins and inflammatory bowel disease. Postgrad. Med. J. 2000, 76, 473–478. [Google Scholar] [CrossRef]
- Taupin, D.; Podolsky, D.K. Trefoil factors: Initiators of mucosal healing. Nat. Rev. Mol. Cell Biol. 2003, 4, 721–732. [Google Scholar] [CrossRef] [PubMed]



| Ingredient (g) | High-Fat Diet |
|---|---|
| Casein, lactic | 258.45 |
| L-Cystine | 3.88 |
| Corn starch | - |
| Maltodextrin | 161.53 |
| Sucrose | 94.08 |
| Cellulose | 64.61 |
| Lard | 316.60 |
| Soybean oil | 32.31 |
| Mineral mix 1 | 64.61 |
| Choline bitartrate | 2.58 |
| Vitamin mix 2 | 1.29 |
| Dye | 0.06 |
| Total | 1000 |
| Protein/Fat/Carbohydrates (%) | 20/60/20 |
| Energy density (kcal/g) | 5.21 |
| Gene Name | Primer Sequence (5′ → 3′) | Accession No. |
|---|---|---|
| AhR | F: GGCTTTCAGCAGTCTGATGTC | AF405563.1 |
| R: CATGAAAGAAGCGTTCTCTGG | ||
| CYP1A1 | F: CAATGAGTTTGGGGAGGTTACTG | NM_009992.4 |
| R: CCCTTCTCAAATGTCCTGTAGTG | ||
| CYP27B1 | F: GGTTCTCCGGAGCTTGTCTG | NM_010009.2 |
| R: AAACTGTGCGAAGTGTCCCA | ||
| IL-22 | F: TTTCCTGACCAAACTCAGCA | XM_006513865.4 |
| R: TCTGGATGTTCTGGTCGTCA | ||
| IL-22Rα | F: CACACCGGTCCTCTCGGAAG | NM_178257.2 |
| R: GGCACTTTCCTTGGACAATATCGG | ||
| Muc2 | F: ATGCCCACCTCCTCAAAGAC | BC039285.1 |
| R: GTAGTTTCCGTTGGAACAGTGAA | ||
| Tff3 | F: TAATGCTGTTGGTGGTCCTG | NM_011575.2 |
| R: CAGCCACGGTTGTTACACTG | ||
| VDR | F: ACCAGCTCTACGCCAAGATG | NM_009504.4 |
| R: CTTCATGCTGTTCTCCGGCT | ||
| GAPDH | F: AACGACCCCTTCATTGAC | M32599.1 |
| R: TCCACGACATACTCAGCAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, C.-L.; Wu, J.-M.; Chen, K.-Y.; Wu, M.-H.; Yang, P.-J.; Lee, P.-C.; Chen, P.-D.; Yeh, S.-L.; Lin, M.-T. Effects of Different Routes and Forms of Vitamin D Administration on Mesenteric Lymph Node CD4+ T Cell Polarization and Intestinal Injury in Obese Mice Complicated with Polymicrobial Sepsis. Nutrients 2022, 14, 3557. https://doi.org/10.3390/nu14173557
Yeh C-L, Wu J-M, Chen K-Y, Wu M-H, Yang P-J, Lee P-C, Chen P-D, Yeh S-L, Lin M-T. Effects of Different Routes and Forms of Vitamin D Administration on Mesenteric Lymph Node CD4+ T Cell Polarization and Intestinal Injury in Obese Mice Complicated with Polymicrobial Sepsis. Nutrients. 2022; 14(17):3557. https://doi.org/10.3390/nu14173557
Chicago/Turabian StyleYeh, Chiu-Li, Jin-Ming Wu, Kuen-Yuan Chen, Ming-Hsun Wu, Po-Jen Yang, Po-Chu Lee, Po-Da Chen, Sung-Ling Yeh, and Ming-Tsan Lin. 2022. "Effects of Different Routes and Forms of Vitamin D Administration on Mesenteric Lymph Node CD4+ T Cell Polarization and Intestinal Injury in Obese Mice Complicated with Polymicrobial Sepsis" Nutrients 14, no. 17: 3557. https://doi.org/10.3390/nu14173557
APA StyleYeh, C.-L., Wu, J.-M., Chen, K.-Y., Wu, M.-H., Yang, P.-J., Lee, P.-C., Chen, P.-D., Yeh, S.-L., & Lin, M.-T. (2022). Effects of Different Routes and Forms of Vitamin D Administration on Mesenteric Lymph Node CD4+ T Cell Polarization and Intestinal Injury in Obese Mice Complicated with Polymicrobial Sepsis. Nutrients, 14(17), 3557. https://doi.org/10.3390/nu14173557