Abstract
Early-life gut microbiota plays a role in determining the health and risk of developing diseases in later life. Various perinatal factors have been shown to contribute to the development and establishment of infant gut microbiota. One of the important factors influencing the infant gut microbial colonization and composition is the mode of infant feeding. While infant formula milk has been designed to resemble human milk as much as possible, the gut microbiome of infants who receive formula milk differs from that of infants who are fed human milk. A diverse microbial population in human milk and the microbes seed the infant gut microbiome. Human milk contains nutritional components that promote infant growth and bioactive components, such as human milk oligosaccharides, lactoferrin, and immunoglobulins, which contribute to immunological development. In an attempt to encourage the formation of a healthy gut microbiome comparable to that of a breastfed infant, manufacturers often supplement infant formula with prebiotics or probiotics, which are known to have a bifidogenic effect and can modulate the immune system. This review aims to elucidate the roles of human milk and formula milk on infants’ gut and health.
1. Introduction
The gut microbiome composition in neonates is closely connected with events such as how they are born (full-term or preterm), the mode of delivery (vagina delivery or caesarean section), what neonates are fed (human or formula milk), and how neonates are cared for (mother’s care or at neonatal intensive care unit (NICU)) []. The early microbiome colonization in the infants’ gut plays a vital role in shaping and maintaining future health outcomes [,,]. Studies have suggested that early-life microbiota can predict the risk of developing illnesses such as atopic diseases, obesity, and type 1 diabetes [,,]. It was previously believed that the onset of microbial colonization of the baby’s gut is at birth. However, recent evidence indicates the presence of microbial communities in the placenta, amniotic fluid, umbilical cord, and meconium, challenging the traditional view of the sterile in utero environment [,,,]. While still controversial, these findings suggest that the infant’s onset of microbial transfer and gut colonization process may begin prenatally [,].
The development of infants’ gut microbiota begins at birth and continues to be shaped up until two–three years, reaching a relatively stable and typical adult microbial taxonomic makeup []. The neonates are first exposed to the maternal microbiome community. The maternal microbiome reservoir colonizes the gut through vertical transmission and microbial taxa obtained from the external surroundings [,]. After birth, the neonatal gut microbiome is briefly dominated by Staphylococcaceae or Enterobacteriaceae before Bifidobacteria become predominant []. Bifidobacterium has long been known to confer health benefits to the host. A higher level of Bifidobacterium in the infant’s gut is associated with a lower risk of childhood infections, atopic disorders, and obesity [,]. After weaning, the infant gut microbiome transitions from a Bifidobacteriaceae-dominated microbiota to an adult-like composition. The key factor influencing these changes in microbiota composition is breastfeeding cessation rather than the introduction of complementary feeding [,]. By the age of three, a stable adult-type gut microbiota, which is clustered into three enterotypes—Bacteroides, Prevotella, Ruminococcus—is acquired [,].
Soon after birth, the infants are fed with human milk or formula milk. It is widely agreed that human milk is the best food for babies, with health effects driven by the combined nutritional and bioactive components. The short-term and long-term health benefits from breastfeeding based on the nutritional, physiological, and development perspectives are well established. The World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF) strongly recommend breastfeeding babies within the first hour of birth and continuing breastfeeding for the first six months, without any other solid food or water [,]. Likewise, the American Academy of Pediatrics (AAP) also suggests breastfeeding for 12 months or beyond while the infant is started on complementary food []. Human milk comprises beneficial bacterial species that contribute to establishing the baby’s gut microbiome and play a part in infection prevention and immunomodulation []. Besides containing all the essential nutrients to fulfil the nutritional requirements for optimal growth of the infant, human milk also contains bioactive components—oligosaccharides, immunoglobulins, hormones, growth factors, cytokines and chemokines—that play important roles in the microbiome and immune system development, as well as maintaining the gut mucosal barrier function []. Given their implications for health and immune development and their potential for therapeutic manipulation, the human milk microbiome and bioactive compounds have become an area of interest for research []. Although human milk is the best for babies, alternatives are sought when human milk is insufficient, babies who cannot be fed with human milk, or should not receive milk from their mothers (due to health reasons) []. In these circumstances, babies are fed with formula milk that mimics the composition of human milk in terms of micronutrients and macronutrient content. Hence, this narrative review aims to discuss the role of human milk and formula milk on infants’ gut and health.
2. Factors Influencing Infants’ Gut
2.1. Mode of Delivery
Many perinatal variables have been shown to contribute to infant gut microbiota development, such as mode of delivery, gestational age, infant diet, use of antibiotics, and infant hospitalization. The initial microbes that colonize the infants’ gut come from their mother and, if vaginally born, then the microbes are from the vaginal microbiome of the mother. Dominguez-Bello and colleagues studied bacteria sampled from infants right after birth and compared them with samples from different maternal body sites. They revealed that all the babies shared similar microbiome composition. If vaginally born, then the bacteria source is from the mother’s vagina []. However, Ferreti et al. reported that neonates’ oral and gut microbiomes at one–three days postpartum do not resemble the microbiome taxa from a specific mother’s body site. Some neonates had taxonomic makeup that resembled the vaginal microbiome, while some had the same composition as the mothers’ faecal microbiome. The early colonization is suggested to be influenced by stochastic events []. Infants delivered by vaginal or caesarean are usually exposed to different microbiome communities. In the first three weeks of life, vaginally born babies are exposed to their own mothers’ vaginal microbiomes and are enriched with Bifidobacterium, Escherichia, Lactobacillus and Bacteroides species. Whereas caesarean-delivered babies are exposed to microbiome species of the skin of parents and potentially colonized by species from hospital environments. Caesarean-born babies have a gut that is commonly colonized by Enterobacter, S. epidermidis, K. pneumoniae, E. coli and Klebsiella [,,].
The effect of birth mode on gut microbial colonization and diversity is most significant during the first six months of life, albeit decreasing with age [,]. Wampach et al. studied the microbial taxa in 33 mother–infant pairs (MIPs) at an interval of the first, third, and fifth days postpartum. They observed the vaginally born infants had a higher number of vertically transmitted strains compared to those born via caesarean. The MIPs shared 23 taxa mostly Bacteroides and Bifidobacterium in vaginally delivered infants []. Similarly, another study by Makino and colleagues found that the majority of vaginally born babies share a Bifidobacterium strain profile with the maternal gut microbiome. The caesarean-born babies did not exhibit any strain sharing profiles with the maternal gut []. A study also found that at the early stage of vaginal delivery, the infant’s gut is enriched with Lactobacillus, and then the Bacteroides will increase in the second week. These microbial patterns are not observed in caesarean-born infants []. Based on these studies, delivery mode does play a significant role in enriching the gut microbiota of infants.
2.2. Gestational Age and Administration of Antibiotics
The gestational age at birth is another crucial factor in the development of neonates’ gut microbiome. The intestines of preterm neonates born around 22 to 36 weeks of gestation are more permeable than term neonates (37 to 42 weeks of gestation). A lower abundance of Bifidobacterium and Bacteroidetes and a higher abundance of Enterobacteriaceae, Enterococcaceae, and Lactobacillaceae were observed in preterm neonates compared to term neonates []. Korpela et al. studied the faecal samples from 45 preterm neonates to explore the gut microbiota properties and development in preterm neonates. The study found that preterm neonates’ gut exhibited a lower number of predominant bacteria genera such as Bifidobacterium, Enterobacter, Staphylococcus, or Enterococcus. They suggested that breastfeeding may help preterm infants in the hospital acquire a normal gut microbiota similar to that of infants who are born to term [].
Perinatal antibiotic usage is also known to affect the gut microbial composition, as indicated by a reduced gut microbiome diversity and stability [,]. A study comparing the gut microbial colonization in infants exposed to perinatal antibiotics and infants who had no antibiotics exposure reported the differences in gut microbiome between the exposure groups and control group, which persist at six months of age and are not prevented by consumption of probiotic Lactobacillus reuteri []. Studies have shown a reduced Bifidobacterium abundance in term babies upon antibiotic treatment for Group B Streptococcus. The other bacteria community (Lactobacillus sp., Bacteroides fragilis, and Clostridium difficile) were not affected [,].
2.3. Feeding Mode
Another essential factor influencing the infant’s gut microbial colonization and composition is the mode of infant feeding. While infant formula milk has been designed to resemble breast milk as much as possible, the gut microbiome composition in breastfed and formula-fed infants remains distinct. Several studies have demonstrated the beneficial effects of human milk on the disrupted microbiome caused by other aforementioned perinatal factors. For example, limited restoration of the disturbed gut microbiota was demonstrated by Liu et al. in infants born through caesarean section by exclusive breastfeeding compared to partial breastfeeding []. Cong et al. studied the influence of feeding on the early life gut microbial composition of preterm babies and found that human milk enriched microbial diversity and helped establish a balanced microbial community structure []. In breastfed term infants, a higher abundance of Bifidobacterium was observed, whereas formula milk fed infants had an increase in Enterobacteriaceae, Bacteroidaceae, and Clostridiaceae [,].
3. Human Milk
3.1. Human Milk Composition
Human milk contains both nutritive and non-nutritive components, which promote normal growth and contribute to immunological development. The nutritional components are classified into macronutrients (carbohydrate, protein, and fat) and micronutrients (minerals and vitamins) []. Human milk contains approximately 7.0% lactose, which is a disaccharide. As the predominant carbohydrate in human milk, lactose contributes to 40% of the milk’s gross energy []. In smaller amounts, other carbohydrates in milk are monosaccharides, such as glucose and galactose; disaccharides, such as lactulose; oligosaccharides and some polysaccharides []. While oligosaccharides in human milk are non-nutritive and indigestible, they play an important role in infant gut microbiome development [,]. The protein content in human milk is estimated to be 0.9%–1.2% [,]. Approximately 30% of the total protein is casein, and 70% is whey proteins primarily constituted by lactoferrin, alpha-lactalbumin, and secretory IgA []. The mean fat concentration in mature milk is approximately 3.8%, contributing to about half of the total energy provided by human milk (Table 1) [,].

Table 1.
The human milk constituents and concentrations [,,].
The composition of breast milk is dynamic and changes in response to the baby’s needs. A study by Paulaviciene et al. showed that human milk exhibits marked circadian fluctuations in the composition of macronutrients, particularly protein and fat, and the diurnal variations are more evident in the breast milk of mothers of premature babies []. The human milk nutritional composition also changes within each feed; for example, there are more fat and energy contents in hindmilk compared to foremilk [,]. Human milk has three stages, and its composition varies across the different lactation stages. The first milk secreted immediately after delivery of a newborn is known as colostrum, which differs from the milk produced later by containing higher concentrations of proteins and immunological components such as lactoferrin, leukocytes, immunoglobulins, and growth factors, as well as containing lower concentrations of carbohydrates and fats [,,,]. The second stage lasts from day 6 to day 14 postpartum, and the milk produced is known as transitional milk []. There is an increase in milk production, carbohydrate, and lipid content in this stage, such that the infant’s nutritional requirements can be met for rapid growth and development [,]. The milk is considered mature by day 15 to day 30 postpartum, and its composition fluctuations are relatively less []. Mature milk is richer in lactose and fat, but its protein is at a lower concentration than colostrum []. Some studies have demonstrated higher carbohydrate, protein, fat, and energy contents in preterm milk compared to term milk. However, some authors found higher protein levels in preterm milk, whereas other macronutrient contents were not significantly influenced by gestational age [,,,]. Two recent studies have reported that the macronutrient composition is not affected by the degree of prematurity [,]. Feeding practices may also be predictors of the nutrient composition as indicated by lower fat concentration, lower total calorie content, and higher carbohydrate and protein contents in the milk of mixed-feeding mothers compared to those who exclusively breastfed [].
3.1.1. Oligosaccharides
Human milk oligosaccharides (HMOs), the third most abundant human milk component, are present at approximately 0.8–1.4% []. The concentration of HMOs may vary with the stage of lactation, as reflected by a higher level of most HMOs in colostrum, and reduces as lactation progresses [,,,]. A higher level of HMOs is also found in the milk of mothers of preterm babies than in mothers of term babies []. While they are indigestible by infants, HMOs have prebiotic effects and are metabolized by certain gut bacteria, promoting their growth and colonization within the infant’s gut []. De Leoz et al. demonstrated a shift in the infant gut microbiota from non-HMO-utilizing bacteria to HMO-utilizing bacteria after receiving breast milk for a few weeks. There was also a reduction in faecal HMOs as the abundance of HMO-consumers increased, confirming the utilization of HMOs by the gut bacteria []. Bifidobacteria and Bacteroides strains have a high capacity in metabolizing HMOs and utilizing them as a source of energy. In contrast, other species such as Enterococcus, Clostridium, Escherichia coli, Staphylococcus, and Streptococcus are inefficient in HMOs metabolism [,]. By selectively encouraging the growth of beneficial bacteria over potential pathogens, HMOs may help to prevent infections.
Furthermore, oligosaccharides fermentation by gut bacteria leads to the production of short-chain fatty acids (SCFAs) such as acetate and organic acids such as lactate, generating a lower pH environment that restrains the growth of enteric pathogens and promotes the absorption of nutrients [,]. In addition, Bifidobacterium grown on HMOs is associated with increased anti-inflammatory cytokine and decreased inflammatory gene expression, indicating that HMO growth possesses anti-inflammatory properties [,]. The ability of bacteria to adhere to the intestinal epithelium determines the microbial colonization of the infant’s gut. HMOs have been shown to modulate and enhance the ability of Bifidobacterium infantis to adhere to intestinal epithelium, which may enhance the bacteria’s colonization ability [,,]. In contrast, HMOs exert anti-adhesive properties against potential pathogens. Studies have demonstrated that some HMOs reduce the level of adhesion of Campylobacter jejuni, Clostridium butyricum, Escherichia coli, Pseudomonas aeruginosa, and Norovirus to epithelial cells [,,,].
3.1.2. Lactoferrin
Lactoferrin, an iron-binding protein, is one of the essential bioactive factors present in human milk []. Its concentration changes throughout lactation, with the highest level, found in colostrum and reduces in the later milk until a relatively constant level is reached in mature milk [,,]. Mastromarino et al. established that human milk is the primary source of lactoferrin in the infant’s gut as the level of faecal lactoferrin in breastfed infants was significantly associated with the level of lactoferrin in human milk []. Woodman et al. have shown that lactoferrin levels are higher in human milk than formula milk and human lactoferrin showed greater effectiveness in preventing growth of pathogens []. Lactoferrin has been shown to exhibit antimicrobial effects on both Gram-positive and Gram-negative bacteria []. Tian et al. demonstrated the ability of lactoferrin to inhibit the growth of pathogenic bacteria, such as Staphylococcus aureus, Listeria monocytogenes, Salmonella enterica, and Escherichia coli. In contrast, the development of probiotic bacteria such as Lactobacillus was not affected [].
Several mechanisms of the antimicrobial activity of lactoferrin have been described. Mechanisms that have long been known include its iron-binding ability that results in sequestrating and depriving bacteria of iron required for their growth, as well as its ability to interact with lipopolysaccharide of Gram-negative bacteria thus impeding their growth [,]. More recent studies have shown that lactoferrin can eliminate biofilms formed by potential pathogens, preventing interactions between microbes and the gut epithelium [,]. These characteristics of lactoferrin aid in the enrichment of a healthy gut microbiota in infants.
3.1.3. Immunoglobulins
Immunoglobulins (Ig) are bioactive factors in human milk that offer passive immunological protection to neonates. IgG antibodies cross the human placenta and hence can provide passive immunity to the foetus in utero and the infant postnatally. The IgA and IgM are unable to cross the human placenta; therefore, human milk is an important source of these antibodies, particularly secretory IgA as infants have very low levels of their own IgA at birth and it only gradually rises in the first few months of life when the immune system develops []. IgA is the main immunoglobulin in human milk, constituting more than 90% of all milk antibodies, whereas IgM and IgG are present at significantly lower concentrations. The mean concentration of IgG, IgM, and secretory IgA in human milk during the first year was 14.71 mg/L, 3.0 mg/L, and 2.12 g/L, respectively, to Czosnykowska-Łukacka et al. []. The levels of IgA and IgM are highest in colostrum and reduce to a relatively stable level in mature milk, whereas the concentration of IgG remains similar throughout the first six months of lactation []. A study by Berdi et al. demonstrated a positive correlation between prepregnancy excessive maternal weight and human milk IgM concentration in the first few days of lactation, and smoking during pregnancy is negatively correlated with IgM and IgG2 concentration []. Breastfeeding practices may also have an effect on immunoglobulin levels in mother’s milk as evidenced by a higher IgG concentration in the milk of mothers who exclusively breastfed compared to mothers who did not exclusively breastfeed in a study by Abuidhail et al., supporting the recommendation of the World Health Organization to exclusively breastfeed babies for the first six months []. IgA and total protein concentrations in human milk have been found to increase after the first year of lactation, indicating that breastfeeding even after introducing food, offers nutritional and immunological benefits to the child. Therefore, prolonged lactation should be encouraged in keeping with the preference of the mother and child [,]. IgA helps establish a healthy gut microbiome by enhancing the ability of Bacteroides and probiotic strains, such as Bifidobacterium and Lactobacillus, to adhere to gut epithelium, thereby promoting their colonization [,]. Lack of secretory IgA from human milk has been associated with an altered intestinal microbiome and gene expression in the gut epithelium, which may result in greater susceptibility to gut inflammation at a later age [].
3.2. Extracellular Vesicles
Extracellular vesicles (EVs) with their cargos can be detected in any tissue or biofluids, including breast milk. They have been shown to shape the gut microbiome and influence the gut immune response []. In vitro study demonstrated intestinal cell uptake of human milk EVs, indicating that EVs are modes of transfer of immunomodulatory genetic material from mother to child []. EVs carry a variety of biologically active compounds, such as proteins, lipids, and RNAs [,]. Proteomic analysis by van Herwijnen et al. discovered 1963 proteins in EVs from breast milk, and the proteins in EVs were involved in inflammatory signalling pathways []. Breast milk was found to have the highest total RNA concentration among other bodily fluids []. Human milk contains high levels of immune-related microRNAs, as reported by Kosaka et al. The molecules were found to be stable in very acidic environments, suggesting that miRNAs can endure the gut environment of infants []. Zhou et al. also demonstrated that immune-related miRNAs packaged within exosomes in human milk remain impervious to a certain extent when subjected to extreme conditions. It is postulated that these miRNAs are passed to infants from maternal milk through the gastrointestinal tract and are involved in immune system modulation []. Alsaweed et al. found a limited number of mature human miRNAs in cow’s milk and soy-based formula, which are expressed at lower levels than those in human milk, indicating that human milk is a richer source of miRNAs for infant immunity and development []. EVs in human milk may have therapeutic potential in preventing necrotizing enterocolitis in premature infants. In a study using rat intestinal epithelial cell culture models, there was a significant reduction in the occurrence and severity of experimental necrotizing enterocolitis resulting from administration of EVs derived from breast milk []. In addition, Wang et al. and Martin et al. showed that EVs derived from human milk promote gut epithelial cell proliferation and protect intestinal cells from oxidative stress, which may protect infants from necrotizing enterocolitis [,].
3.3. Human Milk Microbiota
A diverse microbial population is found in human milk, and its microbial composition varies considerably between mothers []. A recent study by Kim and Yi detected 392 genera in human milk []. Many studies have found Staphylococcus and Streptococcus to be the predominant genera in human milk [,,,,,]. Pannaraj et al. demonstrated that the divergence in human milk microbiota between mothers increases during the first 24 weeks postpartum and reduces after that when infants are no longer primarily breastfed. The study also showed that in infants who are primarily breastfed (mother’s milk accounts for at least 75% of daily milk intake) during the first month of life, about 40% of the gut microbiota originates from mother’s milk and areolar skin, emphasizing the significance of human milk as a source of bacteria and in determining the infant gut microbiome [].
Some studies have suggested that factors such as birth mode, gestational age, stages of lactation, and breastfeeding practices may play a role in determining the microbial composition of breast milk. A lower microbiota diversity has been detected in breast milk from women who delivered by caesarean section than those who gave birth by vaginal delivery [,]. Gestational age has also been shown to affect the microbial composition of human milk, as evidenced by a much lower abundance of Bifidobacteria in preterm breast milk than in term milk []. However, a study by Urbaniak et al. found no differences in the human milk microbial profiles attributable to the mode of delivery or gestational age []. Regarding the mode of breastfeeding, indirect breastfeeding and pump expression compared to direct breastfeeding and manual expression, respectively, are associated with reduced milk microbiota diversity and richness, higher rates of colonization by potential pathogens, and decreased abundance of Bifidobacteria []. Gonzalez et al. compared the human milk microbiome in two lactation stages. They found that the predominant species in the early stage were Streptococcus and Staphylococcus, whereas in the late lactation stage Pseudomonas and Sphingobium were more abundant []. A few studies have shown that microbial richness and diversity in human milk reduce over time from colostrum to mature milk [,,].
4. Infant Formula Milk
4.1. Types of Infant Formula
Different infant formula products are available, including cow’s milk, soy-based, and specialized formulas such as hypoallergenic and lactose-free. Cow’s milk formula is the most commonly consumed infant formula among other types []. Bovine milk contains approximately 3.4% protein, which is significantly higher than human milk, and has a different whey-to-casein ratio of 20:80. Compared to human milk, lactose and lactoferrin levels are lower in bovine milk. Furthermore, human milk has a two-fold higher concentration of alpha-lactalbumin. It does not contain beta-lactoglobulin, whereas beta-lactoglobulin is the dominating protein in the whey fraction of bovine milk []. Thirteen oligosaccharides present in human milk were identified in bovine colostrum by Aldredge et al., indicating that bovine milk could be a source of oligosaccharides with bioactivities comparable to those in human milk []. However, the concentration and diversity of oligosaccharides in bovine milk are lower than in human milk []. Therefore, infant formula manufacturers modify or reformulate cow’s milk to reproduce a nutrient profile similar to human milk [].
Cow’s milk protein allergy is widely regarded as one of the most common food allergies in the paediatric population []. As stated in the food allergy and anaphylaxis guidelines by the European Academy of Allergy and Clinical Immunology, the culprit food allergen should be eliminated from the diet of children with cow’s milk protein allergy. Thus, a therapeutic formula is required. Soy-based formula, extensively hydrolysed formula and amino acid-based formula are alternative infant formulas that can be used in place of cow’s milk formula. While the extensively hydrolysed formula is commonly used as a substitute for cow’s milk, the amino acid-based formula is nonallergenic and may be a substitute when an extensively hydrolysed formula is ineffective []. Soy-based formula should only be considered in infants over six months, and if they cannot tolerate extensively hydrolysed formula, if parents cannot afford other specialized formulas, or if the parents strongly prefer it due to veganism []. The presence of high concentrations of isoflavones in soy formula has raised concerns about its potential estrogenic effect on infants’ development. Andres et al. evaluated the developmental status of infants receiving different types of milk and revealed that infants fed with soy formula had normal growth and did not differ from cow’s milk formula. However, breastfed infants exhibited better cognitive development than formula-fed infants [].
4.2. Addition of Prebiotics and Probiotics to Infant Formula
To promote a gut microbiome formation comparable to that of a breastfed infant, manufacturers often supplement infant formula with prebiotics and probiotics, which are known to have a bifidogenic effect and can modulate the immune system []. Studies have demonstrated that adding prebiotic oligosaccharides to infant formula is well-tolerated in healthy infants and results in softer stools compared to unsupplemented formula [,,,]. Puccio et al. studied the outcome of supplementing infant formula with two common HMOs—2′fucosyllactose and lacto-N-neotetraose—and reported that the supplemented formula could support proper growth of the infants []. Adding these two prebiotic oligosaccharides to infant formula has also been reported to promote the growth of Bifidobacterium and produce gut microbial composition closer to breastfed infants []. Infant formula supplemented with galacto-oligosaccharides and fructo-oligosaccharides is associated with lower faecal pH and greater abundance of Bifidobacteria compared to the unsupplemented formula [,]. Castanet et al. revealed that infant formula containing bovine milk-derived oligosaccharides exhibits a bifidogenic effect, helps to modulate the infant gut microbiome, and enhances gut maturation [].
Probiotics are live microorganisms that provide health benefits to the host when administered in sufficient and appropriate amounts []. Supplementing infant formula with probiotic with Bifidobacterium sp. and/or lactic acid bacteria such as Lactobacillus strains is safe and well-tolerated in healthy term infants [,,]. The supplementation of probiotics increases IgE level in cord blood and promotes TGF-β production. TGF-β is closely related to IgA that is specific to food antigens; thus, elevated TGF-β plays a vital role in atopy prevention in infant early life []. The addition of probiotics to infant formula may have beneficial effects on the immunity of infants, as indicated by a decrease in the incidence of the upper respiratory tract and gastrointestinal infections []. Infants receiving formula milk supplemented with Lactobacillus fermentum had a lower incidence rate of gastrointestinal infections [,]. Chi et al. reported that infant formula supplemented with probiotic Bifidobacterium lactis promotes enrichment of beneficial bacteria in the gut microbiome of low birth weight infants []. In a study by Radke et al., infants receiving formula supplemented with both prebiotics (bovine milk-derived oligosaccharides) and probiotics (Bifidobacterium animalis sp. lactis) have higher levels of Bifidobacterium and Lactobacilli, lower faecal pH, and higher faecal secretory IgA compared to infants receiving unsupplemented formula. The Committee on Nutrition of the European Society for Paediatric Gastroenterology and Nutrition (ESPGHAN) and the ESPGHAN Working Group for Probiotics and Prebiotics recommends the use of L. rhamnosus GG (LGG) ATCC 53103 (at a daily dose ranging from 1 × 109 CFU to 6 × 109 CFU) and the combination of B. infantis Bb-02, B. lactis Bb-12, and Str. thermophilus TH-4 (at a daily dose of 3.0 to 3.5 × 108 CFU of each strain) to reduce necrotizing enterocolitis (NEC) stage 2 or 3 in preterm infants []. These suggestions that adding prebiotics and probiotics to infant formula may bring the gut microbiome closer to that of breastfed infants and positively influence the immune system []. As the postbiotic effect dependents on the bacterial strain, thus, the safety, suitability, and specificity of the bacteria strain in formula milk should be further studied.
4.3. Differences in the Gut Microbial Composition between Breastfed and Formula-Fed Infants
The gut microbiome of infants who receive human milk differs from that of infants fed formula milk. Formula feeding in term infants has increased microbial diversity [,,]. Studies have reported higher levels of Bifidobacteria—an essential inhabitant of a healthy microbiota, in breastfed infants compared to formula-fed infants [,,,]. Consequently, breastfed and formula-fed infants have different levels of faecal SCFAs, the main metabolites of HMOs fermentation, with a higher level in breastfed infants []. However, Wang et al. reported that although the overall microbiota in breastfed and formula-fed infants are different, the levels of Bifidobacteria in both breastfeeding and formula-feeding groups are similar, suggesting that some formula milk is capable of supporting the growth of Bifidobacteria []. In an effort to create a gut microbiota profile that is comparable to that in breastfed infants, advances have been made in the formulation and manufacturing of infant formula milk, such as optimizing the whey-to-casein ratio and supplementing the formula milk with prebiotics, which has been proven to increase the abundance of Bifidobacterium in formula-fed babies [,].
5. Impact of the Early-Life Gut Microbiome on Health and Diseases
5.1. Necrotising Enterocolitis
Necrotising enterocolitis (NEC) is a serious inflammatory disease of the gastrointestinal tract predominantly affecting premature infants []. The earlier the gestational age, the higher the mortality due to NEC []. Dysbiosis and delayed gut microbiota maturation in preterm infants are associated with a higher risk of necrotising enterocolitis [,,]. Prematurity is an independent factor associated with an altered gut microbiome []. Moreover, preterm infants are often treated with antibiotics and prolonged hospital care, which may directly influence the gut microbiota []. Cong et al. investigated the stool samples of 29 stable/healthy preterm infants at the NICU. The study found that the Proteobacteria was the most abundant phylum. There was an increasing pattern of Clostridium and Bacteroides, and decreasing Staphylococcus and Haemophilus observed over time during early life (Table 2) [].

Table 2.
Summary of early-life gut microbiome on health and diseases.
A similar bacteria phylum was observed in a study by Arboleya and colleagues. The study compared faecal samples from full-term vaginal delivered breast-fed infants (FTVDBF) and VLBW preterm infants. The preterm infants had higher levels of Firmicutes and Proteobacteria, and decreased levels of Bacteroidetes and Actinobacteria when compared with FTVDBF infants. The concentration of faecal SCFAs is also lower in premature infants, suggesting an alteration in the functionality of the preterm infant gut microbiome besides the composition []. McMurtry et al. reported that a reduced gut microbial diversity and the absence or significantly low abundance of certain classes of bacteria, for instance, Clostridia, are associated with an increased risk and severity of NEC. This suggests that a diverse microbiota and specific taxa may reduce the inflammatory response leading to the disease [].
Human milk appears to protect against NEC as human milk (maternal or donor) is associated with decreased incidence of NEC compared to the preterm formula [,,,]. Kimak et al. recommended a longer term of exclusive human milk intake to decrease the risk of developing necrotising enterocolitis as the occurrence of NEC was higher in premature infants who received human milk for less than a week compared to those who exclusively received breast milk for more than a week []. Pourcyrous et al. found significant levels of total faecal SCFAs in preterm babies who received only expressed human milk compared to those who received preterm formula and suggested that the increased concentration of SCFAs in infants receiving human milk is associated with reduced risk of NEC []. Gopalakrishna et al. revealed that IgA from human milk plays a vital role in developing infant gut microbiota in preterm infants and preventing NEC []. Specific HMOs in human milk may also contribute to the protection against NEC, as 2′-fucosyllactose and disialyllacto-N-tetraose have been shown to lessen the severity of the disease and improve survival in animal models [,]. In addition, a study by Autran et al. found a significantly lower concentration of disialyllacto-N-tetraose in human milk samples received by VLBW infants who developed NEC. The concentration of this particular HMO could predict the risk of NEC occurrence [].
Probiotics have been used to prevent necrotising enterocolitis in very low birth weight preterm babies; however, there are contradicting results concerning their effectiveness, which might be attributable to the modes of infant feeding and probiotic strains. For example, Braga et al. and Chowdhury et al. reported that human milk supplemented with probiotics (Bifidobacterium and Lactobacillus) decreased the incidence of necrotising enterocolitis [,]. A recent study by Robertson et al. also showed that probiotics supplementation with Bifidobacterium bifidum and Lactobacillus acidophilus is associated with a reduction in risk of NEC and late-onset sepsis, irrespective of mode of feeding []. In contrast, Demirel et al. found probiotic supplementation (Saccharomyces boulardii) in human or formula milk ineffective in decreasing NEC incidence or death []. Saengtawesin et al. found no difference in the incidence of NEC between infants receiving human milk or formula milk supplemented with Bifidobacterium bifidum and Lactobacillus acidophilus and the group that did not receive probiotics supplementation []. Dang et al. compared the outcomes of preterm infants before and after commencing probiotics supplementation consisting of Lactobacillus rhamnosus and Bifidobacterium infantis. It was reported that probiotics resulted in better feeding tolerance and reduced extra-uterine growth restriction; however, there was no significant difference in the occurrence of NEC []. The European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) Committee on Nutrition and the Working Group for Probiotics and Prebiotics reported that only a small number of the investigated probiotic strains or combinations of strains were found to be efficacious in decreasing morbidity and mortality. Although there is low certainty in evidence, the ESPGHAN position paper conditionally recommended using Lactobacillus rhamnosus GG ATCC53103 or the combination of Bifidobacterium infantis Bb-02, Bifidobacterium lactis Bb-12, and Streptococcus thermophilus TH-4 in lowering the risk of necrotising enterocolitis [].
5.2. Obesity
The increasing prevalence of infant and childhood obesity is of concern, as obesity can harm health and quality of life. Moreover, children who are overweight or obese are more prone to becoming obese adults and developing chronic diseases []. It has been found that infant gut microbiota may predict the risk of excessive weight gain in childhood []. A large study by Scheepers et al. reported that early infancy gut microbial composition, particularly the abundance of Bacteroides fragilis, is significantly correlated with weight gain in children []. In addition, the levels of Streptococcus in infants have been found to positively correlate with childhood body mass index (BMI), whereas the levels of Bifidobacterium are negatively correlated with the later BMI [,,]. Factors influencing the gut microbiome development in infancy, including mode of delivery and perinatal antibiotic exposure, have associations with childhood adiposity [,]. Caesarean section and use of antibiotics during the first six months of age are associated with higher body mass in later years as reported by Blustein et al. and Trasande et al., respectively [,]. However, Li et al. suggested that rather than antibiotic exposure, untreated infection—which can also disrupt the gut microbiome—during infancy is correlated with excessive weight gain in childhood [].
Many studies have demonstrated that breastfeeding has a protective effect on childhood obesity, while some have provided contradictory results. Several studies have reported that breastfed children for at least six months have a lower risk of overweight or obesity in childhood compared to those who have never received breast milk [,,]. Some studies revealed a dose-response relationship between the duration of breastfeeding during infancy and child overweight or obesity risk [,,]. In contrast, some studies found no association and dose-response effect between breastfeeding and obesity in later years [,]. On the other hand, studies on formula-fed infants demonstrated that different types of infant formula might also affect the pattern of weight gain due to the energy balance mechanisms. A study by Weber et al. revealed that compared to infants who received formula milk with higher protein content, those who received formula with lower protein content had lower BMI and lowered risk of obesity at older age []. Early-life consumption of higher protein content has been associated with more rapid weight gain, resulting in higher adiposity []. In addition, a more significant proportion of infants fed with cow’s milk formula were fast weight gainers compared to infants receiving extensively hydrolysed formula []. There is an association between rapid weight gain during infancy and high BMI later in life [].
5.3. Atopy
Atopy is characterized by a tendency to develop hypersensitivity reactions in which there is elevated immunoglobulin E (IgE) production in response to antigens or allergens. Some common atopic conditions include allergic rhinitis, asthma, atopic dermatitis, and food allergy []. The development of atopic disorders may be influenced by early-life gut microbiota. Studies have revealed that reduced gut microbial diversity in early infancy is associated with an increased risk of developing atopic diseases [,,]. Penders et al. showed that Clostridia colonization in 5 and 13-week-old infants was associated with a higher risk of developing atopic dermatitis []. West et al. demonstrated that infants with IgE-associated atopic dermatitis have lower Gram-positive Ruminococcaceae, which is associated with excessive TLR2 response, suggesting that the infant gut microbial composition may be correlated with susceptibility to eczema through immune signalling modulation []. Ta et al. revealed that infants with atopic dermatitis have an altered developmental trajectory in their gut microbiome. There was an enrichment of Enterobacteriaceae at three weeks of life and a delay in Bacteroidaceae colonization, leading to an increase in Enterobacteriaceae/Bacteroidaceae ratio in infants with atopic dermatitis []. Arrieta et al. demonstrated a transient dysbiosis of the intestinal microbiota in children at risk of asthma during their first few months. These children have a lower abundance of Lachnospira, Veillonella, Rothia, and Faecalibacterium in their gut microbiome at three months old. Furthermore, inoculation of germ-free mice with these four microbes have been shown to reduce airway inflammation, suggesting that they may play a protective role in asthma development [].
Studies have shown that human milk confers protection against the development of atopic diseases. A study involving 3296 children from the Canadian Healthy Infant Longitudinal Development birth cohort reported that formula feeding and mixed feeding are associated with a higher risk of asthma by three years of age compared to exclusive breastfeeding during the first three months of life []. While the risk of developing childhood asthma was higher in infants delivered by caesarean section without medical indication, exclusive breastfeeding in the first six months of life may reduce this risk []. Elbert et al. found a slight increase in the risk of developing atopic dermatitis in children who were breastfed for a shorter duration and those who were nonexclusively breastfed []. A recent clinical report from the American Academy of Paediatrics concluded that exclusively breastfed infants during the first three to four months of life had a reduced risk of atopic dermatitis up to two years of age. In addition, breastfeeding for a more extended period, regardless of exclusivity, confers protection against asthma even beyond age five. There was no evidence that exclusive breastfeeding for more than three to four months offers any benefits in preventing atopic disorders []. There is conflicting evidence on the preventive effect of partially hydrolysed whey formula, as supplement or substitute to human milk, on atopic disorders in high-risk children [,,].
Evidence on the efficacy of prebiotic supplementation in atopic disorders prevention is inconsistent. Arslanoglu et al. revealed that extensively hydrolysed whey formula supplemented with short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides (9:1; 8 g/L) significantly decreased the risk of any atopic manifestations and atopic dermatitis up to five years of age []. Wopereis et al. demonstrated that partially hydrolysed formula supplemented with neutral short-chain galacto-oligosaccharides, long-chain fructo-oligosaccharides (9:1; 0.68 g/100mL) and pectin-derived acidic oligosaccharide (0.12 g/100mL) brought the gut microbiome of formula-fed infants closer to that of breast-fed infants []. However, Boyle et al. reported that adding a specific prebiotic mixture to partially hydrolysed whey formula was ineffective in preventing atopic dermatitis in high-risk children in the first year of life [].
The use of probiotic supplementation to prevent infants’ allergies remains controversial. Rautava et al. evaluated the effect of maternal probiotic supplementation with Lactobacillus rhamnosus LPR + Bifidobacterium longum BL999 or Lactobacillus paracasei ST11 + Bifidobacterium longum BL999 during the last two months of pregnancy and the subsequent two months during breastfeeding. The study reported that the risk of infants developing atopic dermatitis in the first two years of life was significantly decreased []. Wickens et al. showed that Lactobacillus rhamnosus HN001 protected infants against atopic dermatitis if administered to mothers from 35 weeks to 6 months postpartum if breastfeeding and to the infants during their first two years of life []. Conversely, Allen et al. found that probiotics (Lactobacillus salivarius CUL61, Lactobacillus paracasei CUL08, Bifidobacterium animalis subspecies lactis CUL34, and Bifidobacterium bifidum CUL20) given to mothers from 36 weeks of pregnancy until delivery and to infants until 6 months of age did not prevent atopic dermatitis in the first two years of life []. Using different probiotic strains, Loo et al. and Cabana et al. evaluated the effect of infant probiotic supplementation during the first six months of life. They found that early probiotic supplementation did not prevent atopic diseases at two and five years of age [,]. In short, it is still uncertain whether prebiotics or probiotics supplementation helps prevent atopic diseases owing to the heterogeneity between studies, particularly concerning the types of prebiotics or probiotics used and the duration of supplementation.
6. Conclusions
The infant gut microbiota is complex and can be modulated by many endogenous and exogenous factors (Figure 1). The feeding mode during infancy has been demonstrated to be one of the most important factors in determining the development and establishment of gut microbial composition. Human milk is an excellent source of infant nutrition and has been shown to provide many health benefits; thus, breastfeeding should be encouraged. However, in some circumstances, mothers may not be able to breastfeed or decide to formula feed their babies. Therefore, continuous advances and innovations in infant formula development are important to produce the best alternative for infants who are not breastfed.
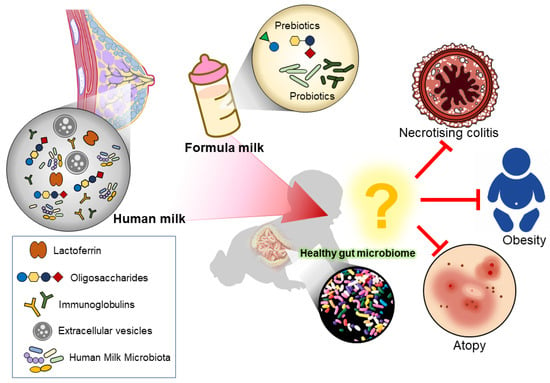
Figure 1.
Illustration of how the different feeding mode modulates the infant gut microbiome. Human milk naturally contains lactoferrin, oligosaccharides, immunoglobulins, extracellular vesicles, and human milk microbiota, which aids in modulating a healthy infant gut. While formula milk often has additional supplements added to mimic human milk.
Studies have suggested that supplementing prebiotics or probiotics in infant formula brings the gut microbiome of formula-fed infants closer to that of breastfed infants. However, its clinical efficacy in preventing diseases, such as necrotising enterocolitis and atopic disorders, should be further explored and studied. The contradictory results concerning their effectiveness might be attributable to different oligosaccharides or probiotic strains in various studies. Therefore, more evidence is needed to establish its clinical efficacy and determine the best prebiotic or probiotic strains, their doses, and duration of supplementation.
Author Contributions
H.-Y.C. performed the literature search, critical data analysis and writing of this manuscript; L.T.-H.T., J.W.-F.L., K.-W.H., V.R., N.-S.A.M., L.-H.L. and V.L. provided vital technical support, proofreading, and comprehensive editing; V.L. and L.-H.L. provided support in resources and supervision; V.L. and L.-H.L. founded this writing project. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the Jeffrey Cheah School of Medicine and Health Sciences Strategic Grant 2022 (Vote Number: STG-000108) awarded to J.W.-F.L., External Industry Grant from Biomerge Sdn Bhd (Vote no. BMRG2018-01) awarded to L.-H.L., and Jeffrey Cheah School of Medicine and Health Sciences Early Career Researcher Grant 2021 (Vote Number: ECR-000021) awarded to V.L.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Shajahan Yasin, Head of School, School Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Healy, D.B.; Ryan, C.A.; Ross, R.P.; Stanton, C.; Dempsey, E.M. Clinical implications of preterm infant gut microbiome development. Nat. Microbiol. 2022, 7, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Thye, A.Y.-K.; Law, J.W.-F.; Tan, L.T.-H.; Thurairajasingam, S.; Chan, K.-G.; Letchumanan, V.; Lee, L.-H. Exploring the Gut Microbiome in Myasthenia Gravis. Nutrients 2022, 14, 1647. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.W.Y.; Tan, L.T.-H.; Ab Mutalib, N.-S.; Wong, S.H.; Letchumanan, V.; Lee, L.-H. The chemistry of gut microbiome in health and diseases. Prog. Microbes Mol. Biol. 2021, 4, 1–40. [Google Scholar] [CrossRef]
- Shen, X.; Wang, M.; Zhang, X.; He, M.; Li, M.; Cheng, G.; Wan, C.; He, F. Dynamic construction of gut microbiota may influence allergic diseases of infants in Southwest China. BMC Microbiol. 2019, 19, 123. [Google Scholar] [CrossRef] [PubMed]
- Cerdó, T.; Ruiz, A.; Campoy, C. Human gut microbiota and obesity during development. In Adiposity—Omics and Molecular Understanding; InTechOpen: London, UK, 2017; pp. 265–285. [Google Scholar]
- Uusitalo, U.; Liu, X.; Yang, J.; Aronsson, C.A.; Hummel, S.; Butterworth, M.; Lernmark, Å.; Rewers, M.; Hagopian, W.; She, J.-X. Association of early exposure of probiotics and islet autoimmunity in the TEDDY study. JAMA Pediatrics 2016, 170, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, E.; Fernández, L.; Marín, M.L.; Martín, R.; Odriozola, J.M.; Nueno-Palop, C.; Narbad, A.; Olivares, M.; Xaus, J.; Rodríguez, J.M. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr. Microbiol. 2005, 51, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Rautava, S.; Aakko, J.; Isolauri, E.; Salminen, S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep. 2016, 6, 23129. [Google Scholar] [CrossRef] [PubMed]
- Gosalbes, M.; Llop, S.; Valles, Y.; Moya, A.; Ballester, F.; Francino, M. Meconium microbiota types dominated by lactic acid or enteric bacteria are differentially associated with maternal eczema and respiratory problems in infants. Clin. Exp. Allergy 2013, 43, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Perez-Muñoz, M.E.; Arrieta, M.-C.; Ramer-Tait, A.E.; Walter, J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: Implications for research on the pioneer infant microbiome. Microbiome 2017, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Ihekweazu, F.D.; Versalovic, J. Development of the pediatric gut microbiome: Impact on health and disease. Am. J. Med. Sci. 2018, 356, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Crossman, D.K.; Morrow, C.D. Strain tracking to identify individualized patterns of microbial strain stability in the developing infant gut ecosystem. Front. Pediatrics 2020, 8, 549844. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ryan, C.A.; Boyaval, P.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Maternal vertical transmission affecting early-life microbiota development. Trends Microbiol. 2020, 28, 28–45. [Google Scholar] [CrossRef]
- Van Daele, E.; Knol, J.; Belzer, C. Microbial transmission from mother to child: Improving infant intestinal microbiota development by identifying the obstacles. Crit. Rev. Microbiol. 2019, 45, 613–648. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, T.; Yahagi, K.; Mori, H.; Matsumoto, H.; Hara, T.; Tajima, S.; Ogawa, E.; Kodama, H.; Yamamoto, K.; Yamada, T. A key genetic factor for fucosyllactose utilization affects infant gut microbiota development. Nat. Commun. 2016, 7, 11939. [Google Scholar] [CrossRef]
- Akay, H.K.; Bahar Tokman, H.; Hatipoglu, N.; Hatipoglu, H.; Siraneci, R.; Demirci, M.; Borsa, B.A.; Yuksel, P.; Karakullukcu, A.; Kangaba, A.A.; et al. The relationship between bifidobacteria and allergic asthma and/or allergic dermatitis: A prospective study of 0–3 years-old children in Turkey. Anaerobe 2014, 28, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Dogra, S.; Sakwinska, O.; Soh, S.-E.; Ngom-Bru, C.; Brück, W.M.; Berger, B.; Brüssow, H.; Lee, Y.S.; Yap, F.; Chong, Y.-S. Dynamics of infant gut microbiota are influenced by delivery mode and gestational duration and are associated with subsequent adiposity. MBio 2015, 6, e02419-14. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Bergström, A.; Skov, T.H.; Bahl, M.I.; Roager, H.M.; Christensen, L.B.; Ejlerskov, K.T.; Mølgaard, C.; Michaelsen, K.F.; Licht, T.R. Establishment of intestinal microbiota during early life: A longitudinal, explorative study of a large cohort of Danish infants. Appl. Environ. Microbiol. 2014, 80, 2889–2900. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- World Health Organization. Exclusive Breastfeeding for Six Months Best for Babies Everywhere. Available online: https://www.who.int/news/item/15-01-2011-exclusive-breastfeeding-for-six-months-best-for-babies-everywhere (accessed on 18 April 2022).
- Moubareck, C.A. Human milk microbiota and oligosaccharides: A glimpse into benefits, diversity, and correlations. Nutrients 2021, 13, 1123. [Google Scholar] [CrossRef] [PubMed]
- Section on Breastfeeding; Eidelman, A.I.; Schanler, R.J.; Johnston, M.; Landers, S.; Noble, L.; Szucs, K.; Viehmann, L. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar]
- Fernández, L.; Langa, S.; Martín, V.; Maldonado, A.; Jiménez, E.; Martín, R.; Rodríguez, J.M. The human milk microbiota: Origin and potential roles in health and disease. Pharmacol. Res. 2013, 69, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatric Clin. 2013, 60, 49–74. [Google Scholar]
- Le Doare, K.; Holder, B.; Bassett, A.; Pannaraj, P.S. Mother’s milk: A purposeful contribution to the development of the infant microbiota and immunity. Front. Immunol. 2018, 9, 361. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Shamir, R. Standards for infant formula milk. BMJ 2006, 332, 621–622. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, P.; Pasolli, E.; Tett, A.; Asnicar, F.; Gorfer, V.; Fedi, S.; Armanini, F.; Truong, D.T.; Manara, S.; Zolfo, M. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe 2018, 24, 133–145.e135. [Google Scholar] [CrossRef] [PubMed]
- Reyman, M.; van Houten, M.A.; van Baarle, D.; Bosch, A.A.; Man, W.H.; Chu, M.L.J.; Arp, K.; Watson, R.L.; Sanders, E.A.; Fuentes, S. Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat. Commun. 2019, 10, 4997. [Google Scholar] [CrossRef] [PubMed]
- Hesla, H.M.; Stenius, F.; Jäderlund, L.; Nelson, R.; Engstrand, L.; Alm, J.; Dicksved, J. Impact of lifestyle on the gut microbiota of healthy infants and their mothers–the ALADDIN birth cohort. FEMS Microbiol. Ecol. 2014, 90, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Forster, S.C.; Tsaliki, E.; Vervier, K.; Strang, A.; Simpson, N.; Kumar, N.; Stares, M.D.; Rodger, A.; Brocklehurst, P. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 2019, 574, 117–121. [Google Scholar] [CrossRef]
- Hill, C.J.; Lynch, D.B.; Murphy, K.; Ulaszewska, M.; Jeffery, I.B.; O’Shea, C.A.; Watkins, C.; Dempsey, E.; Mattivi, F.; Tuohy, K. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome 2017, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Wampach, L.; Heintz-Buschart, A.; Fritz, J.V.; Ramiro-Garcia, J.; Habier, J.; Herold, M.; Narayanasamy, S.; Kaysen, A.; Hogan, A.H.; Bindl, L. Birth mode is associated with earliest strain-conferred gut microbiome functions and immunostimulatory potential. Nat. Commun. 2018, 9, 5091. [Google Scholar] [CrossRef] [PubMed]
- Makino, H.; Kushiro, A.; Ishikawa, E.; Kubota, H.; Gawad, A.; Sakai, T.; Oishi, K.; Martin, R.; Ben-Amor, K.; Knol, J. Mother-to-infant transmission of intestinal bifidobacterial strains has an impact on the early development of vaginally delivered infant’s microbiota. PLoS ONE 2013, 8, e78331. [Google Scholar] [CrossRef] [PubMed]
- Arboleya, S.; Binetti, A.; Salazar, N.; Fernández, N.; Solís, G.; Hernandez-Barranco, A.; Margolles, A.; de Los Reyes-Gavilán, C.G.; Gueimonde, M. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol. Ecol. 2012, 79, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Korpela, K.; Blakstad, E.W.; Moltu, S.J.; Strømmen, K.; Nakstad, B.; Rønnestad, A.E.; Brække, K.; Iversen, P.O.; Drevon, C.A.; de Vos, W. Intestinal microbiota development and gestational age in preterm neonates. Sci. Rep. 2018, 8, 2453. [Google Scholar] [CrossRef] [PubMed]
- Eck, A.; Rutten, N.B.; Singendonk, M.M.; Rijkers, G.T.; Savelkoul, P.H.; Meijssen, C.B.; Crijns, C.E.; Oudshoorn, J.H.; Budding, A.E.; Vlieger, A.M. Neonatal microbiota development and the effect of early life antibiotics are determined by two distinct settler types. PLoS ONE 2020, 15, e0228133. [Google Scholar] [CrossRef]
- Yassour, M.; Vatanen, T.; Siljander, H.; Hämäläinen, A.-M.; Härkönen, T.; Ryhänen, S.J.; Franzosa, E.A.; Vlamakis, H.; Huttenhower, C.; Gevers, D. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci. Transl. Med. 2016, 8, 343ra381. [Google Scholar] [CrossRef] [PubMed]
- Tapiainen, T.; Koivusaari, P.; Brinkac, L.; Lorenzi, H.A.; Salo, J.; Renko, M.; Pruikkonen, H.; Pokka, T.; Li, W.; Nelson, K. Impact of intrapartum and postnatal antibiotics on the gut microbiome and emergence of antimicrobial resistance in infants. Sci. Rep. 2019, 9, 10635. [Google Scholar] [CrossRef]
- Aloisio, I.; Mazzola, G.; Corvaglia, L.T.; Tonti, G.; Faldella, G.; Biavati, B.; Di Gioia, D. Influence of intrapartum antibiotic prophylaxis against group B Streptococcus on the early newborn gut composition and evaluation of the anti-Streptococcus activity of Bifidobacterium strains. Appl. Microbiol. Biotechnol. 2014, 98, 6051–6060. [Google Scholar] [CrossRef] [PubMed]
- Corvaglia, L.; Tonti, G.; Martini, S.; Aceti, A.; Mazzola, G.; Aloisio, I.; Di Gioia, D.; Faldella, G. Influence of intrapartum antibiotic prophylaxis for group B streptococcus on gut microbiota in the first month of life. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, S.; Song, Y.; Feng, Y.; Lv, N.; Xue, Y.; Liu, F.; Wang, S.; Zhu, B.; Ma, J.; et al. The Perturbation of Infant Gut Microbiota Caused by Cesarean Delivery Is Partially Restored by Exclusive Breastfeeding. Front. Microbiol. 2019, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Judge, M.; Xu, W.; Diallo, A.; Janton, S.; Brownell, E.A.; Maas, K.; Graf, J. Influence of infant feeding type on gut microbiome development in hospitalized preterm infants. Nurs. Res. 2017, 66, 123. [Google Scholar] [CrossRef] [PubMed]
- Adlerberth, I.; Wold, A. Establishment of the gut microbiota in Western infants. Acta Paediatr. 2009, 98, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishna, K.P.; Hand, T.W. Influence of maternal milk on the neonatal intestinal microbiome. Nutrients 2020, 12, 823. [Google Scholar] [CrossRef]
- Guo, M. Human Milk Biochemistry and Infant Formula Manufacturing Technology, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2020; p. 422. [Google Scholar]
- Gnoth, M.J.; Kunz, C.; Kinne-Saffran, E.; Rudloff, S. Human milk oligosaccharides are minimally digested in vitro. J. Nutr. 2000, 130, 3014–3020. [Google Scholar] [CrossRef]
- Walsh, C.; Lane, J.A.; van Sinderen, D.; Hickey, R.M. Human milk oligosaccharides: Shaping the infant gut microbiota and supporting health. J. Funct. Foods 2020, 72, 104074. [Google Scholar] [CrossRef]
- Czosnykowska-Łukacka, M.; Królak-Olejnik, B.; Orczyk-Pawiłowicz, M. Breast milk macronutrient components in prolonged lactation. Nutrients 2018, 10, 1893. [Google Scholar] [CrossRef]
- Thakore, V.; Jain, N.K. Protein and fat examination from the raw milk of different mammalian species (Cow, buffalo, goat, and human) with successive lactation days. Pharma Innov. 2018, 7, 506–510. [Google Scholar]
- Kim, S.Y.; Yi, D.Y. Components of human breast milk: From macronutrient to microbiome and microRNA. Clin. Exp. Pediatrics 2020, 63, 301. [Google Scholar] [CrossRef]
- Dror, D.K.; Allen, L.H. Overview of nutrients in human milk. Adv. Nutr. 2018, 9, 278S–294S. [Google Scholar] [CrossRef]
- Paulaviciene, I.J.; Liubsys, A.; Molyte, A.; Eidukaite, A.; Usonis, V. Circadian changes in the composition of human milk macronutrients depending on pregnancy duration: A cross-sectional study. Int. Breastfeed. J. 2020, 15, 49. [Google Scholar] [CrossRef]
- Saarela, T.; Kokkonen, J.; Koivisto, M. Macronutrient and energy contents of human milk fractions during the first six months of lactation. Acta Paediatr. 2005, 94, 1176–1181. [Google Scholar] [CrossRef]
- Khan, S.; Hepworth, A.R.; Prime, D.K.; Lai, C.T.; Trengove, N.J.; Hartmann, P.E. Variation in fat, lactose, and protein composition in breast milk over 24 hours: Associations with infant feeding patterns. J. Hum. Lact. 2013, 29, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Castellote, C.; Casillas, R.; Ramírez-Santana, C.; Pérez-Cano, F.J.; Castell, M.; Moretones, M.G.; López-Sabater, M.C.; Franch, À. Premature delivery influences the immunological composition of colostrum and transitional and mature human milk. J. Nutr. 2011, 141, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Gu, F.; Ye, W.; Ren, Y.; Guo, S. Colostral and mature breast milk protein compositional determinants in Qingdao, Wuhan and Hohhot: Maternal food culture, vaginal delivery and neonatal gender. Asia Pac. J. Clin. Nutr. 2019, 28, 800. [Google Scholar]
- Moltó-Puigmartí, C.; Castellote, A.I.; Carbonell-Estrany, X.; López-Sabater, M.C. Differences in fat content and fatty acid proportions among colostrum, transitional, and mature milk from women delivering very preterm, preterm, and term infants. Clin. Nutr. 2011, 30, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Sever, O.; Mandel, D.; Mimouni, F.B.; Marom, R.; Cohen, S.; Lubetzky, R. Macronutrients in human milk: Colostrum lactose but not fat or protein predicts mature human milk content. ICAN Infant Child Adolesc. Nutr. 2015, 7, 162–165. [Google Scholar] [CrossRef]
- Butte, N.F.; Lopez-Alarcon, M.G.; Garza, C. Nutrient Adequacy of Exclusive Breastfeeding for the Term Infant during the First Six Months of Life; World Health Organization: Geneva, Switzerland, 2002; p. 47. [Google Scholar]
- Yang, T.; Zhang, L.; Bao, W.; Rong, S. Nutritional composition of breast milk in Chinese women: A systematic review. Asia Pac. J. Clin. Nutr. 2018, 27, 491–502. [Google Scholar]
- Bauer, J.; Gerss, J. Longitudinal analysis of macronutrients and minerals in human milk produced by mothers of preterm infants. Clin. Nutr. 2011, 30, 215–220. [Google Scholar] [CrossRef]
- Caldeo, V.; Downey, E.; O’Shea, C.-A.; Affolter, M.; Volger, S.; Courtet-Compondu, M.-C.; De Castros, C.A.; O’Mahony, J.A.; Ryan, C.A.; Kelly, A.L. Protein levels and protease activity in milk from mothers of pre-term infants: A prospective longitudinal study on human milk macronutrient composition. Clin. Nutr. 2020, 40, 3567–3577. [Google Scholar] [CrossRef] [PubMed]
- Mills, L.; Coulter, L.; Savage, E.; Modi, N. Macronutrient content of donor milk from a regional human milk bank: Variation with donor mother–infant characteristics. Br. J. Nutr. 2019, 122, 1155–1167. [Google Scholar] [CrossRef] [PubMed]
- Maly, J.; Burianova, I.; Vitkova, V.; Ticha, E.; Navratilova, M.; Cermakova, E. Preterm human milk macronutrient concentration is independent of gestational age at birth. Arch. Dis. Child.-Fetal Neonatal Ed. 2019, 104, F50–F56. [Google Scholar] [CrossRef] [PubMed]
- Prentice, P.; Ong, K.K.; Schoemaker, M.H.; van Tol, E.A.; Vervoort, J.; Hughes, I.A.; Acerini, C.L.; Dunger, D.B. Breast milk nutrient content and infancy growth. Acta Paediatr. 2016, 105, 641–647. [Google Scholar] [CrossRef]
- Austin, S.; De Castro, C.A.; Bénet, T.; Hou, Y.; Sun, H.; Thakkar, S.K.; Vinyes-Pares, G.; Zhang, Y.; Wang, P. Temporal change of the content of 10 oligosaccharides in the milk of Chinese urban mothers. Nutrients 2016, 8, 346. [Google Scholar] [CrossRef]
- Thurl, S.; Munzert, M.; Henker, J.; Boehm, G.; Müller-Werner, B.; Jelinek, J.; Stahl, B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br. J. Nutr. 2010, 104, 1261–1271. [Google Scholar] [CrossRef]
- Elwakiel, M.; Hageman, J.; Wang, W.; Szeto, I.; Van Goudoever, J.; Hettinga, K.; Schols, H. Human milk oligosaccharides in colostrum and mature milk of Chinese mothers: Lewis positive secretor subgroups. J. Agric. Food Chem. 2018, 66, 7036–7043. [Google Scholar] [CrossRef]
- Sprenger, N.; Lee, L.Y.; De Castro, C.A.; Steenhout, P.; Thakkar, S.K. Longitudinal change of selected human milk oligosaccharides and association to infants’ growth, an observatory, single center, longitudinal cohort study. PLoS ONE 2017, 12, e0171814. [Google Scholar] [CrossRef]
- Gabrielli, O.; Zampini, L.; Galeazzi, T.; Padella, L.; Santoro, L.; Peila, C.; Giuliani, F.; Bertino, E.; Fabris, C.; Coppa, G.V. Preterm milk oligosaccharides during the first month of lactation. Pediatrics 2011, 128, e1520–e1531. [Google Scholar] [CrossRef]
- Seferovic, M.D.; Mohammad, M.; Pace, R.M.; Engevik, M.; Versalovic, J.; Bode, L.; Haymond, M.; Aagaard, K.M. Maternal diet alters human milk oligosaccharide composition with implications for the milk metagenome. Sci. Rep. 2020, 10, 22092. [Google Scholar] [CrossRef] [PubMed]
- De Leoz, M.L.A.; Kalanetra, K.M.; Bokulich, N.A.; Strum, J.S.; Underwood, M.A.; German, J.B.; Mills, D.A.; Lebrilla, C.B. Human milk glycomics and gut microbial genomics in infant feces show a correlation between human milk oligosaccharides and gut microbiota: A proof-of-concept study. J. Proteome Res. 2015, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Marcobal, A.; Barboza, M.; Froehlich, J.W.; Block, D.E.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Consumption of human milk oligosaccharides by gut-related microbes. J. Agric. Food Chem. 2010, 58, 5334–5340. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-T.; Chen, C.; Newburg, D.S. Utilization of major fucosylated and sialylated human milk oligosaccharides by isolated human gut microbes. Glycobiology 2013, 23, 1281–1292. [Google Scholar] [CrossRef]
- Rubio-del-Campo, A.; Alcántara, C.; Collado, M.C.; Rodríguez-Díaz, J.; Yebra, M.J. Human milk and mucosa-associated disaccharides impact on cultured infant fecal microbiota. Sci. Rep. 2020, 10, 11845. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Chichlowski, M.; Guillaume De Lartigue, J.; Raybould, H.E.; Mills, D.A. Bifidobacteria isolated from infants and cultured on human milk oligosaccharides affect intestinal epithelial function. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 321. [Google Scholar] [CrossRef]
- Wickramasinghe, S.; Pacheco, A.R.; Lemay, D.G.; Mills, D.A. Bifidobacteria grown on human milk oligosaccharides downregulate the expression of inflammation-related genes in Caco-2 cells. BMC Microbiol. 2015, 15, 172. [Google Scholar] [CrossRef]
- Kavanaugh, D.W.; O’Callaghan, J.; Butto, L.F.; Slattery, H.; Lane, J.; Clyne, M.; Kane, M.; Joshi, L.; Hickey, R.M. Exposure of Bifidobacterium longum subsp. infantis to milk oligosaccharides increases adhesion to epithelial cells and induces a substantial transcriptional response. PLoS ONE 2013, 8, e67224. [Google Scholar] [CrossRef]
- Musilova, S.; Modrackova, N.; Doskocil, I.; Svejstil, R.; Rada, V. Influence of human milk oligosaccharides on adherence of bifidobacteria and clostridia to cell lines. Acta Microbiol. Immunol. Hung. AMicr 2017, 64, 415. [Google Scholar] [CrossRef][Green Version]
- Weichert, S.; Jennewein, S.; Hüfner, E.; Weiss, C.; Borkowski, J.; Putze, J.; Schroten, H. Bioengineered 2′-fucosyllactose and 3-fucosyllactose inhibit the adhesion of Pseudomonas aeruginosa and enteric pathogens to human intestinal and respiratory cell lines. Nutr. Res. 2013, 33, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Weichert, S.; Koromyslova, A.; Singh, B.K.; Hansman, S.; Jennewein, S.; Schroten, H.; Hansman, G.S. Structural basis for norovirus inhibition by human milk oligosaccharides. J. Virol. 2016, 90, 4843–4848. [Google Scholar] [CrossRef] [PubMed]
- Facinelli, B.; Marini, E.; Magi, G.; Zampini, L.; Santoro, L.; Catassi, C.; Monachesi, C.; Gabrielli, O.; Coppa, G.V. Breast milk oligosaccharides: Effects of 2′-fucosyllactose and 6′-sialyllactose on the adhesion of Escherichia coli and Salmonella fyris to Caco-2 cells. J. Matern.-Fetal Neonatal Med. 2019, 32, 2950–2952. [Google Scholar] [CrossRef] [PubMed]
- Groves, M.L. The isolation of a red protein from Milk2. J. Am. Chem. Soc. 1960, 82, 3345–3350. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, R.; Chen, Q.; Wang, J.; Duan, Y.; Pang, X.; Jiang, S.; Bi, Y.; Zhang, H.; Lönnerdal, B. Concentration of lactoferrin in human milk and its variation during lactation in different Chinese populations. Nutrients 2018, 10, 1235. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodenas, C.L.; De Castro, C.A.; Jenni, R.; Thakkar, S.K.; Beauport, L.; Tolsa, J.-F.; Fischer-Fumeaux, C.J.; Affolter, M. Temporal changes of major protein concentrations in preterm and term human milk. A prospective cohort study. Clin. Nutr. 2019, 38, 1844–1852. [Google Scholar] [CrossRef] [PubMed]
- Trend, S.; Strunk, T.; Lloyd, M.L.; Kok, C.H.; Metcalfe, J.; Geddes, D.T.; Lai, C.T.; Richmond, P.; Doherty, D.A.; Simmer, K. Levels of innate immune factors in preterm and term mothers’ breast milk during the 1st month postpartum. Br. J. Nutr. 2016, 115, 1178–1193. [Google Scholar] [CrossRef] [PubMed]
- Mastromarino, P.; Capobianco, D.; Campagna, G.; Laforgia, N.; Drimaco, P.; Dileone, A.; Baldassarre, M.E. Correlation between lactoferrin and beneficial microbiota in breast milk and infant’s feces. Biometals 2014, 27, 1077–1086. [Google Scholar] [CrossRef]
- Woodman, T.; Strunk, T.; Patole, S.; Hartmann, B.; Simmer, K.; Currie, A. Effects of lactoferrin on neonatal pathogens and Bifidobacterium breve in human breast milk. PLoS ONE 2018, 13, e0201819. [Google Scholar] [CrossRef]
- Jahani, S.; Shakiba, A.; Jahani, L. The Antimicrobial effect of lactoferrin on Gram-negative and Gram-positive bacteria. Int. J. Infect. 2015, 2, e27954. [Google Scholar] [CrossRef]
- Tian, H.; Maddox, I.S.; Ferguson, L.R.; Shu, Q. Influence of bovine lactoferrin on selected probiotic bacteria and intestinal pathogens. Biometals 2010, 23, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Oram, J.; Reiter, B. Inhibition of bacteria by lactoferrin and other iron-chelating agents. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1968, 170, 351–365. [Google Scholar] [CrossRef]
- Brandenburg, K.; Jürgens, G.; Müller, M.; Fukuoka, S.; Koch, M.H. Biophysical characterization of lipopolysaccharide and lipid A inactivation by lactoferrin. Biol. Chem. 2001, 382, 1215–1225. [Google Scholar] [CrossRef]
- Garbe, J.; Sjögren, J.; Cosgrave, E.F.; Struwe, W.B.; Bober, M.; Olin, A.I.; Rudd, P.M.; Collin, M. EndoE from Enterococcus faecalis hydrolyzes the glycans of the biofilm inhibiting protein lactoferrin and mediates growth. PLoS ONE 2014, 9, e91035. [Google Scholar] [CrossRef] [PubMed]
- Angulo-Zamudio, U.A.; Vidal, J.E.; Nazmi, K.; Bolscher, J.G.; Leon-Sicairos, C.; Antezana, B.S.; Canizalez-Roman, A.; León-Sicairos, N. Lactoferrin disaggregates pneumococcal biofilms and inhibits acquisition of resistance through its DNase activity. Front. Microbiol. 2019, 10, 2386. [Google Scholar] [CrossRef]
- Wambach, K.; Spencer, B. Breastfeeding and Human Lactation, 6th ed.; Jones & Bartlett Learning: Burlington, MA, USA, 2019; p. 820. [Google Scholar]
- Czosnykowska-Łukacka, M.; Lis-Kuberka, J.; Królak-Olejnik, B.; Orczyk-Pawiłowicz, M. Changes in human milk immunoglobulin profile during prolonged lactation. Front. Pediatrics 2020, 8, 428. [Google Scholar] [CrossRef] [PubMed]
- Goonatilleke, E.; Huang, J.; Xu, G.; Wu, L.; Smilowitz, J.T.; German, J.B.; Lebrilla, C.B. Human milk proteins and their glycosylation exhibit quantitative dynamic variations during lactation. J. Nutr. 2019, 149, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Berdi, M.; de Lauzon-Guillain, B.; Forhan, A.; Castelli, F.A.; Fenaille, F.; Charles, M.A.; Heude, B.; Junot, C.; Adel-Patient, K.; EDEN Mother-Child Cohort Study Group. Immune components of early breastmilk: Association with maternal factors and with reported food allergy in childhood. Pediatr. Allergy Immunol. 2019, 30, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Abuidhail, J.; Al-Shudiefat, A.A.R.; Darwish, M. Alterations of immunoglobulin G and immunoglobulin M levels in the breast milk of mothers with exclusive breastfeeding compared to mothers with non-exclusive breastfeeding during 6 months postpartum: The Jordanian cohort study. Am. J. Hum. Biol. 2019, 31, e23197. [Google Scholar] [CrossRef] [PubMed]
- Perrin, M.T.; Fogleman, A.D.; Newburg, D.S.; Allen, J.C. A longitudinal study of human milk composition in the second year postpartum: Implications for human milk banking. Matern. Child Nutr. 2017, 13, e12239. [Google Scholar] [CrossRef] [PubMed]
- Mathias, A.; Duc, M.; Favre, L.; Benyacoub, J.; Blum, S.; Corthésy, B. Potentiation of polarized intestinal Caco-2 cell responsiveness to probiotics complexed with secretory IgA. J. Biol. Chem. 2010, 285, 33906–33913. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.P.; Ladinsky, M.S.; Yu, K.B.; Sanders, J.G.; Yoo, B.; Chou, W.-C.; Conner, M.; Earl, A.; Knight, R.; Bjorkman, P. Gut microbiota utilize immunoglobulin A for mucosal colonization. Science 2018, 360, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Rogier, E.W.; Frantz, A.L.; Bruno, M.E.; Wedlund, L.; Cohen, D.A.; Stromberg, A.J.; Kaetzel, C.S. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc. Natl. Acad. Sci. USA 2014, 111, 3074–3079. [Google Scholar] [CrossRef]
- Van Bergenhenegouwen, J.; Kraneveld, A.D.; Rutten, L.; Kettelarij, N.; Garssen, J.; Vos, A.P. Extracellular vesicles modulate host-microbe responses by altering TLR2 activity and phagocytosis. PLoS ONE 2014, 9, e89121. [Google Scholar]
- Liao, Y.; Du, X.; Li, J.; Lönnerdal, B. Human milk exosomes and their microRNAs survive digestion in vitro and are taken up by human intestinal cells. Mol. Nutr. Food Res. 2017, 61, 1700082. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Fahner, C.J.; Reid, G.E.; Simpson, R.J. ExoCarta 2012: Database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012, 40, D1241–D1244. [Google Scholar] [CrossRef] [PubMed]
- Lässer, C.; Alikhani, V.S.; Ekström, K.; Eldh, M.; Paredes, P.T.; Bossios, A.; Sjöstrand, M.; Gabrielsson, S.; Lötvall, J.; Valadi, H. Human saliva, plasma and breast milk exosomes contain RNA: Uptake by macrophages. J. Transl. Med. 2011, 9, 9. [Google Scholar] [CrossRef]
- van Herwijnen, M.J.; Zonneveld, M.I.; Goerdayal, S.; Nolte, E.N.; Garssen, J.; Stahl, B.; Altelaar, A.M.; Redegeld, F.A.; Wauben, M.H. Comprehensive proteomic analysis of human milk-derived extracellular vesicles unveils a novel functional proteome distinct from other milk components. Mol. Cell. Proteom. 2016, 15, 3412–3423. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; How Huang, K.; Jen Lee, M.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, N.; Izumi, H.; Sekine, K.; Ochiya, T. microRNA as a new immune-regulatory agent in breast milk. Silence 2010, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Li, M.; Wang, X.; Li, Q.; Wang, T.; Zhu, Q.; Zhou, X.; Wang, X.; Gao, X.; Li, X. Immune-related microRNAs are abundant in breast milk exosomes. Int. J. Biol. Sci. 2012, 8, 118. [Google Scholar] [CrossRef]
- Alsaweed, M.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Human milk miRNAs primarily originate from the mammary gland resulting in unique miRNA profiles of fractionated milk. Sci. Rep. 2016, 6, 20680. [Google Scholar] [CrossRef] [PubMed]
- Pisano, C.; Galley, J.; Elbahrawy, M.; Wang, Y.; Farrell, A.; Brigstock, D.; Besner, G.E. Human breast milk-derived extracellular vesicles in the protection against experimental necrotizing enterocolitis. J. Pediatr. Surg. 2020, 55, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, X.; Zhang, L.; Cai, J.; Zhou, Y.; Liu, H.; Hu, Y.; Chen, W.; Xu, S.; Liu, P. Identification and peptidomic profiling of exosomes in preterm human milk: Insights into necrotizing enterocolitis prevention. Mol. Nutr. Food Res. 2019, 63, 1801247. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Patel, M.; Williams, S.; Arora, H.; Sims, B. Human breast milk-derived exosomes attenuate cell death in intestinal epithelial cells. Innate Immun. 2018, 24, 278–284. [Google Scholar] [CrossRef]
- Ward, T.L.; Hosid, S.; Ioshikhes, I.; Altosaar, I. Human milk metagenome: A functional capacity analysis. BMC Microbiol. 2013, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Yi, D.Y. Analysis of the human breast milk microbiome and bacterial extracellular vesicles in healthy mothers. Exp. Mol. Med. 2020, 52, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Rubio, R.; Mira-Pascual, L.; Mira, A.; Collado, M. Impact of mode of delivery on the milk microbiota composition of healthy women. J. Dev. Orig. Health Dis. 2016, 7, 54–60. [Google Scholar] [CrossRef]
- Jost, T.; Lacroix, C.; Braegger, C.; Chassard, C. Assessment of bacterial diversity in breast milk using culture-dependent and culture-independent approaches. Br. J. Nutr. 2013, 110, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, E.; de Andrés, J.; Manrique, M.; Pareja-Tobes, P.; Tobes, R.; Martínez-Blanch, J.F.; Codoñer, F.M.; Ramón, D.; Fernández, L.; Rodríguez, J.M. Metagenomic analysis of milk of healthy and mastitis-suffering women. J. Hum. Lact. 2015, 31, 406–415. [Google Scholar] [CrossRef]
- Hunt, K.M.; Foster, J.A.; Forney, L.J.; Schütte, U.M.; Beck, D.L.; Abdo, Z.; Fox, L.K.; Williams, J.E.; McGuire, M.K.; McGuire, M.A. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS ONE 2011, 6, e21313. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.E.; Carrothers, J.M.; Lackey, K.A.; Beatty, N.F.; York, M.A.; Brooker, S.L.; Shafii, B.; Price, W.J.; Settles, M.L.; McGuire, M.A. Human milk microbial community structure is relatively stable and related to variations in macronutrient and micronutrient intakes in healthy lactating women. J. Nutr. 2017, 147, 1739–1748. [Google Scholar] [PubMed]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatrics 2017, 171, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Hermansson, H.; Kumar, H.; Collado, M.C.; Salminen, S.; Isolauri, E.; Rautava, S. Breast milk microbiota is shaped by mode of delivery and intrapartum antibiotic exposure. Front. Nutr. 2019, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Khodayar-Pardo, P.; Mira-Pascual, L.; Collado, M.; Martínez-Costa, C. Impact of lactation stage, gestational age and mode of delivery on breast milk microbiota. J. Perinatol. 2014, 34, 599–605. [Google Scholar] [CrossRef]
- Urbaniak, C.; Angelini, M.; Gloor, G.B.; Reid, G. Human milk microbiota profiles in relation to birthing method, gestation and infant gender. Microbiome 2016, 4, 1. [Google Scholar] [CrossRef]
- Moossavi, S.; Sepehri, S.; Robertson, B.; Bode, L.; Goruk, S.; Field, C.J.; Lix, L.M.; de Souza, R.J.; Becker, A.B.; Mandhane, P.J. Composition and variation of the human milk microbiota are influenced by maternal and early-life factors. Cell Host Microbe 2019, 25, 324–335.e324. [Google Scholar] [CrossRef]
- Gonzalez, E.; Brereton, N.J.; Li, C.; Lopez Leyva, L.; Solomons, N.W.; Agellon, L.B.; Scott, M.E.; Koski, K.G. Distinct Changes Occur in the Human Breast Milk Microbiome Between Early and Established Lactation in Breastfeeding Guatemalan Mothers. Front. Microbiol. 2021, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Jiang, J.; Lu, M.; Tong, W.; Zhou, R.; Li, J.; Yuan, J.; Wang, F.; Li, D. Human milk microbiota development during lactation and its relation to maternal geographic location and gestational hypertensive status. Gut Microbes 2020, 11, 1438–1449. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Curley, D.; O’Callaghan, T.F.; O’Shea, C.-A.; Dempsey, E.M.; O’Toole, P.W.; Ross, R.P.; Ryan, C.A.; Stanton, C. The composition of human milk and infant faecal microbiota over the first three months of life: A pilot study. Sci. Rep. 2017, 7, 40597. [Google Scholar] [CrossRef]
- Cabrera-Rubio, R.; Collado, M.C.; Laitinen, K.; Salminen, S.; Isolauri, E.; Mira, A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am. J. Clin. Nutr. 2012, 96, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Rossen, L.M.; Simon, A.E.; Herrick, K.A. Types of infant formulas consumed in the United States. Clin. Pediatr. 2016, 55, 278–285. [Google Scholar] [CrossRef]
- Park, Y.W.; Haenlein, G.F. Milk and Dairy Products in Human Nutrition: Production, Composition and Health; John Wiley & Sons: Chichester, UK, 2013; p. 728. [Google Scholar]
- Aldredge, D.L.; Geronimo, M.R.; Hua, S.; Nwosu, C.C.; Lebrilla, C.B.; Barile, D. Annotation and structural elucidation of bovine milk oligosaccharides and determination of novel fucosylated structures. Glycobiology 2013, 23, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Fong, B.; Ma, K.; McJarrow, P. Quantification of bovine milk oligosaccharides using liquid chromatography–selected reaction monitoring–mass spectrometry. J. Agric. Food Chem. 2011, 59, 9788–9795. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Brozek, J.; Schünemann, H.; Bahna, S.L.; Von Berg, A.; Beyer, K.; Bozzola, M.; Bradsher, J.; Compalati, E.; Ebisawa, M. World Allergy Organization (WAO) diagnosis and rationale for action against cow’s milk allergy (DRACMA) guidelines. World Allergy Organ. J. 2010, 3, 57–161. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; Dutoit, G.; Eigenmann, P. EAACI food allergy and anaphylaxis guidelines: Diagnosis and management of food allergy. Allergy 2014, 69, 1008–1025. [Google Scholar] [CrossRef]
- Koletzko, S.; Niggemann, B.; Arató, A.; Dias, J.; Heuschkel, R.; Husby, S.; Mearin, M.; Papadopoulou, A.; Ruemmele, F.; Staiano, A. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 221–229. [Google Scholar] [CrossRef]
- Andres, A.; Cleves, M.A.; Bellando, J.B.; Pivik, R.; Casey, P.H.; Badger, T.M. Developmental status of 1-year-old infants fed breast milk, cow’s milk formula, or soy formula. Pediatrics 2012, 129, 1134–1140. [Google Scholar] [CrossRef]
- Borewicz, K.; Suarez-Diez, M.; Hechler, C.; Beijers, R.; de Weerth, C.; Arts, I.; Penders, J.; Thijs, C.; Nauta, A.; Lindner, C. The effect of prebiotic fortified infant formulas on microbiota composition and dynamics in early life. Sci. Rep. 2019, 9, 2434. [Google Scholar] [CrossRef]
- Scalabrin, D.M.; Mitmesser, S.H.; Welling, G.W.; Harris, C.L.; Marunycz, J.D.; Walker, D.C.; Bos, N.A.; Tölkkö, S.; Salminen, S.; Vanderhoof, J.A. New prebiotic blend of polydextrose and galacto-oligosaccharides has a bifidogenic effect in young infants. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 343–352. [Google Scholar] [CrossRef]
- Ashley, C.; Johnston, W.H.; Harris, C.L.; Stolz, S.I.; Wampler, J.L.; Berseth, C.L. Growth and tolerance of infants fed formula supplemented with polydextrose (PDX) and/or galactooligosaccharides (GOS): Double-blind, randomized, controlled trial. Nutr. J. 2012, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.; Choe, Y.; Price, P.; Katz, G.; Suarez, F.; Paule, C.; Mackey, A. Tolerance of formulas containing prebiotics in healthy, term infants. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Piemontese, P.; Giannì, M.L.; Braegger, C.P.; Chirico, G.; Grüber, C.; Riedler, J.; Arslanoglu, S.; van Stuijvenberg, M.; Boehm, G.; Jelinek, J. Tolerance and safety evaluation in a large cohort of healthy infants fed an innovative prebiotic formula: A randomized controlled trial. PLoS ONE 2011, 6, e28010. [Google Scholar]
- Puccio, G.; Alliet, P.; Cajozzo, C.; Janssens, E.; Corsello, G.; Sprenger, N.; Wernimont, S.; Egli, D.; Gosoniu, L.; Steenhout, P. Effects of infant formula with human milk oligosaccharides on growth and morbidity: A randomized multicenter trial. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 624. [Google Scholar] [CrossRef] [PubMed]
- Steenhout, P.; Sperisen, P.; Martin, F.P.; Sprenger, N.; Wernimont, S.; Pecquet, S.; Berger, B. Term Infant Formula Supplemented with Human Milk Oligosaccharides (2′ Fucosyllactose and Lacto-N-neotetraose) Shifts Stool Microbiota and Metabolic Signatures Closer to that of Breastfed Infants. FASEB J. 2016, 30, 275.7. [Google Scholar]
- Holscher, H.D.; Faust, K.L.; Czerkies, L.A.; Litov, R.; Ziegler, E.E.; Lessin, H.; Hatch, T.; Sun, S.; Tappenden, K.A. Effects of prebiotic-containing infant formula on gastrointestinal tolerance and fecal microbiota in a randomized controlled trial. J. Parenter. Enter. Nutr. 2012, 36, 95S–105S. [Google Scholar] [CrossRef]
- Veereman-Wauters, G.; Staelens, S.; Van de Broek, H.; Plaskie, K.; Wesling, F.; Roger, L.; McCartney, A.; Assam, P. Physiological and bifidogenic effects of prebiotic supplements in infant formulae. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 763–771. [Google Scholar] [CrossRef]
- Castanet, M.; Costalos, C.; Haiden, N.; Hascoet, J.-M.; Berger, B.; Sprenger, N.; Grathwohl, D.; Brüssow, H.; De Groot, N.; Steenhout, P. Early effect of supplemented infant formulae on intestinal biomarkers and microbiota: A randomized clinical trial. Nutrients 2020, 12, 1481. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Gil-Campos, M.; López, M.Á.; Rodriguez-Benítez, M.V.; Romero, J.; Roncero, I.; Linares, M.D.; Maldonado, J.; López-Huertas, E.; Berwind, R.; Ritzenthaler, K.L. Lactobacillus fermentum CECT 5716 is safe and well tolerated in infants of 1–6 months of age: A randomized controlled trial. Pharmacol. Res. 2012, 65, 231–238. [Google Scholar] [CrossRef]
- Scalabrin, D.; Harris, C.; Johnston, W.; Berseth, C. Long-term safety assessment in children who received hydrolyzed protein formulas with Lactobacillus rhamnosus GG: A 5-year follow-up. Eur. J. Pediatr. 2017, 176, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Lobón, J.; Gil-Campos, M.; Maldonado, J.; López-Huertas, E.; Flores-Rojas, K.; Valero, A.; Rodríguez-Benítez, M.; Bañuelos, O.; Lara-Villoslada, F.; Fonollá, J. Long-term safety of early consumption of Lactobacillus fermentum CECT5716: A 3-year follow-up of a randomized controlled trial. Pharmacol. Res. 2015, 95, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Rautava, S.; Kalliomäki, M.; Isolauri, E. Probiotics during pregnancy and breast-feeding might confer immunomodulatory protection against atopic disease in the infant. J. Allergy Clin. Immunol. 2002, 109, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, J.; Cañabate, F.; Sempere, L.; Vela, F.; Sánchez, A.R.; Narbona, E.; López-Huertas, E.; Geerlings, A.; Valero, A.D.; Olivares, M. Human milk probiotic Lactobacillus fermentum CECT5716 reduces the incidence of gastrointestinal and upper respiratory tract infections in infants. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 55–61. [Google Scholar] [CrossRef]
- Chi, C.; Xue, Y.; Liu, R.; Wang, Y.; Lv, N.; Zeng, H.; Buys, N.; Zhu, B.; Sun, J.; Yin, C. Effects of a formula with a probiotic Bifidobacterium lactis supplement on the gut microbiota of low birth weight infants. Eur. J. Nutr. 2019, 59, 1493–1503. [Google Scholar] [CrossRef]
- Salminen, S.; Stahl, B.; Vinderola, G.; Szajewska, H. Infant formula supplemented with biotics: Current knowledge and future perspectives. Nutrients 2020, 12, 1952. [Google Scholar] [CrossRef]
- Radke, M.; Picaud, J.-C.; Loui, A.; Cambonie, G.; Faas, D.; Lafeber, H.N.; de Groot, N.; Pecquet, S.S.; Steenhout, P.G.; Hascoet, J.-M. Starter formula enriched in prebiotics and probiotics ensures normal growth of infants and promotes gut health: A randomized clinical trial. Pediatr. Res. 2017, 81, 622–631. [Google Scholar] [CrossRef]
- Azad, M.B.; Konya, T.; Maughan, H.; Guttman, D.S.; Field, C.J.; Chari, R.S.; Sears, M.R.; Becker, A.B.; Scott, J.A.; Kozyrskyj, A.L. Gut microbiota of healthy Canadian infants: Profiles by mode of delivery and infant diet at 4 months. CMAJ 2013, 185, 385–394. [Google Scholar] [CrossRef]
- Praveen, P.; Jordan, F.; Priami, C.; Morine, M.J. The role of breast-feeding in infant immune system: A systems perspective on the intestinal microbiome. Microbiome 2015, 3, 41. [Google Scholar] [CrossRef]
- Ma, J.; Li, Z.; Zhang, W.; Zhang, C.; Zhang, Y.; Mei, H.; Zhuo, N.; Wang, H.; Wang, L.; Wu, D. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: A study of 91 term infants. Sci. Rep. 2020, 10, 15792. [Google Scholar] [CrossRef]
- Stewart, C.J.; Ajami, N.J.; O’Brien, J.L.; Hutchinson, D.S.; Smith, D.P.; Wong, M.C.; Ross, M.C.; Lloyd, R.E.; Doddapaneni, H.; Metcalf, G.A. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 2018, 562, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Roy, N.C.; Guo, Y.; Jia, H.; Ryan, L.; Samuelsson, L.; Thomas, A.; Plowman, J.; Clerens, S.; Day, L.; et al. Human Breast Milk and Infant Formulas Differentially Modify the Intestinal Microbiota in Human Infants and Host Physiology in Rats. J. Nutr. 2015, 146, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Lim, J.Y.; Kim, B.-S.; Cho, S.J.; Kim, N.Y.; Kim, O.B.; Kim, Y. Comparison of the gut microbiota profile in breast-fed and formula-fed Korean infants using pyrosequencing. Nutr. Res. Pract. 2015, 9, 242. [Google Scholar] [CrossRef]
- Salli, K.; Anglenius, H.; Hirvonen, J.; Hibberd, A.A.; Ahonen, I.; Saarinen, M.T.; Tiihonen, K.; Maukonen, J.; Ouwehand, A.C. The effect of 2′-fucosyllactose on simulated infant gut microbiome and metabolites; a pilot study in comparison to GOS and lactose. Sci. Rep. 2019, 9, 13232. [Google Scholar] [CrossRef]
- Wang, M.; Li, M.; Wu, S.; Lebrilla, C.B.; Chapkin, R.S.; Ivanov, I.; Donovan, S.M. Fecal microbiota composition of breast-fed infants is correlated with human milk oligosaccharides consumed. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 825. [Google Scholar] [CrossRef]
- Hascoët, J.-M.; Hubert, C.; Rochat, F.; Legagneur, H.; Gaga, S.; Emady-Azar, S.; Steenhout, P.G. Effect of formula composition on the development of infant gut microbiota. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 756–762. [Google Scholar] [CrossRef]
- Hackam, D.J. Necrotizing Enterocolitis: Pathogenesis, Diagnosis and Treatment; CRC Press: Boca Raton, FL, USA, 2021; p. 302. [Google Scholar]
- Clark, R.H.; Gordon, P.; Walker, W.M.; Laughon, M.; Smith, P.B.; Spitzer, A.R. Characteristics of patients who die of necrotizing enterocolitis. J. Perinatol. 2012, 32, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Claud, E.C.; Keegan, K.P.; Brulc, J.M.; Lu, L.; Bartels, D.; Glass, E.; Chang, E.B.; Meyer, F.; Antonopoulos, D.A. Bacterial community structure and functional contributions to emergence of health or necrotizing enterocolitis in preterm infants. Microbiome 2013, 1, 20. [Google Scholar] [CrossRef]
- Zhou, Y.; Shan, G.; Sodergren, E.; Weinstock, G.; Walker, W.A.; Gregory, K.E. Longitudinal analysis of the premature infant intestinal microbiome prior to necrotizing enterocolitis: A case-control study. PLoS ONE 2015, 10, e0118632. [Google Scholar] [CrossRef]
- Mai, V.; Torrazza, R.M.; Ukhanova, M.; Wang, X.; Sun, Y.; Li, N.; Shuster, J.; Sharma, R.; Hudak, M.L.; Neu, J. Distortions in development of intestinal microbiota associated with late onset sepsis in preterm infants. PLoS ONE 2013, 8, e52876. [Google Scholar]
- Arboleya, S.; Sánchez, B.; Milani, C.; Duranti, S.; Solís, G.; Fernández, N.; de los Reyes-Gavilán, C.G.; Ventura, M.; Margolles, A.; Gueimonde, M. Intestinal Microbiota Development in Preterm Neonates and Effect of Perinatal Antibiotics. J. Pediatrics 2015, 166, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Ahlén, K.M.; Örtqvist, A.K.; Gong, T.; Wallas, A.; Ye, W.; Lundholm, C.; Almqvist, C. Antibiotic treatment and length of hospital stay in relation to delivery mode and prematurity. PLoS ONE 2016, 11, e0164126. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Xu, W.; Janton, S.; Henderson, W.A.; Matson, A.; McGrath, J.M.; Maas, K.; Graf, J. Gut microbiome developmental patterns in early life of preterm infants: Impacts of feeding and gender. PLoS ONE 2016, 11, e0152751. [Google Scholar] [CrossRef]
- Arboleya, S.; Sánchez, B.; Solís, G.; Fernández, N.; Suárez, M.; Hernández-Barranco, A.M.; Milani, C.; Margolles, A.; los Reyes-Gavilán, D.; Clara, G. Impact of prematurity and perinatal antibiotics on the developing intestinal microbiota: A functional inference study. Int. J. Mol. Sci. 2016, 17, 649. [Google Scholar] [CrossRef]
- McMurtry, V.E.; Gupta, R.W.; Tran, L.; Blanchard, E.E.; Penn, D.; Taylor, C.M.; Ferris, M.J. Bacterial diversity and Clostridia abundance decrease with increasing severity of necrotizing enterocolitis. Microbiome 2015, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Sisk, P.M.; Lambeth, T.M.; Rojas, M.A.; Lightbourne, T.; Barahona, M.; Anthony, E.; Auringer, S.T. Necrotizing enterocolitis and growth in preterm infants fed predominantly maternal milk, pasteurized donor milk, or preterm formula: A retrospective study. Am. J. Perinatol. 2017, 34, 676–683. [Google Scholar]
- Pourcyrous, M.; Nolan, V.; Goodwin, A.; Davis, S.; Buddington, R. Fecal short-chain fatty acids of very-low-birth-weight preterm infants fed expressed breast milk or formula. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 725–731. [Google Scholar] [CrossRef]
- Autran, C.A.; Kellman, B.P.; Kim, J.H.; Asztalos, E.; Blood, A.B.; Spence, E.C.H.; Patel, A.L.; Hou, J.; Lewis, N.E.; Bode, L. Human milk oligosaccharide composition predicts risk of necrotising enterocolitis in preterm infants. Gut 2018, 67, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.; Savva, G.M.; Clapuci, R.; Jones, J.; Maimouni, H.; Brown, E.; Minocha, A.; Hall, L.J.; Clarke, P. Incidence of necrotising enterocolitis before and after introducing routine prophylactic Lactobacillus and Bifidobacterium probiotics. Arch. Dis. Child.-Fetal Neonatal Ed. 2020, 105, 380–386. [Google Scholar] [CrossRef]
- Demirel, G.; Erdeve, O.; Celik, I.H.; Dilmen, U. Saccharomyces boulardii for prevention of necrotizing enterocolitis in preterm infants: A randomized, controlled study. Acta Paediatr. 2013, 102, e560–e565. [Google Scholar] [CrossRef] [PubMed]
- Saengtawesin, V.; Tangpolkaiwalsak, R.; Kanjanapattankul, W. Effect of oral probiotics supplementation in the prevention of necrotizing enterocolitis among very low birth weight preterm infants. J. Med. Assoc. Thai. 2014, 97, S20–S25. [Google Scholar]
- Scheepers, L.; Penders, J.; Mbakwa, C.; Thijs, C.; Mommers, M.; Arts, I. The intestinal microbiota composition and weight development in children: The KOALA Birth Cohort Study. Int. J. Obes. 2015, 39, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Stanislawski, M.A.; Dabelea, D.; Wagner, B.D.; Iszatt, N.; Dahl, C.; Sontag, M.K.; Knight, R.; Lozupone, C.A.; Eggesbø, M. Gut microbiota in the first 2 years of life and the association with body mass index at age 12 in a Norwegian birth cohort. MBio 2018, 9, e01751-18. [Google Scholar] [CrossRef] [PubMed]
- Penders, J.; Gerhold, K.; Stobberingh, E.E.; Thijs, C.; Zimmermann, K.; Lau, S.; Hamelmann, E. Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. J. Allergy Clin. Immunol. 2013, 132, 601–607.e608. [Google Scholar] [CrossRef] [PubMed]
- West, C.E.; Rydén, P.; Lundin, D.; Engstrand, L.; Tulic, M.K.; Prescott, S.L. Gut microbiome and innate immune response patterns in I g E-associated eczema. Clin. Exp. Allergy 2015, 45, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Elbert, N.; Van Meel, E.; Den Dekker, H.; de Jong, N.; Nijsten, T.; Jaddoe, V.; de Jongste, J.; Pasmans, S.; Duijts, L. Duration and exclusiveness of breastfeeding and risk of childhood atopic diseases. Allergy 2017, 72, 1936–1943. [Google Scholar] [CrossRef] [PubMed]
- Arslanoglu, S.; Moro, G.; Boehm, G.; Wienz, F.; Stahl, B.; Bertino, E. Early Neutral Prebiotic Oligosaccharide Supplentation reduces the incidence of some allergic manifestations in the first 5 years of life. J. Biol. Regul. Homeost. Agents 2012, 26, 49–59. [Google Scholar] [PubMed]
- Rautava, S.; Kainonen, E.; Salminen, S.; Isolauri, E. Maternal probiotic supplementation during pregnancy and breast-feeding reduces the risk of eczema in the infant. J. Allergy Clin. Immunol. 2012, 130, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Wickens, K.; Black, P.; Stanley, T.; Mitchell, E.; Barthow, C.; Fitzharris, P.; Purdie, G.; Crane, J. A protective effect of L actobacillus rhamnosus HN 001 against eczema in the first 2 years of life persists to age 4 years. Clin. Exp. Allergy 2012, 42, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Zamrik, S.; Giachero, F.; Heldmann, M.; Hensel, K.O.; Wirth, S.; Jenke, A.C. Impact of an in-house pediatric surgery unit and human milk centered enteral nutrition on necrotizing enterocolitis. BioMed Res. Int. 2018, 2018, 5042707. [Google Scholar] [CrossRef] [PubMed]
- Cristofalo, E.A.; Schanler, R.J.; Blanco, C.L.; Sullivan, S.; Trawoeger, R.; Kiechl-Kohlendorfer, U.; Dudell, G.; Rechtman, D.J.; Lee, M.L.; Lucas, A. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J. Pediatrics 2013, 163, 1592–1595.e1591. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Carroll, K. An exclusively human milk diet reduces necrotizing enterocolitis. Breastfeed. Med. 2014, 9, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Kimak, K.S.; de Castro Antunes, M.M.; Braga, T.D.; Brandt, K.G.; de Carvalho Lima, M. Influence of enteral nutrition on occurrences of necrotizing enterocolitis in very-low-birth-weight infants. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishna, K.P.; Macadangdang, B.R.; Rogers, M.B.; Tometich, J.T.; Firek, B.A.; Baker, R.; Ji, J.; Burr, A.H.; Ma, C.; Good, M. Maternal IgA protects against the development of necrotizing enterocolitis in preterm infants. Nat. Med. 2019, 25, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Good, M.; Sodhi, C.P.; Yamaguchi, Y.; Jia, H.; Lu, P.; Fulton, W.B.; Martin, L.Y.; Prindle, T.; Nino, D.F.; Zhou, Q. The human milk oligosaccharide 2′-fucosyllactose attenuates the severity of experimental necrotising enterocolitis by enhancing mesenteric perfusion in the neonatal intestine. Br. J. Nutr. 2016, 116, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Jantscher-Krenn, E.; Zherebtsov, M.; Nissan, C.; Goth, K.; Guner, Y.S.; Naidu, N.; Choudhury, B.; Grishin, A.V.; Ford, H.R.; Bode, L. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut 2012, 61, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Braga, T.D.; da Silva, G.A.P.; de Lira, P.I.C.; de Carvalho Lima, M. Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: A double-blind, randomized, controlled trial. Am. J. Clin. Nutr. 2011, 93, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, T.; Ali, M.M.; Hossain, M.M.; Singh, J.; Yousuf, A.; Yasmin, F.; Chowdhury, F.R. Efficacy of probiotics versus placebo in the prevention of necrotizing enterocolitis in preterm very low birth weight infants: A double-blind randomized controlled trial. J. Coll. Physicians Surg. Pak. 2016, 26, 770–774. [Google Scholar] [PubMed]
- Dang, S.; Shook, L.; Garlitz, K.; Hanna, M.; Desai, N. Nutritional outcomes with implementation of probiotics in preterm infants. J. Perinatol. 2015, 35, 447–450. [Google Scholar] [CrossRef] [PubMed]
- van den Akker, C.H.; van Goudoever, J.B.; Shamir, R.; Domellöf, M.; Embleton, N.D.; Hojsak, I.; Lapillonne, A.; Mihatsch, W.A.; Canani, R.B.; Bronsky, J. Probiotics and preterm infants: A position paper by the European Society for Paediatric Gastroenterology Hepatology and Nutrition Committee on nutrition and the European Society for Paediatric Gastroenterology Hepatology and Nutrition Working Group for probiotics and prebiotics. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 664–680. [Google Scholar] [PubMed]
- World Health Organization. Report of the Commission on Ending Childhood Obesity; World Health Organization: Geneva, Switzerland, 2016; p. 50. [Google Scholar]
- Korpela, K.; Zijlmans, M.; Kuitunen, M.; Kukkonen, K.; Savilahti, E.; Salonen, A.; De Weerth, C.; De Vos, W. Childhood BMI in relation to microbiota in infancy and lifetime antibiotic use. Microbiome 2017, 5, 26. [Google Scholar] [CrossRef]
- Luoto, R.; Kalliomäki, M.; Laitinen, K.; Delzenne, N.M.; Cani, P.D.; Salminen, S.; Isolauri, E. Initial dietary and microbiological environments deviate in normal-weight compared to overweight children at 10 years of age. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-W.; Xu, J.; Soh, S.E.; Aris, I.M.; Tint, M.-T.; Gluckman, P.D.; Tan, K.H.; Shek, L.P.-C.; Chong, Y.-S.; Yap, F. Implication of gut microbiota in the association between infant antibiotic exposure and childhood obesity and adiposity accumulation. Int. J. Obes. 2020, 44, 1508–1520. [Google Scholar] [CrossRef]
- Mueller, N.T.; Whyatt, R.; Hoepner, L.; Oberfield, S.; Dominguez-Bello, M.G.; Widen, E.; Hassoun, A.; Perera, F.; Rundle, A. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int. J. Obes. 2015, 39, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Blustein, J.; Attina, T.; Liu, M.; Ryan, A.M.; Cox, L.M.; Blaser, M.J.; Trasande, L. Association of caesarean delivery with child adiposity from age 6 weeks to 15 years. Int. J. Obes. 2013, 37, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Trasande, L.; Blustein, J.; Liu, M.; Corwin, E.; Cox, L.; Blaser, M. Infant antibiotic exposures and early-life body mass. Int. J. Obes. 2013, 37, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-K.; Chen, H.; Ferber, J.; Odouli, R. Infection and antibiotic use in infancy and risk of childhood obesity: A longitudinal birth cohort study. Lancet Diabetes Endocrinol. 2017, 5, 18–25. [Google Scholar] [CrossRef]
- Wang, L.; Collins, C.; Ratliff, M.; Xie, B.; Wang, Y. Breastfeeding reduces childhood obesity risks. Child. Obes. 2017, 13, 197–204. [Google Scholar] [CrossRef]
- Scott, J.A.; Ng, S.Y.; Cobiac, L. The relationship between breastfeeding and weight status in a national sample of Australian children and adolescents. BMC Public Health 2012, 12, 107. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, M.; Lee, J.; Kim, Y.J.; Ha, E.; Kim, H.S. The protective effect of exclusive breastfeeding on overweight/obesity in children with high birth weight. J. Korean Med. Sci. 2019, 34, e85. [Google Scholar] [CrossRef] [PubMed]
- McCrory, C.; Layte, R. Breastfeeding and risk of overweight and obesity at nine-years of age. Soc. Sci. Med. 2012, 75, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-L.; Ma, R.-M.; Huang, Y.-K.; Liang, K.; Ding, Z.-B. Effect of breastfeeding on childhood overweight in the offspring of mothers with gestational diabetes mellitus. Zhongguo Dang Dai Er Ke Za Zhi Chin. J. Contemp. Pediatrics 2013, 15, 56–61. [Google Scholar]
- Zheng, J.-S.; Liu, H.; Li, J.; Chen, Y.; Wei, C.; Shen, G.; Zhu, S.; Chen, H.; Zhao, Y.-M.; Huang, T. Exclusive breastfeeding is inversely associated with risk of childhood overweight in a large Chinese cohort. J. Nutr. 2014, 144, 1454–1459. [Google Scholar] [CrossRef]
- Novaes, J.F.; Lamounier, J.A.; Colosimo, E.A.; Franceschini, S.C.; Priore, S.E. Breastfeeding and obesity in Brazilian children. Eur. J. Public Health 2012, 22, 383–389. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martin, R.M.; Patel, R.; Kramer, M.S.; Guthrie, L.; Vilchuck, K.; Bogdanovich, N.; Sergeichick, N.; Gusina, N.; Foo, Y.; Palmer, T.; et al. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: A randomized trial. JAMA 2013, 309, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Grote, V.; Closa-Monasterolo, R.; Escribano, J.; Langhendries, J.-P.; Dain, E.; Giovannini, M.; Verduci, E.; Gruszfeld, D.; Socha, P. Lower protein content in infant formula reduces BMI and obesity risk at school age: Follow-up of a randomized trial. Am. J. Clin. Nutr. 2014, 99, 1041–1051. [Google Scholar] [CrossRef]
- Escribano, J.; Luque, V.; Ferre, N.; Mendez-Riera, G.; Koletzko, B.; Grote, V.; Demmelmair, H.; Bluck, L.; Wright, A.; Closa-Monasterolo, R. Effect of protein intake and weight gain velocity on body fat mass at 6 months of age: The EU Childhood Obesity Programme. Int. J. Obes. 2012, 36, 548–553. [Google Scholar] [CrossRef]
- Mennella, J.A.; Inamdar, L.; Pressman, N.; Schall, J.I.; Papas, M.A.; Schoeller, D.; Stallings, V.A.; Trabulsi, J.C. Type of infant formula increases early weight gain and impacts energy balance: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Salgin, B.; Norris, S.A.; Prentice, P.; Pettifor, J.M.; Richter, L.M.; Ong, K.K.; Dunger, D.B. Even transient rapid infancy weight gain is associated with higher BMI in young adults and earlier menarche. Int. J. Obes. 2015, 39, 939–944. [Google Scholar] [CrossRef]
- Vaillant, A.A.J.; Modi, P.; Jan, A. Atopy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Björkstén, B.; Engstrand, L.; Jenmalm, M.C. Low diversity of the gut microbiota in infants with atopic eczema. J. Allergy Clin. Immunol. 2012, 129, 434–440.e432. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, H.; Li, N.; Bonnelykke, K.; Chawes, B.L.K.; Skov, T.; Paludan-Müller, G.; Stokholm, J.; Smith, B.; Krogfelt, K.A. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J. Allergy Clin. Immunol. 2011, 128, 646–652.e645. [Google Scholar] [CrossRef]
- Abrahamsson, T.; Jakobsson, H.; Andersson, A.F.; Björkstén, B.; Engstrand, L.; Jenmalm, M. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin. Exp. Allergy 2014, 44, 842–850. [Google Scholar] [CrossRef]
- Ta, L.D.H.; Chan, J.C.Y.; Yap, G.C.; Purbojati, R.W.; Drautz-Moses, D.I.; Koh, Y.M.; Tay, C.J.X.; Huang, C.-H.; Kioh, D.Y.Q.; Woon, J.Y. A compromised developmental trajectory of the infant gut microbiome and metabolome in atopic eczema. Gut Microbes 2020, 12, 1801964. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.-C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef]
- Klopp, A.; Vehling, L.; Becker, A.B.; Subbarao, P.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; Daley, D.; Silverman, F. Modes of infant feeding and the risk of childhood asthma: A prospective birth cohort study. J. Pediatrics 2017, 190, 192–199.e192. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Chen, Q.; Chen, Y.; Bao, Y.; Wu, M.; Zhang, J. Cesarean section without medical indication and risk of childhood asthma, and attenuation by breastfeeding. PLoS ONE 2017, 12, e0184920. [Google Scholar] [CrossRef]
- Greer, F.R.; Sicherer, S.H.; Burks, A.W. The effects of early nutritional interventions on the development of atopic disease in infants and children: The role of maternal dietary restriction, breastfeeding, hydrolyzed formulas, and timing of introduction of allergenic complementary foods. Pediatrics 2019, 143, e20190281. [Google Scholar] [CrossRef] [PubMed]
- von Berg, A.; Filipiak-Pittroff, B.; Krämer, U.; Hoffmann, B.; Link, E.; Beckmann, C.; Hoffmann, U.; Reinhardt, D.; Grübl, A.; Heinrich, J. Allergies in high-risk schoolchildren after early intervention with cow’s milk protein hydrolysates: 10-year results from the German Infant Nutritional Intervention (GINI) study. J. Allergy Clin. Immunol. 2013, 131, 1565–1573.e1565. [Google Scholar] [CrossRef]
- Lowe, A.J.; Hosking, C.S.; Bennett, C.M.; Allen, K.J.; Axelrad, C.; Carlin, J.B.; Abramson, M.J.; Dharmage, S.C.; Hill, D.J. Effect of a partially hydrolyzed whey infant formula at weaning on risk of allergic disease in high-risk children: A randomized controlled trial. J. Allergy Clin. Immunol. 2011, 128, 360–365.e364. [Google Scholar] [CrossRef] [PubMed]
- Von Berg, A.; Filipiak-Pittroff, B.; Schulz, H.; Hoffmann, U.; Link, E.; Sußmann, M.; Schnappinger, M.; Brüske, I.; Standl, M.; Krämer, U. Allergic manifestation 15 years after early intervention with hydrolyzed formulas–the GINI Study. Allergy 2016, 71, 210–219. [Google Scholar] [CrossRef]
- Wopereis, H.; Sim, K.; Shaw, A.; Warner, J.O.; Knol, J.; Kroll, J.S. Intestinal microbiota in infants at high risk for allergy: Effects of prebiotics and role in eczema development. J. Allergy Clin. Immunol. 2018, 141, 1334–1342.e1335. [Google Scholar] [CrossRef] [PubMed]
- Boyle, R.; Tang, M.K.; Chiang, W.; Chua, M.; Ismail, I.; Nauta, A.; Hourihane, J.O.B.; Smith, P.; Gold, M.; Ziegler, J. Prebiotic-supplemented partially hydrolysed cow’s milk formula for the prevention of eczema in high-risk infants: A randomized controlled trial. Allergy 2016, 71, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Jordan, S.; Storey, M.; Thornton, C.A.; Gravenor, M.B.; Garaiova, I.; Plummer, S.F.; Wang, D.; Morgan, G. Probiotics in the prevention of eczema: A randomised controlled trial. Arch. Dis. Child. 2014, 99, 1014–1019. [Google Scholar] [CrossRef]
- Loo, E.X.; Llanora, G.V.; Lu, Q.; Aw, M.M.; Lee, B.W.; Shek, L.P. Supplementation with probiotics in the first 6 months of life did not protect against eczema and allergy in at-risk Asian infants: A 5-year follow-up. Int. Arch. Allergy Immunol. 2014, 163, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Cabana, M.D.; McKean, M.; Caughey, A.B.; Fong, L.; Lynch, S.; Wong, A.; Leong, R.; Boushey, H.A.; Hilton, J.F. Early probiotic supplementation for eczema and asthma prevention: A randomized controlled trial. Pediatrics 2017, 140, e20163000. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).