The Role of Food Insecurity and Dietary Diversity on Recovery from Wasting among Hospitalized Children Aged 6–23 Months in Sub-Saharan Africa and South Asia
Abstract
:1. Background
2. Methods
2.1. Study Design
2.2. Outcome
2.3. Exposures
2.4. Statistical Analysis
2.5. Ethics
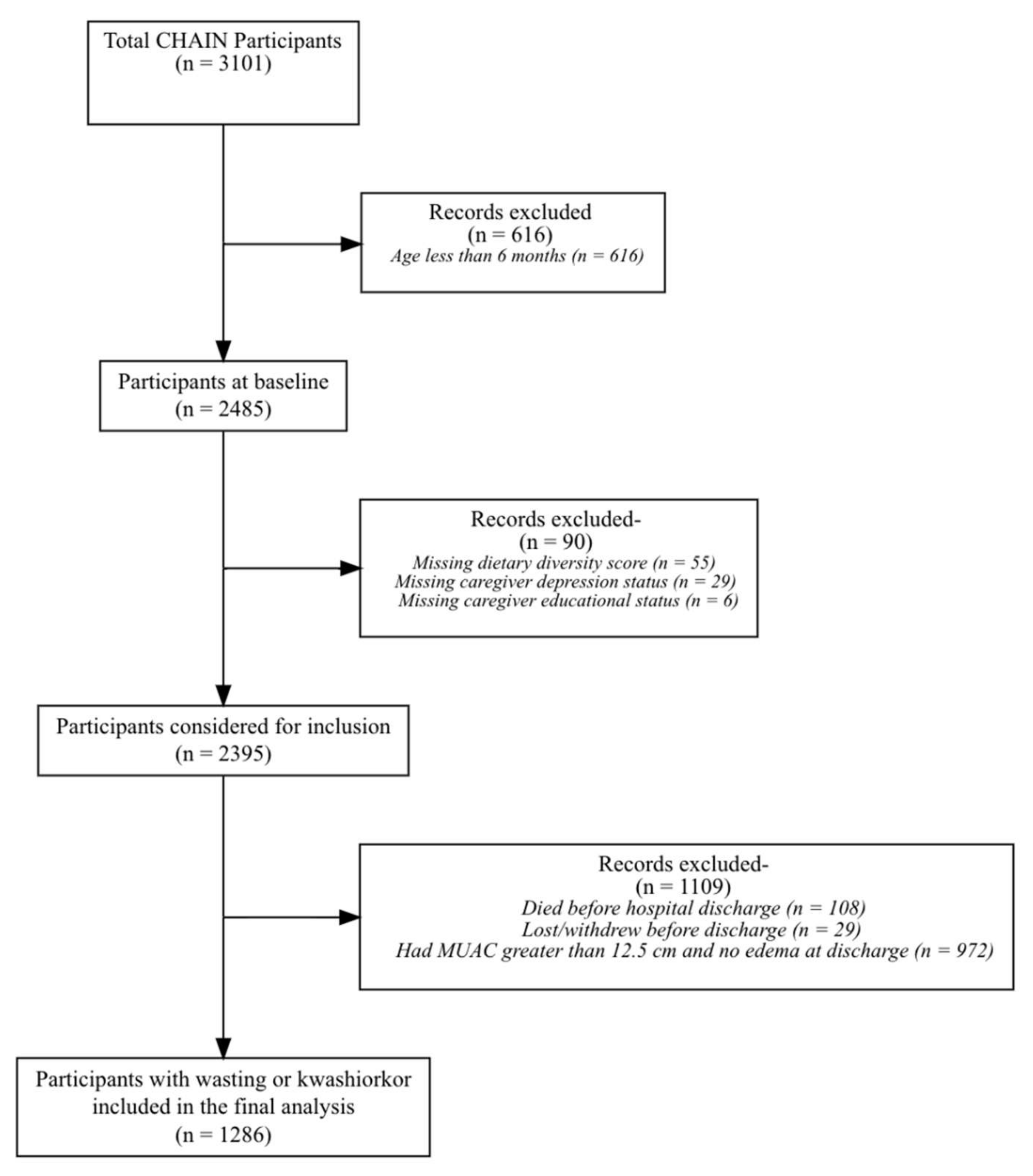
3. Results
3.1. Baseline Characteristics
3.2. Association of Food Insecurity and Dietary Diversity
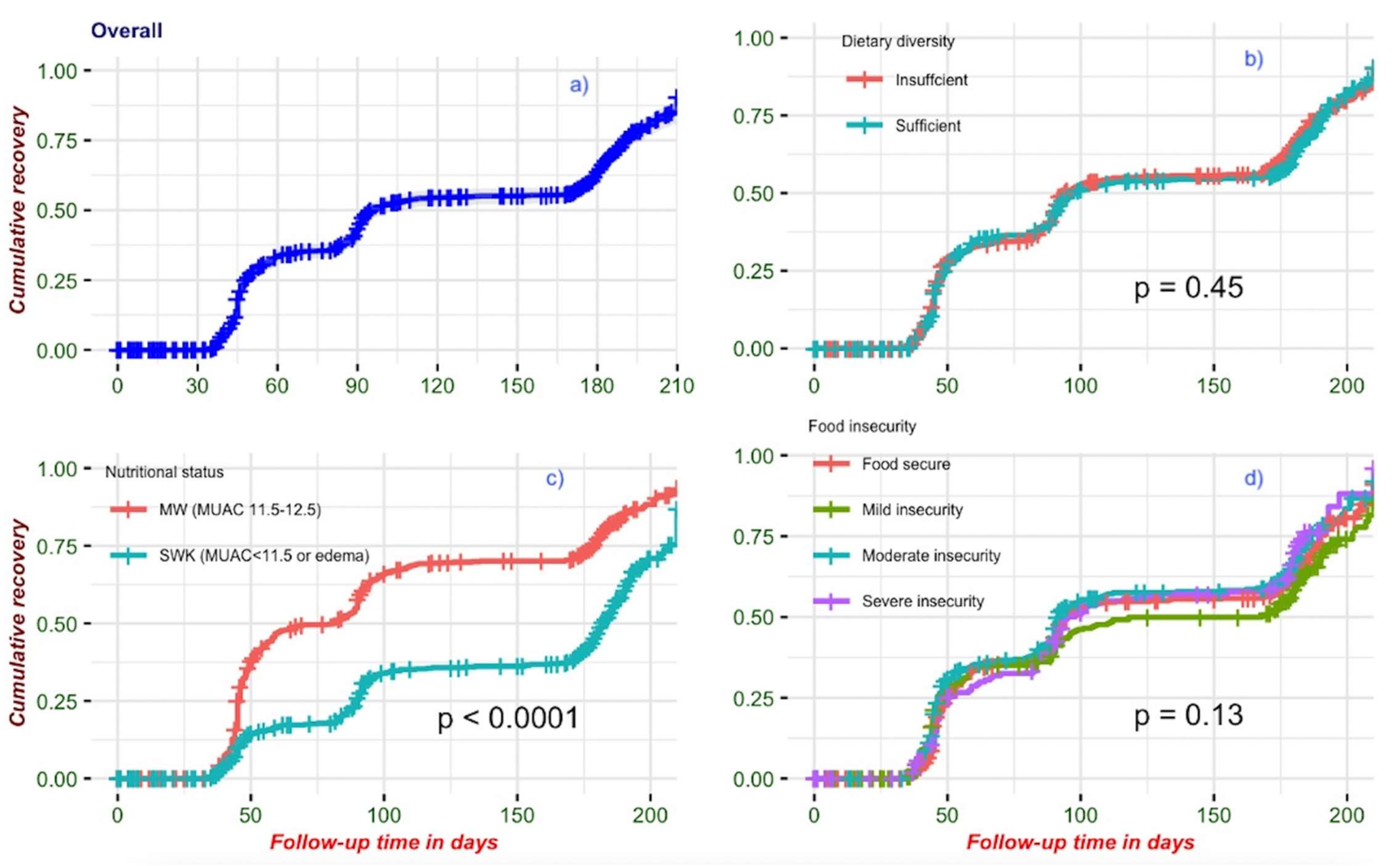
3.3. Recovery from Wasting
3.4. Association of Food Insecurity and Dietary Diversity with Recovery from Wasting
3.5. Association of Specific Food Groups with Recovery from Wasting
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chisti, M.J.; Graham, S.M.; Duke, T.; Ahmed, T.; Faruque, A.S.G.; Ashraf, H.; Bardhan, P.K.; Shahid, A.S.M.S.B.; Shahunja, K.M.; Salam, M.A. Post-Discharge Mortality in Children with Severe Malnutrition and Pneumonia in Bangladesh. PLoS ONE 2014, 9, e107663. [Google Scholar] [CrossRef] [PubMed]
- Desyibelew, H.D.; Bayih, M.T.; Baraki, A.G.; Dadi, A.F. The recovery rate from severe acute malnutrition among under-five years of children remains low in sub-Saharan Africa. A systematic review and meta-analysis of observational studies. PLoS ONE 2020, 15, e0229698. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, A.J.; Walson, J.L. Seeking interventions to reduce post-discharge mortality among children in sub-Saharan Africa. Lancet Glob. Health 2019, 7, e1306–e1307. [Google Scholar] [CrossRef]
- WHO. Guideline: Updates on the Management of Severe Acute Malnutrition in Infants and Children; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Patel, D.; Gupta, P.; Shah, D.; Sethi, K. Home-based rehabilitation of severely malnourished children in resource poor setting. Indian Pediatrics 2010, 47, 694–701. [Google Scholar] [CrossRef]
- Stobaugh, H.C.; Mayberry, A.; McGrath, M.; Bahwere, P.; Zagre, N.M.; Manary, M.J.; Black, R.; Lelijveld, N. Relapse after severe acute malnutrition: A systematic literature review and secondary data analysis. Matern. Child Nutr. 2019, 15, e12702. [Google Scholar] [CrossRef]
- David, S.M.; Ragasudha, P.N.; Taneja, S.; Mohan, S.B.; Iyengar, S.D.; Pricilla, R.A.; Martines, J.; Sachdev, H.S.; Suhalka, V.; Mohan, V.R.; et al. Predictors of recovery in children aged 6 to 59 months with uncomplicated Severe Acute Malnutrition: A multi-center study. Public Health Nutr. 2020, 24, 4899–4907. [Google Scholar] [CrossRef]
- Teshome, G.; Bosha, T.; Gebremedhin, S. Time-to-recovery from severe acute malnutrition in children 6–59 months of age enrolled in the outpatient treatment program in Shebedino, Southern Ethiopia: A prospective cohort study. BMC Pediatrics 2019, 19, 33. [Google Scholar] [CrossRef]
- FAO. The State of Food Security and Nutrition in the World 2020; FAO: Rome, Italy, 2020. [Google Scholar]
- Khan, A.; Khan, S.; Zia-ul-Islam, S.; Tauqeer, A.M.; Khan, M. Causes, sign and symptoms of malnutrition among the children. J. Nutr. Hum. Health 2017, 1. [Google Scholar] [CrossRef]
- Black, M.M.; Lutter, C.K.; Trude, A.C.B. All children surviving and thriving: Re-envisioning UNICEF’s conceptual framework of malnutrition. Lancet Glob. Health 2020, 8, e766–e767. [Google Scholar] [CrossRef]
- Noriega, K.E.; Lindshield, B.L. Is the inclusion of animal source foods in fortified blended foods justified? Nutrients 2014, 6, 3516–3535. [Google Scholar] [CrossRef] [Green Version]
- Scherbaum, V.; Purwestri, R.C.; Stuetz, W.; Inayati, D.A.; Suryantan, J.; Bloem, M.A.; Biesalski, H.K. Locally produced cereal/nut/legume-based biscuits versus peanut/milk-based spread for treatment of moderately to mildly wasted children in daily programmes on Nias Island, Indonesia: An issue of acceptance and compliance? Asia Pac. J. Clin. Nutr. 2015, 24, 152–161. [Google Scholar] [PubMed]
- Madzorera, I.; Duggan, C.; Berthé, F.; Grais, R.F.; Isanaka, S. The role of dietary diversity in the response to treatment of uncomplicated severe acute malnutrition among children in Niger: A prospective study. BMC Nutr. 2018, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Darapheak, C.; Takano, T.; Kizuki, M.; Nakamura, K.; Seino, K. Consumption of animal source foods and dietary diversity reduce stunting in children in Cambodia. Int. Arch. Med. 2013, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- James, P.; Sadler, K.; Wondafrash, M.; Argaw, A.; Luo, H.; Geleta, B.; Kedir, K.; Getnet, Y.; Belachew, T.; Bahwere, P. Children with Moderate Acute Malnutrition with No Access to Supplementary Feeding Programmes Experience High Rates of Deterioration and No Improvement: Results from a Prospective Cohort Study in Rural Ethiopia. PLoS ONE 2016, 11, e0153530. [Google Scholar] [CrossRef] [PubMed]
- Abitew, D.B.; Yalew, A.W.; Bezabih, A.M.; Bazzano, A.N. Predictors of relapse of acute malnutrition following exit from community-based management program in Amhara region, Northwest Ethiopia: An unmatched case-control study. PLoS ONE 2020, 15, e0231524. [Google Scholar] [CrossRef]
- Stobaugh, H.C.; Rogers, B.L.; Webb, P.; Rosenberg, I.H.; Thakwalakwa, C.; Maleta, K.M.; Trehan, I.; Manary, M.J. Household-level factors associated with relapse following discharge from treatment for moderate acute malnutrition. Br. J. Nutr. 2018, 119, 1039–1046. [Google Scholar] [CrossRef]
- Diallo, A.H.; Bin Shahid, A.S.M.S.; Khan, A.F.; Saleem, A.F.; Singa, B.O.; Gnoumou, B.S.; Tigoi, C.; Otieno, C.A.; Bourdon, C.; Oduol, C.O.; et al. Childhood mortality during and after acute illness in Africa and south Asia: A prospective cohort study. Lancet Glob. Health 2022, 10, e673–e684. Available online: https://linkinghub.elsevier.com/retrieve/pii/S2214109X22001188 (accessed on 27 April 2022). [CrossRef]
- Childhood, T.; Illness, A.; Network, N. Childhood Acute Illness and Nutrition (CHAIN) Network: A protocol for a multi-site prospective cohort study to identify modifiable risk factors for mortality among acutely ill children in Africa and Asia. BMJ Open 2019, 9, e028454. [Google Scholar]
- CHAIN CRF. Available online: https://chainnetwork.org/wp-content/uploads/2022/01/CHAIN-Enrollment-CRF_-2nd-July-2018-v1.64.pdf (accessed on 26 March 2021).
- Wambogo, E.A.; Ghattas, H.; Leonard, K.L.; Sahyoun, N.R. Validity of the Food Insecurity Experience Scale for Use in Sub-Saharan Africa and Characteristics of Food-Insecure Individuals. Curr. Dev. Nutr. 2018, 2, nzy062. [Google Scholar] [CrossRef]
- Global Nutrition Monitoring Framework. Operational Guidance for Tracking Progress in Meeting Targets for 2025; WHO: Geneva, Switzerland, 2017; p. 77. Available online: http://apps.who.int/iris/bitstream/handle/10665/259904/9789241513609-eng.pdf;jsessionid=82B08433379C3E3E69B3F8D4F2690C34?sequence=1%0Awww.who.int/nutrition (accessed on 30 March 2021).
- Rutstein, S.O.; Johnson, K. The DHS Wealth Index. In DHS Comparative Reports; DHS: Calverton, MD, USA, 2004. [Google Scholar]
- Kohlmann, K.; Callaghan-Gillespie, M.; Gauglitz, J.M.; Steiner-Asiedu, M.; Saalia, K.; Edwards, C.; Manary, M.J. Alternative Ready-To-Use Therapeutic Food Yields Less Recovery Than the Standard for Treating Acute Malnutrition in Children from Ghana. Available online: www.ghspjournal.org (accessed on 3 February 2022).
- Hendrixson, D.T.; Godbout, C.; Los, A.; Callaghan-Gillespie, M.; Mui, M.; Wegner, D.; Bryant, T.; Koroma, A.; Manary, M.J. Treatment of severe acute malnutrition with oat or standard ready-to-use therapeutic food: A triple-blind, randomised controlled clinical trial. Gut 2020, 69, 2143–2149. [Google Scholar] [CrossRef]
- Headey, D.; Hirvonen, K.; Hoddinott, J. Animal sourced foods and child stunting. Am. J. Agric. Econ. 2018, 100, 1302–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, C.E.; Whaley, S.E.; Crespi, C.M.; Wang, M.C.; Chaparro, M.P. Every month matters: Longitudinal associations between exclusive breastfeeding duration, child growth and obesity among WIC-participating children. J. Epidemiol. Community Health 2020, 74, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, T.; Kumar, S.; Sinha, S.; Haque, M.A.; Singh, V.; Sinha, S. Benefits of Breastfeeding for Early Growth and Long Term Obesity: A Summarized Review Acute Care Rehabilitation bed View project Case Report View project. Int. J. Med. Sci. Diagn. Res. (IJMSDR) 2019, 3, 190–194. [Google Scholar]
- Prell, C.; Koletzko, B. Stillen und Beikost: Empfehlungen für die Säuglingsernährung. Dtsch. Arztebl. Int. 2016, 113, 435–444. [Google Scholar] [PubMed]
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.; Mis, N.F.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A.; et al. Complementary Feeding: A Position Paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 119–132. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; Franca, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Mwangome, M.; Murunga, S.; Kahindi, J.; Gwiyo, P.; Mwasho, G.; Talbert, A.; Kiige, L.; Samburu, B.; Mturi, N.; Abubakar, A.; et al. Individualized breastfeeding support for acutely ill, malnourished infants under 6 months old. Matern. Child Nutr. 2020, 16, e12868. [Google Scholar] [CrossRef]
- Deroover, K.; Bucher, T.; Vandelanotte, C.; De Vries, H.; Duncan, M.J. Practical Nutrition Knowledge Mediates the Relationship Between Sociodemographic Characteristics and Diet Quality in Adults: A Cross-Sectional Analysis. Am. J. Health Promot. 2020, 34, 59–62. [Google Scholar] [CrossRef]
- Fadare, O.; Amare, M.; Mavrotas, G.; Akerele, D.; Ogunniyi, A. Mother’s nutrition-related knowledge and child nutrition outcomes: Empirical evidence from Nigeria. PLoS ONE 2019, 14, e0212775. [Google Scholar]




| Characteristic | MW a n = 6991 | SKW b n = 5871 | Overall n = 1286 |
|---|---|---|---|
| Age | |||
| 6–11 months | 330 (47%) | 297 (51%) | 627 (49%) |
| 12–17 months | 250 (36%) | 186 (32%) | 436 (34%) |
| 18–23 months | 119 (17%) | 104 (18%) | 223 (17%) |
| Sex | |||
| Female | 318 (45%) | 286 (49%) | 604 (47%) |
| Male | 381 (55%) | 301 (51%) | 682 (53%) |
| MUACc | 12.01 (0.32) | 10.60 (0.89) | 11.37 (0.95) |
| Food security status | |||
| Food-secure | 264 (38%) | 196 (33%) | 460 (36%) |
| Mild food insecurity | 180 (26%) | 127 (22%) | 307 (24%) |
| Moderate food insecurity | 180 (26%) | 169 (29%) | 349 (27%) |
| Severe food insecurity | 75 (11%) | 95 (16%) | 170 (13%) |
| Sufficient Dietary diversity | 394 (56%) | 228 (39%) | 622 (48%) |
| Kwashiorkor at admission | 66 (9.4%) | 133 (23%) | 199 (15%) |
| Kwashiorkor at discharge | 0 (0%) | 37 (6.3%) | 37 (2.9%) |
| Stunting * | |||
| Severe stunting | 185 (26%) | 316 (54%) | 501 (39%) |
| Moderate stunting | 215 (31%) | 156 (27%) | 371 (29%) |
| No stunting | 299 (43%) | 112 (19%) | 411 (32%) |
| Unknown | 0 | 3 | 3 |
| Breastfeeding | |||
| Exclusive | 472 (68%) | 308 (52%) | 780 (61%) |
| No | 200 (29%) | 258 (44%) | 458 (36%) |
| Partial | 27 (3.9%) | 21 (3.6%) | 48 (3.7%) |
| HIV status | |||
| Negative | 676 (97%) | 543 (93%) | 1219 (95%) |
| Positive | 23 (3.3%) | 44 (7.5%) | 67 (5.2%) |
| Primary caregiver educational status | |||
| None | 196 (28%) | 180 (31%) | 376 (29%) |
| Primary | 284 (41%) | 260 (44%) | 544 (42%) |
| Secondary and above | 217 (31%) | 145 (25%) | 362 (28%) |
| Unknown | 2 | 2 | 4 |
| Caregiver depression status (PHQ9) | |||
| None/minimal | 231 (33%) | 183 (32%) | 414 (33%) |
| Mild | 294 (42%) | 234 (40%) | 528 (41%) |
| Moderate | 129 (19%) | 105 (18%) | 234 (18%) |
| Severe | 40 (5.8%) | 57 (9.8%) | 97 (7.6%) |
| Unknown | 5 | 8 | 13 |
| Wealth quintiles | |||
| Poorest | 127 (18%) | 118 (20%) | 245 (19%) |
| Second | 134 (19%) | 118 (20%) | 252 (20%) |
| Middle | 142 (20%) | 127 (22%) | 269 (21%) |
| Fourth | 157 (22%) | 123 (21%) | 280 (22%) |
| Least poor | 139 (20%) | 101 (17%) | 240 (19%) |
| Urban residence | |||
| Rural | 357 (51%) | 302 (51%) | 659 (51%) |
| Urban | 342 (49%) | 285 (49%) | 627 (49%) |
| Country ** | |||
| Bangladesh | 200 (29%) | 150 (26%) | 350 (27%) |
| Burkina Faso | 102 (15%) | 94 (16%) | 196 (15%) |
| Kenya | 144 (21%) | 137 (23%) | 281 (22%) |
| Malawi | 47 (6.7%) | 45 (7.7%) | 92 (7.2%) |
| Pakistan | 71 (10%) | 72 (12%) | 143 (11%) |
| Uganda | 135 (19%) | 89 (15%) | 224 (17%) |
| Characteristics | N | Person Time (Months) | Recovered (n) | Recovery Rate (95% CI) (Per 100 Person-Months) | The Proportion of Recovered (%) |
|---|---|---|---|---|---|
| Overall | 1286 | 4610 | 825 | 18(17, 19) | 64 |
| Food groups | |||||
| Grains | 1099 | 3898 | 717 | 18(17, 20) | 65 |
| Breast Milk | 828 | 3027 | 515 | 17(16, 19) | 62 |
| Milk and Dairy products | 754 | 2722 | 491 | 18(16, 20) | 65 |
| Fruits and Vegetables | 693 | 2489 | 453 | 18(17, 20) | 65 |
| Root and Tubers | 617 | 2257 | 398 | 18(16, 19) | 65 |
| Legumes and Nuts | 557 | 1931 | 383 | 20(18, 22) | 69 |
| Meats | 545 | 1938 | 375 | 19(17, 21) | 69 |
| Egg | 371 | 1385 | 237 | 17(15, 19) | 64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsegaye, A.T.; Pavlinac, P.B.; Turyagyenda, L.; Diallo, A.H.; Gnoumou, B.S.; Bamouni, R.M.; Voskuijl, W.P.; van den Heuvel, M.; Mbale, E.; Lancioni, C.L.; et al. The Role of Food Insecurity and Dietary Diversity on Recovery from Wasting among Hospitalized Children Aged 6–23 Months in Sub-Saharan Africa and South Asia. Nutrients 2022, 14, 3481. https://doi.org/10.3390/nu14173481
Tsegaye AT, Pavlinac PB, Turyagyenda L, Diallo AH, Gnoumou BS, Bamouni RM, Voskuijl WP, van den Heuvel M, Mbale E, Lancioni CL, et al. The Role of Food Insecurity and Dietary Diversity on Recovery from Wasting among Hospitalized Children Aged 6–23 Months in Sub-Saharan Africa and South Asia. Nutrients. 2022; 14(17):3481. https://doi.org/10.3390/nu14173481
Chicago/Turabian StyleTsegaye, Adino Tesfahun, Patricia B. Pavlinac, Lynnth Turyagyenda, Abdoulaye H. Diallo, Blaise S. Gnoumou, Roseline M. Bamouni, Wieger P. Voskuijl, Meta van den Heuvel, Emmie Mbale, Christina L. Lancioni, and et al. 2022. "The Role of Food Insecurity and Dietary Diversity on Recovery from Wasting among Hospitalized Children Aged 6–23 Months in Sub-Saharan Africa and South Asia" Nutrients 14, no. 17: 3481. https://doi.org/10.3390/nu14173481
APA StyleTsegaye, A. T., Pavlinac, P. B., Turyagyenda, L., Diallo, A. H., Gnoumou, B. S., Bamouni, R. M., Voskuijl, W. P., van den Heuvel, M., Mbale, E., Lancioni, C. L., Mupere, E., Mukisa, J., Lwanga, C., Atuhairwe, M., Chisti, M. J., Ahmed, T., Shahid, A. S. M. S. B., Saleem, A. F., Kazi, Z., ... Tickell, K. D. (2022). The Role of Food Insecurity and Dietary Diversity on Recovery from Wasting among Hospitalized Children Aged 6–23 Months in Sub-Saharan Africa and South Asia. Nutrients, 14(17), 3481. https://doi.org/10.3390/nu14173481







