Fasting Glucose for the Diagnosis of Gestational Diabetes Mellitus (GDM) during the COVID-19 Pandemic
Abstract
:1. Introduction
2. Material and Methods
2.1. Outcomes
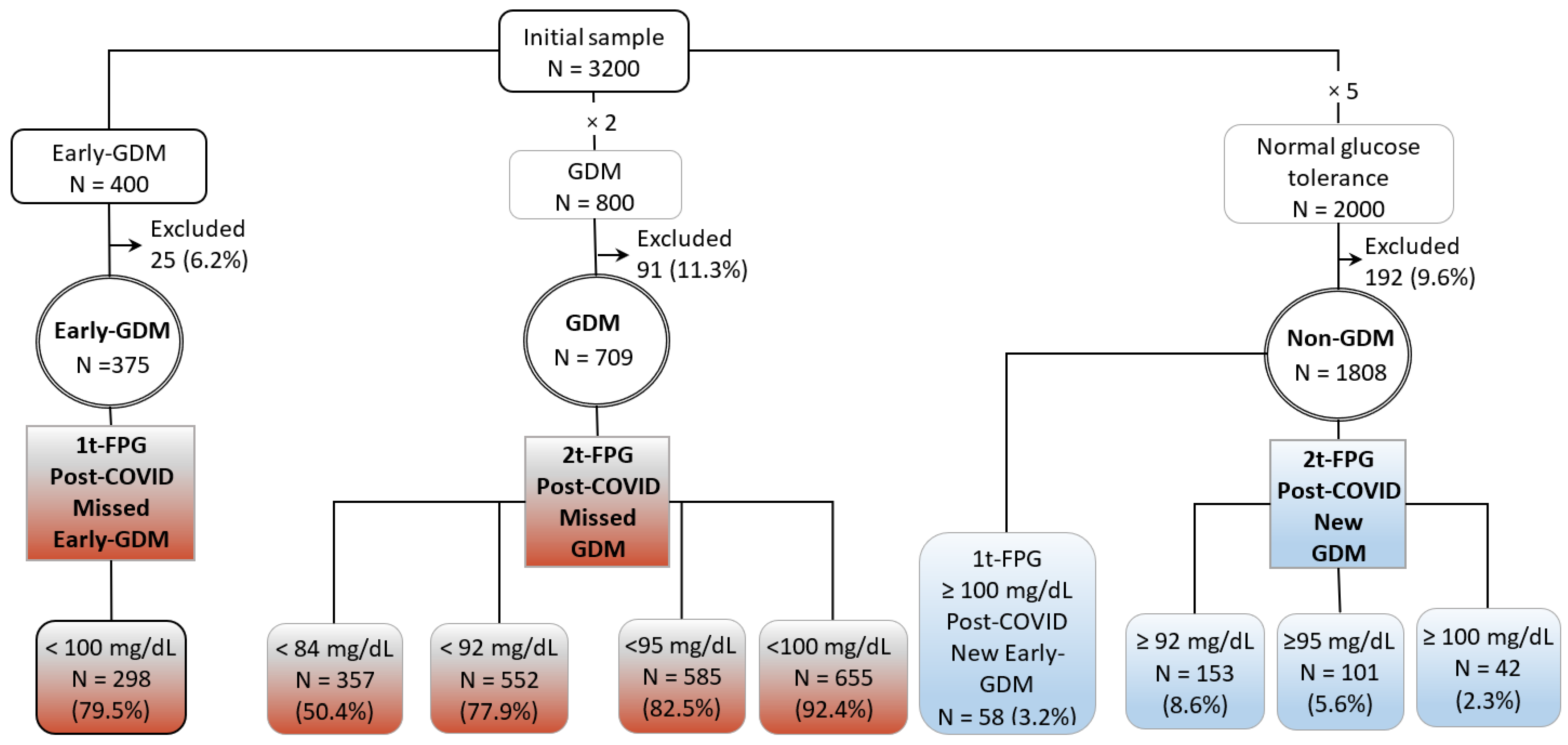
2.2. Subgroups and Comparisons
- Post-COVID Early-GDM was defined using 1t-FPG ≥ 100 mg/dL, according to the recommendations of Spain [4] for the diagnosis of gestational hyperglycemia, GDM, in the first trimester. The following subgroups were identified:
- -
- Post-COVID Missed Early-GDM group: Pregnant women with Early-GDM and 1t-FPG < 100 mg/dL. This group was compared with the Non-GDM group, and
- -
- Post-COVID New Early-GDM group: Pregnant women with normal glucose tolerance and 1t-FPG ≥ 100 mg/dL. This group was compared with the Early-GDM group.
- Post-COVID GDM was defined using 2t-FPG as diagnostic test.The following subgroups were identified according to the glucose threshold recommended by Australia (1), Italy (2) Spain (4), and the United Kingdom (5) and the European Society of Endocrinology (3) (100 mg/dL) to diagnose or rule out GDM.
- -
- Post-COVID Missed-GDM groups: Pregnant women with GDM and 2t-FPG < 84 mg/dL, <92 mg/dL, <95 mg/dL, and <100mg/dL. Each of these groups was compared with the non-GDM group, and
- -
- Post-COVID New GDM groups: Pregnant women with normal glucose tolerance, Non-GDM, and 2t-FPG ≥ 92 mg/dL, ≥95 mg/dL, and >100 mg/dL. Each of these groups was compared with the GDM group.
2.3. Statistical Methods
3. Results in Which Parity Was Also Included
3.1. Diagnosis of Early-GDM during the COVID Pandemic
3.2. Diagnosis of GDM during the COVID Pandemic
3.2.1. Recommendations of Australia
3.2.2. Recommendation of Italy
3.2.3. Recommendation of Spain
3.2.4. Recommendations of the United Kingdom and the European Society of Endocrinologists
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Australasian Diabetes in Pregnancy Society (ADIPS); The Australian Diabetes Society (ADS); The Australian Diabetes Educators Association (ADEA); Diabetes Australia (DA). Diagnostic Testing for Gestational Diabetes Mellitus (GDM) during the COVID 19. Available online: https://www.adips.org/documents/COVID19GDMDiagnosis030420ADIPSADSADEADA (accessed on 12 May 2021).
- Sculli, M.A.; Succurro, E.; Sciacca, L.; Di Bartolo, P.; Purrello, F.; Lapolla, A. Italian recommendations for the diagnosis of gestational diabetes during COVID-19 pandemic: Position statement of the Italian Association of Clinical Diabetologists (AMD) and the Italian Diabetes Society (SID), diabetes, and pregnancy study group. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1418–1422. [Google Scholar]
- Thangaratinam, S.; Cooray, S.D.; Sukumar, N.; Huda, M.S.B.; Devlieger, R.; Benhalima, K.; McAuliffe, F.; Saravanan, P.; Teede, H.J. Endocrinology in the time of COVID-19. Diagnosis and management of gestational diabetes mellitus. Eur. J. Endocrinol. 2020, 183, G49–G56. [Google Scholar] [CrossRef] [PubMed]
- Codina, M.; Corcoy, R.; Goya, M.M.; on behalf of the Spanish Diabetes and Pregnancy Group (GEDE). Update of the hyperglycemia Gestational diagnosis during the COVID-19 pandemic. Endocrinol. Diabetes Y Nutr. 2020, 67, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Bourne, T.; Kyriacou, C.; Coomarasamy, A.; Kirk, E.; Condous, G.; Leonardi, M. Guidance for maternal medicine services in the evolving coronavirus (COVID-19) pandemic. Royal College of Obstetricians and Gynaecologists. In Information for Healthcare Professionals; RCOG: London, UK, 30 March 2020; pp. 1–40. [Google Scholar]
- Yamamoto, J.; Donovan, L.; Feig, D.; Berger, H.; for Diabetes Canada Clinical Practice Guidelines Steering Committee and the Society of Obstetricians and Gynecologists of Canada. Urgent Update. Temporary Alternative Screening Strategy for Gestational Diabetes Screening During the COVID-19 Pandemic. A Joint Consensus Statement from the Diabetes Canada Clinical Practice Guidelines Steering Committee and the Society of Obstetricians and Gynecologists.Canada. 2020. Available online: https://www.sogc.org/common/Uploaded%20files/GDMCOVID19%20temporary%20screening%20guidelines%20-%2020200402%20Agreed%20Final.pdf (accessed on 12 May 2021).
- New Zealand Society for the Study of Diabetes. Screening for GDM during COVID Restrictions—Recommendations from New Zealand Society for the Study of Diabetes. 2020. Available online: https://nzssd.org.nz/covid-19.html (accessed on 12 May 2021).
- World Health Organization. Diagnostic Criteria and Classification of Hyperglycemia First Detected in Pregnancy. Available online: https://apps.who.int/iris/bitstream/handle/10665/85975/WHO_NMH_MND_13.2_eng.pdf;jsessionid=EB989B9FFA849410E012B3577C9C0909?sequence=1. (accessed on 17 May 2021).
- American College of Obstetricians and Gynecologists (ACOG). Gestational diabetes mellitus. ACOG Practice Bulletin No. 190. Obstet. Gynecol. Obstet. Gynecol. 2018, 131, e49–e64. [Google Scholar]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43 (Suppl. S1), S14–S31. [Google Scholar] [CrossRef] [Green Version]
- McIntyre, H.D.; Gibbons, K.S.; Ma, R.C.W.; Tam, W.H.; Sacks, D.A.; Lowe, J.; Madsen, L.R.; Catalano, P.M. Testing for gestational diabetes during the COVID-19 pandemic. An evaluation of proposed protocols for the United Kingdom, Canada and Australia. Diabetes Res. Clin. Pract. 2020, 167, 108353. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Meehan, T.; Veerasingham, M.; Sivanesan, K. COVID-19 pandemic gestational diabetes screening guidelines: A retrospective study in Australian women. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Nachtergaele, C.; Vicaut, E.; Pinto, S.; Tatulashvili, S.; Bihan, H.; Sal, M.; Berkane, N.; Allard, L.; Baudry, C.; Carbillon, L.; et al. COVID-19 pandemic: Can fasting plasma glucose and HbA1c replace the oral glucose tolerance test to screen for hyperglycaemia in pregnancy? Diabetes Res. Clin. Pract. 2021, 172, 108640. [Google Scholar] [CrossRef]
- Gemert, T.E.; Moses, R.G.; Pape, A.V.; Morris, G.J. Gestational diabetes mellitus testing in the COVID-19 pandemic: The problems with simplifying the diagnostic process. Aust N. Zeal. J. Obstet. Gynaecol. 2020, 60, 671–674. [Google Scholar] [CrossRef]
- Meek, C.L.; Lindsay, R.S.; Scott, E.M.; Aiken, C.E.; Myers, J.; Reynolds, R.M.; Simmons, D.; Yamamoto, J.M.; McCance, D.R.; Murphy, H.R. Approaches to screening for hyperglycaemia in pregnant women during and after the COVID-19 pandemic. Diabet. Med. 2021, 38, e14380. [Google Scholar] [CrossRef]
- Crowther, C.A.; Hiller, J.E.; Moss, J.R.; McPhee, A.J.; Jeffries, W.S.; Robinson, J.S.; Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N. Engl. J. Med. 2005, 352, 2477–2486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landon, M.B.; Spong, C.Y.; Thom, E.; Carpenter, M.W.; Ramin, S.M.; Casey, B.; Wapner, R.J.; Varner, M.W.; Rouse, D.J.; Thorp, J.M., Jr.; et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. A multicenter, randomized trial of treatment for mild gestational diabetes. N. Engl. J. Med. 2009, 361, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Natl. Diabetes Data Group Diabetes 1979, 28, 1039–1057. [Google Scholar]
- Grupo Español de Diabetes y Embarazo (GEDE). Asistencia a la Gestante con Diabetes. Guía de Práctica Clínica Actualizada en 2014. Av. Diabetol. 2015, 31, 45–59. [Google Scholar] [CrossRef]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S.; et al. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef] [Green Version]
- González González, N.L.; González Dávila, E.; González Martín A García Hernández, J.A. Maternal thinness and obesity and customized fetal weight charts. Fetal Diagn. Ther. 2021, 48, 551–559. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. S1), S15–S33. [Google Scholar] [CrossRef]
- Riskin-Mashiah, S.; Younes, G.; Damti, A.; Auslender, R. First-trimester fasting hyperglycemia and adverse outcomes. Diabetes Care 2009, 32, 1639–1643. [Google Scholar] [CrossRef] [Green Version]
- International Association of Diabetes and Pregnancy Study Groups (IADPSG). International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.W.; Yang, H.X.; Wei, Y.M.; Yan, J.; Wang, Z.L.; Li, X.L.; Wu, H.R.; Li, N.; Zhang, M.H.; Liu, X.H.; et al. Evaluation of the value of fasting plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in China. Diabetes Care 2013, 36, 586–590. [Google Scholar] [CrossRef] [Green Version]
- Immanuel, J.; Simmons, D. Screening and Treatment for Early-Onset Gestational Diabetes Mellitus: A Systematic Review and Meta-analysis. Curr. Diab. Rep. 2017, 17, 115. [Google Scholar] [CrossRef] [PubMed]
| First Prenatal Visit. Early GDM | GDM | |
|---|---|---|
| Glucose Measured before 20 Weeks | Glucose Measured after 24 Weeks | |
| Australia [1] | HbA1c ≥ 5.9% (41 mmol/mL) | FPG: <84 mg/dL (<4.7 mmol/L) → Non GDM FPG: 84–91 mg/dL (4.7–5 mmol/L) → OGTT FPG: ≥92 mg/dL (5.1mmol/L) → GDM |
| Italy [2] | FPG ≥ 92 mg/dL (5.1 mmol/L) | FPG ≥ 92 mg/dL (5.1 mmol/L) |
| European Society of Endocrinologists [3] | HbA1c: 5.9–6.4% (41–47 mmol/mL) or RPG: 162–200 mg/dL (9–11 mmol/L) | HbA1c ≥ 5.7% (≥ 39 mmol/L) or RPG ≥ 162 mg/dL (≥ 9 mmol/L) FPG ≥ 100 mg/dL (≥ 5.6 mmol/L) |
| Spain [4] | HbA1c: 5.9–6.4% (41–47 mmol/mL) or RPG: 165–199 mg/dL (9.2–11 mmol/L) or FPG: ≥100 mg/dL (≥5.6 mmol/L) | HbA1c ≥ 5.7% (≥39 mmol/L) or RPG: 165–199 mg/dL or FPG ≥ 95 mg/dL (≥5.3 mmol/L) |
| United Kingdom [5] | HbA1c: 5.9–6.4% (41–47 mmol/mL) or RPG: 162–200 mg/dL (9–11 mmol/L) | HbA1c ≥ 5.7% (≥39 mmol/L) or RPG ≥ 162 mg/dL (≥9 mmol/L) FPG ≥ 100 mg/dL (≥5.6 mmol/L) |
| Canada [6] | HbA1c ≥ 5.7% (≥39 mmol/L) or RPG ≥ 200 mg/dL (≥11.1 mmol/L) | |
| New Zealand [7] | HbA1c ≥ 5.8% (40 mmol/mL) | FPG: <81 mg/dL (<4.5 mmol/L) → Non GDM FPG: 81–90 mg/dL (4.7–5 mmol/L) → CGT FPG: ≥90 mg/dL (≥5 mmol/L) → GDM |
| Early-GDM | Non-GDM | p-Values | ||||
|---|---|---|---|---|---|---|
| 1t-FPG < 100 mg/dL | Total | 1t-FPG ≥ 100 mg/dL | Total | Missed Early-GDM vs. Total Non-GDM | New-Early-GDM vs. Total Early GDM | |
| Post-COVID Missed Early-GDM | Post-COVID- New Early GDM | |||||
| N = 298 (79.5%) | N = 375 | N = 58 (3.2%) | N = 1789 | |||
| Maternal age (years) | 35.2 ± 4.7 | 35.2 ± 4.6 | 31.3 ± 6.4 | 30.1 ± 6.0 | <0.001 | <0.001 |
| BMI (kg/m2) | 28.6 ± 6.4 | 29.4 ± 6.6 | 28.6 ± 5.6 | 25.7 ± 5.0 | <0.001 | 0.341 |
| Parity > 1, n (%) | 161 (54.0) | 212 (56.5) | 41 (70.7) | 789 (44.1) | 0.002 | 0.046 |
| Chronic Hypertension, n (%) | 29 (9.6) | 35 (9.4) | 2 (3.4) | 22 (1.2) | <0.001 | 0.184 |
| Insulin, n (%) | 144 (48.3) | 189 (50.4) | 0 | 0 | - | - |
| Perinatal outcomes | ||||||
| Preeclampsia, n (%) | 13 (4.4) | 17 (4.5) | 1 (1.7) | 27 (1.5) | 0.152 | 0.360 |
| Prematurity, n (%) | 30 (10.1) | 41 (10.9) | 5 (8.6) | 108 (6.0) | 0.114 | 0.731 |
| Caesarean section, n (%) | 90 (30.5) | 114 (30.6) | 8 (13.8) | 233 (13.0) | 0.004 | 0.113 |
| LGA, n (%) | 45 (15.1) | 62 (16.5) | 7 (12.1) | 205 (11.5) | 0.648 | 0.725 |
| SGA, n (%) | 31 (10.4) | 38 (10.1) | 8 (13.8) | 205 (11.5) | 0.899 | 0.904 |
| 1m Apgar test ≤ 7, n (%) | 33 (11.4) | 41 (11.2) | 8 (13.8) | 136 (7.6) | 0.068 | 0.553 |
| 5m Apgar test ≤ 7, n (%) | 1 (0.3) | 4 (1.1) | 1 (1.7) | 31 (1.7) | 0.217 | 0.902 |
| pH artery < 7, n (%) | 3 (1.0) | 5 (1.3) | 1 (1.7) | 28 (1.6) | 0.329 | 0.860 |
| NICU, n (%) | 40 (13.4) | 48 (12.8) | 7 (12.1) | 138 (7.7) | 0.001 | 0.889 |
| Composite adverse outcome n (%) | 145 (48.7) | 189 (50.4) | 23 (39.7) | 633 (35.4) | 0.044 | 0.595 |
| Total | GDM | p-Values | GDM | Non-GDM | p-Values | |||
|---|---|---|---|---|---|---|---|---|
| GDM | Non-GDM | 2t-FPG < 84 mg/dL | Missed- GDM vs. Total Non-GDM | 2t-FPG < 92 mg/dL | 2t FPG ≥ 92 mg/dL | Missed- GDM vs. Total Non-GDM | New-GDM vs. Total -GDM | |
| Post-COVID Missed | Post-COVID Missed | Post-COVID New-GDM | ||||||
| N = 709 | N = 1789 | N = 357 (50.4%) | N = 552 (77.9%) | N = 153 (8.6%) | ||||
| Maternalage (years) | 34.1 ± 5.0 | 30.1 ± 6.0 | 33.7 ± 5.1 | <0.001 | 34.1 ± 5.1 | 31.2 ± 5.6 | <0.001 | <0.001 |
| BMI (kg/m2) | 26.8 ± 6.0 | 25.7 ± 5.0 | 25.3 ± 5.4 | 0.127 | 26.1 ± 5.6 | 28.5 ± 5.3 | 0.227 | 0.001 |
| Parity > 1, n (%) | 335 (47.2) | 789 (44.1) | 136 (38.1) | 0.040 | 247 (44.7) | 81 (52.9) | 0.806 | 0.212 |
| Chronic Hypertension, n (%) | 42 (6.0) | 22 (1.2) | 16 (4.6) | 0.001 | 28 (5.1) | 6 (3.9) | <0.001 | 0.421 |
| 2t-FPG (mg/dL) | 84.3 ± 10.1 | 80.2 ± 8.2 | 76.3 ± 5.1 | <0.001 | 80.2 ± 6.8 | 97.5 ± 6.1 | 0.815 | <0.001 |
| Perinatal outcomes | ||||||||
| Preeclampsia, n (%) | 30 (4.2) | 27 (1.5) | 8 (2.2) | 0.497 | 16 (2.9) | 4 (2.6) | 0.252 | 0.190 |
| Prematurity, n (%) | 61 (8.6) | 108 (6.0) | 23 (6.4) | 0.938 | 41 (7.4) | 14 (9.2) | 0.325 | 0.925 |
| Cesarean, n (%) | 169 (23.9) | 233 (13.0) | 82 (23.0) | <0.001 | 127 (23.0) | 26 (17.0) | <0.001 | 0.109 |
| LGA, n (%) | 107 (15.1) | 205 (11.5) | 46 (12.9) | 0.220 | 78 (14.1) | 27 (17.6) | 0.071 | 0.580 |
| SGA, n (%) | 75 (10.6) | 205 (11.5) | 38 (10.6) | 0.586 | 60 (10.9) | 17 (11.1) | 0.962 | 0.808 |
| 1m Apgar ≤ 7 n (%) | 52 (7.5) | 136 (7.6) | 27 (7.8) | 0.691 | 37 (6.9) | 10 (6.5) | 0.878 | 0.464 |
| 5m Apgar ≤ 7, n (%) | 10 (1.5) | 31 (1.7) | 5 (1.5) | 0.535 | 8 (1.5) | 3 (1.9) | 0.638 | 0.402 |
| pH artery < 7, n (%) | 8 (1.1) | 28 (1.6) | 4 (1.1) | 0.800 | 5 (0.9) | 3 (2.0) | 0.551 | 0.351 |
| NICU, n (%) | 59 (8.3) | 138 (7.7) | 27 (7.6) | 0.900 | 41 (7.4) | 18 (11.8) | 0.385 | 0.366 |
| Composite adverse outcome n(%) | 301 (42.5) | 633 (35.4) | 147 (41.2) | 0.077 | 227 (41.1) | 69 (45.1) | 0.038 | 0.685 |
| GDM | Non-GDM | p-Values | ||||
|---|---|---|---|---|---|---|
| 2t-FPG < 95 mg/dL | Total | 2t FPG ≥ 95 mg/dL | Total | Missed-GDM vs. Total Non-GDM | New-GDM vs. Total GDM | |
| Post-COVID Missed-GDM | Post-COVID New-GDM | |||||
| N = 585 (82.5%) | N = 709 | N = 101 (5.6%) | N = 1789 | |||
| Maternal age (years) | 34.1 ± 5.0 | 34.1 ± 5.0 | 31.6 ± 5.7 | 30.1 ± 6.0 | <0.001 | <0.001 |
| BMI (kg/m2) | 26.2 ± 5.7 | 26.8 ± 6.0 | 28.7 ± 5.8 | 25.7 ± 5.0 | 0.078 | 0.003 |
| Parity > 1, n (%) | 266 (45.5) | 335 (47.2) | 52 (51.5) | 789 (44.1) | 0.566 | 0.457 |
| Chronic Hypertension, n (%) | 29 (5.0) | 42 (6.0) | 4 (4.0) | 22 (1.2) | <0.001 | 0.636 |
| 2t-FPG (mg/dL) | 80.9 ± 7.2 | 84.3 ± 10.1 | 99.8 ± 6.3 | 80.2 ± 8.2 | 0.092 | <0.001 |
| Perinatal outcomes | ||||||
| Preeclampsia, n (%) | 19 (3.2) | 30 (4.2) | 3 (3.0) | 27 (1.5) | 0.097 | 0.330 |
| Prematurity, n (%) | 49 (8.4) | 61 (8.6) | 10 (9.9) | 108 (6.0) | 0.067 | 0.870 |
| Caesarean section, n (%) | 136 (23.3) | 169 (23.9) | 18 (17.8) | 233 (13.0) | <0.001 | 0.184 |
| LGA, n (%) | 83 (14.2) | 107 (15.1) | 19 (18.8) | 205 (11.5) | 0.064 | 0.458 |
| SGA, n (%) | 63 (10.8) | 75 (10.6) | 11 (10.9) | 205 (11.5) | 0.847 | 0.933 |
| 1m Apgar test ≤ 7, n (%) | 38 (6.7) | 52 (7.5) | 8 (7.9) | 136 (7.6) | 0.743 | 0.812 |
| 5m Apgar test ≤ 7, n (%) | 8 (1.4) | 10 (1.5) | 2 (2.0) | 31 (1.7) | 0.531 | 0.497 |
| pH artery < 7, n (%) | 5 (0.9) | 8 (1.1) | 2 (2.0) | 28 (1.6) | 0.454 | 0.384 |
| NICU, n (%) | 47 (8.0) | 59 (8.3) | 10 (9.9) | 138 (7.7) | 0.165 | 0.895 |
| Composite adverse outcome n (%) | 242 (41.4) | 301 (42.5) | 50 (49.5) | 633 (35.4) | 0.023 | 0.298 |
| GDM | Non-GDM | p-Values | ||||
|---|---|---|---|---|---|---|
| 2t-FPG < 100 mg/dL | Total | 2t FPG ≥ 100 mg/dL | Total | Missed-GDM vs. Total Non-GDM | New-GDM vs. Total GDM | |
| Post-COVID-Missed-GDM | Post-COVID-New Late GDM | |||||
| N = 655 (92.4%) | N = 709 | N = 42 (2.3%) | N = 1789 | |||
| Maternal age (years) | 34.1 ± 5.0 | 34.1 ± 5.0 | 32.5 ± 4.8 | 30.1 ± 6.0 | <0.001 | 0.050 |
| BMI (kg/m2) | 26.5 ± 5.9 | 26.8 ± 6.0 | 29.2 ± 5.6 | 25.7 ± 5.0 | 0.004 | 0.013 |
| Parity > 1, n (%) | 304 (46.4) | 335 (47.2) | 23 (54.8) | 789 (44.1) | 0.313 | 0.427 |
| Chronic Hypertension, n (%) | 38 (5.8) | 42 (6.0) | 1 (2.4) | 22 (1.2) | <0.001 | 0.500 |
| 2t-FPG (mg/dL) | 82.5 ± 8.3 | 84.3 ± 10.1 | 103.8 ± 8.2 | 80.2 ± 8.2 | <0.001 | <0.001 |
| Perinatal outcomes | ||||||
| Preeclampsia, n (%) | 26 (4.0) | 30 (4.2) | 1 (2.4) | 27 (1.5) | 0.021 | 0.402 |
| Prematurity, n (%) | 54 (8.2) | 61 (8.6) | 4 (9.5) | 108 (6.0) | 0.067 | 0.957 |
| Caesarean section, n (%) | 154 (23.5) | 169 (23.9) | 6 (14.3) | 233 (13.0) | <0.001 | 0.099 |
| LGA, n (%) | 99 (15.1) | 107 (15.1) | 10 (23.8) | 205 (11.5) | 0.028 | 0.183 |
| SGA, n (%) | 70 (10.7) | 75 (10.6) | 4 (9.5) | 205 (11.5) | 0.721 | 0.937 |
| 1m Apgar test ≤ 7, n (%) | 47 (7.4) | 52 (7.5) | 3 (7.1) | 136 (7.6) | 0.983 | 0.633 |
| 5 m Apgar test ≤ 7, n (%) | 10 (1.6) | 10 (1.5) | - | 31 (1.7) | 0.611 | 0.999 |
| pH artery < 7, n (%) | 7 (1.1) | 8 (1.1) | 1 (2.4) | 28 (1.6) | 0.334 | 0.475 |
| NICU, n (%) | 55 (8.4) | 59 (8.3) | 3 (7.1) | 138 (7.7) | 0.207 | 0.546 |
| Composite adverse outcome, n (%) | 278 (42.4) | 301 (42.5) | 21 (50.0) | 633 (35.4) | 0.020 | 0.513 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González González, N.L.; González Dávila, E.; Bugatto, F.; Vega-Guedes, B.; Pintado, P.; Tascón, L.; Villalba Martin, N.; Plasencia, W.; Megía, A. Fasting Glucose for the Diagnosis of Gestational Diabetes Mellitus (GDM) during the COVID-19 Pandemic. Nutrients 2022, 14, 3432. https://doi.org/10.3390/nu14163432
González González NL, González Dávila E, Bugatto F, Vega-Guedes B, Pintado P, Tascón L, Villalba Martin N, Plasencia W, Megía A. Fasting Glucose for the Diagnosis of Gestational Diabetes Mellitus (GDM) during the COVID-19 Pandemic. Nutrients. 2022; 14(16):3432. https://doi.org/10.3390/nu14163432
Chicago/Turabian StyleGonzález González, Nieves Luisa, Enrique González Dávila, Fernando Bugatto, Begoña Vega-Guedes, Pilar Pintado, L. Tascón, Nazaret Villalba Martin, Walter Plasencia, and Ana Megía. 2022. "Fasting Glucose for the Diagnosis of Gestational Diabetes Mellitus (GDM) during the COVID-19 Pandemic" Nutrients 14, no. 16: 3432. https://doi.org/10.3390/nu14163432
APA StyleGonzález González, N. L., González Dávila, E., Bugatto, F., Vega-Guedes, B., Pintado, P., Tascón, L., Villalba Martin, N., Plasencia, W., & Megía, A. (2022). Fasting Glucose for the Diagnosis of Gestational Diabetes Mellitus (GDM) during the COVID-19 Pandemic. Nutrients, 14(16), 3432. https://doi.org/10.3390/nu14163432






