Effects of Gestational Sleep Patterns and Their Changes on Maternal Glycemia and Offspring Physical Growth in Early Life
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Samples
2.2. Assessment of Sleep Pattern during Pregnancy
2.3. Diagnostic Criteria for GDM
2.4. Anthropometric Measures
2.5. Covariates
2.6. Statistical Analyses
3. Results
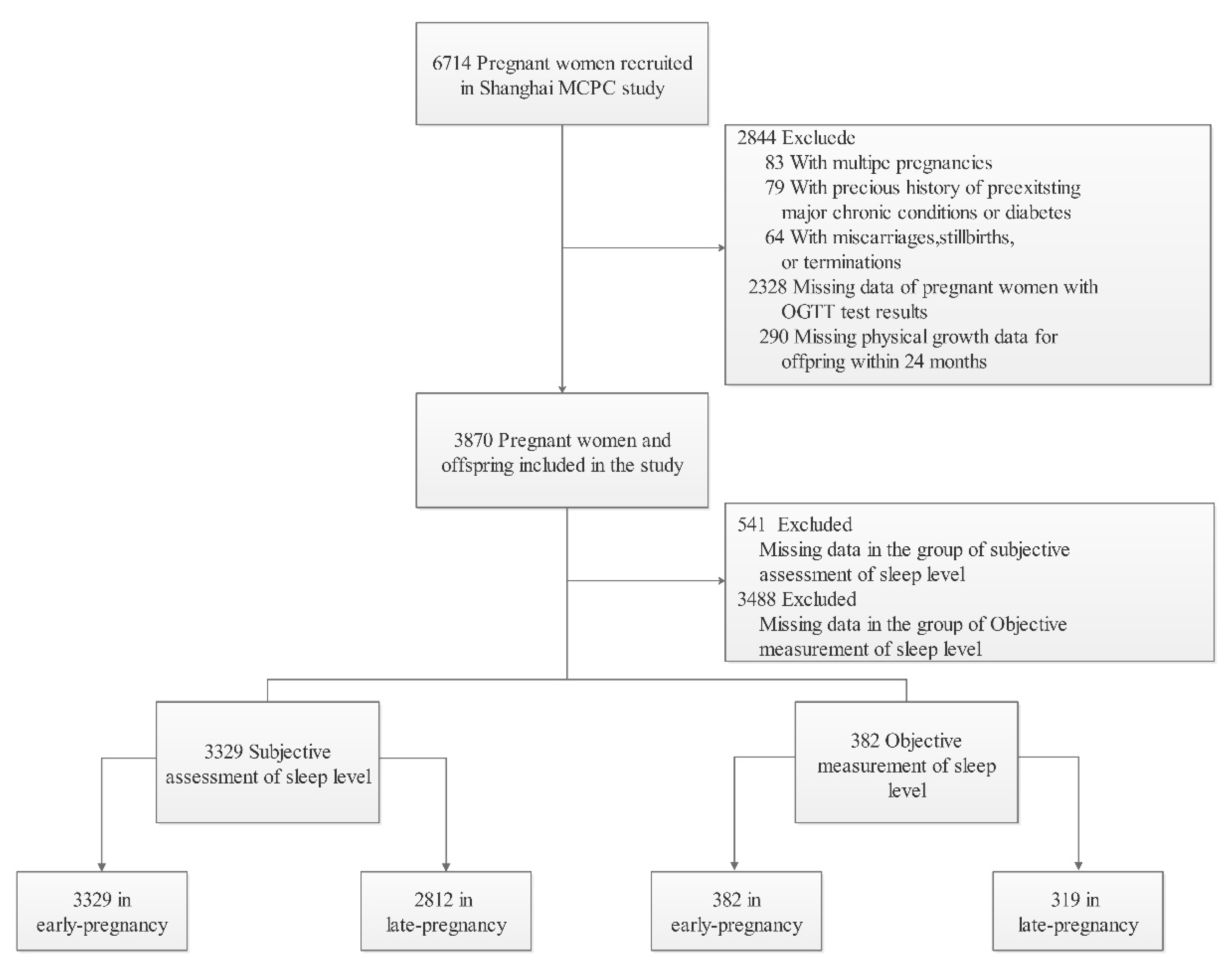
3.1. Participants
3.2. Distribution of Maternal Sleep Patterns and Its Effects on GDM
3.3. Maternal Sleep Patterns on Offspring Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simmonds, M.; Llewellyn, A.; Owen, C.G.; Woolacott, N. Predicting adult obesity from childhood obesity: A systematic review and meta-analysis. Obes. Rev. 2016, 17, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Aris, I.M.; Chen, L.W.; Tint, M.T.; Pang, W.W.; Soh, S.E.; Saw, S.M.; Shek, L.P.C.; Tan, K.H.; Gluckman, P.D.; Chong, Y.S.; et al. Body mass index trajectories in the first two years and subsequent childhood cardio-metabolic outcomes: A prospective multi-ethnic Asian cohort study. Sci. Rep. 2017, 7, 8424. [Google Scholar] [CrossRef] [PubMed]
- Huijing, S.H.I. Strengthening researches in early-life course risk factors for childhood overweight and obesity. Chin. J. Sch. Health 2022, 43, 801–804. [Google Scholar] [CrossRef]
- O’Donnell, K.J.; Meaney, M.J. Fetal Origins of Mental Health: The Developmental Origins of Health and Disease Hypothesis. Am. J. Psychiatry 2017, 174, 319–328. [Google Scholar] [CrossRef]
- Catalano, P.; deMouzon, S.H. Maternal obesity and metabolic risk to the offspring: Why lifestyle interventions may have not achieved the desired outcomes. Int. J. Obes. 2015, 39, 642–649. [Google Scholar] [CrossRef]
- Anothaisintawee, T.; Reutrakul, S.; Van Cauter, E.; Thakkinstian, A. Sleep disturbances compared to traditional risk factors for diabetes development: Systematic review and meta-analysis. Sleep Med. Rev. 2016, 30, 11–24. [Google Scholar] [CrossRef]
- Sweet, L.; Arjyal, S.; Kuller, J.A.; Dotters-Katz, S. A Review of Sleep Architecture and Sleep Changes During Pregnancy. Obstet. Gynecol. Surv. 2020, 75, 253–262. [Google Scholar] [CrossRef]
- Twedt, R.; Bradley, M.; Deiseroth, D.; Althouse, A.; Facco, F. Sleep Duration and Blood Glucose Control in Women with Gestational Diabetes Mellitus. Obstet. Gynecol. 2015, 126, 326–331. [Google Scholar] [CrossRef]
- Shan, Z.; Ma, H.; Xie, M.; Yan, P.; Guo, Y.; Bao, W.; Rong, Y.; Jackson, C.L.; Hu, F.B.; Liu, L. Sleep duration and risk of type 2 diabetes: A meta-analysis of prospective studies. Diabetes Care 2015, 38, 529–537. [Google Scholar] [CrossRef]
- Clausen, T.D.; Mathiesen, E.R.; Hansen, T.; Pedersen, O.; Jensen, D.M.; Lauenborg, J.; Schmidt, L.; Damm, P. Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J. Clin. Endocrinol. Metab. 2009, 94, 2464–2470. [Google Scholar] [CrossRef]
- Reutrakul, S.; Anothaisintawee, T.; Herring, S.J.; Balserak, B.I.; Marc, I.; Thakkinstian, A. Short sleep duration and hyperglycemia in pregnancy: Aggregate and individual patient data meta-analysis. Sleep Med. Rev. 2018, 40, 31–42. [Google Scholar] [CrossRef]
- Xu, Y.H.; Shi, L.; Bao, Y.P.; Chen, S.J.; Shi, J.; Zhang, R.L.; Lu, L. Association between sleep duration during pregnancy and gestational diabetes. Sleep Med. 2018, 52, 67–74. [Google Scholar] [CrossRef]
- Zhong, C.; Chen, R.; Zhou, X.; Xu, S.; Li, Q.; Cui, W.; Wang, W.; Li, X.; Wu, J.; Liu, C.; et al. Poor sleep during early pregnancy increases subsequent risk of gestational diabetes mellitus. Sleep Med. 2018, 46, 20–25. [Google Scholar] [CrossRef]
- Izci-Balserak, B.; Keenan, B.T.; Corbitt, C.; Staley, B.; Perlis, M.; Pien, G.W. Changes in Sleep Characteristics and Breathing Parameters During Sleep in Early and Late Pregnancy. J. Clin. Sleep Med. 2018, 14, 1161–1168. [Google Scholar] [CrossRef]
- Balserak, B.I. Sleep-disordered breathing in pregnancy. Breathe 2015, 11, 268–277. [Google Scholar] [CrossRef]
- O’Keeffe, M.; St-Onge, M.P. Sleep duration and disorders in pregnancy: Implications for glucose metabolism and pregnancy outcomes. Int. J. Obes. 2013, 37, 765–770. [Google Scholar] [CrossRef]
- Ma, X.M.; Wang, Y.; Hu, H.; Grant, T.X.; Zhang, Y.H.; Shi, H.J. The impact of resilience on prenatal anxiety and depression among pregnant women in Shanghai. J. Affect. Disord. 2019, 250, 57–64. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Zhao, X.; Mao, Z.; Abdulai, T.; Liu, X.; Tu, R.; Wang, Y.; Qian, X.; Jiang, J.; et al. The association between PSQI score and hypertension in a Chinese rural population: The Henan Rural Cohort Study. Sleep Med. 2019, 58, 27–34. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Shang, M.; Lin, L.; Ma, L.; Yin, L. Investigation on the suitability of the International Association of Diabetes and Pregnancy Study Group diagnostic criteria for gestational diabetes mellitus in China. J. Obstet. Gynaecol. 2014, 34, 141–145. [Google Scholar] [CrossRef]
- Wang, G.; Johnson, S.; Gong, Y.; Polk, S.; Divall, S.; Radovick, S.; Moon, M.; Paige, D.; Hong, X.; Caruso, D.; et al. Weight Gain in Infancy and Overweight or Obesity in Childhood across the Gestational Spectrum: A Prospective Birth Cohort Study. Sci. Rep. 2016, 6, 29867. [Google Scholar] [CrossRef]
- Ong, K.K.L.; Ahmed, M.L.; Emmett, P.M.; Preece, M.A.; Dunger, D.B.; Pregnancy, A.L.S. Association between postnatal catch-up growth and obesity in childhood: Prospective cohort study. Brit. Med. J. 2000, 320, 967–971. [Google Scholar] [CrossRef]
- Zhou, B. Predictive values of body mass index and waist circumference to risk factors of related diseases in Chinese adult population. Chin. J. Epidemiol. 2002, 23, 5–10. [Google Scholar]
- Zou, J.J.; Yang, Y.T.; Wei, Q.; Zhang, Y.H.; Shi, H.J. Longitudinal Association of Maternal Pre-Pregnancy BMI and Third-Trimester Glycemia with Early Life Growth of Offspring: A Prospective Study among GDM-Negative Pregnant Women. Nutrients 2021, 13, 3971. [Google Scholar] [CrossRef]
- Ma, Y.; Olendzki, B.C.; Merriam, P.A.; Chiriboga, D.E.; Culver, A.L.; Li, W.; Hébert, J.R.; Ockene, I.S.; Griffith, J.A.; Pagoto, S.L. A randomized clinical trial comparing low-glycemic index versus ADA dietary education among individuals with type 2 diabetes. Nutrition 2008, 24, 45–56. [Google Scholar] [CrossRef]
- Tan, L.; Zou, J.; Zhang, Y.; Yang, Q.; Shi, H. A Longitudinal Study of Physical Activity to Improve Sleep Quality during Pregnancy. Nat. Sci. Sleep 2020, 12, 431–442. [Google Scholar] [CrossRef]
- Mindell, J.A.; Cook, R.A.; Nikolovski, J. Sleep patterns and sleep disturbances across pregnancy. Sleep Med. 2015, 16, 483–488. [Google Scholar] [CrossRef]
- Sedov, I.D.; Cameron, E.E.; Madigan, S.; Tomfohr-Madsen, L.M. Sleep quality during pregnancy: A meta-analysis. Sleep Med. Rev. 2018, 38, 168–176. [Google Scholar] [CrossRef]
- Plancoulaine, S.; Flori, S.; Bat-Pitault, F.; Patural, H.; Lin, J.S.; Franco, P. Sleep Trajectories Among Pregnant Women and the Impact on Outcomes: A Population-Based Cohort Study. Matern. Child Health J. 2017, 21, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Frederick, I.O.; Sorensen, T.K.; Enquobahrie, D.A.; Williams, M.A. Sleep duration and plasma leptin concentrations in early pregnancy among lean and overweight/obese women: A cross sectional study. BMC Res. Notes 2014, 7, 20. [Google Scholar] [CrossRef]
- Wang, H.; Leng, J.; Li, W.; Wang, L.; Zhang, C.; Li, W.; Liu, H.; Zhang, S.; Chan, J.; Hu, G.; et al. Sleep duration and quality, and risk of gestational diabetes mellitus in pregnant Chinese women. Diabet. Med. 2017, 34, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Enquobahrie, D.; Frederick, I.O.; Abetew, D.; Williams, M.A. Glucose intolerance and gestational diabetes risk in relation to sleep duration and snoring during pregnancy: A pilot study. BMC Womens Health 2010, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Reutrakul, S.; Van Cauter, E. Interactions between sleep, circadian function, and glucose metabolism: Implications for risk and severity of diabetes. Ann. Ny. Acad. Sci. 2014, 1311, 151–173. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, J.; Zhou, R.; Liu, J.; Dai, Z.; Liu, D.; Wang, Y.; Zhang, H.; Li, Y.; Zeng, G. Sleep disturbances during pregnancy are associated with cesarean delivery and preterm birth. J. Matern. Fetal Neonatal Med. 2017, 30, 733–738. [Google Scholar] [CrossRef]
- Howe, L.D.; Signal, T.L.; Paine, S.J.; Sweeney, B.; Priston, M.; Muller, D.; Lee, K.; Huthwaite, M.; Gander, P. Self-reported sleep in late pregnancy in relation to birth size and fetal distress: The E Moe, Mama prospective cohort study. BMJ Open 2015, 5, e008910. [Google Scholar] [CrossRef]
- Kong, L.; Norstedt, G.; Schalling, M.; Gissler, M.; Lavebratt, C. The Risk of Offspring Psychiatric Disorders in the Setting of Maternal Obesity and Diabetes. Pediatrics 2018, 142, e20180776. [Google Scholar] [CrossRef]
- Stamatakis, K.A.; Punjabi, N.M. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest 2010, 137, 95–101. [Google Scholar] [CrossRef]
- Buchanan, T.A.; Xiang, A.H. Gestational diabetes mellitus. J. Clin. Investig. 2005, 115, 485–491. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Chang, A.M.; Callaway, L.K.; Cowley, D.M.; Dyer, A.R.; Radaelli, T.; Farrell, K.A.; Huston-Presley, L.; Amini, S.B.; Kirwan, J.P.; et al. Hormonal and metabolic factors associated with variations in insulin sensitivity in human pregnancy. Diabetes Care 2010, 33, 356–360. [Google Scholar] [CrossRef][Green Version]
- Izci-Balserak, B.; Pien, G.W. The Relationship and Potential Mechanistic Pathways Between Sleep Disturbances and Maternal Hyperglycemia. Curr. Diabetes Rep. 2014, 14, 459. [Google Scholar] [CrossRef]


| Variables | Participants, n (%). | ||
|---|---|---|---|
| Non-GDM (n = 2529) | GDM (n = 800) | p Value | |
| Parents characteristics | |||
| Maternal age, mean (SD), (Years) | 28.43 (3.95) | 30.08 (4.28) | <0.001 |
| Gestational weight gain, mean (SD), (Kg) | 15.09 (5.00) | 13.78 (5.61) | <0.001 |
| FPG in middle pregnancy, mean (SD), (mmol/L) | 4.39 (0.31) | 4.81 (0.59) | <0.001 |
| 1h plasma glucose level, mean (SD), (mmol/L) | 7.12 (1.40) | 9.49 (1.90) | <0.001 |
| 2h plasma glucose level, mean (SD), (mmol/L) | 6.30 (1.06) | 7.75 (1.73) | <0.001 |
| Pre-pregnancy BMI (Kg/m2) | <0.001 | ||
| 18.5~23.9 | 1714 (67.77) | 523 (65.38) | |
| <18.5 | 438 (17.32) | 94 (11.75) | |
| ≥24.0 | 377 (14.91) | 183 (22.88) | |
| Education level > 12 (years) | 2267 (89.64) | 728 (91.00) | 0.27 |
| Total family income ≤ ¥200 thousand (RMB) | 1868 (73.86) | 574 (71.75) | 0.13 |
| Parity—primiparous | 1469 (58.09) | 409 (51.12) | <0.001 |
| Depression in late pregnancy | 312 (12.34) | 97 (12.12) | 0.87 |
| Anxiety in late pregnancy | 306 (12.10) | 87 (10.88) | 0.35 |
| Energy intake in late-pregnancy, mean (SD), (kcal) | 2254.62 (1182.63) | 2020.78 (1072.19) | <0.001 |
| Complications | 769 (30.41) | 141 (17.62) | 0.16 |
| PA level in late pregnancy—low | 1271 (40.50) | 375 (38.15) | 0.41 |
| Father’s age, mean (SD) | 29.54 (4.53) | 31.01 (5.01) | <0.001 |
| Father’s BMI, mean (SD), (kg/m2) | 23.82 (3.48) | 23.98 (3.49) | 0.26 |
| Offspring characteristics | |||
| Sex—male | 1250 (49.43) | 398 (49.75) | 0.87 |
| Gestational weeks at delivery, mean (SD), | 39.17 (1.30) | 38.90 (1.31) | <0.001 |
| Delivery mode—Cesarean section | 1294 (51.17) | 432 (54.00) | 0.16 |
| Feeding practice within the first 6 months | |||
| Exclusive breastfeeding | 1141 (45.12) | 333 (41.62) | 0.17 |
| Mixed feeding | 796 (31.47) | 277 (34.62) | |
| Bottle-feeding | 592 (23.41) | 190 (23.75) | |
| Breastfeeding duration, mean (SD), (months) | 9.66 (5.09) | 9.85 (5.07) | 0.37 |
| Variables | Participants, n (%) | OR (95% CI) | ||
|---|---|---|---|---|
| Non-GDM | GDM | p Value | ||
| Subjective Assessment (n1 = 2937, n2 = 933) | ||||
| Total PSQI score in early pregnancy, mean (SD) | 5.70 (2.78) | 6.04 (2.90) | 0.003 | 1.03 (1.01, 1.07) |
| Sleep quality in early pregnancy | 0.17 | |||
| Good | 1278 (50.53) | 382 (47.75) | Ref. | |
| Poor | 1251 (49.47) | 418 (52.25) | 1.09 (0.91, 1.31) | |
| Changes in sleep quality during pregnancy | 0.007 | |||
| Always good | 472 (24.69) | 166 (26.39) | Ref. | |
| Always poor | 715 (37.39) | 214 (34.02) | 0.81 (0.61, 1.07) | |
| From good to poor | 489 (25.58) | 141 (22.42) | 0.90 (0.67, 1.22) | |
| From poor to good | 236 (12.34) | 108 (17.17) | 1.48 (1.06, 2.07) | |
| Objective measurement (n1 = 289, n2 = 93) | ||||
| Sleep duration in early pregnancy, continuous, (hour) | 7.80 (0.81) | 7.72 (1.07) | 0.44 | 1.03 (0.77, 1.39) |
| Sleep duration in early pregnancy, categorical, mean (SD), (hour) | 0.13 | |||
| 7.5~8.5 | 144 (49.83) | 37 (39.78) | Ref. | |
| <7.5 | 97 (33.56) | 33 (35.48) | 1.09 (0.60, 1.99) | |
| ≥8.5 | 48 (16.61) | 23 (24.73) | 1.93 (1.01, 3.81) | |
| Changes in sleep duration during pregnancy | 0.28 | |||
| Shortened sleep duration | 177 (73.75) | 63 (79.75) | Ref. | |
| Prolonged sleep duration | 63 (26.25) | 16 (20.25) | 0.81 (0.41, 1.16) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, J.; Wei, Q.; Ye, P.; Shi, Y.; Zhang, Y.; Shi, H. Effects of Gestational Sleep Patterns and Their Changes on Maternal Glycemia and Offspring Physical Growth in Early Life. Nutrients 2022, 14, 3390. https://doi.org/10.3390/nu14163390
Zou J, Wei Q, Ye P, Shi Y, Zhang Y, Shi H. Effects of Gestational Sleep Patterns and Their Changes on Maternal Glycemia and Offspring Physical Growth in Early Life. Nutrients. 2022; 14(16):3390. https://doi.org/10.3390/nu14163390
Chicago/Turabian StyleZou, Jiaojiao, Qian Wei, Peiqi Ye, Yuyang Shi, Yunhui Zhang, and Huijing Shi. 2022. "Effects of Gestational Sleep Patterns and Their Changes on Maternal Glycemia and Offspring Physical Growth in Early Life" Nutrients 14, no. 16: 3390. https://doi.org/10.3390/nu14163390
APA StyleZou, J., Wei, Q., Ye, P., Shi, Y., Zhang, Y., & Shi, H. (2022). Effects of Gestational Sleep Patterns and Their Changes on Maternal Glycemia and Offspring Physical Growth in Early Life. Nutrients, 14(16), 3390. https://doi.org/10.3390/nu14163390







