Vitamin D and Male Reproduction: Updated Evidence Based on Literature Review
Abstract
:1. Introduction
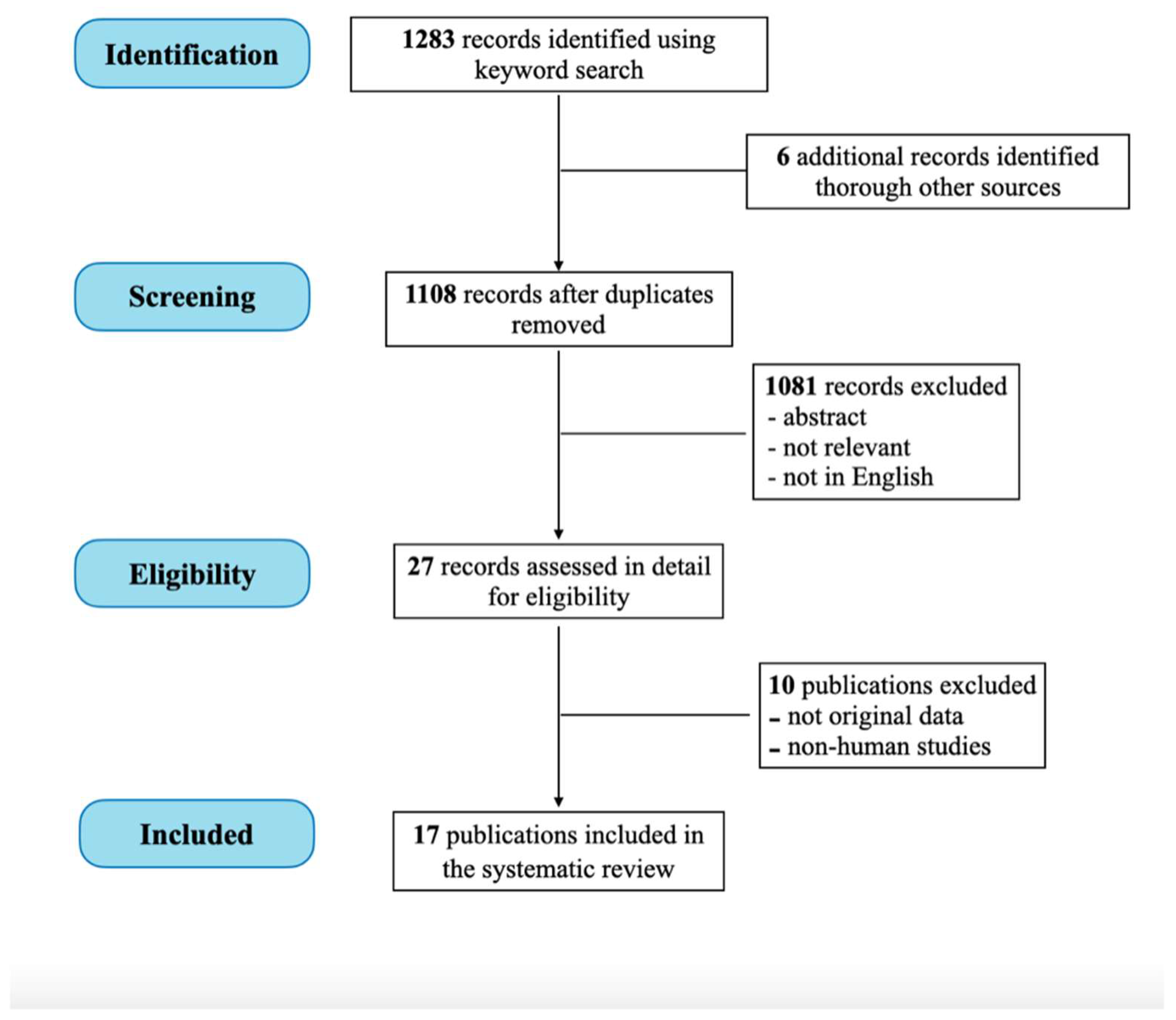
2. Materials and Methods
3. Results
3.1. VD Molecular Mechanism
3.2. VD Level and SQ
3.3. VD Supplementation and SQ
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Prosser, D.; Jones, G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem. Sci. 2004, 29, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Lips, P. Vitamin D physiology. Prog. Biophys. Mol. Biol. 2006, 92, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Garzon, S.; Fichera, M.; Panella, M.M.; Catena, U.; Schiattarella, A.; de Franciscis, P.; Vilos, G.; Tesarik, J.; Török, P.; et al. Vitamin D and gestational diabetes mellitus: Is there a link? Antioxidants 2019, 8, 511. [Google Scholar] [CrossRef] [Green Version]
- Muscogiuri, G.; Altieri, B.; Annweiler, C.; Balercia, G.; Pal, H.B.; Boucher, B.J.; Cannell, J.J.; Foresta, C.; Grübler, M.R.; Kotsa, K.; et al. Vitamin D and chronic diseases: The current state of the art. Arch. Toxicol. 2017, 91, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Haimi, M.; Kremer, R. Vitamin D deficiency/insufficiency from childhood to adulthood: Insights from a sunny country. World J. Clin. Pediatr. 2017, 6, 1. [Google Scholar] [CrossRef]
- Holick, M.F. The Vitamin D Deficiency Pandemic: A Forgotten Hormone Important for Health. Public Health Rev. 2010, 32, 267–283. [Google Scholar] [CrossRef] [Green Version]
- De Angelis, C.; Galdiero, M.; Pivonello, C.; Garifalos, F.; Menafra, D.; Cariati, F.; Salzano, C.; Galdiero, G.; Piscopo, M.; Vece, A.; et al. The role of vitamin D in male fertility: A focus on the testis. Rev. Endocr. Metab. Disord. 2017, 18, 285–305. [Google Scholar] [CrossRef]
- Fedullo, A.L.; Schiattarella, A.; Morlando, M.; Raguzzini, A.; Toti, E.; De Franciscis, P.; Peluso, I. Mediterranean Diet for the Prevention of Gestational Diabetes in the COVID-19 Era: Implications of Il-6 In Diabesity. Int. J. Mol. Sci. 2021, 22, 1213. [Google Scholar] [CrossRef]
- Blomberg Jensen, M.; Nielsen, J.E.; Jorgensen, A.; Rajpert-De Meyts, E.; Kristensen, D.M.; Jorgensen, N.; Skakkebaek, N.E.; Juul, A.; Leffers, H. Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum. Reprod. 2010, 25, 1303–1311. [Google Scholar] [CrossRef] [Green Version]
- Nangia, A.K.; Hill, O.; Waterman, M.D.; Schwender, C.E.B.; Memoli, V. Testicular Maturation Arrest to Testis Cancer: Spectrum of Expression of the Vitamin D Receptor and Vitamin D Treatment In Vitro. J. Urol. 2007, 178, 1092–1096. [Google Scholar] [CrossRef]
- Blomberg Jensen, M.; Jørgensen, A.; Nielsen, J.E.; Bjerrum, P.J.; Skalkam, M.; Petersen, J.H.; Egeberg, D.L.; Bangsbøll, S.; Andersen, A.N.; Skakkebaek, N.E.; et al. Expression of the vitamin D metabolizing enzyme CYP24A1 at the annulus of human spermatozoa may serve as a novel marker of semen quality. Int. J. Androl. 2012, 35, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Rehman, R.; Lalani, S.; Baig, M.; Nizami, I.; Rana, Z.; Gazzaz, Z.J. Association Between Vitamin D, Reproductive Hormones and Sperm Parameters in Infertile Male Subjects. Front. Endocrinol. 2018, 9, 607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blomberg Jensen, M.; Gerner Lawaetz, J.; Andersson, A.-M.; Petersen, J.H.; Nordkap, L.; Bang, A.K.; Ekbom, P.; Joensen, U.N.; Prætorius, L.; Lundstrøm, P.; et al. Vitamin D deficiency and low ionized calcium are linked with semen quality and sex steroid levels in infertile men. Hum. Reprod. 2016, 31, 1875–1885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammoud, A.O.; Meikle, A.W.; Peterson, C.M.; Stanford, J.; Gibson, M.; Carrell, D.T. Association of 25-hydroxy-vitamin D levels with semen and hormonal parameters. Asian J. Androl. 2012, 14, 855–859. [Google Scholar] [CrossRef] [Green Version]
- Tartagni, M.; Matteo, M.; Baldini, D.; Tartagni, M.V.; Alrasheed, H.; De Salvia, M.A.; Loverro, G.; Montagnani, M. Males with low serum levels of vitamin D have lower pregnancy rates when ovulation induction and timed intercourse are used as a treatment for infertile couples: Results from a pilot study. Reprod. Biol. Endocrinol. 2015, 13, 127. [Google Scholar] [CrossRef] [Green Version]
- Neville, G.; Martyn, F.; Kilbane, M.; O’Riordan, M.; Wingfield, M.; McKenna, M.; McAuliffe, F.M. Vitamin D status and fertility outcomes during winter among couples undergoing in vitro fertilization/intracytoplasmic sperm injection. Int. J. Gynecol. Obstet. 2016, 135, 172–176. [Google Scholar] [CrossRef]
- Blomberg Jensen, M.; Lawaetz, J.G.; Petersen, J.H.; Juul, A.; Jørgensen, N. Effects of Vitamin D Supplementation on Semen Quality, Reproductive Hormones, and Live Birth Rate: A Randomized Clinical Trial. J. Clin. Endocrinol. Metab. 2017, 103, 870–881. [Google Scholar] [CrossRef]
- Jiang, L.; Ji, L.; Song, J.; Qian, K. The effect of serum vitamin D levels in couples on embryo development and clinical outcomes. Reprod. Biomed. Online 2019, 38, 699–710. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [Green Version]
- Oxford International Programme in Evidence-Based Health Care. BMJ Evid.-Based Med. 2009, 14, 69. [CrossRef]
- Waud, K.; Bocca, S. Role of Vitamin D Deficiency in Male Fertility. Fertil. Steril. 2015, 103, e39. [Google Scholar] [CrossRef]
- Abbasihormozi, S.; Kouhkan, A.; Alizadeh, A.R.; Shahverdi, A.H.; Nasr-Esfahani, M.H.; Sadighi Gilani, M.A.; Salman Yazdi, R.; Matinibehzad, A.; Zolfaghari, Z. Association of vitamin D status with semen quality and reproductive hormones in Iranian subfertile men. Andrology 2016, 5, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Tirabassi, G.; Cutini, M.; Muscogiuri, G.; delli Muti, N.; Corona, G.; Galdiero, M.; Pivonello, R.; Colao, A.; Balercia, G. Association between vitamin D and sperm parameters: Clinical evidence. Endocrine 2016, 58, 194–198. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.-L.; Xu, Q.-F.; Li, S.-X.; Wei, Y.-C.; Zhu, G.-C.; Yang, C.; Shi, Y.-C. Investigation of serum vitamin D levels in Chinese infertile men. Andrologia 2016, 48, 1261–1266. [Google Scholar] [CrossRef]
- Akhavizadegan, H.; Karbakhsh, M. Comparison of Serum Vitamin D between Fertile and Infertile Men in a Vitamin D Deficient Endemic Area: A Case-Control Study. Urol. J. 2017, 84, 218–220. [Google Scholar] [CrossRef]
- Jueraitetibaike, K.; Ding, Z.; Wang, D.-D.; Peng, L.-P.; Jing, J.; Chen, L.; Ge, X.; Qiu, X.-H.; Yao, B. The effect of vitamin D on sperm motility and the underlying mechanism. Asian J. Androl. 2019, 21, 400–407. [Google Scholar] [CrossRef]
- Wadhwa, L.; Priyadarshini, S.; Fauzdar, A.; Wadhwa, S.N.; Arora, S. Impact of Vitamin D Supplementation on Semen Quality in Vitamin D-Deficient Infertile Males with Oligoasthenozoospermia. J. Obstet. Gynaecol. India 2020, 70, 44–49. [Google Scholar] [CrossRef]
- Blomberg Jensen, M.; Bjerrum, P.J.; Jessen, T.E.; Nielsen, J.E.; Joensen, U.N.; Olesen, I.A.; Petersen, J.H.; Juul, A.; Dissing, S.; Jørgensen, N. Vitamin D is positively associated with sperm motility and increases intracellular calcium in human spermatozoa. Hum. Reprod. 2011, 26, 1307–1317. [Google Scholar] [CrossRef] [Green Version]
- Ramlau-Hansen, C.H.; Moeller, U.K.; Bonde, J.P.; Olsen, J.; Thulstrup, A.M. Are serum levels of vitamin D associated with semen quality? Results from a cross-sectional study in young healthy men. Fertil. Steril. 2011, 95, 1000–1004. [Google Scholar] [CrossRef]
- Yang, B.; Sun, H.; Wan, Y.; Wang, H.; Qin, W.; Yang, L.; Zhao, H.; Yuan, J.; Yao, B. Associations between testosterone, bone mineral density, vitamin D and semen quality in fertile and infertile Chinese men. Int. J. Androl. 2012, 35, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Luk, J.; Torrealday, S.; Neal Perry, G.; Pal, L. Relevance of vitamin D in reproduction. Hum. Reprod. 2012, 27, 3015–3027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aquila, S.; Guido, C.; Middea, E.; Perrotta, I.; Bruno, R.; Pellegrino, M.; Andò, S. Human male gamete endocrinology: 1alpha, 25-dihydroxyvitamin D3 (1,25(OH)2D3) regulates different aspects of human sperm biology and metabolism. Reprod. Biol. Endocrinol. 2009, 7, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blomberg Jensen, M.; Jørgensen, A.; Nielsen, J.E.; Steinmeyer, A.; Leffers, H.; Juul, A.; Rajpert-De Meyts, E. Vitamin D Metabolism and Effects on Pluripotency Genes and Cell Differentiation in Testicular Germ Cell Tumors In Vitro and In Vivo. Neoplasia 2012, 14, 952–963, IN14–IN18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojansky, N.; Brzezinski, A.; Schenker, J.G. Seasonality in human reproduction: An update. Hum. Reprod. 1992, 7, 735–745. [Google Scholar] [CrossRef]
- Maghsoumi-Norouzabad, L.; Labibzadeh, M.; Zare Javid, A.; Ahmad Hosseini, S.; Abbas Kaydani, G.; Dastoorpur, M. The association of vitamin D, semen parameters, and reproductive hormones with male infertility: A cross-sectional study. Int. J. Reprod. Biomed. 2022, 20, 331–338. [Google Scholar] [CrossRef]
- Banks, N.; Sun, F.; Krawetz, S.A.; Coward, R.M.; Masson, P.; Smith, J.F.; Trussell, J.C.; Santoro, N.; Zhang, H.; Steiner, A.Z. Male vitamin D status and male factor infertility. Fertil. Steril. 2021, 116, 973–979. [Google Scholar] [CrossRef]
- Homayouni-Meymandi, M.; Sotoodehnejadnematalahi, F.; Nasr-Esfahani, M.H. Relationship between Serum Vitamin D in Male, Sperm Function and Clinical Outcomes in Infertile Men Candidate for ICSI: A Cohort Study. Int. J. Fertil. Steril. 2022, 16, 115–121. [Google Scholar] [CrossRef]
- Kumari, S.; Singh, K.; Kumari, S.; Nishat, H.; Tiwary, B. Association of Vitamin D and Reproductive Hormones with Semen Parameters in Infertile Men. Cureus 2021, 13, e14511. [Google Scholar] [CrossRef]
| Author | Level of Evidence | Study Design | Details (Author’s Definition) |
|---|---|---|---|
| Blomberg Jensen [10] | 2b | cohort study | prospective, cross-sectional, observational study |
| Blomberg Jensen [12] | 2b | cohort study | prospective, cross-sectional, observational study |
| Rehman [13] | 4 | case-series | prospective, cross-sectional, observational study |
| Blomberg Jensen [14] | 2b | cohort study | prospective, cross-sectional, observational study |
| Hammoud [15] | 2b | cohort study | prospective, cross-sectional, observational study |
| Tartagni [16] | 4 | case-series | prospective, cross-sectional, observational study |
| Blomberg Jensen [18] | 1b | RCT | prospective randomized controlled clinical trial |
| Blomberg Jensen [29] | 2b | cohort study | prospective, cross-sectional, observational study |
| Ramlau-Hansen [30] | 2b | cohort study | prospective, cross-sectional, observational study |
| Yang [31] | 2b | cohort study | prospective, cross-sectional, observational study |
| Waud [22] | 2b | cohort study | prospective, cross-sectional, observational study |
| Abbasihormozi [23] | 2b | cohort study | prospective, cross-sectional, observational study |
| Tirabassi [24] | 4 | case-series | prospective, cross-sectional, observational study |
| Zhu [25] | 3b | case-control | retrospective observational study |
| Akhavizadegan [26] | 3b | case-control | retrospective observational study |
| Jueraitetibaike [27] | 2b | cohort study | prospective, cross-sectional, observational study |
| Wadhwa [28] | 2b | cohort study | prospective, observational study |
| References | Patients (n) | Main End-Points | Methods | Sperm Parameters (TC-MT-MR) | T | FSH-LH | Results |
|---|---|---|---|---|---|---|---|
| Blomberg Jensen [29] | NS (300) |
|
| TC → MT ↑ MR ↑ | NV | FSH → |
|
| Ramlau-Hansen [30] | NS (307) |
|
| TC ↓ MR ↓ | → | FSH → LH → |
|
| Hammoud [15] | NS (127) |
|
| TC ↑ MT ↑ MR → | → | FSH → LH → |
|
| Yang [31] | NS (195); AS (314) * |
|
| MT ↑ MR ↑ | → | NV |
|
| Tartagni [16] | NS (90) |
|
| TC → MT → MR → | NV | NV |
|
| Abbasihormozi [23] | NS (186); AS (92) |
|
| TC → MT ↑ MR → | → | FSH → LH → |
|
| Zhu [25] | NS (79); AS (186) | Comparation VD and SQ |
| TC ↑ MT ↑ | NV | NV | Significant positive association (TC, MT p < 0.05) |
| Blomberg Jensen [14] | AS (1189) |
|
| TC → MT ↑ MR → | → | FSH → LH → |
|
| Tirabassi [24] | AS (104) |
|
| TC → MT ↑ | → | FSH → LH → |
|
| Akhavizadegan [26] | NS (116); AS (114) | Correlation VD and SQ |
| TC ↑ MT ↑ MR ↑ | NV | NV | Significant positive association (TC,MT,MR p < 0.001) |
| Rehman [13] | NS (186); AS (127) |
|
| TC ↑ MT ↑ MR ↑ | ↑ | FSH ↓ LH ↓ |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calagna, G.; Catinella, V.; Polito, S.; Schiattarella, A.; De Franciscis, P.; D’Antonio, F.; Calì, G.; Perino, A.; Cucinella, G. Vitamin D and Male Reproduction: Updated Evidence Based on Literature Review. Nutrients 2022, 14, 3278. https://doi.org/10.3390/nu14163278
Calagna G, Catinella V, Polito S, Schiattarella A, De Franciscis P, D’Antonio F, Calì G, Perino A, Cucinella G. Vitamin D and Male Reproduction: Updated Evidence Based on Literature Review. Nutrients. 2022; 14(16):3278. https://doi.org/10.3390/nu14163278
Chicago/Turabian StyleCalagna, Gloria, Valeria Catinella, Salvatore Polito, Antonio Schiattarella, Pasquale De Franciscis, Francesco D’Antonio, Giuseppe Calì, Antonino Perino, and Gaspare Cucinella. 2022. "Vitamin D and Male Reproduction: Updated Evidence Based on Literature Review" Nutrients 14, no. 16: 3278. https://doi.org/10.3390/nu14163278
APA StyleCalagna, G., Catinella, V., Polito, S., Schiattarella, A., De Franciscis, P., D’Antonio, F., Calì, G., Perino, A., & Cucinella, G. (2022). Vitamin D and Male Reproduction: Updated Evidence Based on Literature Review. Nutrients, 14(16), 3278. https://doi.org/10.3390/nu14163278










