Association between Alcohol Consumption and the Risk of Sarcopenia: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Main and Subgroup Analyses
2.5. Risk of Bias Assessment
2.6. Statistical Analysis
3. Results
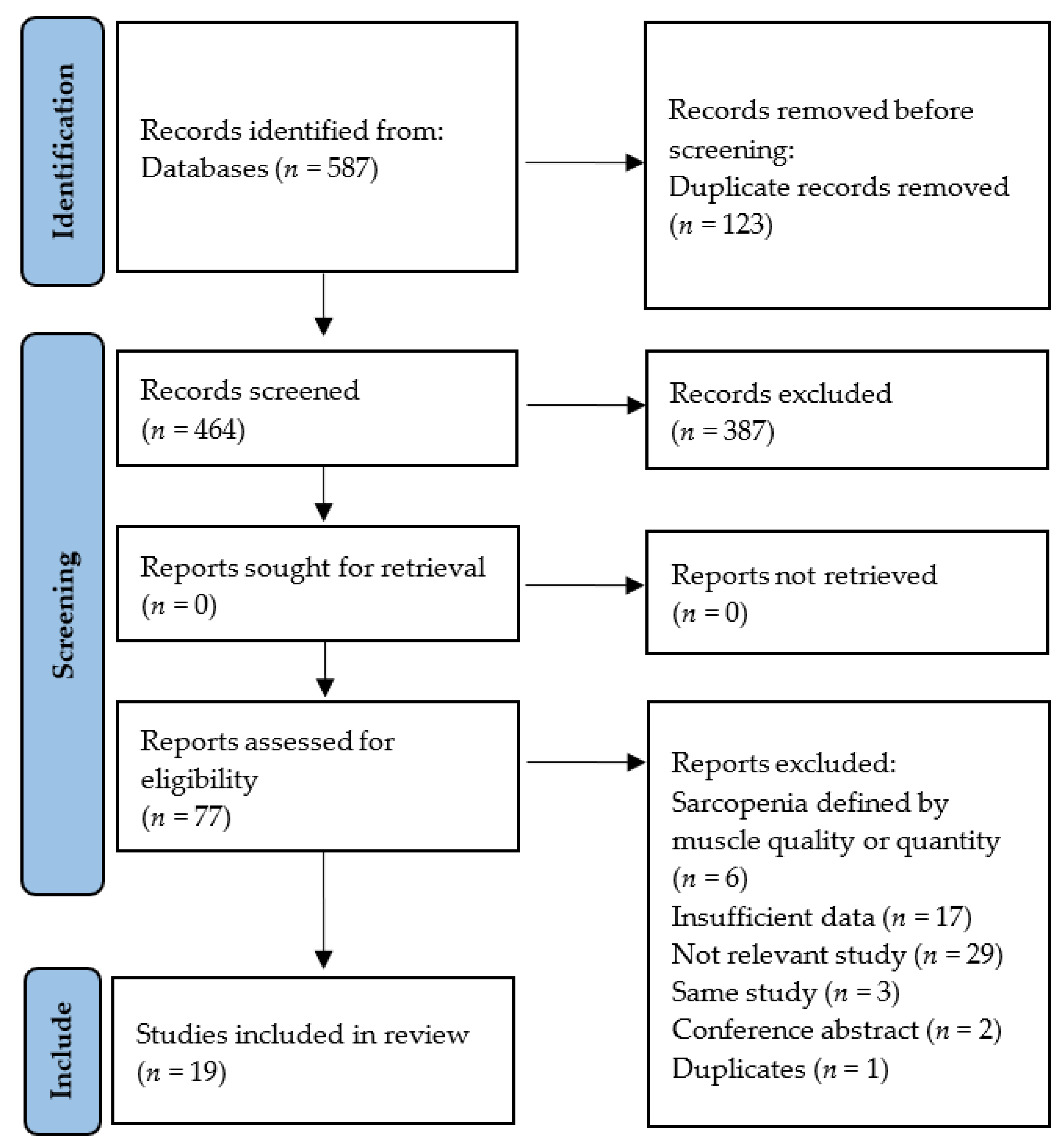
3.1. Selection of Relevant Studies
3.2. Characteristics of Included Studies
3.3. Risk of Bias
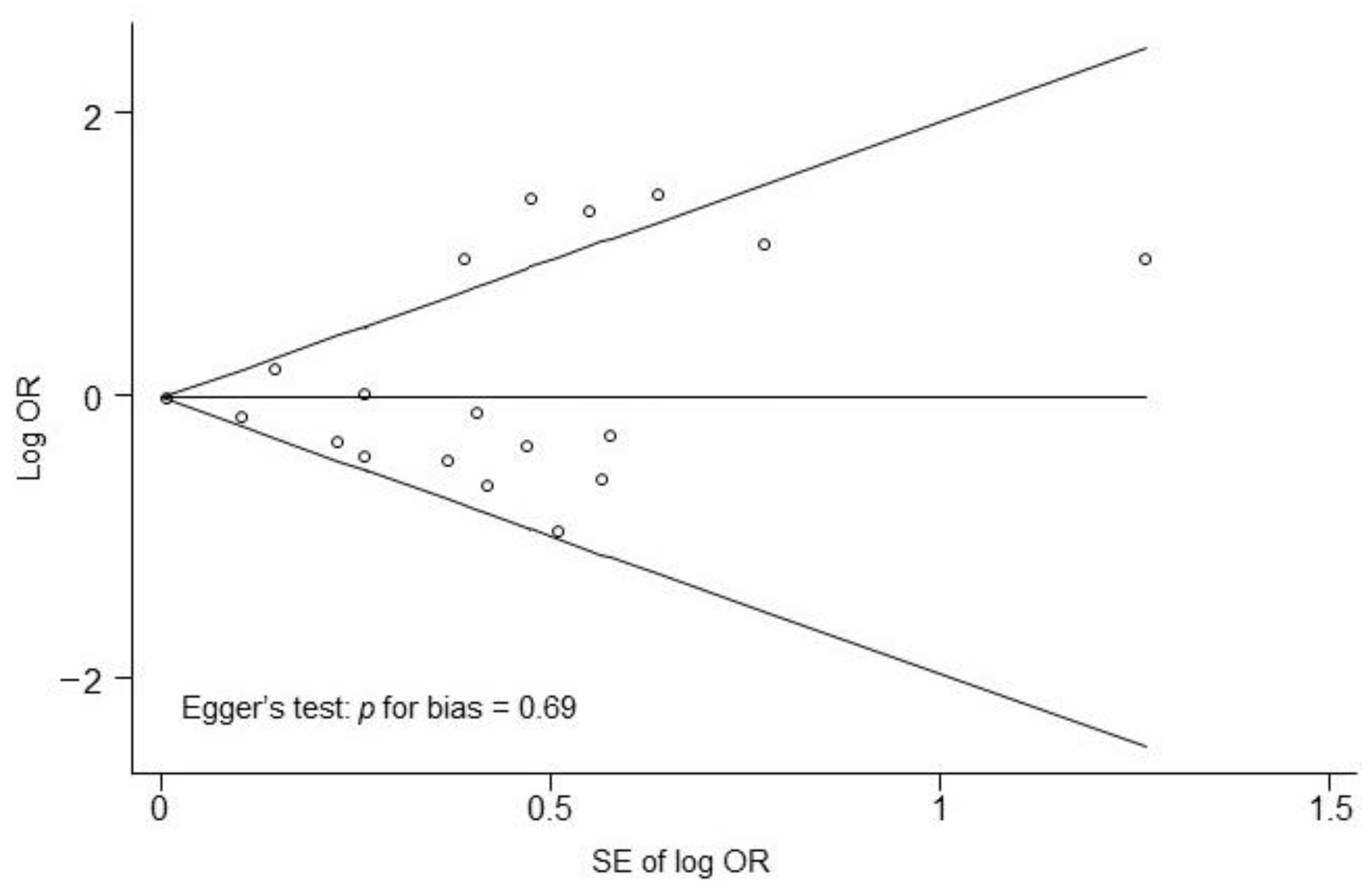
3.4. Result of the Meta-Analysis
3.5. Subgroup Meta-Analyses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AWGS | Asian Working Group for Sarcopenia |
| AWGS 2019 | Asian Working Group for Sarcopenia 2019 |
| CI | confidence interval |
| EWGSOP | European Working Group on Sarcopenia in Older People |
| EWGSOP2 | European Working Group on Sarcopenia in Older People2 |
| FNIH | Foundation for the National Institutes of Health |
| OR | odds ratio |
| NOS | Newcastle–Ottawa Scale |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-analysis |
References
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Xia, L.; Zhao, R.; Wan, Q.; Wu, Y.; Zhou, Y.; Wang, Y.; Cui, Y.; Shen, X.; Wu, X. Sarcopenia and adverse health-related outcomes: An umbrella review of meta-analyses of observational studies. Cancer Med. 2020, 9, 7964–7978. [Google Scholar] [CrossRef] [PubMed]
- Granic, A.; Sayer, A.A.; Robinson, S.M. Dietary patterns, skeletal muscle health, and sarcopenia in older adults. Nutrients 2019, 11, 745. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Niti, M.; Yap, K.B.; Tan, C.T.Y.; Nyunt, M.S.Z.; Feng, L.; Tan, B.Y.; Chan, G.; Khoo, S.A.; Chan, S.M.; et al. Effects of multi-domain lifestyle interventions on sarcopenia measures and blood biomarkers: Secondary analysis of a randomized controlled trial of community-dwelling pre-frail and frail older adults. Aging 2021, 13, 9330–9347. [Google Scholar] [CrossRef] [PubMed]
- Cervo, M.M.; Shivappa, N.; Hebert, J.R.; Oddy, W.H.; Winzenberg, T.; Balogun, S.; Wu, F.; Ebeling, P.; Aitken, D.; Jones, G.; et al. Longitudinal associations between dietary inflammatory index and musculoskeletal health in community-dwelling older adults. Clin. Nutr. 2020, 39, 516–523. [Google Scholar] [CrossRef]
- Van Dronkelaar, C.; van Velzen, A.; Abdelrazek, M.; van der Steen, A.; Weijs, P.J.M.; Tieland, M. Minerals and sarcopenia; The role of calcium, iron, magnesium, phosphorus, potassium, selenium, sodium, and zinc on muscle mass, muscle strength, and physical performance in older adults: A systematic review. J. Am. Med. Dir. Assoc. 2018, 19, 6–11. [Google Scholar] [CrossRef]
- Dupont, J.; Dedeyne, L.; Dalle, S.; Koppo, K.; Gielen, E. The role of omega-3 in the prevention and treatment of sarcopenia. Aging Clin. Exp. Res. 2019, 31, 825–836. [Google Scholar] [CrossRef] [Green Version]
- Steffl, M.; Bohannon, R.W.; Petr, M.; Kohlikova, E.; Holmerova, I. Alcohol consumption as a risk factor for sarcopenia- a meta-analysis. BMC Geriatr. 2016, 16, 99. [Google Scholar] [CrossRef] [Green Version]
- Steffl, M.; Bohannon, R.W.; Petr, M.; Kohlikova, E.; Holmerova, I. Relation between cigarette smoking and sarcopenia: Meta-analysis. Physiol. Res. 2015, 64, 419–426. [Google Scholar] [CrossRef]
- Marway, J.S.; Preedy, V.R.; Peters, T.J. Experimental alcoholic skeletal muscle myopathy is characterised by a rapid and sustained decrease in muscle RNA content. Alcohol Alcohol. 1990, 25, 401–406. [Google Scholar]
- Preedy, V.R.; Keating, J.W.; Peters, T.J. The acute effects of ethanol and acetaldehyde on rates of protein synthesis in type I and type II fibre-rich skeletal muscles of the rat. Alcohol Alcohol. 1992, 27, 241–251. [Google Scholar] [PubMed]
- Preedy, V.R.; Macallan, D.C.; Griffin, G.E.; Cook, E.B.; Palmer, T.N.; Peters, T.J. Total contractile protein contents and gene expression in skeletal muscle in response to chronic ethanol consumption in the rat. Alcohol 1997, 14, 545–549. [Google Scholar] [CrossRef]
- Thapaliya, S.; Runkana, A.; McMullen, M.R.; Nagy, L.E.; McDonald, C.; Naga Prasad, S.V.; Dasarathy, S. Alcohol-induced autophagy contributes to loss in skeletal muscle mass. Autophagy 2014, 10, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Prokopidis, K.; Witard, O.C. Understanding the role of smoking and chronic excess alcohol consumption on reduced caloric intake and the development of sarcopenia. Nutr. Res. Rev. 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fanelli Kuczmarski, M.; Mason, M.A.; Beydoun, M.A.; Allegro, D.; Zonderman, A.B.; Evans, M.K. Dietary patterns and sarcopenia in an urban African American and White population in the United States. J. Nutr. Gerontol. Geriatr. 2013, 32, 291–316. [Google Scholar] [CrossRef] [PubMed]
- Gabat, J.A.L.; Faltado, A.L.; Sedurante, M.B.; Tee, M.L. Association of obesity and sarcopenia among adult Filipinos. Osteoporos. Sarcopenia 2018, 4, 109–113. [Google Scholar] [CrossRef]
- Pang, B.W.J.; Wee, S.L.; Lau, L.K.; Jabbar, K.A.; Seah, W.T.; Ng, D.H.M.; Tan, Q.L.L.; Chen, K.K.; Jagadish, M.U.; Ng, T.P. Prevalence and associated factors of sarcopenia in Singaporean adults—The Yishun Study. J. Am. Med. Dir. Assoc. 2021, 22, 885. [Google Scholar] [CrossRef]
- Ko, Y.C.; Chie, W.C.; Wu, T.Y.; Ho, C.Y.; Yu, W.R. A cross-sectional study about the relationship between physical activity and sarcopenia in Taiwanese older adults. Sci. Rep. 2021, 11, 11488. [Google Scholar] [CrossRef]
- Daskalopoulou, C.; Wu, Y.T.; Pan, W.; Gine Vazquez, I.; Prince, M.; Prina, M.; Tyrovolas, S. Factors related with sarcopenia and sarcopenic obesity among low- and middle-income settings: The 10/66 DRG study. Sci. Rep. 2020, 10, 20453. [Google Scholar] [CrossRef]
- Castillo, E.M.; Goodman-Gruen, D.; Kritz-Silverstein, D.; Morton, D.J.; Wingard, D.L.; Barrett-Connor, E. Sarcopenia in elderly men and women: The Rancho Bernardo study. Am. J. Prev. Med. 2003, 25, 226–231. [Google Scholar] [CrossRef]
- Akune, T.; Muraki, S.; Oka, H.; Tanaka, S.; Kawaguchi, H.; Nakamura, K.; Yoshimura, N. Exercise habits during middle age are associated with lower prevalence of sarcopenia: The ROAD study. Osteoporos. Int. 2014, 25, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, J.; Chen, X.; Hou, L.; Lin, X.; Yang, M. Prevalence and associated factors of sarcopenia in nursing home residents: A systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 2019, 20, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed on 1 May 2022).
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials. 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Lau, E.M.; Lynn, H.S.; Woo, J.W.; Kwok, T.C.; Melton, L.J. Prevalence of and risk factors for sarcopenia in elderly Chinese men and women. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 213–216. [Google Scholar] [CrossRef] [Green Version]
- Domiciano, D.S.; Figueiredo, C.P.; Lopes, J.B.; Caparbo, V.F.; Takayama, L.; Menezes, P.R.; Bonfa, E.; Pereira, R.M.R. Discriminating sarcopenia in community-dwelling older women with high frequency of overweight/obesity: The Sao Paulo Ageing & Health Study (SPAH). Osteoporos. Int. 2013, 24, 595–603. [Google Scholar]
- Lin, C.C.; Lin, W.Y.; Meng, N.H.; Li, C.I.; Liu, C.S.; Lin, C.H.; Chang, C.K.; Lee, Y.D.; Lee, C.C.; Li, T.S. Sarcopenia prevalence and associated factors in an elderly Taiwanese metropolitan population. J. Am. Geriatr. Soc. 2013, 61, 459–462. [Google Scholar] [CrossRef]
- Hai, S.; Wang, H.; Cao, L.; Liu, P.; Zhou, J.; Yang, Y.; Dong, B. Association between sarcopenia with lifestyle and family function among community-dwelling Chinese aged 60 years and older. BMC Geriatr. 2017, 17, 187. [Google Scholar] [CrossRef]
- Han, P.; Kang, L.; Guo, Q.; Wang, J.; Zhang, W.; Shen, S.; Wang, X.; Dong, R.; Ma, Y.; Shi, Y.; et al. Prevalence and factors associated with sarcopenia in suburb-dwelling older Chinese using the Asian Working Group for Sarcopenia Definition. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Samper-Ternent, R.; Reyes-Ortiz, C.; Ottenbacher, K.J.; Cano, C.A. Frailty and sarcopenia in Bogotá: Results from the SABE Bogotá Study. Aging Clin. Exp. Res. 2017, 29, 265–272. [Google Scholar] [CrossRef]
- Wu, C.H.; Chen, K.T.; Hou, M.T.; Chang, Y.F.; Chang, C.S.; Liu, P.Y.; Wu, S.J.; Chiu, C.J.; Jou, I.M.; Chen, C.Y. Prevalence and associated factors of sarcopenia and severe sarcopenia in older Taiwanese living in rural community: The Tianliao Old People study 04. Geriatr. Gerontol. Int. 2014, 14, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Confortin, S.C.; Ono, L.M.; Barbosa, A.R.; d’Orsi, E. Sarcopenia and its association with changes in socioeconomic, behavioral, and health factors: The EpiFloripa Elderly Study. Cad. Saude. Publica 2018, 34, e00164917. [Google Scholar] [CrossRef] [Green Version]
- Petermann-Rocha, F.; Chen, M.; Gray, S.R.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Factors associated with sarcopenia: A cross-sectional analysis using UK Biobank. Maturitas 2020, 133, 60–67. [Google Scholar] [CrossRef]
- Sousa-Santos, A.R.; Afonso, C.; Borges, N.; Santos, A.; Padrão, P.; Moreira, P.; Amaral, T.F. Factors associated with sarcopenia and undernutrition in older adults. Nutr. Diet. 2019, 76, 604–612. [Google Scholar] [CrossRef]
- Su, Y.; Hirayama, K.; Han, T.F.; Izutsu, M.; Yuki, M. Sarcopenia prevalence and risk factors among Japanese community dwelling older adults living in a snow-covered city according to EWGSOP2. J. Clin. Med. 2019, 8, 291. [Google Scholar] [CrossRef] [Green Version]
- Park, E.Y.; Han, K.H.; Chung, T.H.; Kim, N.Y.; Lee, J.M.; Choi, S.J.; Kim, J.K. Association between reproductive span and sarcopenia. Int. J. Environ. Res. Public Health 2020, 18, 154. [Google Scholar] [CrossRef]
- Gavaler, J.S. Alcohol effects on hormone levels in normal postmenopausal women and in postmenopausal women with alcohol-induced cirrhosis. Recent Dev. Alcohol 1995, 12, 199–208. [Google Scholar]
- Enns, D.L.; Tiidus, P.M. The influence of estrogen on skeletal muscle: Sex matters. Sports Med. 2010, 40, 41–58. [Google Scholar] [CrossRef]
- Kitajima, Y.; Ono, Y. Estrogens maintain skeletal muscle and satellite cell functions. J. Endocrinol. 2016, 229, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Playdon, M.C.; Coburn, S.B.; Moore, S.C.; Brinton, L.A.; Wentzensen, N.; Anderson, G.; Wallace, R.; Falk, R.T.; Pfeiffer, R.; Xu, X.; et al. Alcohol and oestrogen metabolites in postmenopausal women in the Women’s Health Initiative Observational Study. Br. J. Cancer 2018, 118, 448–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, Y.J.; Lim, H.J.; Lee, Y.J.; Lee, H.S.; Linton, J.A.; Lee, J.W.; Kang, H.T. Associations between high-risk alcohol consumption and sarcopenia among postmenopausal women. Menopause 2017, 24, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.I.; Ha, Y.C.; Lee, Y.K.; Choi, H.; Yoo, M.J.; Koo, K.H. High prevalence of sarcopenia among binge drinking elderly women: A nationwide population-based study. BMC Geriatr. 2017, 17, 114. [Google Scholar] [CrossRef]
- Wu, X.; Li, X.; Xu, M.; Zhang, Z.; He, L.; Li, Y. Sarcopenia prevalence and associated factors among older Chinese population: Findings from the China Health and Retirement Longitudinal Study. PLoS ONE 2021, 16, e0247617. [Google Scholar] [CrossRef]
- Ferreira, M.P.; Weems, M.K. Alcohol consumption by aging adults in the United States: Health benefits and detriments. J. Am. Diet. Assoc. 2008, 108, 1668–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Boca, F.K.; Darkes, J. The validity of self-reports of alcohol consumption: State of the science and challenges for research. Addiction 2003, 98, 1–12. [Google Scholar] [CrossRef]
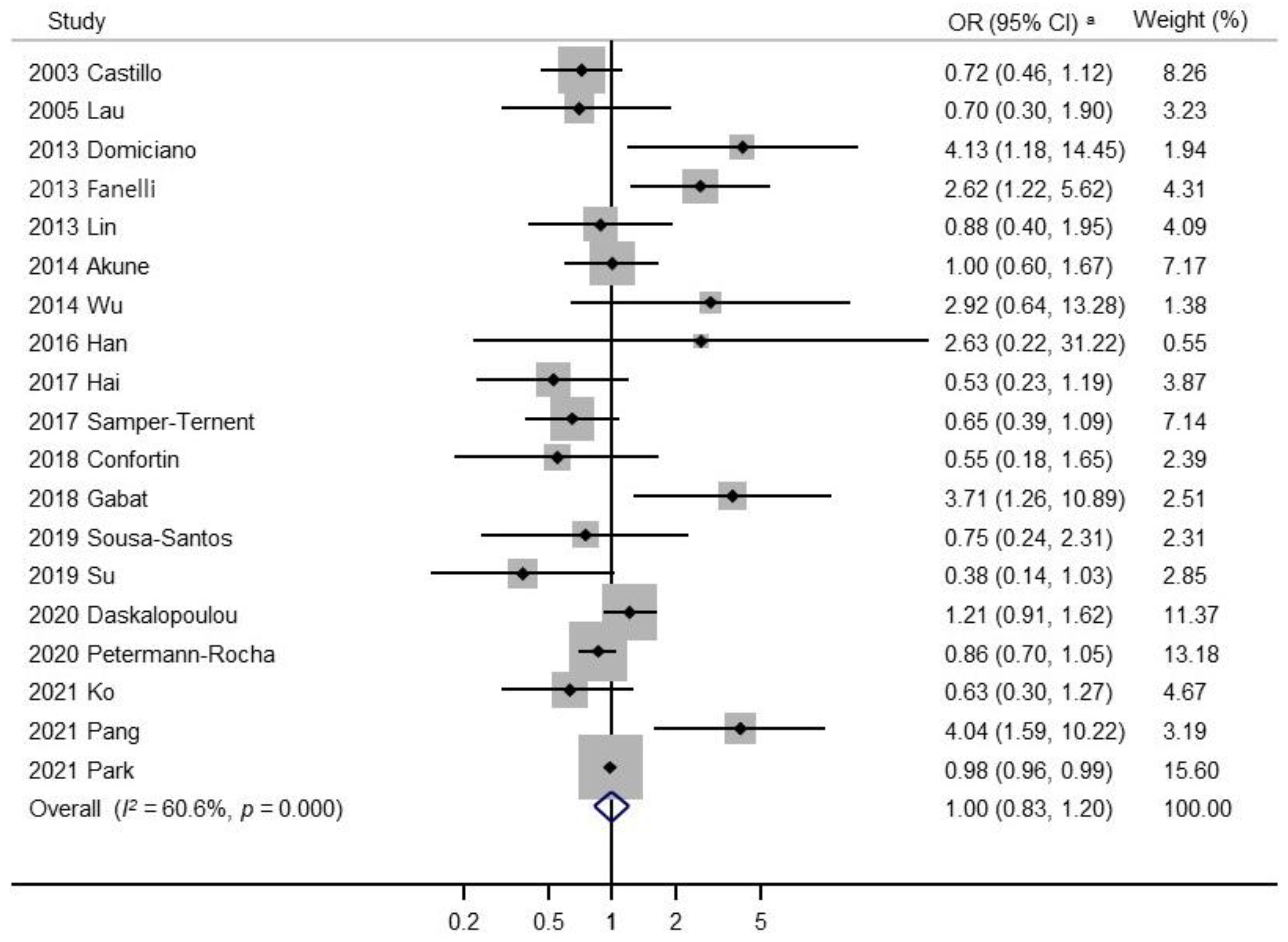

| Study | Country, Study Design | Definition of Sarcopenia | Body Composition | Participants (Sarcopenia/ No Sarcopenia) | Sex (M/W) | Age (Years) | Expose (Highest Category) | Reference (Lowest Category) | OR (95% CI) | Adjusted Variables |
|---|---|---|---|---|---|---|---|---|---|---|
| 2003 Castillo [20] | USA, cross-sectional study | FFM of ≥2.0 SDs below the mean of a young reference group | BIA | 1700 (102/1598) | 694M/1006W | 55–98 | ≥181.0 g/week for men, ≥120.5 g/week for women | <181.0 g/week for men, <120.5 g/week for women | 0.72 (0.46–1.12) | Age, exercise, smoking |
| 2005 Lau [27] | Hong Kong, cross-sectional study | Total adjusted skeletal muscle mass two SDs or more below the mean of young men | DXA | 173 (32/141) | 173M | ≥70 | Daily | Never | 0.70 (0.30–1.90) | Age |
| 2013 Domiciano [28] | Brazil, cross-sectional study | Baumgartner’s criteria (ASM/height2 is less than 5.45 kg/m2) | DXA | 611 (23/588) | 611W | ≥65 | Three or more units daily | No | 4.13 (1.18–14.45) | Age |
| 2013 Fanelli [15] | USA, cross-sectional study | EWGSOP | DXA | 2176 (139/2037) | 945M/1231W | 30–64 | Alcohol drink cluster | Healthy pasta/rice reference cluster | 2.62 (1.22–5.62) | Sex, race, age, socioeconomic status |
| 2013 Lin [29] | Taiwan, cross-sectional study | EWGSOP | DXA | 761 (99/662) | 407M/354W | ≥65 | Current | Never | 0.88 (0.40–1.95) | Age, sex, marital status, regular exercise habit, comorbidity status (diabetes mellitus, stroke, heart disease, cataract, fall history) |
| 2014 Akune [21] | Japan, cross-sectional study | EWGSOP | BIA | 1000 (129/871) | 349M/651W | ≥65 | Yes | No | 1.00 (0.60–1.67) | Age, sex, BMI |
| 2014 Wu [33] | Taiwan, cross-sectional study | EWGSOP | BIA | 549 (70/479) | 285M/264W | ≥65 | Yes | No | 2.92 (0.64–13.28) | None |
| 2016 Han [31] | China, cross-sectional study | AWGS | BIA | 1069 (99/970) | 437M/533W | ≥60 | Daily | Never or former | 2.63 (0.22–31.22) | Age, BMI, widowed, living alone, illiteracy, farming, diabetes, peptic ulcer, pulmonary disease |
| 2017 Hai [30] | China, cross-sectional study | AWGS | BIA | 834 (88/746) | 415M/419W | ≥60 | Drinking ≥2/week | Not drinking | 0.53 (0.23–1.19) | Gender, age, educational level, diabetes, hypertension, heart disease, stroke, MMSE score, GDS score |
| 2017 Samper-Ternent [32] | Colombia, cross-sectional study | EWGSOP | DXA | 1442 (166/1276) | 562M/880W | ≥60 | ≥1 glass per day | No alcohol consumption | 0.65 (0.39–1.09) | Age, sex, education, comorbidities, MMSE score, GDS score, IADL disability, ADL disability, smoking |
| 2018 Confortin [34] | Brazil, cross-sectional study | Baumgartner’s criteria (ASMI: <7.26 kg/m2 for men and <5.5 kg/m2 for women) | DXA | 598 (126/472) | 207M/391W | ≥60 | Continued consuming or started consuming alcohol | Continued not consuming or stopped consuming alcohol | 0.55 (0.18–1.65) | Age, schooling, income, marital status, family arrangement, smoking, physical activity, social support, self-rated health |
| 2018 Gabat [16] | Philippine, cross-sectional study | EWGSOP | FBCM | 164 (10/154) | 37M/127W | ≥40 | Yes | No | 3.71 (1.26–10.89) | Controlling possible confounders |
| 2019 Sousa-Santos [36] | Portugal, cross-sectional study | EWGSOP2 | MAMC | 1500 (66/1434) | 628M/872W | ≥65 | Women >1/day: men >2/day | None | 0.75 (0.24–2.31) | Sex, age, residential status, regional area, educational level, marital status, BMI, physical activity level, undernutrition status |
| 2019 Su [37] | Japan, cross-sectional study | EWGSOP2 | BIA | 310 (25/285) | 89M/221W | ≥65 | Consumes alcohol | None | 0.38 (0.14–1.03) | None |
| 2020 Daskalopoulou [19] | LMICs., Multicenter population study | FINH | Body fat percent (%BF) | 8694 (-/-) | 8694MW | ≥65 | 1–14 units/week for women and 1–21 units/week for men | No/heavy | 1.21 (0.91–1.62) | Dementia, depression, diabetes, stroke |
| 2020 Petermann-Rocha [35] | UK, cross-sectional study | EWGSOP2 | BIA | 396283 (1678/394605) | 187046M/209237W | 38–73 | Higher | Lower | 0.86 (0.70–1.05) | Age, sex, deprivation, education attainment |
| 2021 Ko [18] | Taiwan, cross-sectional study | AWGS 2019 | BIA | 500 (138/362) | 235M/265W | ≥65 | Yes | No | 0.63 (0.30–1.27) | Sex, institutionalization, age, BMI, albumin, hemoglobin, HDL-C levels, history of cardiovascular disease, education level |
| 2021 Pang [17] | Singapore, cross-sectional study | AWGS 2019 | DXA | 536 (132/404) | 226M/310W | 21–90 | Yes | No | 4.04 (1.59–10.22) | None |
| 2021 Park [38] | Korea, cross-sectional study | AWGS 2019 | DXA | 3970 (704/3266) | 3970W | ≥40 | Yes | No | 0.98 (0.96–0.99) | None |
| Study | Selection | Comparability | Exposure | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5A | 5B | 6 | 7 | 8 | ||
| 2003 Castillo [20] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 7 |
| 2005 Lau [27] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 5 |
| 2013 Domiciano [28] | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 6 |
| 2013 Fanelli [15] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 7 |
| 2013 Lin [29] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 5 |
| 2014 Akune [21] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 8 |
| 2014 Wu [33] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 6 |
| 2016 Han [31] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 7 |
| 2017 Hai [30] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 7 |
| 2017 Samper-Ternent [32] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 7 |
| 2018 Confortin [34] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 8 |
| 2018 Gabat [16] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 7 |
| 2019 Sousa-Santos [36] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 7 |
| 2019 Su [37] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 5 |
| 2020 Daskalopoulou [19] | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 7 |
| 2020 Petermann-Rocha [35] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 7 |
| 2021 Ko [18] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 7 |
| 2021 Pang [17] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 7 |
| 2021 Park [38] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 7 |
| Factors | Number of Studies | Summary OR (95% CI) | Heterogeneity, I2 (%) |
|---|---|---|---|
| Age | |||
| 40 years and older [16,20,38] | 3 | 1.07 (0.65–1.74) | 74.0 |
| 60 years and older [30,31,32,34] | 4 | 0.63 (0.42–0.94) | 0.0 |
| 65 years and older [15,18,19,21,28,29,33,36,37] | 9 | 0.97 (0.69–1.36) | 44.8 |
| 65 years and younger [27] | 1 | 2.62 (1.22–5.62) | 100 |
| Definition of sarcopenia | |||
| AWGS [30,31] | 2 | 0.76 (0.21–2.80) | 30.9 |
| AWGS 2019 [17,18,38] | 3 | 1.24 (0.58–2.65) | 80.7 |
| EWGSOP [15,16,21,29,32,33] | 6 | 1.38 (0.79–2.41) | 68.5 |
| EWGSOP2 [35,36,37] | 3 | 0.76 (0.52–1.12) | 20.1 |
| Region | |||
| America [15,20,28,32,34] | 5 | 1.12 (0.58–2.16) | 75.7 |
| Asia [16,17,18,21,27,29,30,31,33,37,38] | 11 | 1.03 (0.74–1.45) | 60.0 |
| Europe [35,36] | 2 | 0.86 (0.70–1.05) | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.-H.; Bae, Y.-J. Association between Alcohol Consumption and the Risk of Sarcopenia: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 3266. https://doi.org/10.3390/nu14163266
Hong S-H, Bae Y-J. Association between Alcohol Consumption and the Risk of Sarcopenia: A Systematic Review and Meta-Analysis. Nutrients. 2022; 14(16):3266. https://doi.org/10.3390/nu14163266
Chicago/Turabian StyleHong, Seung-Hee, and Yun-Jung Bae. 2022. "Association between Alcohol Consumption and the Risk of Sarcopenia: A Systematic Review and Meta-Analysis" Nutrients 14, no. 16: 3266. https://doi.org/10.3390/nu14163266
APA StyleHong, S.-H., & Bae, Y.-J. (2022). Association between Alcohol Consumption and the Risk of Sarcopenia: A Systematic Review and Meta-Analysis. Nutrients, 14(16), 3266. https://doi.org/10.3390/nu14163266






