The Effects of Persimmon (Diospyros kaki L.f.) Oligosaccharides on Features of the Metabolic Syndrome in Zebrafish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Extraction of D. kaki Fruit Extract and Source of the Oligosaccharides
2.2. The Investigation of the Chemical Constituent of D. kaki Fruit Extract
2.3. Animals
2.4. Anti-Insulin Resistance in Zebrafish
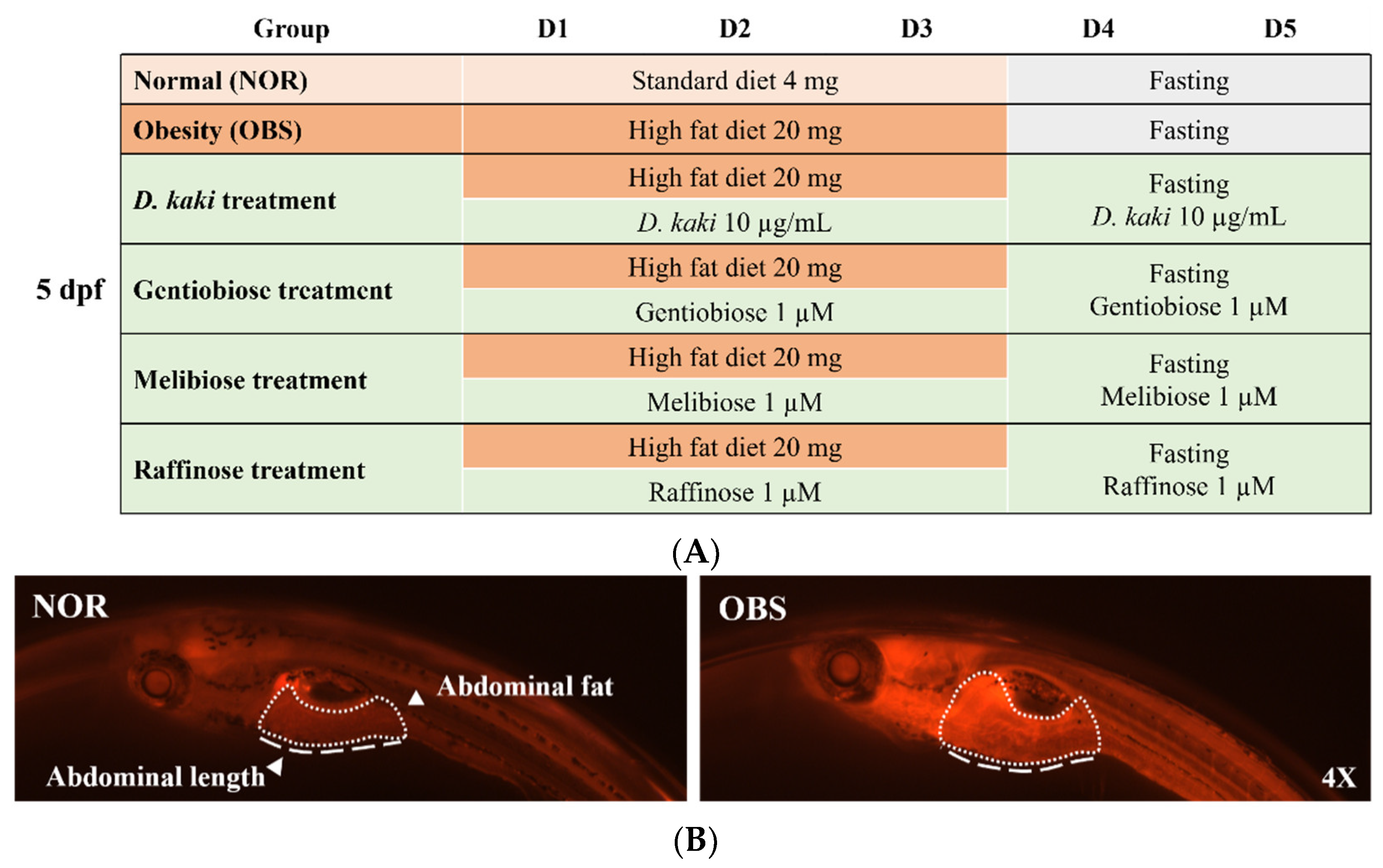
2.5. Obesity-Preventing Effect in Zebrafish
2.6. Molecular Docking of Isolated Compounds and PTP1B
2.7. Enzyme Activity Assays of PTP1B
2.8. Real Time-Quantitative Polymerase Chain Reaction (RT-qPCR)
2.9. Statistical Analysis
3. Results and Discussions
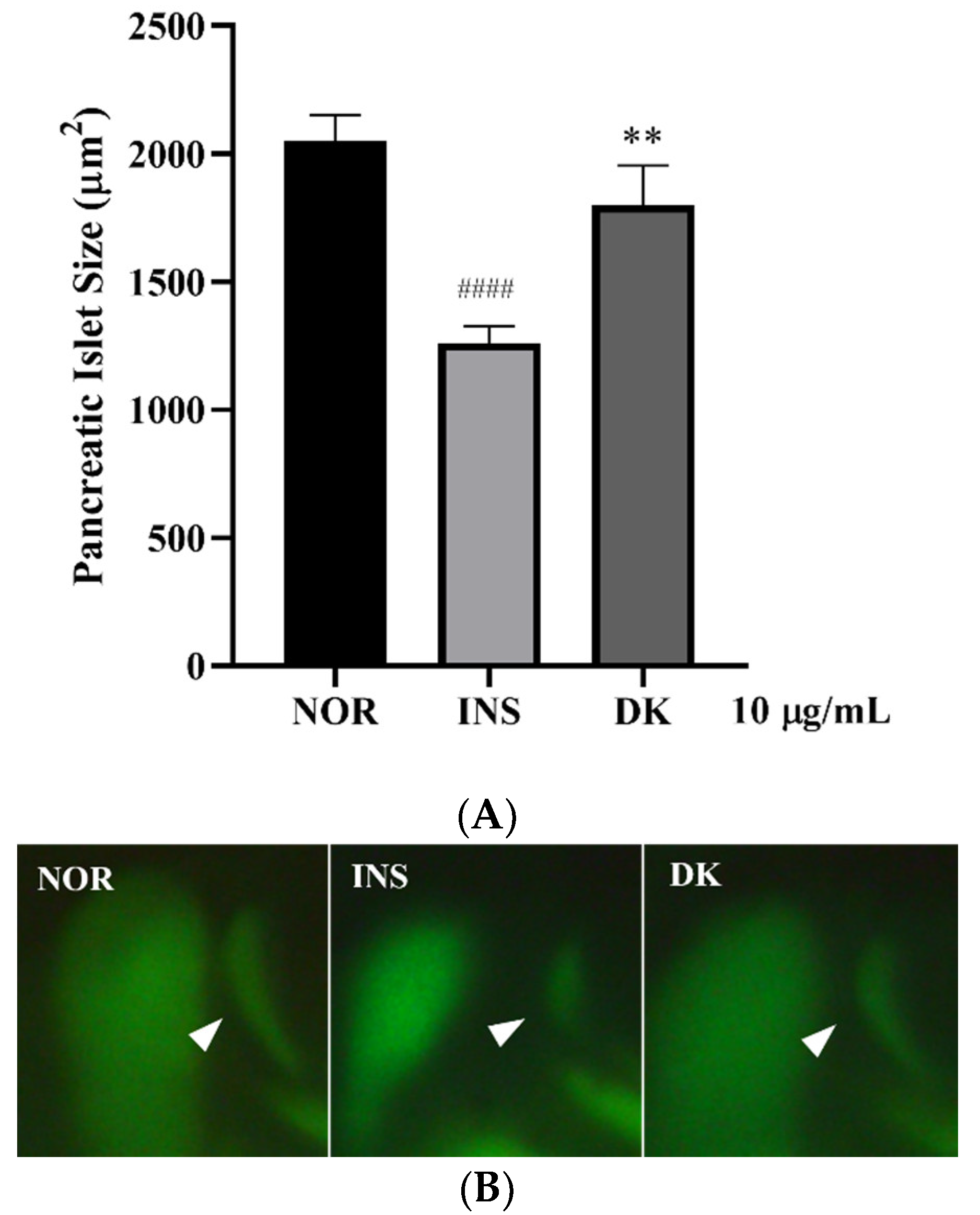
3.1. Screening of D. kaki Fruits Extracts for Anti-Insulin Resistance in Zebrafish
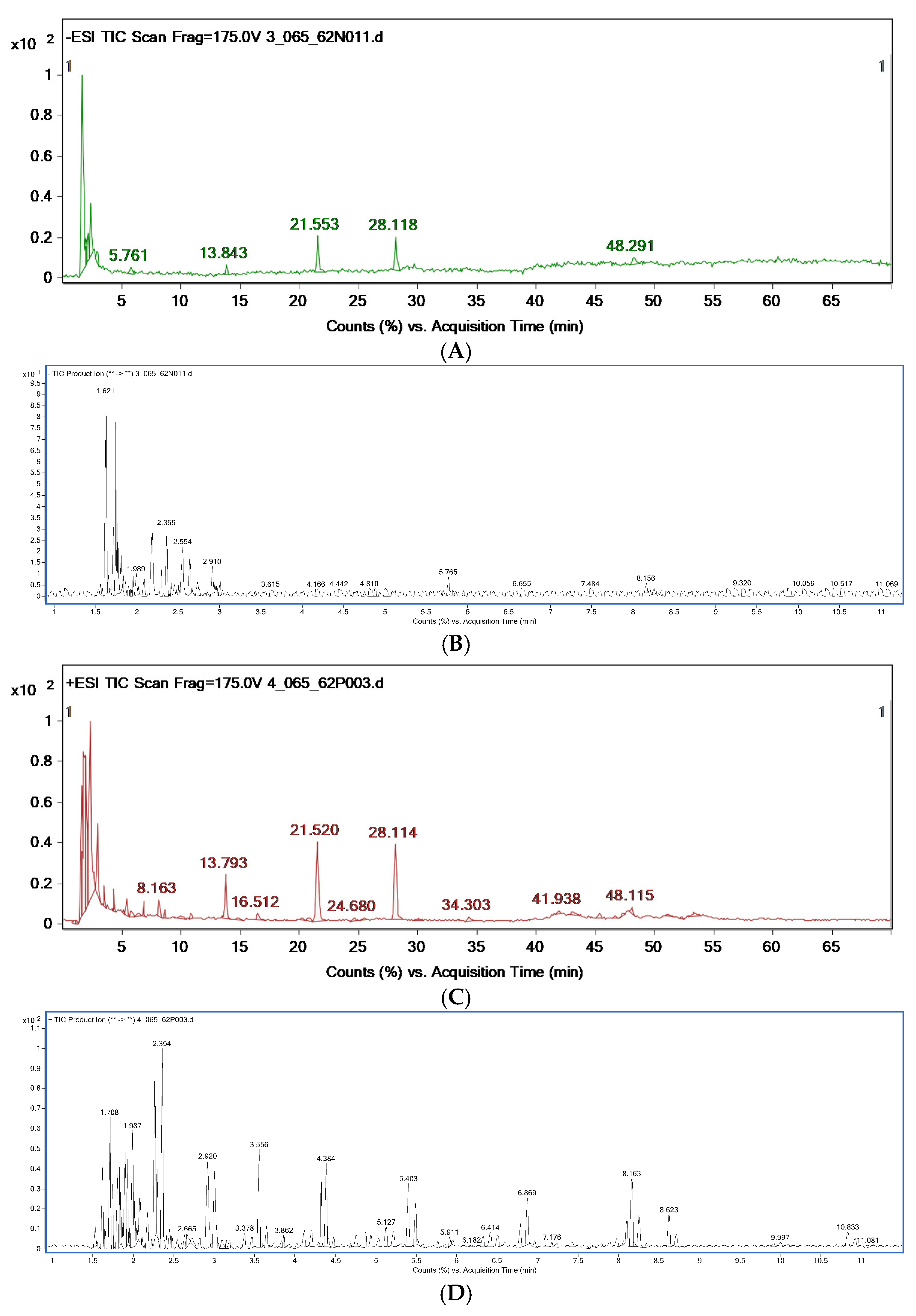
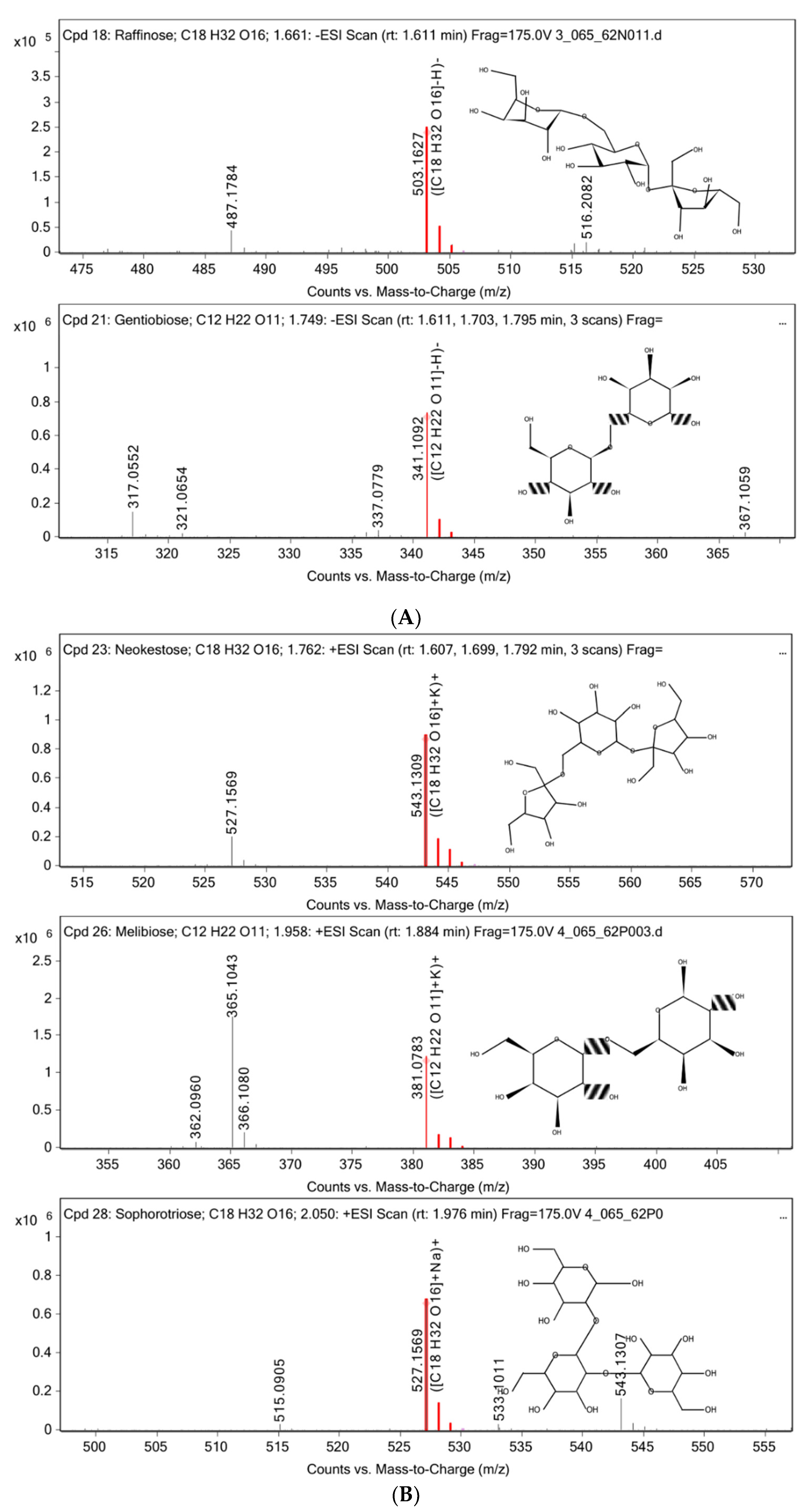
3.2. Chemical Constituent Analysis by HPLC-MS/MS
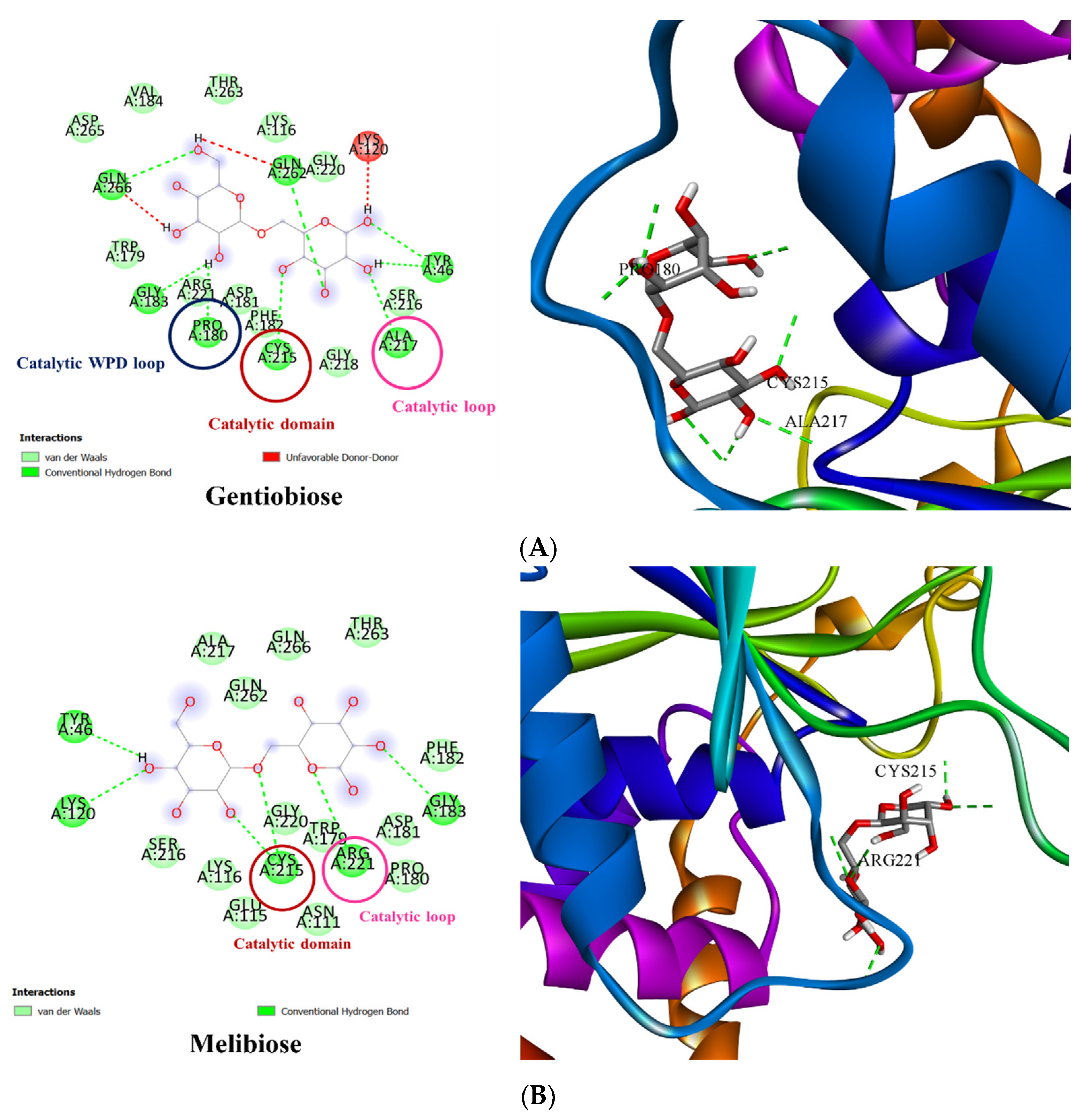
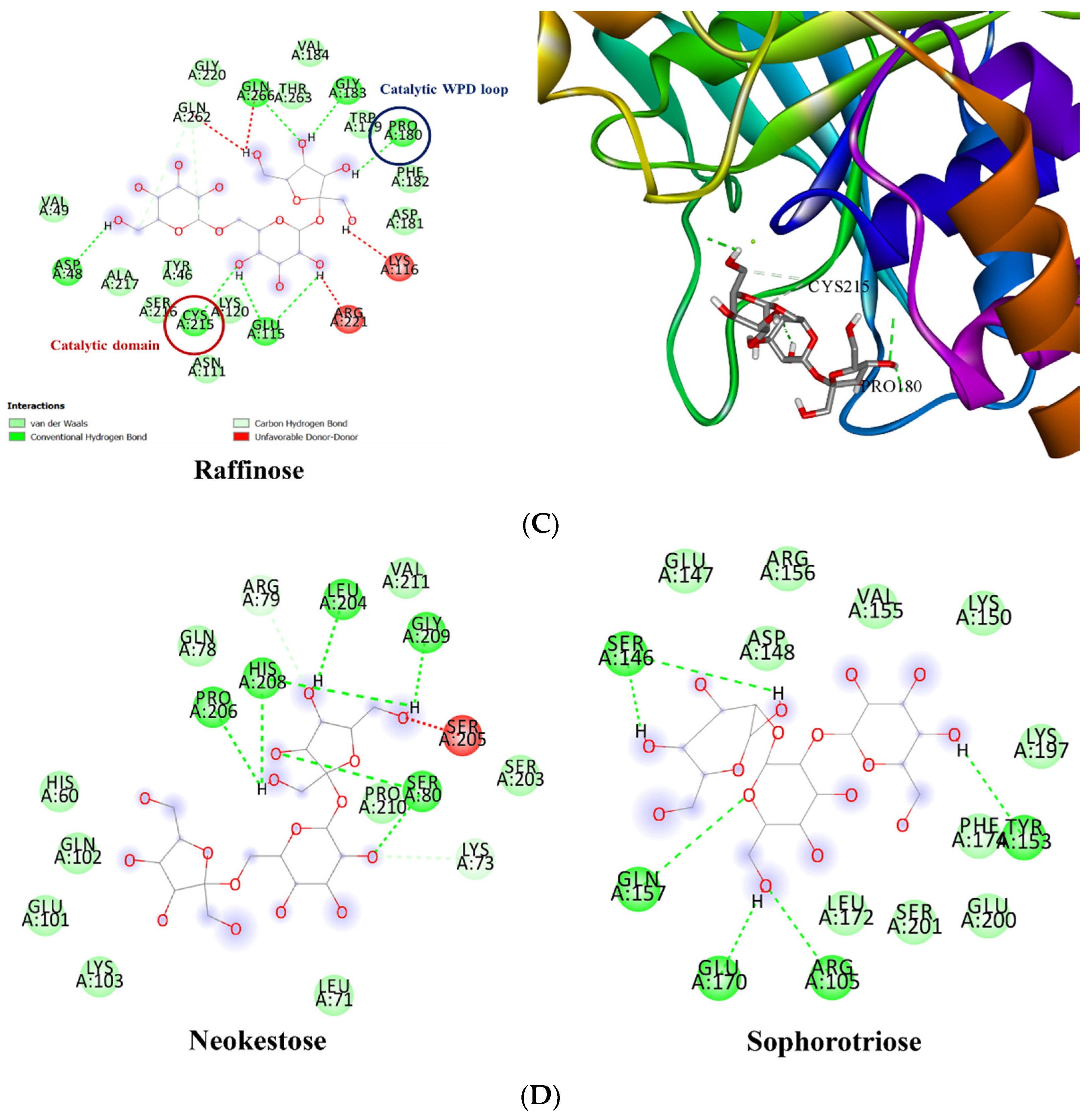
3.3. Molecular Docking of PTP1B to the Oligosaccharide from D. kaki Fruit
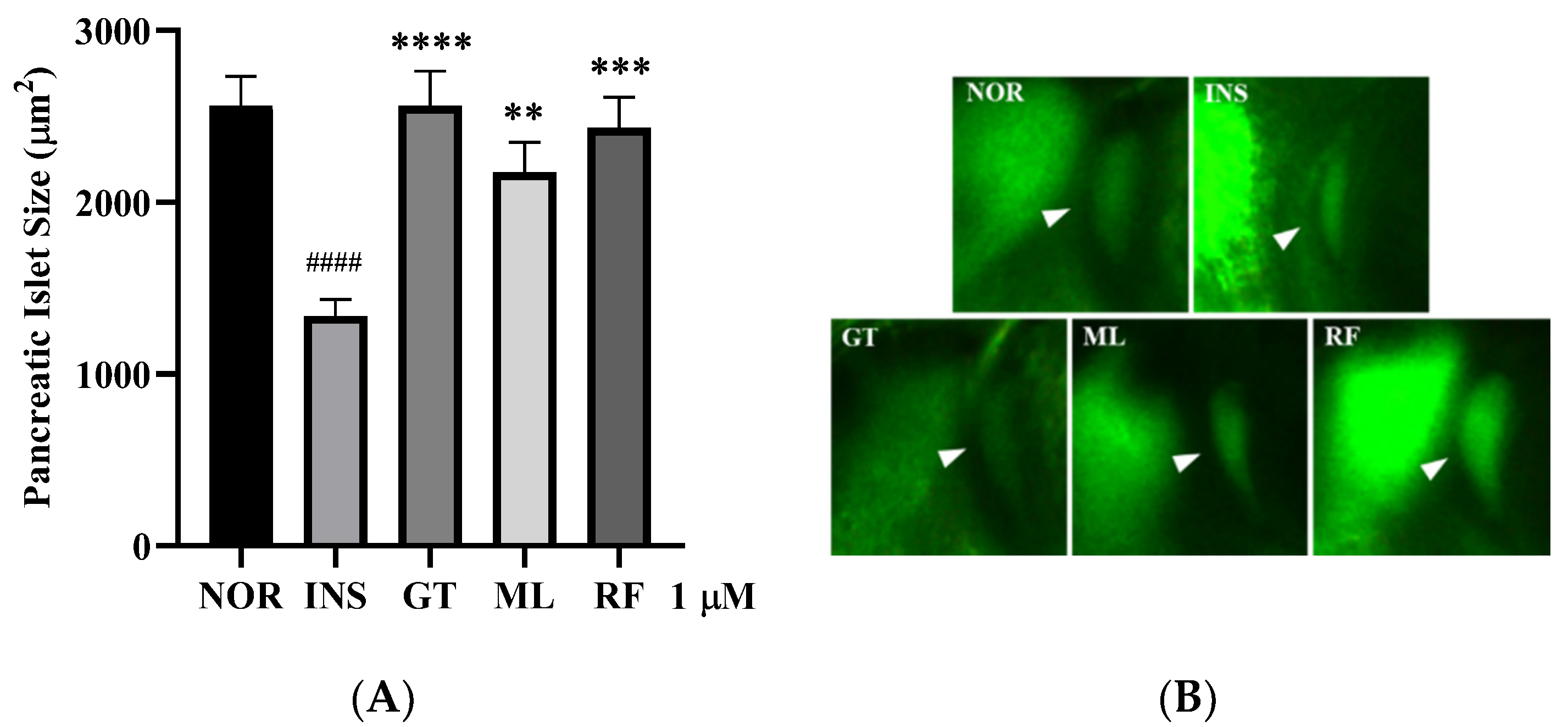
3.4. Anti-Insulin Resistance in Zebrafish Induced by Oligosaccharides from D. kaki Fruit
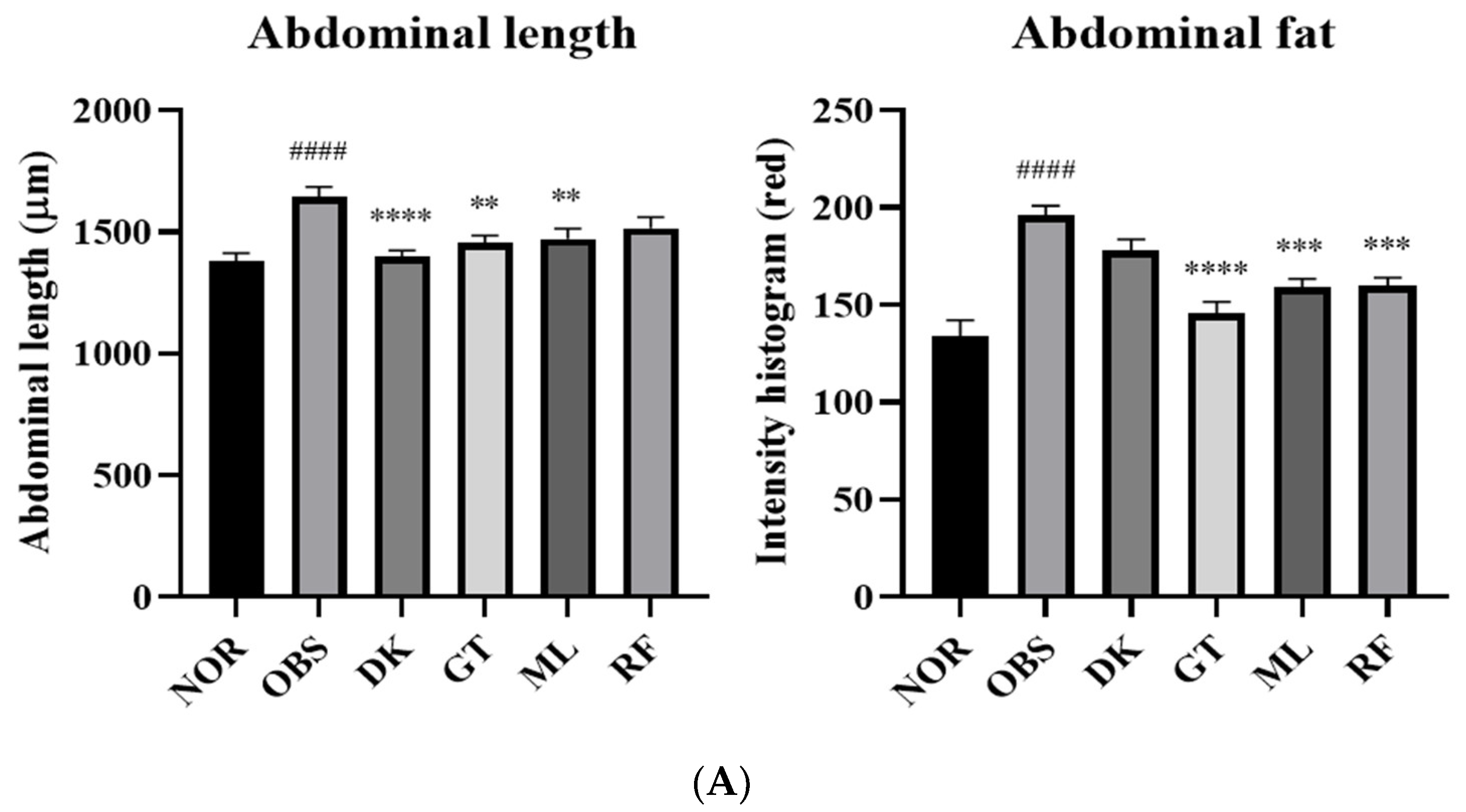
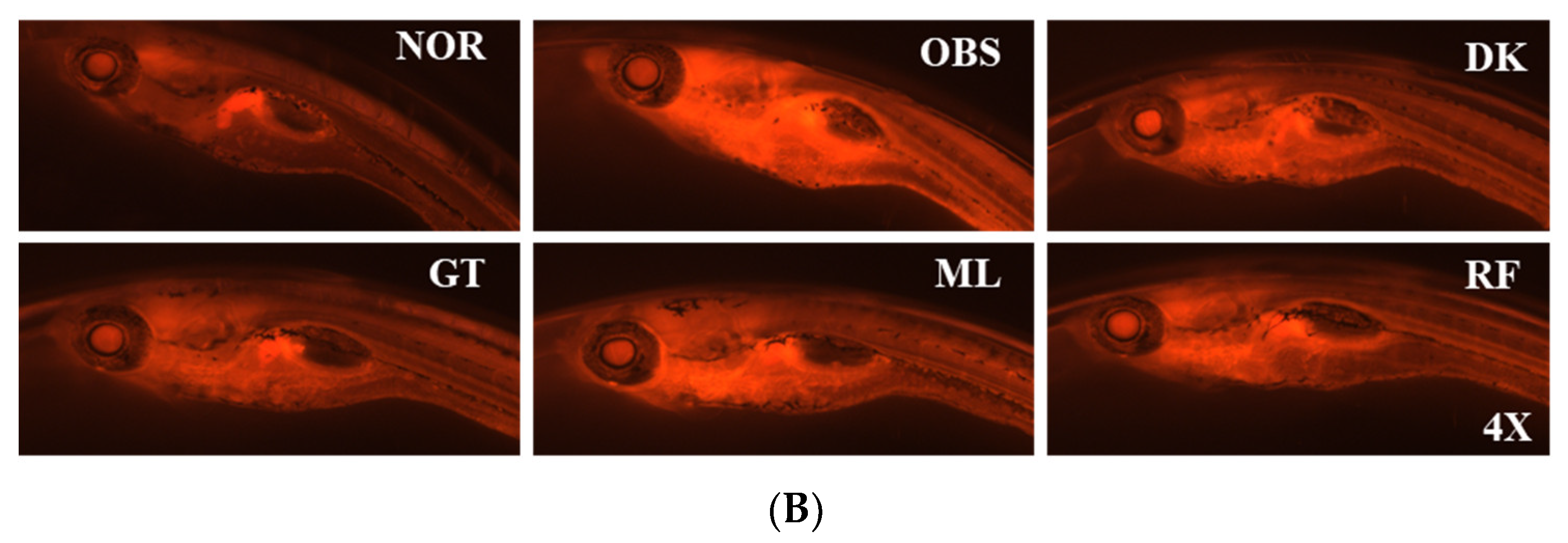
3.5. The Investigation of the Obesity-Preventing Effect of the D. kaki Fruit Extract and Its Oligosaccharides
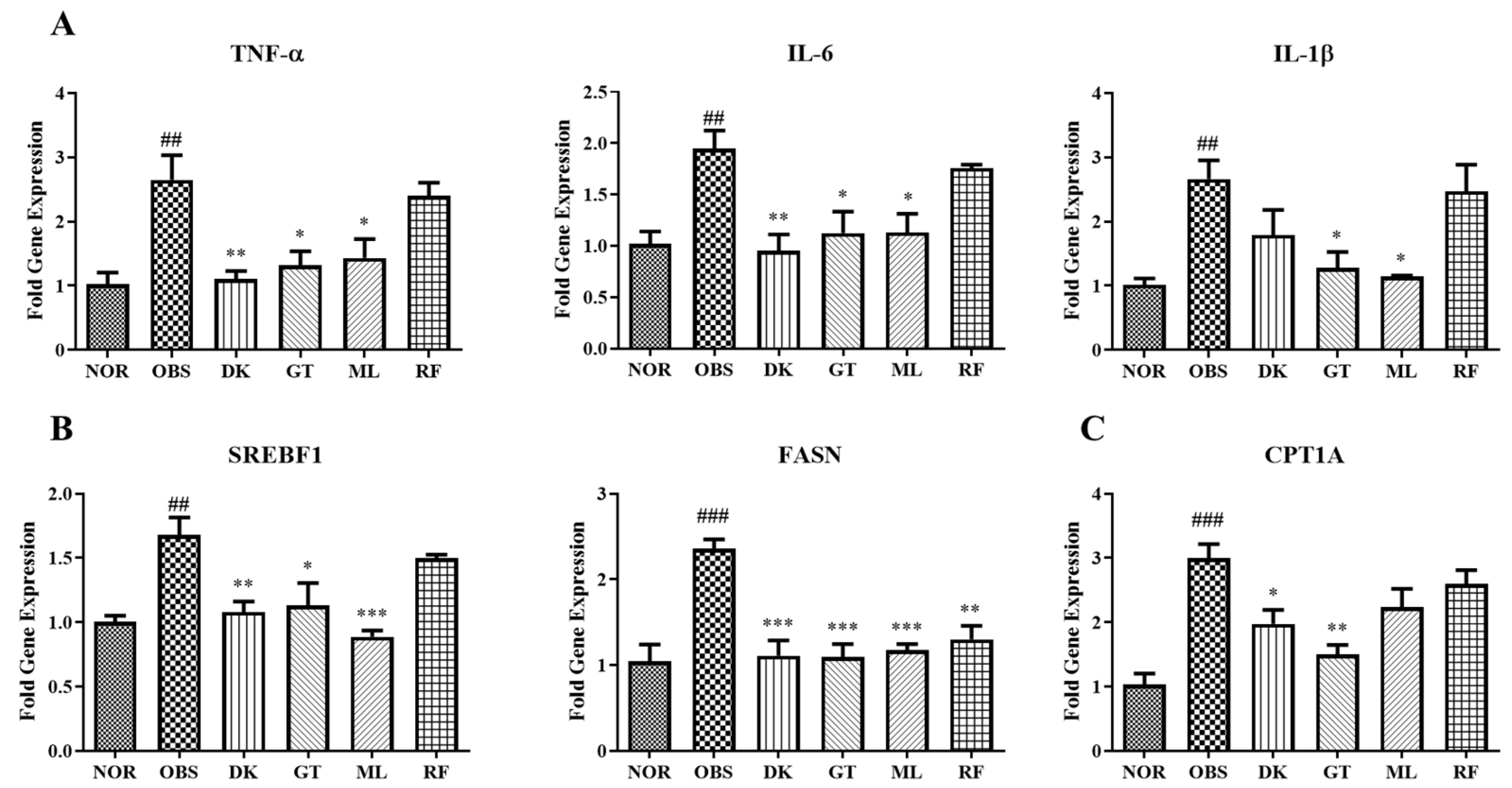
3.6. Genes Expression on High-Fat-Diet-Induced Obesity in Zebrafish Larvae
3.7. Enzyme Activity Assays of PTP1B to D. kaki Fruit and Oligosaccharides
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grundy, S.M. Metabolic syndrome pandemic. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 629–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef] [Green Version]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Martyn, J.A.; Kaneki, M.; Yasuhara, S. Obesity-induced insulin resistance and hyperglycemia: Etiologic factors and molecular mechanisms. Anesthesiology 2008, 109, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.I.; Ketsawatsomkron, P.; Belin de Chantemele, E.J.; Mintz, J.D.; Muta, K.; Salet, C.; Black, S.N.; Tremblay, M.L.; Fulton, D.V.; Marrero, M.B.; et al. Deletion of Protein Tyrosine Phosphatase 1b Improves Peripheral Insulin Resistance and Vascular Function in Obese, Leptin-Resistant Mice via Reduced Oxidant Tone. Circ. Res. 2009, 105, 1013–1022. [Google Scholar] [CrossRef]
- Banno, R.; Zimmer, D.; De Jonghe, B.C.; Atienza, M.; Rak, K.; Yang, W.; Bence, K.K. PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. J. Clin. Investig. 2010, 120, 720–734. [Google Scholar] [CrossRef] [Green Version]
- Yip, S.C.; Saha, S.; Chernoff, J. PTP1B: A double agent in metabolism and oncogenesis. Trends Biochem. Sci. 2010, 35, 442–449. [Google Scholar] [CrossRef] [Green Version]
- Guillot, R.; Cortés, R.; Navarro, S.; Mischitelli, M.; García-Herranz, V.; Sánchez, E.; Cal, L.; Navarro, J.C.; Míguez, J.M.; Afanasyev, S.; et al. Behind melanocortin antagonist overexpression in the zebrafish brain: A behavioral and transcriptomic approach. Horm. Behav. 2016, 82, 87–100. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Li, C.; Yuan, G.; Xie, F. Anatomical and histological observation on the pancreas in adult zebrafish. Pancreas 2007, 34, 120–125. [Google Scholar] [CrossRef]
- Zang, L.; Shimada, Y.; Nishimura, Y.; Tanaka, T.; Nishimura, N. Repeated blood collection for blood tests in adult zebrafish. J. Vis. Exp. 2015, 102, e53272. [Google Scholar] [CrossRef]
- Anderson, J.L.; Carten, J.D.; Farber, S.A. Zebrafish lipid metabolism: From mediating early patterning to the metabolism of dietary fat and cholesterol. Methods Cell Biol. 2011, 101, 111–141. [Google Scholar]
- Moss, J.B.; Koustubhan, P.; Greenman, M.; Parsons, M.J.; Walter, I.; Moss, L.G. Regeneration of the pancreas in adult zebrafish. Diabetes 2009, 58, 1844–1851. [Google Scholar] [CrossRef] [Green Version]
- Intine, R.V.; Olsen, A.S.; Sarras, M.P. A zebrafish model of diabetes mellitus and metabolic memory. J. Vis. Exp. 2013, 72, e50232. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.A.; Cho, E.J.; Tanaka, T.; Yokozawa, T. Inhibitory activities of proanthocyanidins from persimmon against oxidative stress and digestive enzymes related to diabetes. J. Nutr. Sci. Vitaminol. 2007, 53, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Ramasubbu, N.; Ragunath, C.; Mishra, P.J.; Thomas, L.M.; Gyémánt, G.; Kandra, L. Human salivary alpha-amylase Trp58 situated at subsite -2 is critical for enzyme activity. Eur. J. Biochem. 2004, 271, 2517–2529. [Google Scholar] [CrossRef]
- Thuong, P.T.; Lee, C.H.; Dao, T.T.; Nguyen, P.H.; Kim, W.G.; Lee, S.J.; Oh, W.K. Triterpenoids from the leaves of Diospyros kaki (persimmon) and their inhibitory effects on protein tyrosine phosphatase 1B. J. Nat. Prod. 2008, 71, 1775–1778. [Google Scholar] [CrossRef]
- Lee, Y.A.; Kim, Y.J.; Cho, E.J.; Yokozawa, T. Ameliorative effects of proanthocyanidin on oxidative stress and inflammation in streptozotocin-induced diabetic rats. J. Agrie. Food Chem. 2008, 55, 9395–9400. [Google Scholar] [CrossRef]
- Zhu, D.; Yan, Q.; Liu, J.; Wu, X.; Jiang, Z. Can functional oligosaccharides reduce the risk of diabetes mellitus? FASEB J. 2019, 33, 11655–11667. [Google Scholar] [CrossRef] [Green Version]
- Swennen, K.; Courtin, C.M.; Delcour, J.A. Non-digestible oligosaccharides with prebiotic properties. Crit. Rev. Food Sci. Nutr. 2006, 46, 459–471. [Google Scholar] [CrossRef]
- Nuankaew, W.; Heemman, A.; Wattanapiromsakul, C.; Shi, J.H.; Kim, N.W.; Yasmin, T.; Jeong, S.Y.; Nam, Y.H.; Hong, B.N.; Dej-adisai, S.; et al. Anti-insulin resistance effect of constituents from Senna siamea on zebrafish model, its molecular docking, and structure–activity relationships. J. Nat. Med. 2021, 75, 520–531. [Google Scholar] [CrossRef]
- Ha, M.; Seong, S.; Nguyen, T.; Cho, W.; Ah, K.; Ma, J.; Woo, M.; Choi, J.; Min, B. Chalcone derivatives from the root bark of Morus alba L. act as inhibitors of PTP1B and α-glucosidase. Phytochemistry 2018, 155, 114–125. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Walsh, A.B.; Wu, L.; McNamarai, D.J.; Dobrusini, E.M.; Miller, W.T. Determinants of substrate recognition in the protein-tyrosine phosphatase, PTP1. J. Biol. Chem. 1996, 271, 5386–5392. [Google Scholar] [CrossRef] [Green Version]
- Jin, T.; Yu, H.; Huang, X.F. Selective binding modes and allosteric inhibitory effects of lupane triterpenes on protein tyrosine phosphatase 1B. Sci. Rep. 2016, 6, 20766. [Google Scholar] [CrossRef] [Green Version]
- Tingaud-Sequeira, A.; Ouadah, N.; Babin, P.J. Zebrafish obesogenic test: A tool for screening molecules that target adiposity. J. Lipid Res. 2011, 52, 1765–1772. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Xu, Y.Q.; Guo, S.Y.; Li, C.Q. Rapid analysis of hypolipidemic drugs in a live zebrafish assay. J. Pharmacol. Toxicol. Methods 2015, 72, 47–52. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Z.Y. PTP1B as a drug target: Recent developments in PTP1B inhibitor discovery. Drug Discov. Today 2007, 12, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.O.; Ermolieff, J.; Jirousek, M.R. Protein tyrosine phosphatase 1B inhibitors for diabetes. Nat. Rev. Drug Discov. 2002, 1, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Wiesmann, C.; Barr, K.; Kung, J.; Zhu, J.; Erlanson, D.A.; Shen, W.; Fahr, B.J.; Zhong, M.; Taylor, L.; Randal, M.; et al. Allosteric inhibition of protein tyrosine phosphatase 1B. Nat. Struct. Mol. Biol. 2004, 11, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, J.; Lu, S.; Huang, W.; Geng, L.; Shen, Q.; Zhang, J. The mechanism of allosteric inhibition of protein tyrosine phosphatase 1B. PLoS ONE 2014, 9, e97668. [Google Scholar]
- Cooper, J.M. The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Del Fabbro, S.; Calder, P.; Childs, C. Microbiota-independent immunological effects of non-digestible oligosaccharides in the context of inflammatory bowel diseases. Proc. Nutr. Soc. 2020, 79, 468–478. [Google Scholar] [CrossRef]
- Kaarel, A.; Signe, A.; Karin, E.; Anneli, L.; Tiia, V.; Madis, J.; Triinu, V.; Tiina, A. Composition and metabolism of fecal microbiota from normal and overweight children are differentially affected by melibiose, raffinose and raffinose-derived fructans. Anaerobe 2018, 52, 100–110. [Google Scholar]
- Padmanaban, M.; Gopal, T.; Rajendran, A.B.; Baddireddi, S.L. Raffinose from Costus speciosus attenuates lipid synthesis through modulation of PPARs/SREBP1c and improves insulin sensitivity through PI3K/AKT. Chem. Biol. Interact. 2018, 284, 80–89. [Google Scholar]
- Oka, T.; Nishimura, Y.; Zang, L.; Hirano, M.; Shimada, Y.; Wang, Z.; Umemoto, N.; Kuroyanagi, J.; Nishimura, N.; Tanaka, T. Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiol. 2010, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Kern, L.; Mittenbühler, M.J.; Vesting, A.J.; Ostermann, A.L.; Wunderlich, C.M.; Wunderlich, F.T. Obesity-Induced TNFα and IL-6 Signaling: The Missing Link between Obesity and Inflammation—Driven Liver and Colorectal Cancers. Cancers 2019, 11, 24. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Bell, E.H.; Mischel, P.; Chakravarti, A. Targeting SREBP-1-driven lipid metabolism to treat cancer. Curr. Pharm. Des. 2014, 20, 2619–2626. [Google Scholar] [CrossRef] [Green Version]
- Das, M.; Sha, J.; Hidalgo, B.; Aslibekyan, S.; Do, A.N.; Zhi, D.; Sun, D.; Zhang, T.; Li, S.; Chen, W.; et al. Association of DNA Methylation at CPT1A Locus with Metabolic Syndrome in the Genetics of Lipid Lowering Drugs and Diet Network (GOLDN) Study. PLoS ONE 2016, 11, e0145789. [Google Scholar] [CrossRef]
- Donath, M.Y.; Dalmas, E.; Sauter, N.S.; Böni-Schnetzler, M. Inflammation in obesity and diabetes: Islet dysfunction and therapeutic opportunity. Cell Metab. 2013, 17, 860–872. [Google Scholar] [CrossRef] [Green Version]
| Gene | Forward Sequence | Reverse Sequence |
|---|---|---|
| TNF-α | GCT TAT GAG CCA TGC AGT GA | TGC CCA GTC TGT CTC CTT CT |
| IL6 | AAG GGG TCA GGA TCA GCA C | GCT GTA GAT TCG CGT TAG ACA TC |
| IL1B | TGG CGA ACG TCA TCC AAG | GGA GCA CTG GGC GAC GCA TA |
| SREBF1 | CAT CCA CAT GGC TCT GAG TG | CTC ATC CAC AAA GAA GCG GT |
| FASN | GAG AAA GCT TGC CAA ACA GG | GAG GGT CTT GCA GGA GAC AG |
| CPT1A | CAT GAG GCT CTT CGG CAA | AAG AGC AGG CCT AAG GAT G |
| RT | Height | Mass | Identification Result |
|---|---|---|---|
| LC-ESI-MS/MS chromatograms | |||
| 1.661 | 497,154 | 504.17 | Raffinose |
| 1.749 | 4,200,859 | 342.1166 | Gentiobiose |
| 1.991 | 482,479 | 342.1165 | Gentiobiose |
| 2.188 | 1,329,997 | 646.1966 | beta-D-4-Deoxy-delta4-GlcA-(1->4)-beta-D-Glc-(1->4)-alpha-L-Rha-(1->3)-beta-D-Glc |
| 2.318 | 294,628 | 342.1169 | Gentiobiose |
| 2.33 | 73,904 | 192.0268 | 2,5-Didehydro-D-gluconate |
| 2.464 | 252,822 | 219.1112 | Pantothenic Acid |
| 2.602 | 1,051,653 | 224.0327 | Dehydrochorismic acid |
| 2.811 | 145,798 | 342.1157 | D-(+)-Cellobiose |
| 5.836 | 415,037 | 382.1839 | 1,2,10-Trihydroxydihydro-trans-linalyl oxide 7-O-beta-D-glucopyranoside |
| 46.14 | 208,962 | 294.1834 | Myrsinone |
| 48.25 | 409,723 | 328.2251 | (9R,10S,12Z)-9,10-Dihydroxy-8-oxo-12-octadecenoic acid |
| LC+ESI-MS/MS chromatograms | |||
| 1.59 | 142,819 | 317.1212 | N-(1-Deoxy-1-fructosyl) histidine |
| 1.762 | 4,633,119 | 504.1678 | Neokestose |
| 1.933 | 6,561,444 | 261.1204 | Epidermin |
| 1.958 | 1,734,862 | 342.1152 | Melibiose |
| 2.05 | 1,517,744 | 504.1676 | Sophorotriose |
| 2.234 | 735,399 | 438.1151 | Loquatoside |
| 2.301 | 6,245,852 | 271.1647 | Prolyl-Arginine |
| 2.519 | 998,209 | 342.1153 | Sucrose |
| 2.703 | 1,104,006 | 366.1413 | N-(1-Deoxy-1-fructosyl) tryptophan |
| 3.013 | 6,275,606 | 187.063 | 3-Amino-2-naphthoic acid |
| 3.486 | 1,147,920 | 422.2117 | 6alpha-Fluoro-17-hydroxycorticosterone 21-acetate |
| 3.615 | 266,556 | 418.1823 | Glu Arg Asp |
| 3.841 | 595,699 | 478.1672 | Kelampayoside A |
| 4.406 | 758,433 | 444.2559 | Arg Asn Arg |
| 4.912 | 1,230,160 | 361.233 | Lys Lys Ser |
| 5.13 | 1,574,844 | 510.2643 | Nebramycin factor 5′ |
| 5.498 | 4,669,055 | 436.2273 | Flurandrenolide |
| 5.82 | 568,261 | 404.1644 | Asp Asp Arg |
| 6.418 | 1,185,915 | 532.3082 | 5β-Cyprinolsulfate |
| 6.879 | 3,665,061 | 458.2718 | Arg Gln Arg |
| 8.719 | 2,455,730 | 486.3239 | Docosahexaenoyl Serotonin |
| 10.941 | 1,224,227 | 568.3056 | Ceanothine E |
| 16.685 | 162,831 | 538.2945 | Euphorbia factor Ti2 |
| 21.609 | 2,415,031 | 672.4863 | PE-Cer(d14:2(4E,6E)/20:1(11Z)(2OH)) |
| 28.06 | 5,609,787 | 775.6128 | PE(21:0/17:0) |
| 42.038 | 1,513,417 | 273.2659 | C16 Sphinganine |
| 43.05 | 1,422,944 | 229.24 | Xestoaminol C |
| 48.205 | 1,502,361 | 317.2921 | Phytosphingosine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuankaew, W.; Lee, H.K.; Nam, Y.H.; Shim, J.H.; Kim, N.W.; Shin, S.W.; Kim, M.C.; Shin, S.Y.; Hong, B.N.; Dej-adisai, S.; et al. The Effects of Persimmon (Diospyros kaki L.f.) Oligosaccharides on Features of the Metabolic Syndrome in Zebrafish. Nutrients 2022, 14, 3249. https://doi.org/10.3390/nu14163249
Nuankaew W, Lee HK, Nam YH, Shim JH, Kim NW, Shin SW, Kim MC, Shin SY, Hong BN, Dej-adisai S, et al. The Effects of Persimmon (Diospyros kaki L.f.) Oligosaccharides on Features of the Metabolic Syndrome in Zebrafish. Nutrients. 2022; 14(16):3249. https://doi.org/10.3390/nu14163249
Chicago/Turabian StyleNuankaew, Wanlapa, Hyo Kyu Lee, Youn Hee Nam, Ji Heon Shim, Na Woo Kim, Sung Woo Shin, Min Cheol Kim, Seung Yeon Shin, Bin Na Hong, Sukanya Dej-adisai, and et al. 2022. "The Effects of Persimmon (Diospyros kaki L.f.) Oligosaccharides on Features of the Metabolic Syndrome in Zebrafish" Nutrients 14, no. 16: 3249. https://doi.org/10.3390/nu14163249
APA StyleNuankaew, W., Lee, H. K., Nam, Y. H., Shim, J. H., Kim, N. W., Shin, S. W., Kim, M. C., Shin, S. Y., Hong, B. N., Dej-adisai, S., Kwak, J. H., & Kang, T. H. (2022). The Effects of Persimmon (Diospyros kaki L.f.) Oligosaccharides on Features of the Metabolic Syndrome in Zebrafish. Nutrients, 14(16), 3249. https://doi.org/10.3390/nu14163249






