Abstract
Lately, studies have shown that patients with Parkinson’s disease (PD) report a strong craving for sweets and consume significantly more fast-acting carbohydrates than healthy controls. Consuming food with a high-sugar content is assumed to lead to an increase in insulin concentration, which could positively influence dopamine concentration in the brain and unconsciously be used by patients as kind of “self-medication” to compensate for a lack of dopamine in PD. On the other hand, high-sugar intake could also lead to insulin resistance and diabetes, which is discussed as a causative factor for progressive neurodegeneration in PD. In this critical appraisal, we discuss the role of sugar intake and insulin on dopamine metabolism in patients with PD and how this could influence the potential neurodegeneration mediated by insulin resistance.
1. Introduction
Many patients with Parkinson’s disease (PD) report a change in eating behavior with an excessive craving for sweets [1,2], sometimes already occurring before the manifestation of the cardinal motor symptoms. Lately, some studies confirmed that patients with PD prefer eating sweet foods [1,3], including chocolate [4], cakes [5] and ice cream [6]. Preferring sweet foods seems to reflect a craving or a need for fast-acting sugar more than a need for certain tastes or ingredients [7]. In fact, patients with PD consume significantly more (fast-acting) carbohydrates compared to healthy controls [1,2,8]. More precisely, they have a higher consumption of free sugar. Interestingly, a higher consumption of sugar in PD is not necessarily accompanied by an increase in weight, even in the early phases of the disease, in which increases in muscle tone, tremor or dyskinesia do not reach a degree that requires higher energy consumption [8]. In fact, many patients even lose weight [9,10]. Some studies indicate that although patients consume more fast-acting carbohydrates, there are no differences in total energy intake [1,2]. However, the reason for this change in preference has not yet been explained. Especially for patients, the effect of this change in eating habits on disease progression remains unclear. It has been suggested that a higher intake of sugar might increase dopamine (DA) concentration in the brain. Hence, increased sugar consumption in patients with PD can be seen as a form of “self-treatment” [3,11]. On the other hand, it has been shown that a high intake of fast-acting carbohydrates also affects insulin metabolism, which has lately been discussed as a factor that potentially influences progressive neurodegeneration in PD [12,13]. Therefore, it remains unclear whether increased sugar intake in PD results in benefits for the patient or, in contrast, might be a culprit triggering the progression of neurodegeneration. Taken together, the frequently observed intake of increased amounts of free sugar in patients with PD might influence pathophysiology, and thus may also hold therapeutic options.
In this critical appraisal, we give an overview of the potential mechanisms leading to increased sugar intake in patients with PD and discuss the role of insulin on DA metabolism and neurodegeneration, potentially mediated by central and peripheral insulin resistance.
2. Methods
To find suitable information, a search in Medline was conducted. Articles including animal and human studies, reviews and comments were identified using the terms “Parkinson’s disease” and “insulin” or “diabetes” or “diabetes mellitus” or “metformin” or “glitazones” or “glucagon-like peptide-1 receptor agonists” or “dipeptidyl peptidase 4 inhibitors” or “insulin resistance” or “sugar” or “carbohydrates” or “dopamine”. Suitable articles were also detected in the citation lists of the papers identified by the literature search. Only articles published in English up until November 2021 were evaluated in this critical appraisal.
3. Sugar Intake, Dopamine and Insulin in Parkinson’s Disease
3.1. Effects of Sugar Intake on Dopamine Concentrations in the Brain via Insulin
Primarily, the intake of sugar leads to an increase in blood glucose, which triggers insulin release in the pancreatic ß-cells (reviewed by [14]). Insulin then acts via peripheral and central insulin receptors (reviewed by [15]). In rat brains, insulin receptors are highly represented in the substantia nigra [16,17,18]. The application of intravenous glucose has been shown to lead to a transient increase in DA release in rodent substantia nigra cells [19] via several mechanisms. Thus, insulin leads to a higher firing frequency of dopaminergic neurons [20]. Additionally, insulin seems to increase the excitability of striatal cholinergic interneurons via insulin receptors, leading to an increase in striatal DA release [21]. Moreover, insulin delays the degradation of DA by reducing the expression of monoamine-oxidase (MAO). Finally, it increases DA uptake by increasing dopamine reuptake transporters (DAT) expression [22,23].
When examining post-mortem brain tissue from patients with PD, a loss of insulin receptor immunoreactivity as well as tyrosine hydroxylase protein was observed, which potentially indicates limited DA production via this pathway [24,25,26]. However, there are no further human post-mortem studies examining these possible coherences.
Following the evidence from animal models, it can be hypothesized that patients with PD unconsciously consume higher amounts of sugar to increase brain DA concentration through an insulin peak as a kind of “self-medication” to counteract the disease-related low DA concentration and consecutive symptoms [3,11]. However, it remains unclear whether an insulin peak really increases DA concentrations and thereby decreases symptoms in patients with PD as there are only few studies concerning this issue. One study showed an improvement in motor symptoms one hour after chocolate intake [7], while another study showed an association of higher sugar consumption with increased non-motor symptom burden including depression, dementia and REM behavior sleep disorder (RBD), as well as poorer quality of life in patients with PD [1]. One may argue that patients with a more severe progression of the disease, and thus more overall symptoms may crave more fast-acting sugar [1]. Conversely, higher sugar intake could lead to a more rapid disease progression. However, other studies could not confirm this association of symptom severity and increase in sugar intake [3,7]. Due to differences in study populations regarding disease duration and study design (retrospective [3] and prospective [1] observational studies, one interventional study [7]), further investigations are needed.
3.2. Potential Interactions between Insulin Metabolism and Neurodegeneration in PD
Although the short-term effects of high intake of sugar and increased insulin concentration in patients with PD have not been determined yet, it is essential to consider long-term effects of increased sugar consumption, as there are indices that this eating habit may be disadvantageous for patients with PD. Over time, carbohydrates with a high glycemic index are associated with inflammation, insulin resistance and diabetes [27,28,29], which are discussed as potential factors contributing to progressive neurodegeneration in PD [30]. Aside from glucose, fructose, which is often contained in the sweeteners used in processed food, has a massive impact on insulin resistance (reviewed by [31]) and needs to be studied in this respect.
In fact, several studies indicate that patients with diabetes mellitus, including type 1 and 2, have a higher risk of developing PD [32,33,34,35,36]. This risk increases with a disease duration of diabetes of more than five years [37,38]. In one study, the risk of developing PD was more pronounced in women with diabetes [39]. However, results are inconsistent as other studies could not show a higher risk of developing PD in patients with diabetes [40,41,42,43]. This can be explained in part by different study designs.
Besides these indications for an increased risk of PD in patients with diabetes, there is evidence indicating a common genetic predisposition for diabetes and PD in at least one subgroup of patients with shared genetic pathways, evidenced by several common genetic loci. One major shared pathway relates to a role in immune function, which is demonstrated by the fact that several changes occur in genes coding for the human leucocyte antigen (HLA) system. Another pathway relates to common changes in the microtubule-associated protein tau (MAPT) [44]. Additionally, patients with PD and patients with type 1 and 2 diabetes share abnormal concentrations of some microRNAs that are important for epigenetic modification (reviewed by [45]).
Finally, some studies found greater symptom severity in patients with PD and diabetes, in particular regarding postural instability, gait difficulties and a generally faster progression of motor symptoms [44,46]. While posture- and gait-related symptoms could also be caused by other diabetes-associated disorders—e.g., polyneuropathy caused by diabetes—a faster overall progression may indicate a more direct effect of insulin dysregulation on PD. The observation that patients with PD and dementia suffer more from insulin resistance compared to patients with PD without dementia is important in this respect [47]. Consistent with studies in the general population, there is evidence that patients with PD and diabetes experience greater cognitive decline and worse cognitive function [46,48]. So far, underlying mechanisms remain unclear. In this paper, the negative effects of diabetes on intracerebral small vessels and associated changes in metabolism are discussed, among other factors.
3.3. Insulin Pathways in the Brain
Cell studies and animal models provide evidence that insulin binds to insulin receptors in the brain, which, by phosphorylating substrates of the insulin receptor, activates two pathways associated with neuronal health: (I) the PI3K/AKT pathway, which is demonstrated to play a role in neuronal survival, reduce oxidative stress and reactive oxygen species, as well as contributing to the reduced aggregation of alpha-synuclein and (II) the MAPK pathway, fostering cell growth (reviewed by [49,50]). Supporting this, some studies showed that the inactivation of insulin receptors could lead to more proinflammatory cytokines, increased oxidative stress, as well as the aggregation of alpha-synuclein in PD rodent models [12,51], potentially contributing to progressive neuronal degeneration. Importantly, in rodents, the inactivation of insulin receptors can be caused by insulin resistance [12,52]. Consequently, the high intake of free sugar, including fructose, could contribute to insulin resistance and DA depletion in patients with PD.
On the other hand, in a PD rodent model, a depletion of dopaminergic neurons was also found to alter insulin signaling and was associated with increased markers of insulin resistance, which could lead to a vicious cycle with progressive insulin resistance and a loss of dopaminergic neurons [53].
3.4. Effects of Diabetes Medication on Risk of Developing PD
As there is only little direct evidence regarding the consequences of high-sugar intake and insulin resistance in patients with PD, studies investigating the effect of antidiabetic drugs, which improve insulin resistance and thereby glucose metabolism, are worth considering. The prevalence of PD seems to vary among patients diagnosed with diabetes depending on their diabetes medication. In a retrospective cohort study with more than 100,000 patients with diabetes, individuals treated with dipeptidyl peptidase 4 (DPP4) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists alone or in combination had a significantly lower risk of developing PD compared to patients with diabetes treated with other antidiabetics than DPP4 inhibitors, GLP-1 agonists or glitazones [54]. One other study also showed a significant reduction in PD incidence after use of DPP-4 inhibitors [55]. Moreover, treatment with insulin was also associated with a decreased risk of PD but to a smaller degree compared to DPP4 or GLP-1 agonist treatments [54]. Additionally, a meta-analysis showed a significant reduction in PD incidence in patients with diabetes treated with glitazones [56]. Conversely, three studies found no reduction in PD incidence when treated with glitazones [54,57,58]. Regarding the association of metformin and sulfonylurea with PD prevalence, treatment with sulfonylurea alone seemed to increase the risk of PD in patients with diabetes, while adding metformin seemed to lead to a decreased risk [59].
3.5. Effects of Diabetes Medication on Disease Progression in PD
As summarized in Table 1, many studies that investigated the effects of antidiabetics on the progression of PD used PD animal models. Rodent models are most commonly used. From these studies, causal relations explaining the associations observed in humans can be derived. Several of these studies showed the neuroprotective properties of dopaminergic neurons, less alpha-synuclein aggregation, better mitochondrial function, and anti-inflammatory as well as antioxidant effects after different antidiabetic treatments. However, only a few studies investigated antidiabetic drugs in patients with PD, with so far inconclusive results. As an example, the use of glitazones had a positive influence on disease progression in PD animal models [60,61], which could not be shown in patients with PD [62].

Table 1.
Effects of antidiabetics on PD.
With regard to symptoms, several studies showed improved motor and cognitive functions in animal models using different antidiabetic drugs. In patients with PD and multiple system atrophy (MSA), an improvement of motor and cognitive function was found after application of intranasal insulin or GLP1-agonists [68,94,95,96]. Additionally, patients taking GLP-1 agonists showed increased dopamine transporter density and had a slower increase in L-Dopa use and less L-Dopa-induced dyskinesia [83].
Taken together, several antidiabetic drugs might be associated with slower symptom progression in PD, and thus may have a neuroprotective, disease-modifying, or at least symptomatic effect in PD, with randomized controlled studies still absent. However, this is only indirect evidence and does not replace the need for high-quality studies investigating the short- and long-term effects of sugar intake and insulin resistance in patients with PD.
4. Brain Reward Circuit—Dopamine, Insulin and Depression
Dopamine is also known for its important role in the brain reward system (reviewed by [124]), which is closely linked to depression. In patients with PD, depression is a frequent non-motor symptom and seems to be associated with the abnormal neurotransmitter release of DA and serotonin (reviewed by [125]). Remarkably, evidence showed that patients with PD and depression consume more fast-acting carbohydrates than patients with PD and without depression [1], which might indicate a higher demand of DA in the brain reward system. This is supported by the observation that healthy individuals with genetically reduced amounts of DA receptors and thereby a higher demand of DA, similar to reduced DA concentrations in patients with PD, seem to develop a “reward deficiency syndrome” and use excessive carbohydrate intake as one form of “self-medication” to balance the lack of DA [126,127,128,129].
5. Limitations and Future Directions
Taken together, there is some evidence from animal and cell studies that elevated insulin concentrations can lead to an increased release of DA in the brain following fast-acting carbohydrate intake. It can therefore be hypothesized that patients with PD unconsciously improve motor and potentially even non-motor symptoms by consuming high-sugar-content food (see Figure 1). However, sufficient evidence from clinical studies is still missing to confirm this assumption. On the other hand, increased insulin concentrations, following a high intake of fast-acting carbohydrates over a prolonged time, can lead to insulin resistance and diabetes, which may contribute to neuronal degeneration. Again, high-quality studies in patients with PD are still absent, especially as some of the available studies show inconclusive results. Taken together, previous studies investigating the relation between DA and insulin metabolism could not clarify whether an increased intake of high-sugar foods in PD might have the potential to improve clinical symptoms or, on the contrary, contributes to neurodegenerative processes.
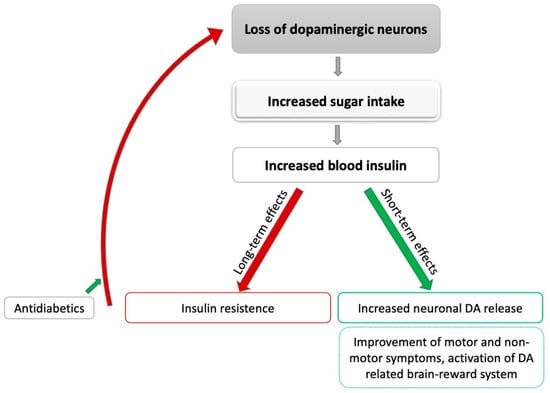
Figure 1.
Potential effects of sugar intake on Parkinson’s disease. DA: dopamine; grey: leads to; green: positive influence; red: negative influence.
Additionally, altered concentrations of insulin and insulin-growth factor (IGF) in the cerebrospinal fluid (CSF) and serum could be of interest regarding the pathophysiology of the neurodegenerative process in patients with PD. However, there are only a few studies investigating this topic. While one study could not show any differences between the insulin concentrations in the CSF of patients with PD and healthy controls [130], another study showed higher IGF-1 concentrations in the blood and CSF of patients with PD compared to the healthy individuals [131]. Moreover, one further study detected higher IGF-1 serum concentrations in patients with PD compared to healthy controls, which did not reach statistical significance [132]. Interestingly, higher IGF serum concentrations seem to be related to low concentrations of alpha-synuclein and tau in the CSF, which is assumed to represent an increased burden of those proteins in the brain tissue. As there are only a few studies investigating the topic with inconclusive results, additional research would be essential to see whether there are relevant changes in insulin and IGF-1 in the serum and CSF and whether they are related to increased concentrations of alpha-synuclein and tau in patients with PD brain tissue.
Interestingly, not every patient with PD reports an increased intake of fast-acting carbohydrates, suggesting a possible subtype of PD that is especially prone to this eating behavior. Further studies are necessary to clarify this subtype hypothesis. Longitudinally designed studies examining changes in sugar intake during disease progression, associations with symptom severity, and the possible development of insulin resistance and diabetes over time would be of high interest.
Overall, the studies discussed here have some limitations that weaken their results. Firstly, most of the studies mentioned in this critical appraisal used cell or animal models, which only offer limited transferability. Only a few studies were conducted in humans, and these studies mostly used a retrospective design. Moreover, there is a lack of more recent studies regarding insulin and DA. In fact, most studies concerning this topic are from the 1990s or early 2000s. Furthermore, it should be mentioned that there are some more aspects in patients with PD that are not fully understood yet and might contribute to changes in eating behavior. Changes in their energy expenditure and hypothalamic function, which might contribute to altered eating behavior, have been observed, among others. Especially orexin and the melanin-concentrating hormone (MCH), which are released in a homeostatic fashion, seem to be reduced in PD [133,134]. However, this seems to be somewhat correlated with a loss of appetite [135,136] and does not explain the higher intake of sugar. Additionally, changes in peripheral signals such as ghrelin and leptin concentrations have been described. However, as ghrelin and leptin regulate contradictory effects (ghrelin induces hunger, while leptin induces satiety), the relevance of these findings remains unclear [137,138]. Finally, changes in eating behavior in patients with PD could be a consequence of gastrointestinal non-motor symptoms, including dysphagia, constipation and defecatory dysfunction [139], although it is unlikely that these symptoms directly affect the intake of sugary foods.
6. Conclusions
In conclusion, evidence explaining the interaction of fast-acting carbohydrate intake, insulin metabolism and DA in patients with PD remains limited, and further research is needed to clarify the role of sugar intake as beneficial or harmful to patients with PD. On the one hand, there is evidence suggesting that sugar intake could improve motor and non-motor symptoms in patients with PD by increasing DA release in the short term, on the other hand, it could also lead to progressive neurodegeneration in the long term. Additionally, a high intake of fast-acting carbohydrates increases the risk for overweight and diabetes mellitus, which impairs patients’ health. Taken together, at the moment, it seems that the disadvantages of high-sugar intake predominate the benefits in the long run. Therefore, in clinical practice, it is recommended that patients are informed about the benefits of a healthy diet to positively influence the development and progression of PD and prevent other diseases [140,141]. Especially diets with a low glycemic index, rich of vitamins and polyphenols, a Mediterranean diet for example, can be recommended [142]. Moreover, patients with PD should be screened for diabetes on a regular basis, and nutrition counselling should be provided. Future research should specifically address pathophysiological mechanisms of fast-acting carbohydrates, and longitudinal observations should include the assessment of markers of carbohydrate metabolism for a better understanding of disease development, progression and, finally, the influence of therapeutic options.
Author Contributions
Conceptualization, J.H., E.S., A.B.-W. and D.B.; methodology, J.H.; validation, E.S., D.B. and A.B.-W.; investigation, J.H.; resources, E.S. and D.B.; data curation, J.H. and E.S.; writing—original draft preparation, J.H. and E.S.; writing—review and editing, A.B.-W. and D.B.; visualization, J.H. and E.S.; supervision, A.B.-W. and D.B.; project administration, E.S. and D.B. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge financial support by DFG within the funding programme Open Access Publikationsfonds.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the corresponding author upon reasonable request.
Conflicts of Interest
J.H. declares no conflict of interest. D.B. is a consultant for Biogen BIAL, UCB Pharma GmbH, AC Immune SA and received honoraria from AbbVie, Biogen, BIAL, UCB Pharma GmbH Zambon, Novartis and grants from the Deutsche Forschungsgemeinschaft (DFG), German Parkinson’s Disease Association (dPV), BMBF, Parkinson Fonds Deutschland gGmbH, UCB Pharma GmbH, UCB Pharma GmbH, EU, Novartis Pharma GmbH, Lundbek, Damp Foundation and the Michael J. Fox Foundation outside the submitted work. A.B.-W. declares no conflict of interest. E.S. received intramural research funding from the University of Kiel, speaker’s honoraria from Bayer Vital GmbH, BIAL GmbH, Movement Disorder Society and Novartis and grants from the German Society for Parkinson’s Disease and Movement Disorders (DPG e.V.) outside the submitted work.
References
- Palavra, N.C.; Lubomski, M.; Flood, V.M.; Davis, R.L.; Sue, C.M. Increased Added Sugar Consumption Is Common in Parkinson’s Disease. Front. Nutr. 2021, 8, 628845. [Google Scholar] [CrossRef] [PubMed]
- Adén, E.; Carlsson, M.; Poortvliet, E.; Stenlund, H.; Linder, J.; Edström, M.; Forsgren, L.; Haglin, L. Dietary Intake and Olfactory Function in Patients with Newly Diagnosed Parkinson’s Disease: A Case-Control Study. Nutr. Neurosci. 2011, 14, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Cassani, E.; Barichella, M.; Ferri, V.; Pinelli, G.; Iorio, L.; Bolliri, C.; Caronni, S.; Faierman, S.A.; Mottolese, A.; Pusani, C. Dietary Habits in Parkinson’s Disease: Adherence to Mediterranean Diet. Parkinsonism Relat. Disord. 2017, 42, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Wolz, M.; Kaminsky, A.; Löhle, M.; Koch, R.; Storch, A.; Reichmann, H. Chocolate Consumption Is Increased in Parkinson’s Disease. J. Neurol. 2009, 256, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Lorefält, B.; Granérus, A.-K.; Unosson, M. Avoidance of Solid Food in Weight Losing Older Patients with Parkinson’s Disease. J. Clin. Nurs. 2006, 15, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Meyers, C.; Amick, M.A.; Friedman, J.H. Ice Cream Preference in Parkinson’s Disease. Med. Health 2010, 93, 66–96. [Google Scholar]
- Wolz, M.; Schleiffer, C.; Klingelhöfer, L.; Schneider, C.; Proft, F.; Schwanebeck, U.; Reichmann, H.; Riederer, P.; Storch, A. Comparison of Chocolate to Cacao-Free White Chocolate in Parkinson’s Disease: A Single-Dose, Investigator-Blinded, Placebo-Controlled, Crossover Trial. J. Neurol. 2012, 259, 2447–2451. [Google Scholar] [CrossRef] [PubMed]
- Wills, A.-M.; Li, R.; Pérez, A.; Ren, X.; Boyd, J. Predictors of Weight Loss in Early Treated Parkinson’s Disease from the NET-PD LS-1 Cohort. J. Neurol. 2017, 264, 1746–1753. [Google Scholar] [CrossRef]
- Akbar, U.; He, Y.; Dai, Y.; Hack, N.; Malaty, I.; McFarland, N.R.; Hess, C.; Schmidt, P.; Wu, S.; Okun, M.S. Weight Loss and Impact on Quality of Life in Parkinson’s Disease. PLoS ONE 2015, 10, e0124541. [Google Scholar] [CrossRef]
- Uc, E.Y.; Struck, L.K.; Rodnitzky, R.L.; Zimmerman, B.; Dobson, J.; Evans, W.J. Predictors of Weight Loss in Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2006, 21, 930–936. [Google Scholar] [CrossRef]
- Craft, S.; Watson, G.S. Insulin and Neurodegenerative Disease: Shared and Specific Mechanisms. Lancet Neurol. 2004, 3, 169–178. [Google Scholar] [CrossRef]
- Morris, J.K.; Bomhoff, G.L.; Stanford, J.A.; Geiger, P.C. Neurodegeneration in an Animal Model of Parkinson’s Disease Is Exacerbated by a High-Fat Diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1082–R1090. [Google Scholar] [CrossRef] [PubMed]
- Shaughness, M.; Acs, D.; Brabazon, F.; Hockenbury, N.; Byrnes, K.R. Role of Insulin in Neurotrauma and Neurodegeneration: A Review. Front. Neurosci. 2020, 14, 940. [Google Scholar] [CrossRef] [PubMed]
- Bratanova-Tochkova, T.K.; Cheng, H.; Daniel, S.; Gunawardana, S.; Liu, Y.-J.; Mulvaney-Musa, J.; Schermerhorn, T.; Straub, S.G.; Yajima, H.; Sharp, G.W. Triggering and Augmentation Mechanisms, Granule Pools, and Biphasic Insulin Secretion. Diabetes 2002, 51, S83–S90. [Google Scholar] [CrossRef]
- Wilcox, G. Insulin and Insulin Resistance. Clin. Biochem. Rev. 2005, 26, 19. [Google Scholar]
- Figlewicz, D.P.; Evans, S.B.; Murphy, J.; Hoen, M.; Baskin, D.G. Expression of Receptors for Insulin and Leptin in the Ventral Tegmental Area/Substantia Nigra (VTA/SN) of the Rat. Brain Res. 2003, 964, 107–115. [Google Scholar] [CrossRef]
- Houten, M.V.; Posner, B.I.; Kopriwa, B.M.; Brawer, J.R. Insulin-Binding Sites in the Rat Brain: In Vivo Localization to the Circumventricular Organs by Quantitative Radioautography. Endocrinology 1979, 105, 666–673. [Google Scholar] [CrossRef]
- Unger, J.W.; Livingston, J.N.; Moss, A.M. Insulin Receptors in the Central Nervous System: Localization, Signalling Mechanisms and Functional Aspects. Prog. Neurobiol. 1991, 36, 343–362. [Google Scholar] [CrossRef]
- Levin, B.E. Glucose-Regulated Dopamine Release from Substantia Nigra Neurons. Brain Res. 2000, 874, 158–164. [Google Scholar] [CrossRef]
- Könner, A.C.; Hess, S.; Tovar, S.; Mesaros, A.; Sánchez-Lasheras, C.; Evers, N.; Verhagen, L.A.; Brönneke, H.S.; Kleinridders, A.; Hampel, B. Role for Insulin Signaling in Catecholaminergic Neurons in Control of Energy Homeostasis. Cell Metab. 2011, 13, 720–728. [Google Scholar]
- Stouffer, M.A.; Woods, C.A.; Patel, J.C.; Lee, C.R.; Witkovsky, P.; Bao, L.; Machold, R.P.; Jones, K.T.; De Vaca, S.C.; Reith, M.E. Insulin Enhances Striatal Dopamine Release by Activating Cholinergic Interneurons and Thereby Signals Reward. Nat. Commun. 2015, 6, 8543. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.C.; Stouffer, M.A.; Mancini, M.; Nicholson, C.; Carr, K.D.; Rice, M.E. Interactions between Insulin and Diet on Striatal Dopamine Uptake Kinetics in Rodent Brain Slices. Eur. J. Neurosci. 2019, 49, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Kleinridders, A.; Cai, W.; Cappellucci, L.; Ghazarian, A.; Collins, W.R.; Vienberg, S.G.; Pothos, E.N.; Kahn, C.R. Insulin Resistance in Brain Alters Dopamine Turnover and Causes Behavioral Disorders. Proc. Natl. Acad. Sci. USA 2015, 112, 3463–3468. [Google Scholar] [CrossRef]
- Kastner, A.; Hirsch, E.C.; Agid, Y.; Javoy-Agid, F. Tyrosine Hydroxylase Protein and Messenger RNA in the Dopaminergic Nigral Neurons of Patients with Parkinson’s Disease. Brain Res. 1993, 606, 341–345. [Google Scholar] [CrossRef]
- Takahashi, M.; Yamada, T.; Tooyama, I.; Moroo, I.; Kimura, H.; Yamamoto, T.; Okada, H. Insulin Receptor MRNA in the Substantia Nigra in Parkinson’s Disease. Neurosci. Lett. 1996, 204, 201–204. [Google Scholar] [CrossRef]
- Moroo, I.; Yamada, T.; Makino, H.; Tooyama, I.; McGeer, P.L.; McGeer, E.G.; Hirayama, K. Loss of Insulin Receptor Immunoreactivity from the Substantia Nigra Pars Compacta Neurons in Parkinson’s Disease. Acta Neuropathol. 1994, 87, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Manson, J.E.; Stampfer, M.J.; Hu, F.B.; Giovannucci, E.; Colditz, G.A.; Hennekens, C.H.; Willett, W.C. A Prospective Study of Whole-Grain Intake and Risk of Type 2 Diabetes Mellitus in US Women. Am. J. Public Health 2000, 90, 1409. [Google Scholar] [PubMed]
- Sahu, S.; Priyan, G.I. Consumption of Sweets as a Risk Factor for Diabetes Mellitus among Adults in Odisha-A Cross-Sectional Study. J. Adv. Med. Med. Res. 2018, 26, 1–5. [Google Scholar] [CrossRef]
- Steyn, N.P.; Mann, J.; Bennett, P.H.; Temple, N.; Zimmet, P.; Tuomilehto, J.; Lindström, J.; Louheranta, A. Diet, Nutrition and the Prevention of Type 2 Diabetes. Public Health Nutr. 2004, 7, 147–165. [Google Scholar] [CrossRef]
- Chohan, H.; Senkevich, K.; Patel, R.K.; Bestwick, J.P.; Jacobs, B.M.; Bandres Ciga, S.; Gan-Or, Z.; Noyce, A.J. Type 2 Diabetes as a Determinant of Parkinson’s Disease Risk and Progression. Mov. Disord. 2021, 36, 1420–1429. [Google Scholar] [CrossRef]
- Softic, S.; Stanhope, K.L.; Boucher, J.; Divanovic, S.; Lanaspa, M.A.; Johnson, R.J.; Kahn, C.R. Fructose and Hepatic Insulin Resistance. Crit. Rev. Clin. Lab. Sci. 2020, 57, 308–322. [Google Scholar] [CrossRef] [PubMed]
- De Pablo-Fernandez, E.; Goldacre, R.; Pakpoor, J.; Noyce, A.J.; Warner, T.T. Association between Diabetes and Subsequent Par-kinson Disease: A Record-Linkage Cohort Study. Neurology 2018, 91, e139–e142. [Google Scholar] [CrossRef] [PubMed]
- Schernhammer, E.; Hansen, J.; Rugbjerg, K.; Wermuth, L.; Ritz, B. Diabetes and the Risk of Developing Parkinson’s Disease in Denmark. Diabetes Care 2011, 34, 1102–1108. [Google Scholar] [CrossRef]
- Xu, Q.; Park, Y.; Huang, X.; Hollenbeck, A.; Blair, A.; Schatzkin, A.; Chen, H. Diabetes and Risk of Parkinson’s Disease. Diabetes Care 2011, 34, 910–915. [Google Scholar] [CrossRef]
- Yang, Y.-W.; Hsieh, T.-F.; Li, C.-I.; Liu, C.-S.; Lin, W.-Y.; Chiang, J.-H.; Li, T.-C.; Lin, C.-C. Increased Risk of Parkinson Disease with Diabetes Mellitus in a Population-Based Study. Medicine 2017, 96, e5921. [Google Scholar] [CrossRef]
- Yue, X.; Li, H.; Yan, H.; Zhang, P.; Chang, L.; Li, T. Risk of Parkinson Disease in Diabetes Mellitus: An Updated Meta-Analysis of Population-Based Cohort Studies. Medicine 2016, 95, e3549. [Google Scholar] [CrossRef] [PubMed]
- De Pablo-Fernandez, E.; Sierra-Hidalgo, F.; Benito-León, J.; Bermejo-Pareja, F. Association between Parkinson’s Disease and Diabetes: Data from NEDICES Study. Acta Neurol. Scand. 2017, 136, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.Y.; Han, K.-D.; Kwon, H.; Park, S.-E.; Park, Y.-G.; Kim, Y.-H.; Yoo, S.-J.; Rhee, E.-J.; Lee, W.-Y. Association between Glycemic Status and the Risk of Parkinson Disease: A Nationwide Population-Based Study. Diabetes Care 2020, 43, 2169–2175. [Google Scholar] [CrossRef]
- Deischinger, C.; Dervic, E.; Kaleta, M.; Klimek, P.; Kautzky-Willer, A. Diabetes Mellitus Is Associated with a Higher Relative Risk for Parkinson’s Disease in Women than in Men. J. Parkinson’s Dis. 2021, 11, 793–800. [Google Scholar] [CrossRef]
- Becker, C.; Brobert, G.P.; Johansson, S.; Jick, S.S.; Meier, C.R. Diabetes in Patients with Idiopathic Parkinson’s Disease. Diabetes Care 2008, 31, 1808–1812. [Google Scholar] [CrossRef]
- Cereda, E.; Barichella, M.; Pedrolli, C.; Klersy, C.; Cassani, E.; Caccialanza, R.; Pezzoli, G. Diabetes and Risk of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Diabetes Care 2011, 34, 2614–2623. [Google Scholar] [CrossRef] [PubMed]
- Palacios, N.; Gao, X.; McCullough, M.L.; Jacobs, E.J.; Patel, A.V.; Mayo, T.; Schwarzschild, M.A.; Ascherio, A. Obesity, Diabetes, and Risk of Parkinson’s Disease. Mov. Disord. 2011, 26, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- Savica, R.; Grossardt, B.R.; Ahlskog, J.E.; Rocca, W.A. Metabolic Markers or Conditions Preceding Parkinson’s Disease: A Case-Control Study. Mov. Disord. 2012, 27, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Kotagal, V.; Albin, R.L.; Müller, M.L.; Koeppe, R.A.; Frey, K.A.; Bohnen, N.I. Diabetes Is Associated with Postural Instability and Gait Difficulty in Parkinson Disease. Parkinsonism Relat. Disord. 2013, 19, 522–526. [Google Scholar] [CrossRef]
- Wang, H. MicroRNAs, Parkinson’s Disease, and Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 2953. [Google Scholar] [CrossRef]
- Pagano, G.; Polychronis, S.; Wilson, H.; Giordano, B.; Ferrara, N.; Niccolini, F.; Politis, M. Diabetes Mellitus and Parkinson Disease. Neurology 2018, 90, e1654–e1662. [Google Scholar] [CrossRef]
- Bosco, D.; Plastino, M.; Cristiano, D.; Colica, C.; Ermio, C.; De Bartolo, M.; Mungari, P.; Fonte, G.; Consoli, D.; Consoli, A. Dementia Is Associated with Insulin Resistance in Patients with Parkinson’s Disease. J. Neurol. Sci. 2012, 315, 39–43. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Kotagal, V.; Müller, M.L.; Koeppe, R.A.; Scott, P.J.; Albin, R.L.; Frey, K.A.; Petrou, M. Diabetes Mellitus Is Inde-pendently Associated with More Severe Cognitive Impairment in Parkinson Disease. Parkinsonism Relat. Disord. 2014, 20, 1394–1398. [Google Scholar] [CrossRef]
- Athauda, D.; Foltynie, T. Insulin Resistance and Parkinson’s Disease: A New Target for Disease Modification? Prog. Neurobiol. 2016, 145, 98–120. [Google Scholar] [CrossRef]
- Cheong, J.L.; de Pablo-Fernandez, E.; Foltynie, T.; Noyce, A.J. The Association between Type 2 Diabetes Mellitus and Parkinson’s Disease. J. Parkinson’s Dis. 2020, 10, 775–789. [Google Scholar] [CrossRef]
- Hong, C.-T.; Chen, K.-Y.; Wang, W.; Chiu, J.-Y.; Wu, D.; Chao, T.-Y.; Hu, C.-J.; Chau, K.-Y.D.; Bamodu, O.A. Insulin Resistance Promotes Parkinson’s Disease through Aberrant Expression of α-Synuclein, Mitochondrial Dysfunction, and Deregulation of the Polo-like Kinase 2 Signaling. Cells 2020, 9, 740. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.-W.; Lin, Y.-T.; Ho, W.-Y.; Lu, P.-J.; Chen, H.-H.; Lai, C.-C.; Sun, G.-C.; Yeh, T.-C.; Hsiao, M.; Tseng, C.-J. Fructose Induced Neurogenic Hypertension Mediated by Overactivation of P38 MAPK to Impair Insulin Signaling Transduction Caused Central Insulin Resistance. Free Radic. Biol. Med. 2017, 112, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.K.; Zhang, H.; Gupte, A.A.; Bomhoff, G.L.; Stanford, J.A.; Geiger, P.C. Measures of Striatal Insulin Resistance in a 6-Hydroxydopamine Model of Parkinson’s Disease. Brain Res. 2008, 1240, 185–195. [Google Scholar] [CrossRef]
- Brauer, R.; Wei, L.; Ma, T.; Athauda, D.; Girges, C.; Vijiaratnam, N.; Auld, G.; Whittlesea, C.; Wong, I.; Foltynie, T. Diabetes Medications and Risk of Parkinson’s Disease: A Cohort Study of Patients with Diabetes. Brain 2020, 143, 3067–3076. [Google Scholar] [CrossRef] [PubMed]
- Svenningsson, P.; Wirdefeldt, K.; Yin, L.; Fang, F.; Markaki, I.; Efendic, S.; Ludvigsson, J.F. Reduced Incidence of Parkinson’s Disease after Dipeptidyl Peptidase-4 Inhibitors—A Nationwide Case-Control Study. Mov. Disord. 2016, 31, 1422–1423. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Singh, A.; Baxi, H.; Taylor, B.; Burgess, J.; Antony, B. Thiazolidinedione Use Is Associated with Reduced Risk of Parkinson’s Disease in Patients with Diabetes: A Meta-Analysis of Real-World Evidence. Neurol. Sci. 2020, 41, 3697–3703. [Google Scholar] [CrossRef]
- Connolly, J.G.; Bykov, K.; Gagne, J.J. Thiazolidinediones and Parkinson Disease: A Cohort Study. Am. J. Epidemiol. 2015, 182, 936–944. [Google Scholar] [CrossRef]
- Wu, H.-F.; Kao, L.-T.; Shih, J.-H.; Kao, H.-H.; Chou, Y.-C.; Li, I.-H.; Kao, S. Pioglitazone Use and Parkinson’s Disease: A Retro-spective Cohort Study in Taiwan. BMJ Open 2018, 8, e023302. [Google Scholar] [CrossRef]
- Wahlqvist, M.L.; Lee, M.-S.; Hsu, C.-C.; Chuang, S.-Y.; Lee, J.-T.; Tsai, H.-N. Metformin-Inclusive Sulfonylurea Therapy Reduces the Risk of Parkinson’s Disease Occurring with Type 2 Diabetes in a Taiwanese Population Cohort. Parkinsonism Relat. Disord. 2012, 18, 753–758. [Google Scholar] [CrossRef]
- Kang, H.; Khang, R.; Ham, S.; Jeong, G.R.; Kim, H.; Jo, M.; Lee, B.D.; Lee, Y.I.; Jo, A.; Park, C. Activation of the ATF2/CREB-PGC-1α Pathway by Metformin Leads to Dopaminergic Neuroprotection. Oncotarget 2017, 8, 48603. [Google Scholar] [CrossRef]
- Saewanee, N.; Praputpittaya, T.; Malaiwong, N.; Chalorak, P.; Meemon, K. Neuroprotective Effect of Metformin on Dopaminergic Neurodegeneration and α-Synuclein Aggregation in C. Elegans Model of Parkinson’s Disease. Neurosci. Res. 2019, 162, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Neurol, L. Pioglitazone in Early Parkinson’s Disease: A Phase 2, Multicentre, Double-Blind, Randomised Trial. Lancet Neurol. 2015, 14, 795–803. [Google Scholar]
- Fine, J.M.; Stroebel, B.M.; Faltesek, K.A.; Terai, K.; Haase, L.; Knutzen, K.E.; Kosyakovsky, J.; Bowe, T.J.; Fuller, A.K.; Frey, W.H. Intranasal Delivery of Low-Dose Insulin Ameliorates Motor Dysfunction and Dopaminergic Cell Death in a 6-OHDA Rat Model of Parkinson’s Disease. Neurosci. Lett. 2020, 714, 134567. [Google Scholar] [CrossRef] [PubMed]
- Iravanpour, F.; Dargahi, L.; Rezaei, M.; Haghani, M.; Heidari, R.; Valian, N.; Ahmadiani, A. Intranasal Insulin Improves Mitochondrial Function and Attenuates Motor Deficits in a Rat 6-OHDA Model of Parkinson’s Disease. CNS Neurosci. Ther. 2021, 27, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, X.; Li, S.; Wang, H.; Zhang, X.; Liu, L.; Xie, A. Intranasal Insulin Ameliorates Cognitive Impairment in a Rat Model of Parkinson’s Disease through Akt/GSK3β Signaling Pathway. Life Sci. 2020, 259, 118159. [Google Scholar] [CrossRef]
- Pang, Y.; Lin, S.; Wright, C.; Shen, J.; Carter, K.; Bhatt, A.; Fan, L.-W. Intranasal Insulin Protects against Substantia Nigra Dopaminergic Neuronal Loss and Alleviates Motor Deficits Induced by 6-OHDA in Rats. Neuroscience 2016, 318, 157–165. [Google Scholar] [CrossRef]
- Ramalingam, M.; Kim, S.-J. The Neuroprotective Role of Insulin Against MPP+-Induced Parkinson’s Disease in Differentiated SH-SY5Y Cells. J. Cell. Biochem. 2016, 117, 917–926. [Google Scholar] [CrossRef]
- Novak, P.; Pimentel Maldonado, D.A.; Novak, V. Safety and Preliminary Efficacy of Intranasal Insulin for Cognitive Impairment in Parkinson Disease and Multiple System Atrophy: A Double-Blinded Placebo-Controlled Pilot Study. PLoS ONE 2019, 14, e0214364. [Google Scholar] [CrossRef]
- Lu, M.; Su, C.; Qiao, C.; Bian, Y.; Ding, J.; Hu, G. Metformin Prevents Dopaminergic Neuron Death in MPTP/P-Induced Mouse Model of Parkinson’s Disease via Autophagy and Mitochondrial ROS Clearance. Int. J. Neuropsychopharmacol. 2016, 19, pyw047. [Google Scholar] [CrossRef]
- Mor, D.E.; Sohrabi, S.; Kaletsky, R.; Keyes, W.; Tartici, A.; Kalia, V.; Miller, G.W.; Murphy, C.T. Metformin Rescues Parkinson’s Disease Phenotypes Caused by Hyperactive Mitochondria. Proc. Natl. Acad. Sci. USA 2020, 117, 26438–26447. [Google Scholar] [CrossRef]
- Patil, S.P.; Jain, P.D.; Ghumatkar, P.J.; Tambe, R.; Sathaye, S. Neuroprotective Effect of Metformin in MPTP-Induced Parkinson’s Disease in Mice. Neuroscience 2014, 277, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.-K.; Go, J.; Park, H.-Y.; Choi, Y.-K.; Seo, Y.J.; Choi, J.H.; Rhee, M.; Lee, T.G.; Lee, C.-H.; Kim, K.-S. Metformin Regulates Astrocyte Reactivity in Parkinson’s Disease and Normal Aging. Neuropharmacology 2020, 175, 108173. [Google Scholar] [CrossRef]
- Fitzgerald, J.C.; Zimprich, A.; Carvajal Berrio, D.A.; Schindler, K.M.; Maurer, B.; Schulte, C.; Bus, C.; Hauser, A.-K.; Kübler, M.; Lewin, R. Metformin Reverses TRAP1 Mutation-Associated Alterations in Mitochondrial Function in Parkinson’s Disease. Brain 2017, 140, 2444–2459. [Google Scholar] [CrossRef] [PubMed]
- El-Ghaiesh, S.H.; Bahr, H.I.; Ibrahiem, A.T.; Ghorab, D.; Alomar, S.Y.; Farag, N.E.; Zaitone, S.A. Metformin Protects from Rotenone–Induced Nigrostriatal Neuronal Death in Adult Mice by Activating AMPK-FOXO3 Signaling and Mitigation of Angiogenesis. Front. Mol. Neurosci. 2020, 13, 84. [Google Scholar] [CrossRef]
- Yan, Q.; Han, C.; Wang, G.; Waddington, J.L.; Zheng, L.; Zhen, X. Activation of AMPK/MTORC1-Mediated Autophagy by Metformin Reverses Clk1 Deficiency-Sensitized Dopaminergic Neuronal Death. Mol. Pharmacol. 2017, 92, 640–652. [Google Scholar] [CrossRef]
- Katila, N.; Bhurtel, S.; Park, P.-H.; Choi, D.-Y. Metformin Attenuates Rotenone-Induced Oxidative Stress and Mitochondrial Damage via the AKT/Nrf2 Pathway. Neurochem. Int. 2021, 148, 105120. [Google Scholar] [CrossRef] [PubMed]
- Katila, N.; Bhurtel, S.; Shadfar, S.; Srivastav, S.; Neupane, S.; Ojha, U.; Jeong, G.-S.; Choi, D.-Y. Metformin Lowers α-Synuclein Phosphorylation and Upregulates Neurotrophic Factor in the MPTP Mouse Model of Parkinson’s Disease. Neuropharmacology 2017, 125, 396–407. [Google Scholar] [CrossRef]
- Ozbey, G.; Nemutlu-Samur, D.; Parlak, H.; Yildirim, S.; Aslan, M.; Tanriover, G.; Agar, A. Metformin Protects Rotenone-Induced Dopaminergic Neurodegeneration by Reducing Lipid Peroxidation. Pharmacol. Rep. 2020, 72, 1397–1406. [Google Scholar] [CrossRef]
- Tayara, K.; Espinosa-Oliva, A.M.; García-Domínguez, I.; Ismaiel, A.A.; Boza-Serrano, A.; Deierborg, T.; Machado, A.; Herrera, A.J.; Venero, J.L.; de Pablos, R.M. Divergent Effects of Metformin on an Inflammatory Model of Parkinson’s Disease. Front. Cell. Neurosci. 2018, 12, 440. [Google Scholar] [CrossRef]
- Wang, D.-X.; Chen, A.-D.; Wang, Q.-J.; Xin, Y.-Y.; Yin, J.; Jing, Y.-H. Protective Effect of Metformin against Rotenone-Induced Parkinsonism in Mice. Toxicol. Mech. Methods 2020, 30, 350–357. [Google Scholar] [CrossRef]
- Ismaiel, A.A.; Espinosa-Oliva, A.M.; Santiago, M.; García-Quintanilla, A.; Oliva-Martín, M.J.; Herrera, A.J.; Venero, J.L.; de Pablos, R.M. Metformin, besides Exhibiting Strong In Vivo Anti-Inflammatory Properties, Increases Mptp-Induced Damage to the Nigrostriatal Dopaminergic System. Toxicol. Appl. Pharmacol. 2016, 298, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, R.M.; Safar, M.M. Neuroprotective Effects of Vildagliptin in Rat Rotenone Parkinson’s Disease Model: Role of RAGE-NF ΚB and Nrf2-Antioxidant Signaling Pathways. J. Neurochem. 2015, 133, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Chung, S.J.; Yoo, H.S.; Hong, N.; Jung, J.H.; Baik, K.; Lee, Y.H.; Sohn, Y.H.; Lee, P.H. Beneficial Effects of Dipeptidyl Peptidase-4 Inhibitors in Diabetic Parkinson’s Disease. Brain 2021, 144, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.; Li, L.; Hölscher, C. Neuroprotective Effects of the Novel GLP-1 Long Acting Analogue Semaglutide in the MPTP Parkinson’s Disease Mouse Model. Neuropeptides 2018, 71, 70–80. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Li, L.; Hölscher, C. Neuroprotective Effects of (Val8) GLP-1-Glu-PAL in the MPTP Parkinson’s Disease Mouse Model. Behav. Brain Res. 2015, 293, 107–113. [Google Scholar] [CrossRef]
- Liu, W.; Jalewa, J.; Sharma, M.; Li, G.; Li, L.; Hölscher, C. Neuroprotective Effects of Lixisenatide and Liraglutide in the 1-Methyl-4-Phenyl-1, 2, 3, 6-Tetrahydropyridine Mouse Model of Parkinson’s Disease. Neuroscience 2015, 303, 42–50. [Google Scholar] [CrossRef]
- Elbassuoni, E.A.; Ahmed, R.F. Mechanism of the Neuroprotective Effect of GLP-1 in a Rat Model of Parkinson’s with Pre-Existing Diabetes. Neurochem. Int. 2019, 131, 104583. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Li, L.; Hölscher, C. Semaglutide Is Neuroprotective and Reduces α-Synuclein Levels in the Chronic MPTP Mouse Model of Parkinson’s Disease. J. Parkinson’s Dis. 2019, 9, 157–171. [Google Scholar] [CrossRef]
- Wang, V.; Kuo, T.-T.; Huang, E.Y.-K.; Ma, K.-H.; Chou, Y.-C.; Fu, Z.-Y.; Lai, L.-W.; Jung, J.; Choi, H.-I.; Choi, D.-S. Sustained Release GLP-1 Agonist PT320 Delays Disease Progression in a Mouse Model of Parkinson’s Disease. ACS Pharmacol. Transl. Sci. 2021, 4, 858–869. [Google Scholar] [CrossRef]
- Aksoy, D.; Solmaz, V.; Çavuşoğlu, T.; Meral, A.; Ateş, U.; Erbaş, O. Neuroprotective Effects of Eexenatide in a Rotenone-Induced Rat Model of Parkinson’s Disease. Am. J. Med. Sci. 2017, 354, 319–324. [Google Scholar] [CrossRef]
- Chen, S.; Yu, S.-J.; Li, Y.; Lecca, D.; Glotfelty, E.; Kim, H.K.; Choi, H.-I.; Hoffer, B.J.; Greig, N.H.; Kim, D.S. Post-Treatment with PT302, a Long-Acting Exendin-4 Sustained Release Formulation, Reduces Dopaminergic Neurodegeneration in a 6-Hydroxydopamine Rat Model of Parkinson’s Disease. Sci. Rep. 2018, 8, 10722. [Google Scholar] [CrossRef] [PubMed]
- Harkavyi, A.; Abuirmeileh, A.; Lever, R.; Kingsbury, A.E.; Biggs, C.S.; Whitton, P.S. Glucagon-like Peptide 1 Receptor Stimulation Reverses Key Deficits in Distinct Rodent Models of Parkinson’s Disease. J. Neuroinflamm. 2008, 5, 19. [Google Scholar] [CrossRef]
- Lin, T.-K.; Lin, K.-J.; Lin, H.-Y.; Lin, K.-L.; Lan, M.-Y.; Wang, P.-W.; Wang, T.-J.; Wang, F.-S.; Tsai, P.-C.; Liou, C.-W. Glucagon-like Peptide-1 Receptor Agonist Ameliorates 1-Methyl-4-Phenyl-1, 2, 3, 6-Tetrahydropyridine (MPTP) Neurotoxicity through Enhancing Mitophagy Flux and Reducing α-Synuclein and Oxidative Stress. Front. Mol. Neurosci. 2021, 14, 697440. [Google Scholar] [CrossRef] [PubMed]
- Aviles-Olmos, I.; Dickson, J.; Kefalopoulou, Z.; Djamshidian, A.; Ell, P.; Soderlund, T.; Whitton, P.; Wyse, R.; Isaacs, T.; Lees, A. Exenatide and the Treatment of Patients with Parkinson’s Disease. J. Clin. Investig. 2013, 123, 2730–2736. [Google Scholar] [CrossRef] [PubMed]
- Aviles-Olmos, I.; Dickson, J.; Kefalopoulou, Z.; Djamshidian, A.; Kahan, J.; Ell, P.; Whitton, P.; Wyse, R.; Isaacs, T.; Lees, A. Motor and Cognitive Advantages Persist 12 Months after Exenatide Exposure in Parkinson’s Disease. J. Parkinson’s Dis. 2014, 4, 337–344. [Google Scholar] [CrossRef]
- Athauda, D.; Maclagan, K.; Skene, S.S.; Bajwa-Joseph, M.; Letchford, D.; Chowdhury, K.; Hibbert, S.; Budnik, N.; Zampedri, L.; Dickson, J. Exenatide Once Weekly versus Placebo in Parkinson’s Disease: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2017, 390, 1664–1675. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Li, Y.; Li, L.; Melchiorsen, J.U.; Rosenkilde, M.; Hölscher, C. The Novel Dual GLP-1/GIP Receptor Agonist DA-CH5 Is Superior to Single GLP-1 Receptor Agonists in the MPTP Model of Parkinson’s Disease. J. Parkinson’s Dis. 2020, 10, 523–542. [Google Scholar] [CrossRef]
- Feng, P.; Zhang, X.; Li, D.; Ji, C.; Yuan, Z.; Wang, R.; Xue, G.; Li, G.; Hölscher, C. Two Novel Dual GLP-1/GIP Receptor Agonists Are Neuroprotective in the MPTP Mouse Model of Parkinson’s Disease. Neuropharmacology 2018, 133, 385–394. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, D.; Feng, P.; Xue, G.; Ji, C.; Li, G.; Hölscher, C. A Novel GLP-1/GIP Dual Agonist Is More Effective than Liraglutide in Reducing Inflammation and Enhancing GDNF Release in the MPTP Mouse Model of Parkinson’s Disease. Eur. J. Pharmacol. 2017, 812, 82–90. [Google Scholar] [CrossRef]
- Li, T.; Tu, L.; Gu, R.; Yang, X.-L.; Liu, X.-J.; Zhang, G.-P.; Wang, Q.; Ren, Y.-P.; Wang, B.-J.; Tian, J.-Y. Neuroprotection of GLP-1/GIP Receptor Agonist via Inhibition of Mitochondrial Stress by AKT/JNK Pathway in a Parkinson’s Disease Model. Life Sci. 2020, 256, 117824. [Google Scholar] [CrossRef]
- Cao, L.; Li, D.; Feng, P.; Li, L.; Xue, G.-F.; Li, G.; Hölscher, C. A Novel Dual GLP-1 and GIP Incretin Receptor Agonist Is Neuroprotective in a Mouse Model of Parkinson’s Disease by Reducing Chronic Inflammation in the Brain. Neuroreport 2016, 27, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Jalewa, J.; Sharma, M.K.; Gengler, S.; Hölscher, C. A Novel GLP-1/GIP Dual Receptor Agonist Protects from 6-OHDA Lesion in a Rat Model of Parkinson’s Disease. Neuropharmacology 2017, 117, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Xue, G.-F.; Lijun, C.; Feng, P.; Li, D.; Li, L.; Li, G.; Hölscher, C. A Novel Dual GLP-1 and GIP Receptor Agonist Is Neuroprotective in the MPTP Mouse Model of Parkinson′ s Disease by Increasing Expression of BNDF. Brain Res. 2016, 1634, 1–11. [Google Scholar] [CrossRef]
- Lv, M.; Xue, G.; Cheng, H.; Meng, P.; Lian, X.; Hölscher, C.; Li, D. The GLP-1/GIP Dual-Receptor Agonist DA5-CH Inhibits the NF-ΚB Inflammatory Pathway in the MPTP Mouse Model of Parkinson’s Disease More Effectively than the GLP-1 Single-Receptor Agonist NLY01. Brain Behav. 2021, 11, e2231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-Y.; Jin, Q.-Q.; Hölscher, C.; Li, L. Glucagon-like Peptide-1/Glucose-Dependent Insulinotropic Polypeptide Dual Receptor Agonist DA-CH5 Is Superior to Exendin-4 in Protecting Neurons in the 6-Hydroxydopamine Rat Parkinson Model. Neural Regen. Res. 2021, 16, 1660. [Google Scholar]
- Breidert, T.; Callebert, J.; Heneka, M.T.; Landreth, G.; Launay, J.M.; Hirsch, E.C. Protective Action of the Peroxisome Proliferator-Activated Receptor-γ Agonist Pioglitazone in a Mouse Model of Parkinson’s Disease. J. Neurochem. 2002, 82, 615–624. [Google Scholar] [CrossRef]
- Dehmer, T.; Heneka, M.T.; Sastre, M.; Dichgans, J.; Schulz, J.B. Protection by Pioglitazone in the MPTP Model of Parkinson’s Disease Correlates with IκBα Induction and Block of NFκB and INOS Activation. J. Neurochem. 2004, 88, 494–501. [Google Scholar] [CrossRef]
- Schintu, N.; Frau, L.; Ibba, M.; Caboni, P.; Garau, A.; Carboni, E.; Carta, A.R. PPAR-Gamma-Mediated Neuroprotection in a Chronic Mouse Model of Parkinson’s Disease. Eur. J. Neurosci. 2009, 29, 954–963. [Google Scholar] [CrossRef]
- Bonato, J.M.; Bassani, T.B.; Milani, H.; Vital, M.A.B.F.; de Oliveira, R.M.W. Pioglitazone Reduces Mortality, Prevents Depressive-like Behavior, and Impacts Hippocampal Neurogenesis in the 6-OHDA Model of Parkinson’s Disease in Rats. Exp. Neurol. 2018, 300, 188–200. [Google Scholar] [CrossRef]
- Machado, M.M.F.; Bassani, T.B.; Cóppola-Segovia, V.; Moura, E.L.R.; Zanata, S.M.; Andreatini, R.; Vital, M.A.B.F. PPAR-γ Agonist Pioglitazone Reduces Microglial Proliferation and NF-ΚB Activation in the Substantia Nigra in the 6-Hydroxydopamine Model of Parkinson’s Disease. Pharmacol. Rep. 2019, 71, 556–564. [Google Scholar] [CrossRef]
- Lee, E.Y.; Lee, J.E.; Park, J.H.; Shin, I.C.; Koh, H.C. Rosiglitazone, a PPAR-γ Agonist, Protects against Striatal Dopaminergic Neurodegeneration Induced by 6-OHDA Lesions in the Substantia Nigra of Rats. Toxicol. Lett. 2012, 213, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.L.; Mounsey, R.B.; Mustafa, S.; Sathe, K.; Teismann, P. Pharmacological Manipulation of Peroxisome Proliferator-Activated Receptor γ (PPARγ) Reveals a Role for Anti-Oxidant Protection in a Model of Parkinson’s Disease. Exp. Neurol. 2012, 235, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, G.K.; Celik, T.; Kayir, H.; Gürsoy, M.; Isik, A.T.; Uzbay, T.I. Effects of Pioglitazone and Retinoic Acid in a Rotenone Model of Parkinson’s Disease. Brain Res. Bull. 2011, 85, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Laloux, C.; Petrault, M.; Lecointe, C.; Devos, D.; Bordet, R. Differential Susceptibility to the PPAR-γ Agonist Pioglitazone in 1-Methyl-4-Phenyl-1, 2, 3, 6-Tetrahydropyridine and 6-Hydroxydopamine Rodent Models of Parkinson’s Disease. Pharmacol. Res. 2012, 65, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Barbiero, J.K.; Santiago, R.M.; Lima, M.M.; Ariza, D.; Morais, L.H.; Andreatini, R.; Vital, M.A. Acute but Not Chronic Administration of Pioglitazone Promoted Behavioral and Neurochemical Protective Effects in the MPTP Model of Parkinson’s Disease. Behav. Brain Res. 2011, 216, 186–192. [Google Scholar] [CrossRef]
- Carta, A.R.; Frau, L.; Pisanu, A.; Wardas, J.; Spiga, S.; Carboni, E. Rosiglitazone Decreases Peroxisome Proliferator Receptor-Gamma Levels in Microglia and Inhibits TNF-Alpha Production: New Evidences on Neuroprotection in a Progressive Parkinson’s Disease Model. Neuroscience 2011, 194, 250–261. [Google Scholar] [CrossRef]
- Hassanzadeh, K.; Rahimi, A.; Moloudi, M.R.; Maccarone, R.; Corbo, M.; Izadpanah, E.; Feligioni, M. Effect of Lobeglitazone on Motor Function in Rat Model of Parkinson’s Disease with Diabetes Co-Morbidity. Brain Res. Bull. 2021, 173, 184–192. [Google Scholar] [CrossRef]
- Swanson, C.; Emborg, M. Expression of Peroxisome Proliferator-Activated Receptor-Gamma in the Substantia Nigra of Hemiparkinsonian Nonhuman Primates. Neurol. Res. 2014, 36, 634–646. [Google Scholar] [CrossRef]
- Pisanu, A.; Lecca, D.; Mulas, G.; Wardas, J.; Simbula, G.; Spiga, S.; Carta, A.R. Dynamic Changes in Pro-and Anti-Inflammatory Cytokines in Microglia after PPAR-γ Agonist Neuroprotective Treatment in the MPTPp Mouse Model of Progressive Parkinson’s Disease. Neurobiol. Dis. 2014, 71, 280–291. [Google Scholar] [CrossRef]
- Jung, T.W.; Lee, J.Y.; Shim, W.S.; Kang, E.S.; Kim, S.K.; Ahn, C.W.; Lee, H.C.; Cha, B.S. Rosiglitazone Protects Human Neuroblastoma SH-SY5Y Cells against MPP+ Induced Cytotoxicity via Inhibition of Mitochondrial Dysfunction and ROS Production. J. Neurol. Sci. 2007, 253, 53–60. [Google Scholar] [CrossRef]
- Pinto, M.; Nissanka, N.; Peralta, S.; Brambilla, R.; Diaz, F.; Moraes, C.T. Pioglitazone Ameliorates the Phenotype of a Novel Par-kinson’s Disease Mouse Model by Reducing Neuroinflammation. Mol. Neurodegener. 2016, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kaundal, R.K.; More, S.; Sharma, S.S. Beneficial Effects of Pioglitazone on Cognitive Impairment in MPTP Model of Parkinson’s Disease. Behav. Brain Res. 2009, 197, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Arab, H.H.; Safar, M.M.; Shahin, N.N. Targeting ROS-Dependent AKT/GSK-3β/NF-ΚB and DJ-1/Nrf2 Pathways by Dapagliflozin Attenuates Neuronal Injury and Motor Dysfunction in Rotenone-Induced Parkinson’s Disease Rat Model. ACS Chem. Neurosci. 2021, 12, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Schultz, W. Dopamine Signals for Reward Value and Risk: Basic and Recent Data. Behav. Brain Funct. 2010, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M. Depression in Parkinson’s Disease: Its Prevalence, Diagnosis, and Neurochemical Background. J. Neurol. 2001, 248, III5–III11. [Google Scholar]
- Blum, K.; Braverman, E.R.; Holder, J.M.; Lubar, J.F.; Monastra, V.J.; Miller, D.; Lubar, J.O.; Chen, T.J.; Comings, D.E. The Reward Deficiency Syndrome: A Biogenetic Model for the Diagnosis and Treatment of Impulsive, Addictive and Compulsive Behaviors. J. Psychoact. Drugs 2000, 32, 1–112. [Google Scholar] [CrossRef]
- Delis, F.; Thanos, P.K.; Rombola, C.; Rosko, L.; Grandy, D.; Wang, G.-J.; Volkow, N.D. Chronic Mild Stress Increases Alcohol Intake in Mice with Low Dopamine D2 Receptor Levels. Behav. Neurosci. 2013, 127, 95. [Google Scholar] [CrossRef]
- Noble, E.P.; Blum, K.; Khalsa, M.E.; Ritchie, T.; Montgomery, A.; Wood, R.C.; Fitch, R.J.; Ozkaragoz, T.; Sheridan, P.J.; Anglin, M.D. Allelic Association of the D2 Dopamine Receptor Gene with Cocaine Dependence. Drug Alcohol Depend. 1993, 33, 271–285. [Google Scholar] [CrossRef]
- Noble, E.P.; Noble, R.E.; Ritchie, T.; Syndulko, K.; Bohlman, M.C.; Noble, L.A.; Zhang, Y.; Sparkes, R.S.; Grandy, D.K. D2 Dopamine Receptor Gene and Obesity. Int. J. Eat. Disord. 1994, 15, 205–217. [Google Scholar] [CrossRef]
- Jimenez-Jimenez, F.J.; Molina, J.A.; Vargas, C.; Gomez, P.; De Bustos, F.; Zurdo, M.; Gomez-Escalonilla, C.; Barcenilla, B.; Berbel, A.; Camacho, A. Normal Cerebrospinal Fluid Levels of Insulin in Patients with Parkinson’s Disease. J. Neural Transm. 2000, 107, 445–449. [Google Scholar] [CrossRef]
- Mashayekhi, F.; Mirzajani, E.; Naji, M.; Azari, M. Expression of Insulin-like Growth Factor-1 and Insulin-like Growth Factor Binding Proteins in the Serum and Cerebrospinal Fluid of Patients with Parkinson’s Disease. J. Clin. Neurosci. 2010, 17, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Ghazi Sherbaf, F.; Mohajer, B.; Ashraf-Ganjouei, A.; Mojtahed Zadeh, M.; Javinani, A.; Sanjari Moghaddam, H.; Shirin Shandiz, M.; Aarabi, M.H. Serum Insulin-like Growth Factor-1 in Parkinson’s Disease; Study of Cerebrospinal Fluid Biomarkers and White Matter Microstructure. Front. Endocrinol. 2018, 9, 608. [Google Scholar] [CrossRef] [PubMed]
- Fronczek, R.; Overeem, S.; Lee, S.Y.; Hegeman, I.M.; Van Pelt, J.; Van Duinen, S.G.; Lammers, G.J.; Swaab, D.F. Hypocretin (Orexin) Loss in Parkinson’s Disease. Brain 2007, 130, 1577–1585. [Google Scholar] [CrossRef]
- Thannickal, T.C.; Lai, Y.-Y.; Siegel, J.M. Hypocretin (Orexin) Cell Loss in Parkinson’s Disease. Brain 2007, 130, 1586–1595. [Google Scholar] [CrossRef]
- Asakawa, A.; Inui, A.; Inui, T.; Katsuura, G.; Fujino, M.A.; Kasuga, M. Orexin Reverses Cholecystokinin-Induced Reduction in Feeding. Diabetes Obes. Metab. 2002, 4, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Tritos, N.A.; Lowell, B.B.; Flier, J.S.; Maratos-Flier, E. Mice Lacking Melanin-Concentrating Hormone Are Hypophagic and Lean. Nature 1998, 396, 670–674. [Google Scholar] [CrossRef]
- Levin, F.; Edholm, T.; Schmidt, P.T.; Gryback, P.; Jacobsson, H.; Degerblad, M.; Hoybye, C.; Holst, J.J.; Rehfeld, J.F.; Hellstrom, P.M. Ghrelin Stimulates Gastric Emptying and Hunger in Normal-Weight Humans. J. Clin. Endocrinol. Metab. 2006, 91, 3296–3302. [Google Scholar] [CrossRef]
- Morton, G.J.; Blevins, J.E.; Williams, D.L.; Niswender, K.D.; Gelling, R.W.; Rhodes, C.J.; Baskin, D.G.; Schwartz, M.W. Leptin Action in the Forebrain Regulates the Hindbrain Response to Satiety Signals. J. Clin. Investig. 2005, 115, 703–710. [Google Scholar] [CrossRef]
- Cersosimo, M.G.; Raina, G.B.; Pecci, C.; Pellene, A.; Calandra, C.R.; Gutiérrez, C.; Micheli, F.E.; Benarroch, E.E. Gastrointestinal Manifestations in Parkinson’s Disease: Prevalence and Occurrence before Motor Symptoms. J. Neurol. 2013, 260, 1332–1338. [Google Scholar] [CrossRef]
- Alcalay, R.N.; Gu, Y.; Mejia-Santana, H.; Cote, L.; Marder, K.S.; Scarmeas, N. The Association between Mediterranean Diet Adherence and Parkinson’s Disease. Mov. Disord. 2012, 27, 771–774. [Google Scholar] [CrossRef]
- Mischley, L.K.; Lau, R.C.; Bennett, R.D. Role of Diet and Nutritional Supplements in Parkinson’s Disease Progression. Oxid. Med. Cell. Longev. 2017, 2017, 6405278. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, C.T.; Guyer, H.; Langa, K.M.; Yaffe, K. Neuroprotective Diets Are Associated with Better Cognitive Function: The Health and Retirement Study. J. Am. Geriatr. Soc. 2017, 65, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).