Investigating Causal Associations of Diet-Derived Circulating Antioxidants with the Risk of Digestive System Cancers: A Mendelian Randomization Study
Abstract
:1. Introduction
2. Materials and Methods
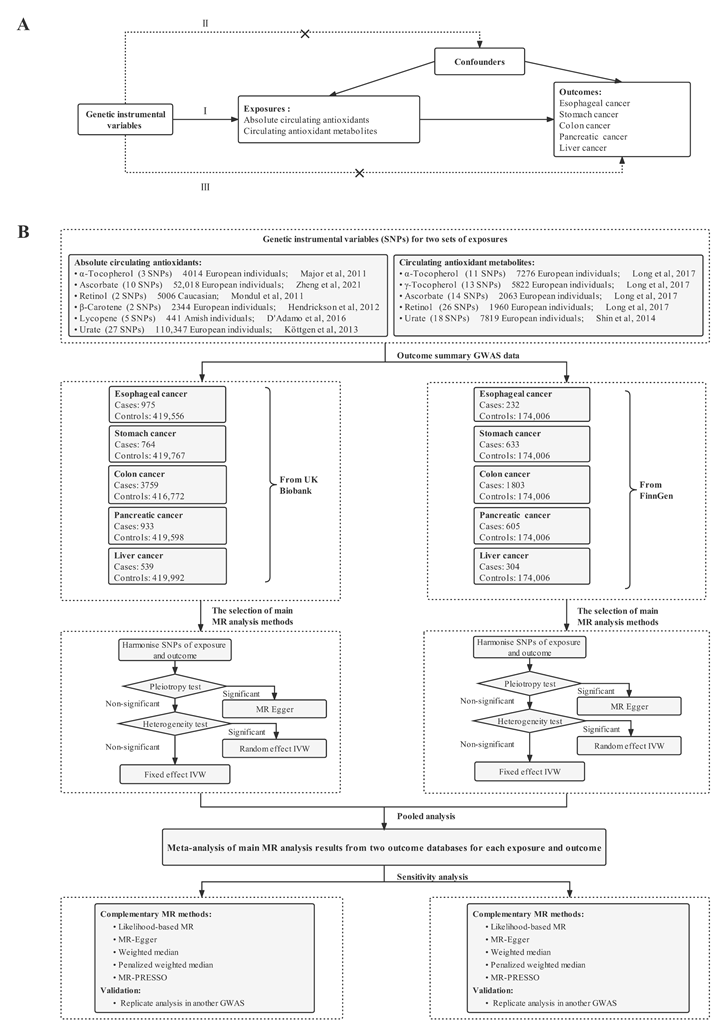
2.1. Overall Study Design
2.2. Genetic Instruments Selection
2.3. Outcome Data Sources
2.4. Statistical Analysis
3. Results
3.1. Strength of Genetic Instruments
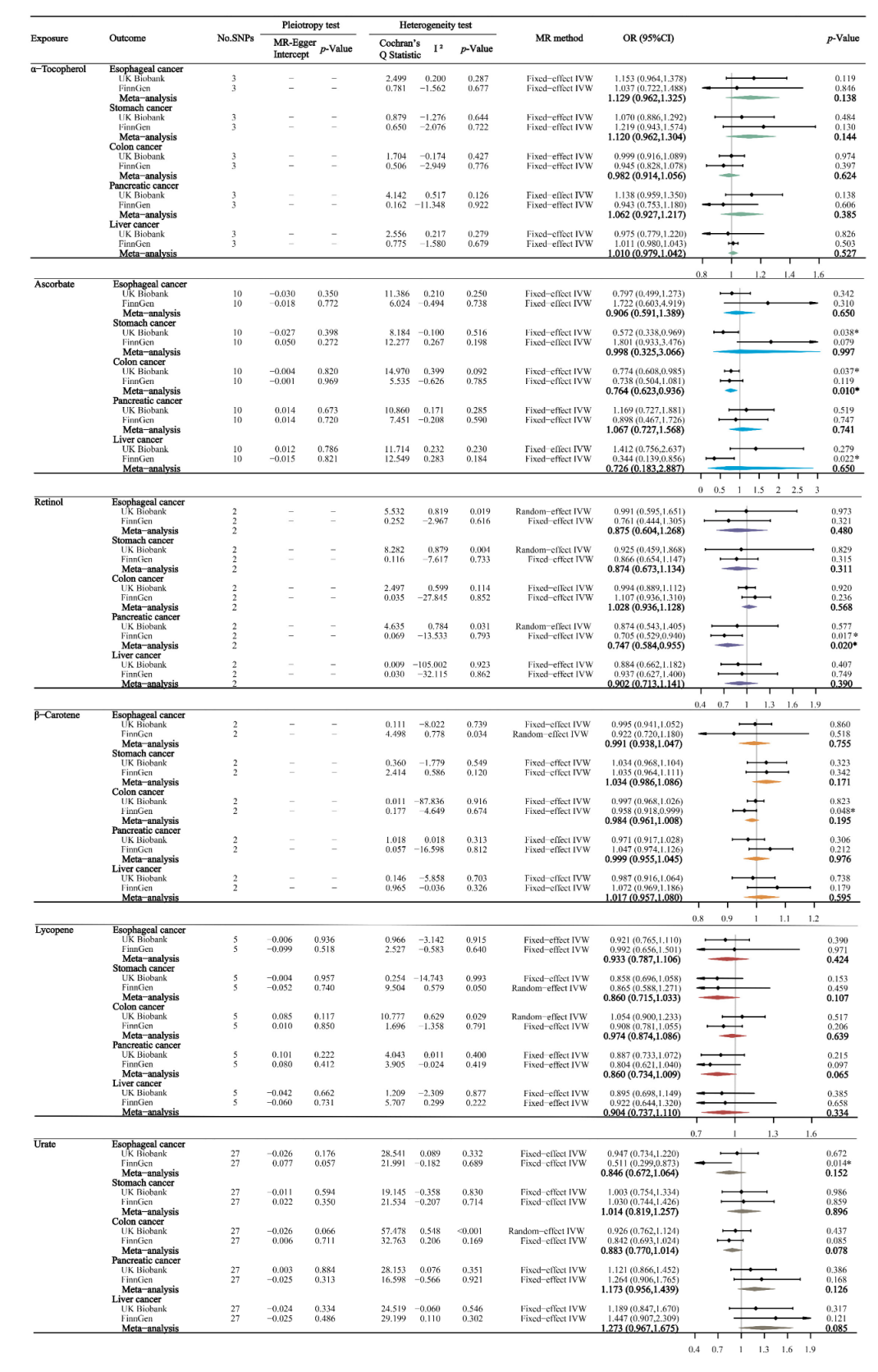
3.2. Absolute Circulating Antioxidants and Digestive System Cancers
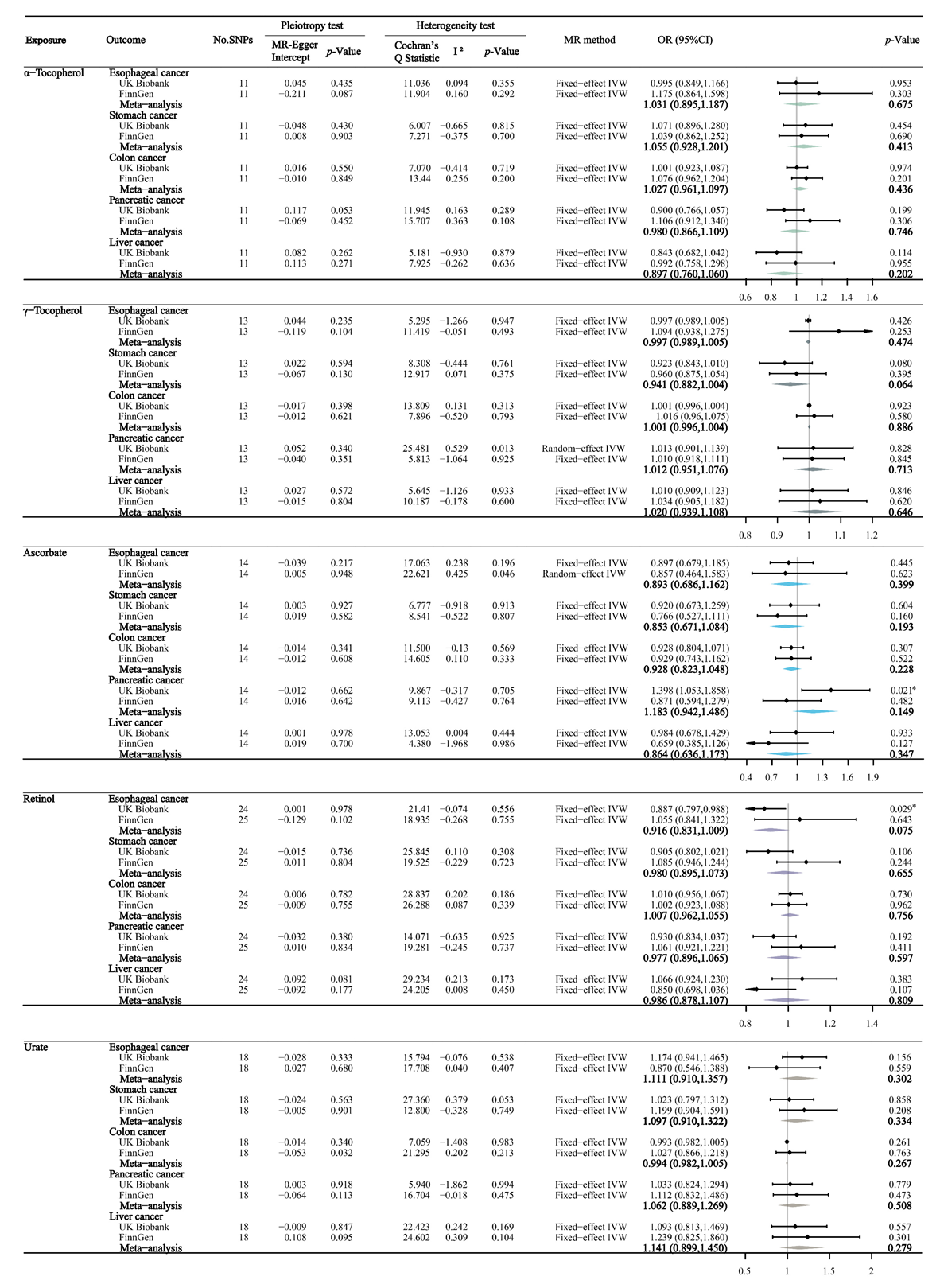
3.3. Circulating Antioxidant Metabolites and Digestive System Cancers
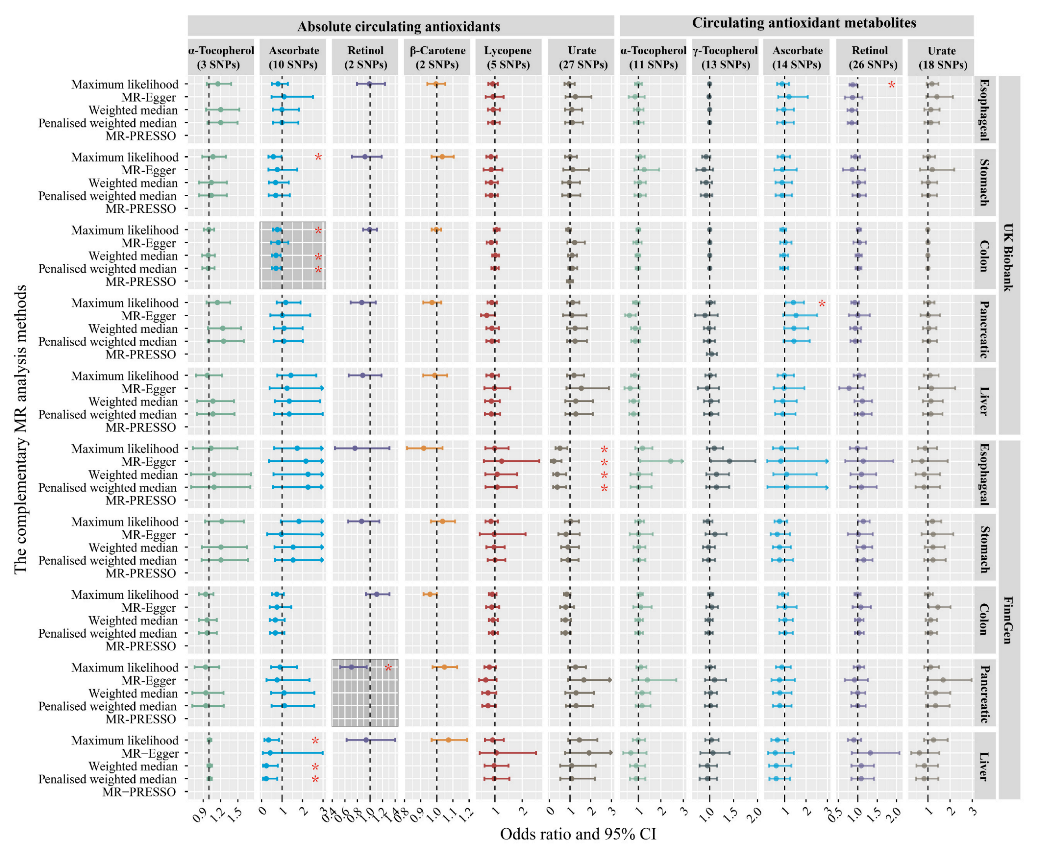
3.4. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Edderkaoui, M.; Hong, P.; Vaquero, E.C.; Lee, J.K.; Fischer, L.; Friess, H.; Buchler, M.W.; Lerch, M.M.; Pandol, S.J.; Gukovskaya, A.S. Extracellular matrix stimulates reactive oxygen species production and increases pancreatic cancer cell survival through 5-lipoxygenase and NADPH oxidase. Am. J. Physiol. Gastrointest Liver Physiol. 2005, 289, G1137–G1147. [Google Scholar] [CrossRef] [PubMed]
- Calvisi, D.F.; Ladu, S.; Hironaka, K.; Factor, V.M.; Thorgeirsson, S.S. Vitamin E down-modulates iNOS and NADPH oxidase in c-Myc/TGF-alpha transgenic mouse model of liver cancer. J. Hepatol. 2004, 41, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Klaunig, J.E. Oxidative Stress and Cancer. Curr. Pharm. Des. 2018, 24, 4771–4778. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free. Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, N.; Yoshida, N.; Nakamura, Y.; Ichikawa, H.; Naito, Y.; Okanoue, T.; Yoshikawa, T. Influence of vitamin E on gastric mucosal injury induced by Helicobacter pylori infection. BioFactors 2006, 28, 9–19. [Google Scholar] [CrossRef]
- Toh, J.W.T.; Wilson, R.B. Pathways of Gastric Carcinogenesis, Helicobacter pylori Virulence and Interactions with Antioxidant Systems, Vitamin C and Phytochemicals. Int. J. Mol. Sci. 2020, 21, 6451. [Google Scholar] [CrossRef]
- Firozi, P.F.; Aboobaker, V.S.; Bhattacharya, R.K. Action of vitamin A on DNA adduct formation by aflatoxin B1 in a microsome catalyzed reaction. Cancer Lett. 1987, 34, 213–220. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, H.; Zhou, J.; Qiu, S.; Cai, T.; Li, H.; Shen, Z.; Hu, Y.; Ding, B.; Luo, M.; et al. Vitamin A and Its Multi-Effects on Pancreas: Recent Advances and Prospects. Front. Endocrinol. 2021, 12, 620941. [Google Scholar] [CrossRef]
- McCarroll, J.A.; Phillips, P.A.; Santucci, N.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Vitamin A inhibits pancreatic stellate cell activation: Implications for treatment of pancreatic fibrosis. Gut 2006, 55, 79–89. [Google Scholar] [CrossRef]
- Bo, Y.; Lu, Y.; Zhao, Y.; Zhao, E.; Yuan, L.; Lu, W.; Cui, L.; Lu, Q. Association between dietary vitamin C intake and risk of esophageal cancer: A dose-response meta-analysis. Int. J. Cancer 2016, 138, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Li, L.; Tian, Y.; Xu, F.; Qiao, T. Association between Dietary Vitamin E Intake and Esophageal Cancer Risk: An Updated Meta-Analysis. Nutrients 2018, 10, 801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Zhang, B. The association of dietary β-carotene and vitamin A intake on the risk of esophageal cancer: A meta-analysis. Rev. Esp. Enferm. Dig. 2020, 112, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Botterweck, A.A.; van den Brandt, P.A.; Goldbohm, R.A. Vitamins, carotenoids, dietary fiber, and the risk of gastric carcinoma: Results from a prospective study after 6.3 years of follow-up. Cancer 2000, 88, 737–748. [Google Scholar] [CrossRef]
- Enger, S.M.; Longnecker, M.P.; Chen, M.J.; Harper, J.M.; Lee, E.R.; Frankl, H.D.; Haile, R.W. Dietary intake of specific carotenoids and vitamins A, C, and E, and prevalence of colorectal adenomas. Cancer Epidemiol. Biomark. Prev. 1996, 5, 147–153. [Google Scholar]
- Heine-Bröring, R.C.; Winkels, R.M.; Renkema, J.M.; Kragt, L.; van Orten-Luiten, A.C.; Tigchelaar, E.F.; Chan, D.S.; Norat, T.; Kampman, E. Dietary supplement use and colorectal cancer risk: A systematic review and meta-analyses of prospective cohort studies. Int. J. Cancer 2015, 136, 2388–2401. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, W.; Shao, L.; Zhong, D.; Wu, Y.; Cai, J. Association between intake of antioxidants and pancreatic cancer risk: A meta-analysis. Int. J. Food Sci. Nutr. 2016, 67, 744–753. [Google Scholar] [CrossRef]
- Lan, Q.Y.; Zhang, Y.J.; Liao, G.C.; Zhou, R.F.; Zhou, Z.G.; Chen, Y.M.; Zhu, H.L. The Association between Dietary Vitamin A and Carotenes and the Risk of Primary Liver Cancer: A Case-Control Study. Nutrients 2016, 8, 624. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, E.J.; Connell, C.J.; McCullough, M.L.; Chao, A.; Jonas, C.R.; Rodriguez, C.; Calle, E.E.; Thun, M.J. Vitamin C, vitamin E, and multivitamin supplement use and stomach cancer mortality in the Cancer Prevention Study II cohort. Cancer Epidemiol. Biomark. Prev. 2002, 11, 35–41. [Google Scholar]
- Bonelli, L.; Puntoni, M.; Gatteschi, B.; Massa, P.; Missale, G.; Munizzi, F.; Turbino, L.; Villanacci, V.; De Censi, A.; Bruzzi, P. Antioxidant supplement and long-term reduction of recurrent adenomas of the large bowel. A double-blind randomized trial. J. Gastroenterol. 2013, 48, 698–705. [Google Scholar] [CrossRef]
- Argyriou, A.A.; Chroni, E.; Koutras, A.; Iconomou, G.; Papapetropoulos, S.; Polychronopoulos, P.; Kalofonos, H.P. A randomized controlled trial evaluating the efficacy and safety of vitamin E supplementation for protection against cisplatin-induced peripheral neuropathy: Final results. Supportive Care Cancer 2006, 14, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Ebrahim, S. ‘Mendelian randomization’: Can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 2003, 32, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgess, S.; Thompson, S.G. Mendelian Randomization: Methods for Using Genetic Variants in Causal Estimation; CRC Press: Boca Raton, FL, USA, 2015; Volume 181, pp. 549–550. [Google Scholar]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.T.; Timpson, N.J.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. Jama 2021, 326, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.M.; Hagg, S.; Pedersen, N.L. Circulating antioxidants and Alzheimer disease prevention: A Mendelian randomization study. Am. J. Clin. Nutr. 2019, 109, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Martens, L.G.; Luo, J.; Willems van Dijk, K.; Jukema, J.W.; Noordam, R.; van Heemst, D. Diet-Derived Antioxidants Do Not Decrease Risk of Ischemic Stroke: A Mendelian Randomization Study in 1 Million People. J. Am. Heart Assoc. 2021, 10, e022567. [Google Scholar] [CrossRef]
- Luo, J.; le Cessie, S.; van Heemst, D.; Noordam, R. Diet-Derived Circulating Antioxidants and Risk of Coronary Heart Disease: A Mendelian Randomization Study. J. Am. Coll. Cardiol. 2021, 77, 45–54. [Google Scholar] [CrossRef]
- Emdin, C.A.; Khera, A.V.; Kathiresan, S. Mendelian Randomization. Jama 2017, 318, 1925–1926. [Google Scholar] [CrossRef]
- Major, J.M.; Yu, K.; Wheeler, W.; Zhang, H.; Cornelis, M.C.; Wright, M.E.; Yeager, M.; Snyder, K.; Weinstein, S.J.; Mondul, A.; et al. Genome-wide association study identifies common variants associated with circulating vitamin E levels. Hum. Mol. Genet. 2011, 20, 3876–3883. [Google Scholar] [CrossRef]
- Zheng, J.S.; Luan, J.; Sofianopoulou, E.; Imamura, F.; Stewart, I.D.; Day, F.R.; Pietzner, M.; Wheeler, E.; Lotta, L.A.; Gundersen, T.E.; et al. Plasma Vitamin C and Type 2 Diabetes: Genome-Wide Association Study and Mendelian Randomization Analysis in European Populations. Diabetes Care 2021, 44, 98–106. [Google Scholar] [CrossRef]
- Mondul, A.M.; Yu, K.; Wheeler, W.; Zhang, H.; Weinstein, S.J.; Major, J.M.; Cornelis, M.C.; Männistö, S.; Hazra, A.; Hsing, A.W.; et al. Genome-wide association study of circulating retinol levels. Hum. Mol. Genet. 2011, 20, 4724–4731. [Google Scholar] [CrossRef]
- Hendrickson, S.J.; Hazra, A.; Chen, C.; Eliassen, A.H.; Kraft, P.; Rosner, B.A.; Willett, W.C. β-Carotene 15,15′-monooxygenase 1 single nucleotide polymorphisms in relation to plasma carotenoid and retinol concentrations in women of European descent. Am. J. Clin. Nutr. 2012, 96, 1379–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Adamo, C.R.; D’Urso, A.; Ryan, K.A.; Yerges-Armstrong, L.M.; Semba, R.D.; Steinle, N.I.; Mitchell, B.D.; Shuldiner, A.R.; McArdle, P.F. A Common Variant in the SETD7 Gene Predicts Serum Lycopene Concentrations. Nutrients 2016, 8, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köttgen, A.; Albrecht, E.; Teumer, A.; Vitart, V.; Krumsiek, J.; Hundertmark, C.; Pistis, G.; Ruggiero, D.; O’Seaghdha, C.M.; Haller, T.; et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat. Genet. 2013, 45, 145–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.Y.; Fauman, E.B.; Petersen, A.K.; Krumsiek, J.; Santos, R.; Huang, J.; Arnold, M.; Erte, I.; Forgetta, V.; Yang, T.P.; et al. An atlas of genetic influences on human blood metabolites. Nat. Genet. 2014, 46, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Long, T.; Hicks, M.; Yu, H.C.; Biggs, W.H.; Kirkness, E.F.; Menni, C.; Zierer, J.; Small, K.S.; Mangino, M.; Messier, H.; et al. Whole-genome sequencing identifies common-to-rare variants associated with human blood metabolites. Nat. Genet. 2017, 49, 568–578. [Google Scholar] [CrossRef]
- Kamat, M.A.; Blackshaw, J.A.; Young, R.; Surendran, P.; Burgess, S.; Danesh, J.; Butterworth, A.S.; Staley, J.R. PhenoScanner V2: An expanded tool for searching human genotype-phenotype associations. Bioinformatics 2019, 35, 4851–4853. [Google Scholar] [CrossRef] [Green Version]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [Green Version]
- Machiela, M.J.; Chanock, S.J. LDlink: A web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics 2015, 31, 3555–3557. [Google Scholar] [CrossRef]
- Schmidt, A.F.; Dudbridge, F. Mendelian randomization with Egger pleiotropy correction and weakly informative Bayesian priors. Int. J. Epidemiol. 2018, 47, 1217–1228. [Google Scholar] [CrossRef] [Green Version]
- Burgess, S.; Bowden, J.; Fall, T.; Ingelsson, E.; Thompson, S.G. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology 2017, 28, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Xue, H.; Shen, X.; Pan, W. Constrained maximum likelihood-based Mendelian randomization robust to both correlated and uncorrelated pleiotropic effects. Am. J. Hum. Genet. 2021, 108, 1251–1269. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [Green Version]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Leenders, M.; Leufkens, A.M.; Siersema, P.D.; van Duijnhoven, F.J.; Vrieling, A.; Hulshof, P.J.; van Gils, C.H.; Overvad, K.; Roswall, N.; Kyrø, C.; et al. Plasma and dietary carotenoids and vitamins A, C and E and risk of colon and rectal cancer in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2014, 135, 2930–2939. [Google Scholar] [CrossRef]
- Ruder, E.H.; Thiébaut, A.C.; Thompson, F.E.; Potischman, N.; Subar, A.F.; Park, Y.; Graubard, B.I.; Hollenbeck, A.R.; Cross, A.J. Adolescent and mid-life diet: Risk of colorectal cancer in the NIH-AARP Diet and Health Study. Am. J. Clin. Nutr. 2011, 94, 1607–1619. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Park, J.S.; Moon, S.; Yeo, J. Effect of Intravenous High Dose Vitamin C on Postoperative Pain and Morphine Use after Laparoscopic Colectomy: A Randomized Controlled Trial. Pain Res. Manag. 2016, 2016, 9147279. [Google Scholar] [CrossRef] [PubMed]
- Riordan, H.D.; Riordan, N.H.; Jackson, J.A.; Casciari, J.J.; Hunninghake, R.; González, M.J.; Mora, E.M.; Miranda-Massari, J.R.; Rosario, N.; Rivera, A. Intravenous vitamin C as a chemotherapy agent: A report on clinical cases. Puerto Rico Health Sci. J. 2004, 23, 115–118. [Google Scholar]
- Fu, Y.; Xu, F.; Jiang, L.; Miao, Z.; Liang, X.; Yang, J.; Larsson, S.C.; Zheng, J.S. Circulating vitamin C concentration and risk of cancers: A Mendelian randomization study. BMC Med. 2021, 19, 171. [Google Scholar] [CrossRef]
- Arranz, N.; Haza, A.I.; García, A.; Delgado, M.E.; Rafter, J.; Morales, P. Inhibition by vitamin C of apoptosis induced by N-nitrosamines in HepG2 and HL-60 cells. J. Appl. Toxicol. 2008, 28, 788–796. [Google Scholar] [CrossRef]
- Yun, J.; Mullarky, E.; Lu, C.; Bosch, K.N.; Kavalier, A.; Rivera, K.; Roper, J.; Chio, I.I.; Giannopoulou, E.G.; Rago, C.; et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 2015, 350, 1391–1396. [Google Scholar] [CrossRef] [Green Version]
- Cenigaonandia-Campillo, A.; Serna-Blasco, R.; Gómez-Ocabo, L.; Solanes-Casado, S.; Baños-Herraiz, N.; Puerto-Nevado, L.D.; Cañas, J.A.; Aceñero, M.J.; García-Foncillas, J.; Aguilera, Ó. Vitamin C activates pyruvate dehydrogenase (PDH) targeting the mitochondrial tricarboxylic acid (TCA) cycle in hypoxic KRAS mutant colon cancer. Theranostics 2021, 11, 3595–3606. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Gao, Y.; Zhi, X.; Ta, N.; Jiang, H.; Zheng, J. Association between vitamin A, retinol and carotenoid intake and pancreatic cancer risk: Evidence from epidemiologic studies. Sci. Rep. 2016, 6, 38936. [Google Scholar] [CrossRef] [PubMed]
- Fabris, C.; Piccoli, A.; Meani, A.; Farini, R.; Vianello, D.; Del Favero, G.; Sturniolo, G.; Brosolo, P.; Naccarato, R. Study of retinol-binding protein in pancreatic cancer. J. Cancer Res. Clin. Oncol. 1984, 108, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Recchia, F.; Sica, G.; Candeloro, G.; Bisegna, R.; Bratta, M.; Bonfili, P.; Necozione, S.; Tombolini, V.; Rea, S. Chemoradioimmunotherapy in locally advanced pancreatic and biliary tree adenocarcinoma: A multicenter phase II study. Pancreas 2009, 38, e163–e168. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, F.; Dalgleish, A.G.; Bissonnette, R.P.; Colston, K.W. Retinoids cause apoptosis in pancreatic cancer cells via activation of RAR-gamma and altered expression of Bcl-2/Bax. Br. J. Cancer 2002, 87, 555–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chronopoulos, A.; Robinson, B.; Sarper, M.; Cortes, E.; Auernheimer, V.; Lachowski, D.; Attwood, S.; García, R.; Ghassemi, S.; Fabry, B.; et al. ATRA mechanically reprograms pancreatic stellate cells to suppress matrix remodelling and inhibit cancer cell invasion. Nat. Commun. 2016, 7, 12630. [Google Scholar] [CrossRef] [Green Version]
- Guan, J.; Zhang, H.; Wen, Z.; Gu, Y.; Cheng, Y.; Sun, Y.; Zhang, T.; Jia, C.; Lu, Z.; Chen, J. Retinoic acid inhibits pancreatic cancer cell migration and EMT through the downregulation of IL-6 in cancer associated fibroblast cells. Cancer Lett. 2014, 345, 132–139. [Google Scholar] [CrossRef]
- Wang, S.M.; Taylor, P.R.; Fan, J.H.; Pfeiffer, R.M.; Gail, M.H.; Liang, H.; Murphy, G.A.; Dawsey, S.M.; Qiao, Y.L.; Abnet, C.C. Effects of Nutrition Intervention on Total and Cancer Mortality: 25-Year Post-trial Follow-up of the 5.25-Year Linxian Nutrition Intervention Trial. J. Natl. Cancer Inst. 2018, 110, 1229–1238. [Google Scholar] [CrossRef] [Green Version]
| Trait | Sample Size | p-Value | LD | No. of SNPs | Explained Variance (R2, %) * | Unit | PMID |
|---|---|---|---|---|---|---|---|
| Absolute circulating antioxidants | |||||||
| α-Tocopherol | 4014 | 5 × 10−8 | 0.001 | 3 | 1.7 | mg/L in log-transformed scale | 21729881 |
| Ascorbate | 52,018 | 5 × 10−8 | 0.001 | 10 | 1.7 | µmol/L | 33203707 |
| Retinol | 5006 | 5 × 10−8 | 0.001 | 2 | 2.3 | µg/L in ln-transformed scale | 21878437 |
| β-Carotene | 2344 | 5 × 10−8 | 0.001 | 2 | 4.8 | µg/L in ln-transformed scale | 23134893 |
| Lycopene | 441 | 5 × 10−8 | 0.001 | 5 | 30.1 | µg/dL | 26861389 |
| Urate | 110,347 | 5 × 10−8 | 0.001 | 27 | 3.7 | mg/dL | 23263486 |
| Circulating antioxidant metabolites | |||||||
| α-Tocopherol | 7725 | 1 × 10−5 | 0.001 | 11 | 6.8 | log10-transfomed metabolite concentration | 24816252 |
| γ-Tocopherol | 6226 | 1 × 10−5 | 0.001 | 13 | 9.8 | log10-transfomed metabolite concentration | 24816252 |
| Ascorbate | 2085 | 1 × 10−5 | 0.001 | 14 | 21.7 | log10-transfomed metabolite concentration | 24816252 |
| Retinol | 1960 | 1 × 10−5 | 0.001 | 26 | 20.6 | log10-transfomed metabolite concentration | 28263315 |
| Urate | 7819 | 1 × 10−5 | 0.001 | 18 | 11.4 | log10-transfomed metabolite concentration | 24816252 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhao, H.; Man, J.; Yin, X.; Zhang, T.; Yang, X.; Lu, M. Investigating Causal Associations of Diet-Derived Circulating Antioxidants with the Risk of Digestive System Cancers: A Mendelian Randomization Study. Nutrients 2022, 14, 3237. https://doi.org/10.3390/nu14153237
Zhang X, Zhao H, Man J, Yin X, Zhang T, Yang X, Lu M. Investigating Causal Associations of Diet-Derived Circulating Antioxidants with the Risk of Digestive System Cancers: A Mendelian Randomization Study. Nutrients. 2022; 14(15):3237. https://doi.org/10.3390/nu14153237
Chicago/Turabian StyleZhang, Xuening, Hao Zhao, Jinyu Man, Xiaolin Yin, Tongchao Zhang, Xiaorong Yang, and Ming Lu. 2022. "Investigating Causal Associations of Diet-Derived Circulating Antioxidants with the Risk of Digestive System Cancers: A Mendelian Randomization Study" Nutrients 14, no. 15: 3237. https://doi.org/10.3390/nu14153237
APA StyleZhang, X., Zhao, H., Man, J., Yin, X., Zhang, T., Yang, X., & Lu, M. (2022). Investigating Causal Associations of Diet-Derived Circulating Antioxidants with the Risk of Digestive System Cancers: A Mendelian Randomization Study. Nutrients, 14(15), 3237. https://doi.org/10.3390/nu14153237






