Effects of Green Tea and Green Tea Incorporated in Nanoparticle Lyotropic Liquid Crystal on Exercise Adaptations: A High-Intensity Interval Training Pre-Clinical Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. GT and NP-LLC Preparation
2.2. Ethics and Animal Care
2.3. Pre-Clinical Experimental Design
2.4. HIIT Training Protocol
2.5. Hematological and Biochemical Analysis
2.6. Redox State Analysis
2.7. Histological Analysis
2.8. Statistical Analysis
3. Results
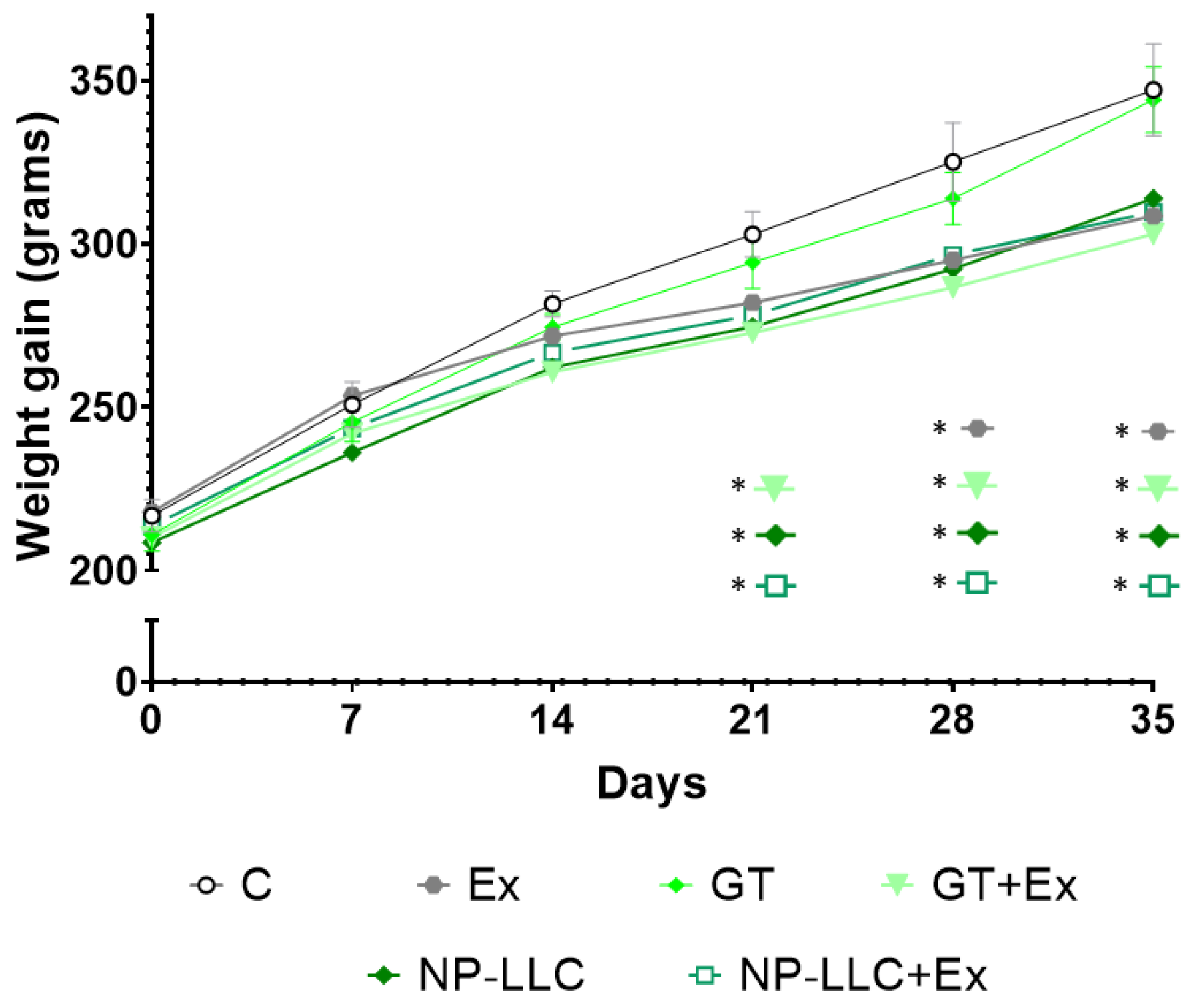
3.1. Body Weight and Physical Conditioning of the Animals
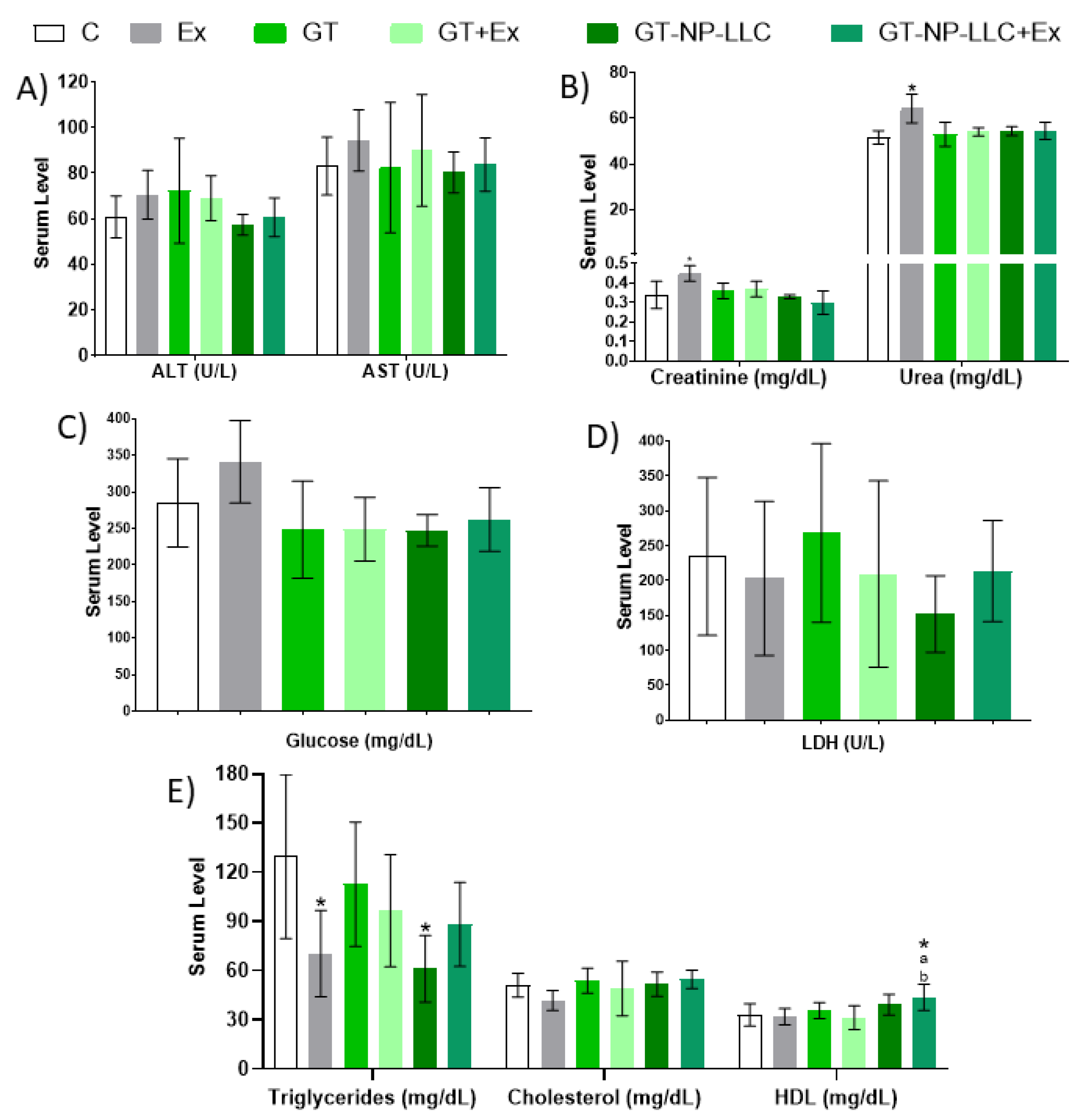
3.2. Hematological and Biochemical Analysis
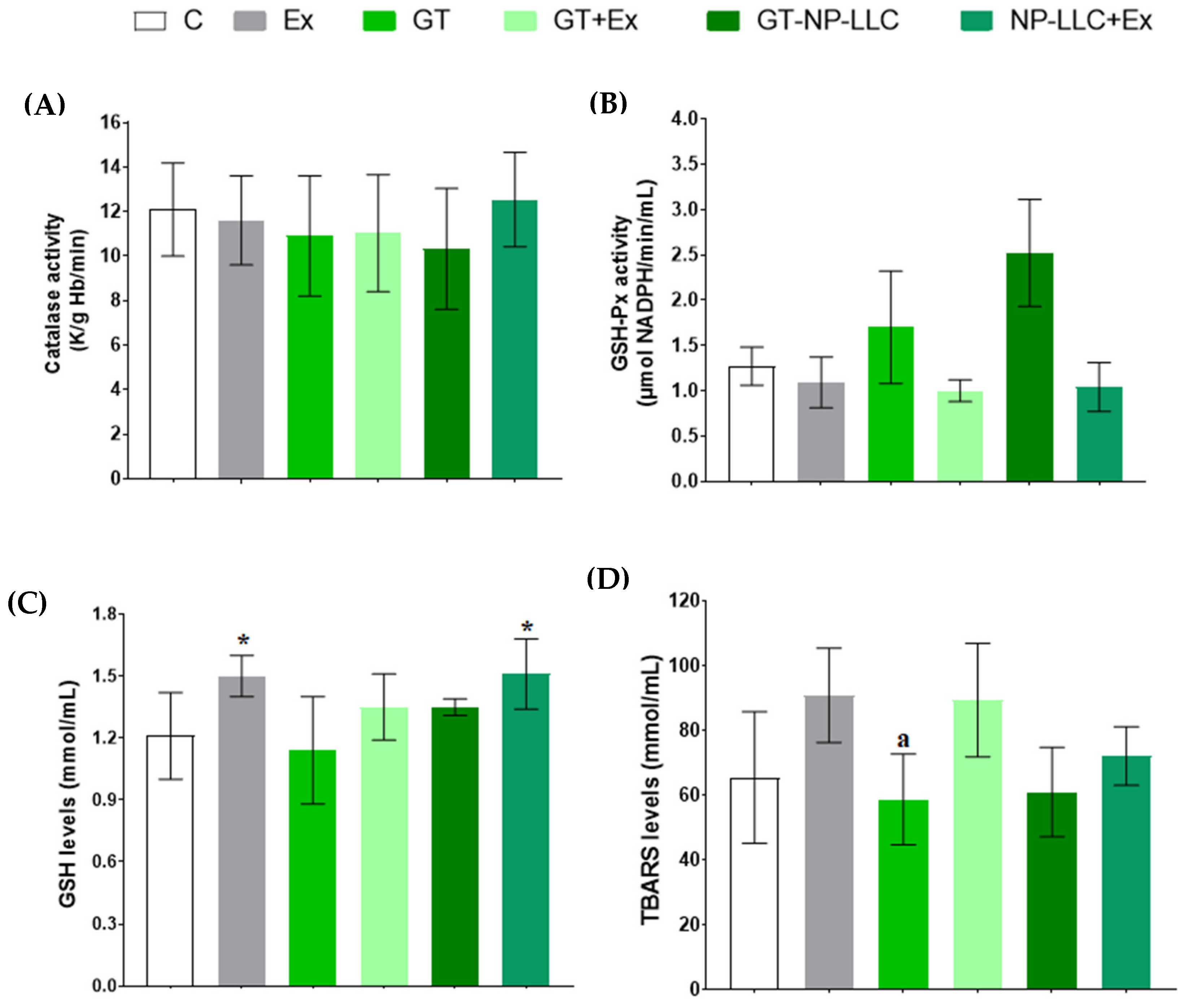
3.3. Oxidative Stress
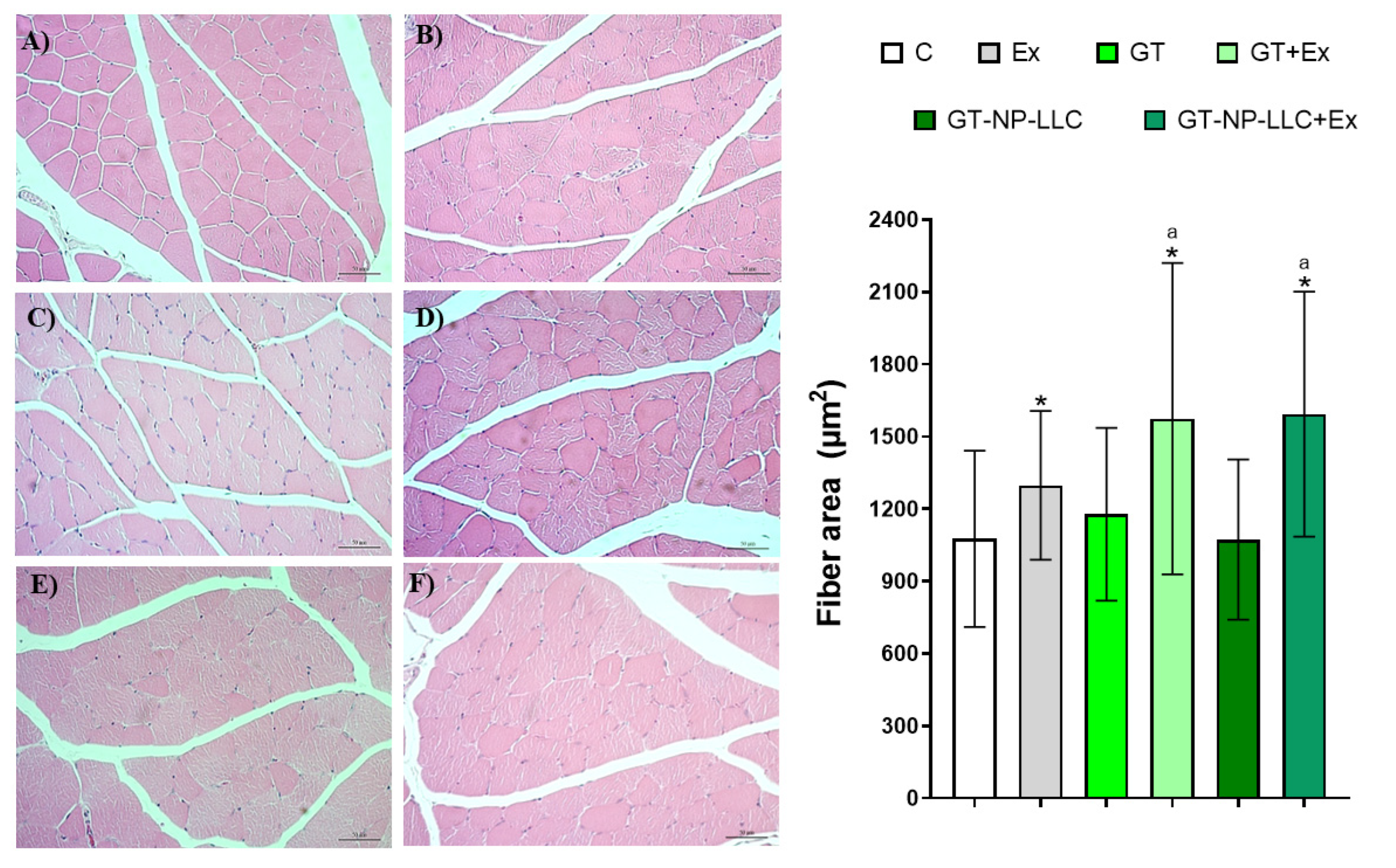
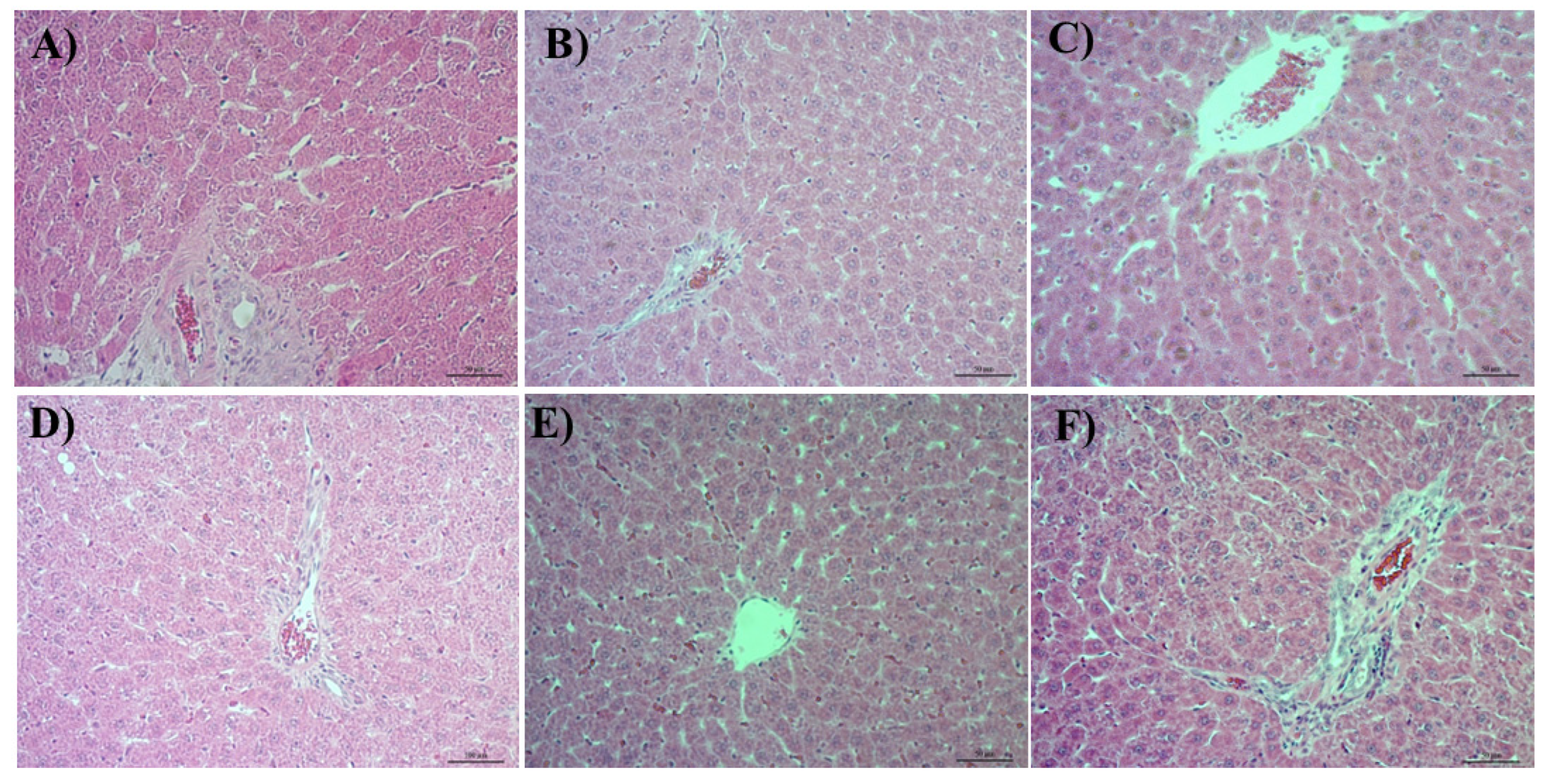
3.4. Histological Analysis
4. Discussion
5. Conclusions
Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tahani, B.; Sabzian, R. Effect of Camellia sinensis plant on decreasing the level of halitosis: A systematic review. Dent. Res. J. 2018, 15, 379–384. [Google Scholar] [CrossRef]
- Gopal, J.; Muthu, M.; Paul, D.; Kim, D.-H.; Chun, S. Bactericidal activity of green tea extracts: The importance of catechin containing nano particles. Sci. Rep. 2016, 6, 19710. [Google Scholar] [CrossRef]
- Hayat, K.; Iqbal, H.; Malik, U.; Bilal, U.; Mushtaq, S. Tea and Its Consumption: Benefits and Risks. Crit. Rev. Food Sci. Nutr. 2015, 55, 939–954. [Google Scholar] [CrossRef]
- Braud, L.; Peyre, L.; De Sousa, G.; Armand, M.; Rahmani, R.; Maixent, J.-M. Effect of Brewing Duration on the Antioxidant and Hepatoprotective Abilities of Tea Phenolic and Alkaloid Compounds in a t-BHP Oxidative Stress-Induced Rat Hepatocyte Model. Molecules 2015, 20, 14985–15002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Couvreur, P. Nanoparticles in drug delivery: Past, present and future. Adv. Drug Deliv. Rev. 2013, 65, 21–23. [Google Scholar] [CrossRef]
- Dierking, I.; Al-Zangana, S. Lyotropic Liquid Crystal Phases from Anisotropic Nanomaterials. Nanomaterials 2017, 7, 305. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Gui, S.; Huang, J.; Cao, J.; Li, Z.; Li, Q.; Chu, X. Characterization of Lipid-Based Lyotropic Liquid Crystal and Effects of Guest Molecules on Its Microstructure: A Systematic Review. AAPS PharmSciTech 2018, 19, 2023–2040. [Google Scholar] [CrossRef] [PubMed]
- De Souza, J.F.; Pontes, K.D.S.; Alves, T.F.R.; Amaral, V.A.; Rebelo, M.D.A.; Hausen, M.A.; Chaud, M.V. Spotlight on Biomimetic Systems Based on Lyotropic Liquid Crystal. Molecules 2017, 22, 419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamal Kumar, C.; Pooja, K.; Nidhi, M. Liquid Crystal Systems in Drug Delivery, in Novel Approaches for Drug Delivery; Raj, K.K., Anil, K.S., Rajesh Kumar, K., Eds.; IGI Global: Hershey, PA, USA, 2017; pp. 217–243. [Google Scholar]
- Nieri, V.; De Souza, J.F.; Chaud, M.V.; Grotto, D. Efeito protetivo de formulação de cristal líquido liotrópico na oxidação do chá verde. Braz. J. Dev. 2020, 6, 14529–14538. [Google Scholar] [CrossRef]
- Feito, Y.; Heinrich, K.; Butcher, S.; Poston, W. High-Intensity Functional Training (HIFT): Definition and Research Implications for Improved Fitness. Sports 2018, 6, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamurtas, A.Z.; Fatouros, I.G.; Deli, C.K.; Georgakouli, K.; Poulios, A.; Draganidis, D.; Papanikolaou, K.; Tsimeas, P.; Chatzinikolaou, A.; Avloniti, A.; et al. The Effects of Acute Low-Volume HIIT and Aerobic Exercise on Leukocyte Count and Redox Status. J. Sports Sci. Med. 2018, 17, 501–508. [Google Scholar] [PubMed]
- Martinez-Ferran, M.; Sanchis-Gomar, F.; Lavie, C.J.; Lippi, G.; Pareja-Galeano, H. Do Antioxidant Vitamins Prevent Exercise-Induced Muscle Damage? A Systematic Review. Antioxidants 2020, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Merry, T.L.; Ristow, M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J. Physiol. 2016, 594, 5135–5147. [Google Scholar] [CrossRef]
- Ferrer, M.; Sureda, A.; Mestre, A.; Tur, J.; Pons, A. The Double Edge of Reactive Oxygen Species as Damaging and Signaling Molecules in HL60 Cell Culture. Cell. Physiol. Biochem. 2010, 25, 241–252. [Google Scholar] [CrossRef]
- Higgins, M.R.; Izadi, A.; Kaviani, M. Antioxidants and Exercise Performance: With a Focus on Vitamin E and C Supplementation. Int. J. Environ. Res. Public Health 2020, 17, 8452. [Google Scholar] [CrossRef] [PubMed]
- Pastor, R. Antioxidant Supplementation and Adaptive Response to Training: A Systematic Review. Curr. Pharm. Des. 2019, 25, 1889–1912. [Google Scholar] [CrossRef]
- Vera-Cruz, M.; Nunes, E.; Mendonça, L.; Chaves, E.; Fernandes, M.L.D.L.A. Efeito do chá verde (Camelia sinensis) em ratos com obesidade induzida por dieta hipercalórica. J. Bras. Patol. Med. Lab. 2010, 46, 407–413. [Google Scholar] [CrossRef]
- Da Silva, P.B.; Calixto, G.M.F.; Júnior, J.A.O.; Bombardelli, R.L.; Fonseca-Santos, B.; Rodero, C.F.; Chorilli, M. Structural Features and the Anti-Inflammatory Effect of Green Tea Extract-Loaded Liquid Crystalline Systems Intended for Skin Delivery. Polymers 2017, 9, 30. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Tolosa, E.M.C.d. Manual de Técnicas para Histologia Normal e Patológica; Behmer, O.A., Ed.; Manole: São Paulo, Brazial, 2003. [Google Scholar]
- Bagheri, R.; Rashidlamir, A.; Ashtary-Larky, D.; Wong, A.; Alipour, M.; Motevalli, M.S.; Chebbi, A.; Laher, I.; Zouhal, H. Does green tea extract enhance the anti-inflammatory effects of exercise on fat loss? Br. J. Clin. Pharmacol. 2019, 86, 753–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gahreman, D.; Heydari, M.; Boutcher, Y.; Freund, J.; Boutcher, S. The Effect of Green Tea Ingestion and Interval Sprinting Exercise on the Body Composition of Overweight Males: A Randomized Trial. Nutrients 2016, 8, 510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Meo, S.; Venditti, P. Evolution of the Knowledge of Free Radicals and Other Oxidants. Oxidative Med. Cell. Longev. 2020, 2020, 9829176. [Google Scholar] [CrossRef]
- Mankowski, R.T.; Anton, S.D.; Buford, T.W.; Leeuwenburgh, C. Dietary Antioxidants as Modifiers of Physiologic Adaptations to Exercise. Med. Sci. Sports Exerc. 2015, 47, 1857–1868. [Google Scholar] [CrossRef] [Green Version]
- Wackerhage, H.; Schoenfeld, B.J.; Hamilton, D.; Lehti, M.; Hulmi, J.J. Stimuli and sensors that initiate skeletal muscle hypertrophy following resistance exercise. J. Appl. Physiol. 2019, 126, 30–43. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Salvador-Pascual, A.; Cabo, H.; Ferrando, B.; Viña, J. Redox modulation of mitochondriogenesis in exercise. Does antioxidant supplementation blunt the benefits of exercise training? Free Radic. Biol. Med. 2015, 86, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Krępa, E.; Domaszewski, P.; Pokora, I.; Żebrowska, A.; Gdańska, A.; Podgórski, T. Effects of medium-term green tea extract supplementation combined with CrossFit workout on blood antioxidant status and serum brain-derived neurotrophic factor in young men: A pilot study. J. Int. Soc. Sports Nutr. 2019, 16, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Week | Sessions | Series per Session | Time | Break |
|---|---|---|---|---|
| 1 | 3 | 5 | 30″ | 1′ |
| 2 | 4 | 5 | 30″ | 1′ |
| 3 | 3 | 6 | 30″ | 1′ |
| 4 | 3 | 7 | 30″ | 1′ |
| 5 | 2 | 8 | 30″ | 1′ |
| Num Jumps | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 |
|---|---|---|---|---|---|
| Ex | 18.5 ± 7.7 | 25.0 ± 8.3 | 26.2 ± 7.0 * | 32.3 ± 14.5 * | 43.6 ± 16.0 * |
| GT+Ex | 18.9 ± 16.3 | 28.1 ± 16.6 | 29.2 ± 22.6 | 30.3 ± 18.0 | 41.2 ± 29.6 * |
| NP-LLC+Ex | 19.2 ± 11.3 | 26.6 ± 13.7 | 27.7 ± 11.2 | 30.5 ± 13.2 * | 46.2 ± 27.4 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieri, V.; de Souza, J.F.; Segato, T.C.M.; Caetano, É.L.A.; Leite, F.G.; Chaud, M.V.; Grotto, D. Effects of Green Tea and Green Tea Incorporated in Nanoparticle Lyotropic Liquid Crystal on Exercise Adaptations: A High-Intensity Interval Training Pre-Clinical Study. Nutrients 2022, 14, 3226. https://doi.org/10.3390/nu14153226
Nieri V, de Souza JF, Segato TCM, Caetano ÉLA, Leite FG, Chaud MV, Grotto D. Effects of Green Tea and Green Tea Incorporated in Nanoparticle Lyotropic Liquid Crystal on Exercise Adaptations: A High-Intensity Interval Training Pre-Clinical Study. Nutrients. 2022; 14(15):3226. https://doi.org/10.3390/nu14153226
Chicago/Turabian StyleNieri, Vitor, Juliana Ferreira de Souza, Talita Cristina Mena Segato, Érika Leão Ajala Caetano, Fernanda Gomes Leite, Marco Vinícius Chaud, and Denise Grotto. 2022. "Effects of Green Tea and Green Tea Incorporated in Nanoparticle Lyotropic Liquid Crystal on Exercise Adaptations: A High-Intensity Interval Training Pre-Clinical Study" Nutrients 14, no. 15: 3226. https://doi.org/10.3390/nu14153226
APA StyleNieri, V., de Souza, J. F., Segato, T. C. M., Caetano, É. L. A., Leite, F. G., Chaud, M. V., & Grotto, D. (2022). Effects of Green Tea and Green Tea Incorporated in Nanoparticle Lyotropic Liquid Crystal on Exercise Adaptations: A High-Intensity Interval Training Pre-Clinical Study. Nutrients, 14(15), 3226. https://doi.org/10.3390/nu14153226







