Positive Effects of α-Lactalbumin in the Management of Symptoms of Polycystic Ovary Syndrome
Abstract
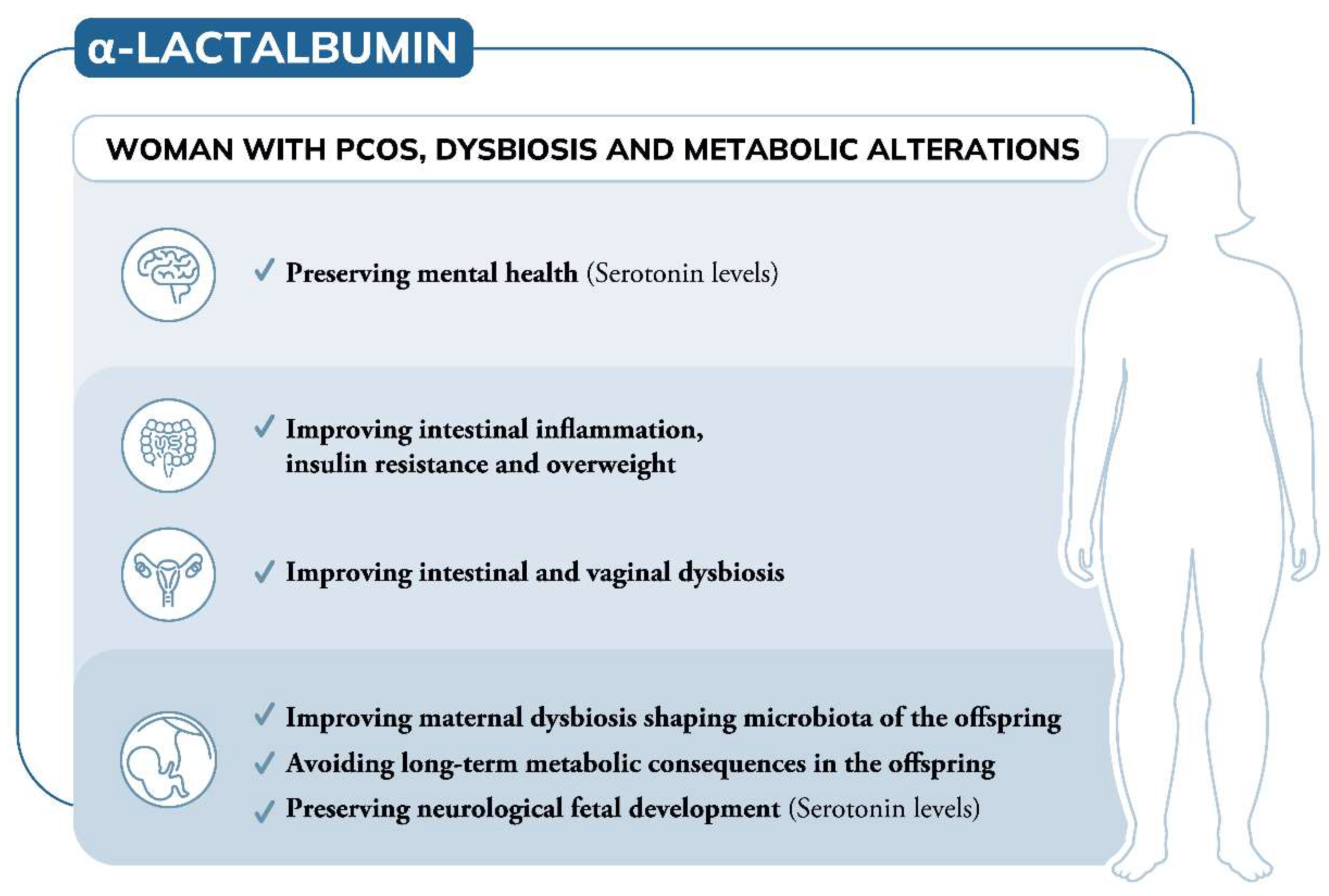
:1. Introduction
2. Microbial and Metabolic Association in the Pathogenesis of PCOS
3. α-Lactalbumin for Improving Dysbiosis
α-Lactalbumin Positively Affects Maternal Dysbiosis Shaping Neonatal Microbiota
4. Intestinal and Mental Health: Working Hypothesis for a Positive Effect of α-Lactalbumin on Mood
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Layman, D.K.; Lonnerdal, B.; Fernstrom, J.D. Applications for alpha-lactalbumin in human nutrition. Nutr. Rev. 2018, 76, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Brew, K.; Castellino, F.J.; Vanaman, T.C.; Hill, R.L. The complete amino acid sequence of bovine alpha-lactalbumin. J. Biol. Chem. 1970, 245, 4570–4582. [Google Scholar] [CrossRef]
- Findlay, J.B.; Brew, K. The complete amino-acid sequence of human α-lactalbumin. Eur. J. Biochem. 1972, 27, 65–86. [Google Scholar] [CrossRef]
- Mburu Kamau, S.C.C.S.; Chen, W.; Liu, X.-M.; Lu, R.R. Alpha-lactalbumin: Its production technologies and bioactive peptides. Compr. Rev. Food Sci. Food Saf. 2010, 9, 197–212. [Google Scholar] [CrossRef]
- Pellegrini, A.; Thomas, U.; Bramaz, N.; Hunziker, P.; von Fellenberg, R. Isolation and identification of three bactericidal domains in the bovine alpha-lactalbumin molecule. Biochim. Biophys. Acta 1999, 1426, 439–448. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Yoshida, K.; Uchida, M. Novel functions of bovine milk-derived α-lactalbumin: Anti-nociceptive and anti-inflammatory activity caused by inhibiting cyclooxygenase-2 and phospholipase A2. Biol. Pharm. Bull. 2009, 32, 366–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshdcawa, M.; Tani, F.; Yoshimura, T.; Chiba, H. Opioid peptides from milk proteins. Agric. Biol. Chem. 1986, 50, 2419–2421. [Google Scholar] [CrossRef]
- Pihlanto-Leppala, A.; Koskinen, P.; Piilola, K.; Tupasela, T.; Korhonen, H. Angiotensin I-converting enzyme inhibitory properties of whey protein digests: Concentration and characterization of active peptides. J. Dairy Res. 2000, 67, 53–64. [Google Scholar] [CrossRef]
- Svensson, M.; Hakansson, A.; Mossberg, A.K.; Linse, S.; Svanborg, C. Conversion of alpha-lactalbumin to a protein inducing apoptosis. Proc. Natl. Acad. Sci. USA 2000, 97, 4221–4226. [Google Scholar] [CrossRef] [Green Version]
- Jaziri, M.; Migliore-Samour, D.; Casabianca-Pignede, M.R.; Keddad, K.; Morgat, J.L.; Jolles, P. Specific binding sites on human phagocytic blood cells for Gly-Leu-Phe and Val-Glu-Pro-Ile-Pro-Tyr, immunostimulating peptides from human milk proteins. Biochim. Biophys. Acta 1992, 1160, 251–261. [Google Scholar] [CrossRef]
- Petschow, B.W.; Talbott, R.D. Response of bifidobacterium species to growth promoters in human and cow milk. Pediatr. Res. 1991, 29, 208–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madureira, A.R.; Tavares, T.; Gomes, A.M.; Pintado, M.E.; Malcata, F.X. Invited review: Physiological properties of bioactive peptides obtained from whey proteins. J. Dairy Sci. 2010, 93, 437–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burokas, A.; Arboleya, S.; Moloney, R.D.; Peterson, V.L.; Murphy, K.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol. Psychiatry 2017, 82, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Orosco, M.; Rouch, C.; Beslot, F.; Feurte, S.; Regnault, A.; Dauge, V. Alpha-lactalbumin-enriched diets enhance serotonin release and induce anxiolytic and rewarding effects in the rat. Behav. Brain Res. 2004, 148, 1–10. [Google Scholar] [CrossRef]
- Parker, J.; O’Brien, C.; Hawrelak, J. A narrative review of the role of gastrointestinal dysbiosis in the pathogenesis of polycystic ovary syndrome. Obstet. Gynecol. Sci. 2022, 65, 14–28. [Google Scholar] [CrossRef]
- Yurtdas, G.; Akdevelioglu, Y. A New Approach to Polycystic Ovary Syndrome: The Gut Microbiota. J. Am. Coll. Nutr. 2020, 39, 371–382. [Google Scholar] [CrossRef]
- Robinson, C.M.; Pfeiffer, J.K. Viruses and the Microbiota. Annu. Rev. Virol. 2014, 1, 55–69. [Google Scholar] [CrossRef] [Green Version]
- Ansorge, M.S.; Morelli, E.; Gingrich, J.A. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J. Neurosci. 2008, 28, 199–207. [Google Scholar] [CrossRef]
- Goeden, N.; Velasquez, J.; Arnold, K.A.; Chan, Y.; Lund, B.T.; Anderson, G.M.; Bonnin, A. Maternal Inflammation Disrupts Fetal Neurodevelopment via Increased Placental Output of Serotonin to the Fetal Brain. J. Neurosci. 2016, 36, 6041–6049. [Google Scholar] [CrossRef] [Green Version]
- Tamboli, C.P.; Neut, C.; Desreumaux, P.; Colombel, J.F. Dysbiosis in inflammatory bowel disease. Gut 2004, 53, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guirro, M.; Costa, A.; Gual-Grau, A.; Herrero, P.; Torrell, H.; Canela, N.; Arola, L. Effects from diet-induced gut microbiota dysbiosis and obesity can be ameliorated by fecal microbiota transplantation: A multiomics approach. PLoS ONE 2019, 14, e0218143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, C.H.; Wu, C.Y. The gut microbiome in obesity. J. Formos. Med. Assoc. 2019, 118 (Suppl. 1), S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ming, Q.; Liang, J.; Zhang, Y.; Zhang, H.; Shen, T. Gut microbiota dysbiosis in polycystic ovary syndrome: Association with obesity—A preliminary report. Can. J. Physiol. Pharmacol. 2020, 98, 803–809. [Google Scholar] [CrossRef]
- Harsch, I.A.; Konturek, P.C. The Role of Gut Microbiota in Obesity and Type 2 and Type 1 Diabetes Mellitus: New Insights into “Old” Diseases. Med. Sci. (Basel) 2018, 6, 32. [Google Scholar] [CrossRef] [Green Version]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Jiang, Y.; Xi, H.; Chen, L.; Feng, X. Exploration of the Relationship Between Gut Microbiota and Polycystic Ovary Syndrome (PCOS): A Review. Geburtshilfe Frauenheilkd 2020, 80, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Kalhoro, D.H.; Kalhoro, M.S.; Metwally, E.; Chughtai, M.I.; Mazhar, M.U.; Khan, S.A. Relationship between gut microbiota and host-metabolism: Emphasis on hormones related to reproductive function. Anim. Nutr. 2021, 7, 1–10. [Google Scholar] [CrossRef]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Tremellen, K.; Pearce, K. Dysbiosis of Gut Microbiota (DOGMA)—A novel theory for the development of Polycystic Ovarian Syndrome. Med. Hypotheses 2012, 79, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.J.; Siakowska, M.; Banaszewska, B.; Pawelczyk, L.; Duleba, A.J.; Kelley, S.T.; Thackray, V.G. Gut Microbial Diversity in Women With Polycystic Ovary Syndrome Correlates With Hyperandrogenism. J. Clin. Endocrinol. Metab. 2018, 103, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Giampaolino, P.; Foreste, V.; Di Filippo, C.; Gallo, A.; Mercorio, A.; Serafino, P.; Improda, F.P.; Verrazzo, P.; Zara, G.; Buonfantino, C.; et al. Microbiome and PCOS: State-of-Art and Future Aspects. Int. J. Mol. Sci. 2021, 22, 2048. [Google Scholar] [CrossRef]
- Qi, X.; Yun, C.; Sun, L.; Xia, J.; Wu, Q.; Wang, Y.; Wang, L.; Zhang, Y.; Liang, X.; Wang, L.; et al. Publisher Correction: Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat. Med. 2019, 25, 1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, F.F.; Li, Y.M. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: A review. J. Ovarian Res. 2020, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Garrido, M.A.; Tena-Sempere, M. Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategies. Mol. Metab. 2020, 35, 100937. [Google Scholar] [CrossRef]
- Kwon, H.; Kim, D.; Kim, J.S. Body Fat Distribution and the Risk of Incident Metabolic Syndrome: A Longitudinal Cohort Study. Sci. Rep. 2017, 7, 10955. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.S.; Norman, R.J.; Davies, M.J.; Moran, L.J. The effect of obesity on polycystic ovary syndrome: A systematic review and meta-analysis. Obes. Rev. 2013, 14, 95–109. [Google Scholar] [CrossRef]
- Vrbikova, J.; Hainer, V. Obesity and polycystic ovary syndrome. Obes. Facts 2009, 2, 26–35. [Google Scholar] [CrossRef]
- Rojas, J.; Chavez, M.; Olivar, L.; Rojas, M.; Morillo, J.; Mejias, J.; Calvo, M.; Bermudez, V. Polycystic ovary syndrome, insulin resistance, and obesity: Navigating the pathophysiologic labyrinth. Int. J. Reprod. Med. 2014, 2014, 719050. [Google Scholar] [CrossRef]
- Bajuk Studen, K.; Pfeifer, M. Cardiometabolic risk in polycystic ovary syndrome. Endocr. Connect. 2018, 7, R238–R251. [Google Scholar] [CrossRef] [PubMed]
- Cooney, L.G.; Dokras, A. Beyond fertility: Polycystic ovary syndrome and long-term health. Fertil. Steril. 2018, 110, 794–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Zhang, C.; Shi, Y.; Zhang, F.; Li, L.; Wang, X.; Ling, Y.; Fu, H.; Dong, W.; Shen, J.; et al. Dysbiosis of Gut Microbiota Associated with Clinical Parameters in Polycystic Ovary Syndrome. Front. Microbiol. 2017, 8, 324. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Qi, Y.; Yang, X.; Zhao, L.; Wen, S.; Liu, Y.; Tang, L. Association between Polycystic Ovary Syndrome and Gut Microbiota. PLoS ONE 2016, 11, e0153196. [Google Scholar] [CrossRef] [Green Version]
- Duan, L.; An, X.; Zhang, Y.; Jin, D.; Zhao, S.; Zhou, R.; Duan, Y.; Zhang, Y.; Liu, X.; Lian, F. Gut microbiota as the critical correlation of polycystic ovary syndrome and type 2 diabetes mellitus. Biomed. Pharmacother. 2021, 142, 112094. [Google Scholar] [CrossRef]
- Rizk, M.G.; Thackray, V.G. Intersection of Polycystic Ovary Syndrome and the Gut Microbiome. J. Endocr. Soc. 2021, 5, bvaa177. [Google Scholar] [CrossRef]
- Kumar, P.S. Sex and the subgingival microbiome: Do female sex steroids affect periodontal bacteria? Periodontol. 2000 2013, 61, 103–124. [Google Scholar] [CrossRef]
- Haro, C.; Rangel-Zuniga, O.A.; Alcala-Diaz, J.F.; Gomez-Delgado, F.; Perez-Martinez, P.; Delgado-Lista, J.; Quintana-Navarro, G.M.; Landa, B.B.; Navas-Cortes, J.A.; Tena-Sempere, M.; et al. Intestinal Microbiota Is Influenced by Gender and Body Mass Index. PLoS ONE 2016, 11, e0154090. [Google Scholar] [CrossRef] [Green Version]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Kelley, S.T.; Skarra, D.V.; Rivera, A.J.; Thackray, V.G. The Gut Microbiome Is Altered in a Letrozole-Induced Mouse Model of Polycystic Ovary Syndrome. PLoS ONE 2016, 11, e0146509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherman, S.B.; Sarsour, N.; Salehi, M.; Schroering, A.; Mell, B.; Joe, B.; Hill, J.W. Prenatal androgen exposure causes hypertension and gut microbiota dysbiosis. Gut Microbes 2018, 9, 400–421. [Google Scholar] [CrossRef] [Green Version]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4680–4687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, X.; Qin, P.; Huang, K.; Ding, X.; Ma, J.; Xuan, Y.; Zhu, X.; Peng, D.; Wang, B. Association between polycystic ovary syndrome and the vaginal microbiome: A case-control study. Clin. Endocrinol. 2020, 93, 52–60. [Google Scholar] [CrossRef]
- Petrova, M.I.; Lievens, E.; Malik, S.; Imholz, N.; Lebeer, S. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front. Physiol. 2015, 6, 81. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Wang, H.; Yang, J.; Zhang, X.; Chen, Y.; Feng, R.; Qian, Y. Changes in Vaginal Microbiome Diversity in Women With Polycystic Ovary Syndrome. Front. Cell. Infect. Microbiol. 2021, 11, 755741. [Google Scholar] [CrossRef]
- Matteuzzi, D.; Swennen, E.; Rossi, M.; Hartman, T.; Lebet, V. Prebiotic effects of a wheat germ preparation in human healthy subjects. Food Microbiol. 2004, 21, 119–124. [Google Scholar] [CrossRef]
- Connolly, M.L.; Tuohy, K.M.; Lovegrove, J.A. Wholegrain oat-based cereals have prebiotic potential and low glycaemic index. Br. J. Nutr. 2012, 108, 2198–2206. [Google Scholar] [CrossRef] [Green Version]
- Jefferson, A.; Adolphus, K. The Effects of Intact Cereal Grain Fibers, Including Wheat Bran on the Gut Microbiota Composition of Healthy Adults: A Systematic Review. Front. Nutr. 2019, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Dinicola, S.; Unfer, V.; Facchinetti, F.; Soulage, C.O.; Greene, N.D.; Bizzarri, M.; Laganà, A.S.; Chan, S.Y.; Bevilacqua, A.; Pkhaladze, L.; et al. Inositols: From Established Knowledge to Novel Approaches. Int. J. Mol. Sci. 2021, 22, 10575. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Marin, I.; Picconi, O.; Laganà, A.S.; Costabile, L.; Unfer, V. A multicenter clinical study with myo-inositol and alpha-lactalbumin in Mexican and Italian PCOS patients. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3316–3324. [Google Scholar] [CrossRef] [PubMed]
- Monastra, G.; Sambuy, Y.; Ferruzza, S.; Ferrari, D.; Ranaldi, G. Alpha-lactalbumin Effect on Myo-inositol Intestinal Absorption: In vivo and In vitro. Curr. Drug Deliv. 2018, 15, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Montanino Oliva, M.; Buonomo, G.; Calcagno, M.; Unfer, V. Effects of myo-inositol plus alpha-lactalbumin in myo-inositol-resistant PCOS women. J. Ovarian Res. 2018, 11, 38. [Google Scholar] [CrossRef]
- Ranaldi, G.; Ferruzza, S.; Natella, F.; Unfer, V.; Sambuy, Y.; Monastra, G. Enhancement of D-chiro-inositol transport across intestinal cells by alpha-Lactalbumin peptides. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10143–10154. [Google Scholar] [CrossRef]
- Szczuko, M.; Kikut, J.; Szczuko, U.; Szydlowska, I.; Nawrocka-Rutkowska, J.; Zietek, M.; Verbanac, D.; Saso, L. Nutrition Strategy and Life Style in Polycystic Ovary Syndrome-Narrative Review. Nutrients 2021, 13, 2452. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; International, P.N. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 2018, 33, 1602–1618. [Google Scholar] [CrossRef] [Green Version]
- Barrea, L.; Arnone, A.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; Salzano, C.; Pugliese, G.; Colao, A.; Savastano, S. Adherence to the Mediterranean Diet, Dietary Patterns and Body Composition in Women with Polycystic Ovary Syndrome (PCOS). Nutrients 2019, 11, 2278. [Google Scholar] [CrossRef] [Green Version]
- Paoli, A.; Mancin, L.; Giacona, M.C.; Bianco, A.; Caprio, M. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med 2020, 27, 104. [Google Scholar] [CrossRef]
- Yao, K.; Zeng, L.; He, Q.; Wang, W.; Lei, J.; Zou, X. Effect of Probiotics on Glucose and Lipid Metabolism in Type 2 Diabetes Mellitus: A Meta-Analysis of 12 Randomized Controlled Trials. Med. Sci. Monit. 2017, 23, 3044–3053. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Bai, L.; Guan, M.; Zhou, X.; Liang, X.; Lv, Y.; Yi, H.; Zhou, H.; Liu, T.; Gong, P.; et al. Potential probiotics Lactobacillus casei K11 combined with plant extracts reduce markers of type 2 diabetes mellitus in mice. J. Appl. Microbiol. 2021, 131, 1970–1982. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Jamilian, M.; Karamali, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z. Probiotic supplementation and the effects on weight loss, glycaemia and lipid profiles in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Hum. Fertil. 2017, 20, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Shoaei, T.; Heidari-Beni, M.; Tehrani, H.G.; Feizi, A.; Esmaillzadeh, A.; Askari, G. Effects of Probiotic Supplementation on Pancreatic beta-cell Function and C-reactive Protein in Women with Polycystic Ovary Syndrome: A Randomized Double-blind Placebo-controlled Clinical Trial. Int. J. Prev. Med. 2015, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Rashad, N.M.; Amal, S.; Amin, A.I.; Soliman, M.H. Effects of probiotics supplementation on macrophage migration inhibitory factor and clinical laboratory feature of polycystic ovary syndrome. J. Funct. Foods 2017, 36, 317–324. [Google Scholar] [CrossRef]
- Ghanei, N.; Rezaei, N.; Amiri, G.A.; Zayeri, F.; Makki, G.; Nasseri, E. The probiotic supplementation reduced inflammation in polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. J. Funct. Foods 2018, 42, 306–311. [Google Scholar] [CrossRef]
- Gao, J.; Song, J.; Du, M.; Mao, X. Bovine α-Lactalbumin Hydrolysates (α-LAH) Ameliorate Adipose Insulin Resistance and Inflammation in High-Fat Diet-Fed C57BL/6J Mice. Nutrients 2018, 10, 242. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Gao, J.; Du, M.; Mao, X. Bovine α-lactalbumin hydrolysates ameliorate obesity-associated endotoxemia and inflammation in high-fat diet-fed mice through modulation of gut microbiota. Food Funct. 2019, 10, 3368–3378. [Google Scholar] [CrossRef]
- Boscaini, S.; Cabrera-Rubio, R.; Speakman, J.R.; Cotter, P.D.; Cryan, J.F.; Nilaweera, K.N. Dietary α-lactalbumin alters energy balance, gut microbiota composition and intestinal nutrient transporter expression in high-fat diet-fed mice. Br. J. Nutr. 2019, 121, 1097–1107. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [Green Version]
- Ferrocino, I.; Ponzo, V.; Gambino, R.; Zarovska, A.; Leone, F.; Monzeglio, C.; Goitre, I.; Rosato, R.; Romano, A.; Grassi, G.; et al. Changes in the gut microbiota composition during pregnancy in patients with gestational diabetes mellitus (GDM). Sci. Rep. 2018, 8, 12216. [Google Scholar] [CrossRef]
- Ponzo, V.; Ferrocino, I.; Zarovska, A.; Amenta, M.B.; Leone, F.; Monzeglio, C.; Rosato, R.; Pellegrini, M.; Gambino, R.; Cassader, M.; et al. The microbiota composition of the offspring of patients with gestational diabetes mellitus (GDM). PLoS ONE 2019, 14, e0226545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zheng, J.; Shi, W.; Du, N.; Xu, X.; Zhang, Y.; Ji, P.; Zhang, F.; Jia, Z.; Wang, Y.; et al. Dysbiosis of maternal and neonatal microbiota associated with gestational diabetes mellitus. Gut 2018, 67, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Chandler-Laney, P.C.; Bush, N.C.; Granger, W.M.; Rouse, D.J.; Mancuso, M.S.; Gower, B.A. Overweight status and intrauterine exposure to gestational diabetes are associated with children’s metabolic health. Pediatr. Obes. 2012, 7, 44–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Nomura, Y.; Bashir, A.; Fernandez-Hernandez, H.; Itzkowitz, S.; Pei, Z.; Stone, J.; Loudon, H.; Peter, I. Diversified microbiota of meconium is affected by maternal diabetes status. PLoS ONE 2013, 8, e78257. [Google Scholar] [CrossRef] [Green Version]
- Hasan, S.; Aho, V.; Pereira, P.; Paulin, L.; Koivusalo, S.B.; Auvinen, P.; Eriksson, J.G. Gut microbiome in gestational diabetes: A cross-sectional study of mothers and offspring 5 years postpartum. Acta Obstet. Gynecol. Scand. 2018, 97, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Collado, M.C.; Isolauri, E.; Laitinen, K.; Salminen, S. Effect of mother’s weight on infant’s microbiota acquisition, composition, and activity during early infancy: A prospective follow-up study initiated in early pregnancy. Am. J. Clin. Nutr. 2010, 92, 1023–1030. [Google Scholar] [CrossRef]
- Collado, M.C.; Isolauri, E.; Laitinen, K.; Salminen, S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am. J. Clin. Nutr. 2008, 88, 894–899. [Google Scholar] [CrossRef]
- Singh, A.; Mittal, M. Neonatal microbiome—A brief review. J. Matern Fetal Neonatal Med. 2020, 33, 3841–3848. [Google Scholar] [CrossRef]
- Abbott, D.H.; Barnett, D.K.; Bruns, C.M.; Dumesic, D.A. Androgen excess fetal programming of female reproduction: A developmental aetiology for polycystic ovary syndrome? Hum. Reprod. Update 2005, 11, 357–374. [Google Scholar] [CrossRef] [Green Version]
- Parker, J.; O’Brien, C.; Hawrelak, J.; Gersh, F.L. Polycystic Ovary Syndrome: An Evolutionary Adaptation to Lifestyle and the Environment. Int J Environ Res Public Health. 2022, 19, 1336. [Google Scholar] [CrossRef]
- Gulan, T.; Yeernuer, T.; Sui, S.; Mayinuer, N. A Rat Model of Maternal Polycystic Ovary Syndrome Shows that Exposure to Androgens in Utero Results in Dysbiosis of the Intestinal Microbiota and Metabolic Disorders of the Newborn Rat. Med. Sci. Monit. 2019, 25, 9377–9391. [Google Scholar] [CrossRef] [PubMed]
- Daan, N.M.; Koster, M.P.; Steegers-Theunissen, R.P.; Eijkemans, M.J.; Fauser, B.C. Endocrine and cardiometabolic cord blood characteristics of offspring born to mothers with and without polycystic ovary syndrome. Fertil. Steril. 2017, 107, 261–268.e263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, J.A.; Kay, A.R.; Navaratnarajah, R.; Iqbal, S.; Bamfo, J.E.; David, A.L.; Hines, M.; Hardiman, P.J. Umbilical vein testosterone in female infants born to mothers with polycystic ovary syndrome is elevated to male levels. J. Obstet. Gynaecol. 2010, 30, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Insenser, M.; Murri, M.; Del Campo, R.; Martinez-Garcia, M.A.; Fernandez-Duran, E.; Escobar-Morreale, H.F. Gut Microbiota and the Polycystic Ovary Syndrome: Influence of Sex, Sex Hormones, and Obesity. J. Clin. Endocrinol. Metab. 2018, 103, 2552–2562. [Google Scholar] [CrossRef]
- Zeng, B.; Lai, Z.; Sun, L.; Zhang, Z.; Yang, J.; Li, Z.; Lin, J.; Zhang, Z. Structural and functional profiles of the gut microbial community in polycystic ovary syndrome with insulin resistance (IR-PCOS): A pilot study. Res. Microbiol. 2019, 170, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Collden, H.; Landin, A.; Wallenius, V.; Elebring, E.; Fandriks, L.; Nilsson, M.E.; Ryberg, H.; Poutanen, M.; Sjogren, K.; Vandenput, L.; et al. The gut microbiota is a major regulator of androgen metabolism in intestinal contents. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E1182–E1192. [Google Scholar] [CrossRef] [PubMed]
- Neuman, H.; Debelius, J.W.; Knight, R.; Koren, O. Microbial endocrinology: The interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev. 2015, 39, 509–521. [Google Scholar] [CrossRef] [Green Version]
- Bhagavata Srinivasan, S.P.; Raipuria, M.; Bahari, H.; Kaakoush, N.O.; Morris, M.J. Impacts of Diet and Exercise on Maternal Gut Microbiota Are Transferred to Offspring. Front. Endocrinol. (Lausanne) 2018, 9, 716. [Google Scholar] [CrossRef]
- Zhou, L.; Xiao, X. The role of gut microbiota in the effects of maternal obesity during pregnancy on offspring metabolism. Biosci. Rep. 2018, 38, BSR20171234. [Google Scholar] [CrossRef] [Green Version]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.; Eriksson, J.G.; Broekman, B.F. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Gohir, W.; Ratcliffe, E.M.; Sloboda, D.M. Of the bugs that shape us: Maternal obesity, the gut microbiome, and long-term disease risk. Pediatr. Res. 2015, 77, 196–204. [Google Scholar] [CrossRef]
- Gambioli, R.; Forte, G.; Buzzaccarini, G.; Unfer, V.; Lagana, A.S. Myo-Inositol as a Key Supporter of Fertility and Physiological Gestation. Pharmaceuticals 2021, 14, 504. [Google Scholar] [CrossRef] [PubMed]
- D’Anna, R.; Di Benedetto, A.; Scilipoti, A.; Santamaria, A.; Interdonato, M.L.; Petrella, E.; Neri, I.; Pintaudi, B.; Corrado, F.; Facchinetti, F. Myo-inositol Supplementation for Prevention of Gestational Diabetes in Obese Pregnant Women: A Randomized Controlled Trial. Obstet. Gynecol. 2015, 126, 310–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Anna, R.; Corrado, F.; Loddo, S.; Gullo, G.; Giunta, L.; Di Benedetto, A. Myoinositol plus α-lactalbumin supplementation, insulin resistance and birth outcomes in women with gestational diabetes mellitus: A randomized, controlled study. Sci. Rep. 2021, 11, 8866. [Google Scholar] [CrossRef] [PubMed]
- Monastra, G.; Unfer, V.; Harrath, A.H.; Bizzarri, M. Combining treatment with myo-inositol and D-chiro-inositol (40:1) is effective in restoring ovary function and metabolic balance in PCOS patients. Gynecol. Endocrinol. 2017, 33, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Minozzi, M.; Nordio, M.; Pajalich, R. The Combined therapy myo-inositol plus D-Chiro-inositol, in a physiological ratio, reduces the cardiovascular risk by improving the lipid profile in PCOS patients. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 537–540. [Google Scholar]
- Elsenbruch, S.; Hahn, S.; Kowalsky, D.; Offner, A.H.; Schedlowski, M.; Mann, K.; Janssen, O.E. Quality of life, psychosocial well-being, and sexual satisfaction in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 5801–5807. [Google Scholar] [CrossRef] [Green Version]
- Deeks, A.A.; Gibson-Helm, M.E.; Teede, H.J. Anxiety and depression in polycystic ovary syndrome: A comprehensive investigation. Fertil. Steril. 2010, 93, 2421–2423. [Google Scholar] [CrossRef]
- Chaudhari, N.; Dawalbhakta, M.; Nampoothiri, L. GnRH dysregulation in polycystic ovarian syndrome (PCOS) is a manifestation of an altered neurotransmitter profile. Reprod. Biol. Endocrinol. 2018, 16, 37. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, L.; Fu, S.; Li, N. Co-involvement of psychological and neurological abnormalities in infertility with polycystic ovarian syndrome. Arch. Gynecol. Obstet. 2011, 284, 773–778. [Google Scholar] [CrossRef]
- Moguilevsky, J.A.; Faigon, M.R.; Rubio, M.C.; Scacchi, P.; Szwarcfarb, B. Sexual differences in the effect of serotonin on LH secretion in rats. Acta Endocrinol. (Cph.) 1985, 109, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, V.D.; Dluzen, D.E. Release of luteinizing hormone-releasing hormone (LHRH) and neuroactive substances in unanesthetized animals as estimated with push-pull cannulae (PPC). Biol. Reprod. 1987, 36, 59–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lokuge, S.; Frey, B.N.; Foster, J.A.; Soares, C.N.; Steiner, M. Depression in women: Windows of vulnerability and new insights into the link between estrogen and serotonin. J. Clin. Psychiatry 2011, 72, e1563–e1569. [Google Scholar] [CrossRef] [PubMed]
- Markus, C.R.; Jonkman, L.M.; Lammers, J.H.; Deutz, N.E.; Messer, M.H.; Rigtering, N. Evening intake of alpha-lactalbumin increases plasma tryptophan availability and improves morning alertness and brain measures of attention. Am. J. Clin. Nutr. 2005, 81, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D.; Langham, K.A.; Marcelino, L.M.; Irvine, Z.L.; Fernstrom, M.H.; Kaye, W.H. The ingestion of different dietary proteins by humans induces large changes in the plasma tryptophan ratio, a predictor of brain tryptophan uptake and serotonin synthesis. Clin. Nutr. 2013, 32, 1073–1076. [Google Scholar] [CrossRef] [Green Version]
- Williams, W.A.; Shoaf, S.E.; Hommer, D.; Rawlings, R.; Linnoila, M. Effects of Acute Tryptophan Depletion on Plasma and Cerebrospinal Fluid Tryptophan and 5-Hydroxyindoleacetic Acid in Normal Volunteers. J. Neurochem. 2001, 72, 1641–1647. [Google Scholar] [CrossRef]
- Sodhi, M.S.; Sanders-Bush, E. Serotonin and brain development. Int. Rev. Neurobiol. 2004, 59, 111–174. [Google Scholar] [CrossRef]
- Shah, R.; Courtiol, E.; Castellanos, F.X.; Teixeira, C.M. Abnormal Serotonin Levels During Perinatal Development Lead to Behavioral Deficits in Adulthood. Front. Behav. Neurosci. 2018, 12, 114. [Google Scholar] [CrossRef] [Green Version]
- Mazer, C.; Muneyyirci, J.; Taheny, K.; Raio, N.; Borella, A.; Whitaker-Azmitia, P. Serotonin depletion during synaptogenesis leads to decreased synaptic density and learning deficits in the adult rat: A possible model of neurodevelopmental disorders with cognitive deficits. Brain Res. 1997, 760, 68–73. [Google Scholar] [CrossRef]
- Segall, P.E.; Timiras, P.S.; Walton, J.R. Low tryptophan diets delay reproductive aging. Mech. Ageing Dev. 1983, 23, 245–252. [Google Scholar] [CrossRef]
- Segall, P.E.; Timiras, P.S. Patho-physiologic findings after chronic tryptophan deficiency in rats: A model for delayed growth and aging. Mech. Ageing Dev. 1976, 5, 109–124. [Google Scholar] [CrossRef]
- Yan, W.; Wilson, C.C.; Haring, J.H. Effects of neonatal serotonin depletion on the development of rat dentate granule cells. Brain Res. Dev. Brain Res. 1997, 98, 177–184. [Google Scholar] [CrossRef]
- Whitaker-Azmitia, P.M. Serotonin and brain development: Role in human developmental diseases. Brain Res. Bull. 2001, 56, 479–485. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardinale, V.; Lepore, E.; Basciani, S.; Artale, S.; Nordio, M.; Bizzarri, M.; Unfer, V. Positive Effects of α-Lactalbumin in the Management of Symptoms of Polycystic Ovary Syndrome. Nutrients 2022, 14, 3220. https://doi.org/10.3390/nu14153220
Cardinale V, Lepore E, Basciani S, Artale S, Nordio M, Bizzarri M, Unfer V. Positive Effects of α-Lactalbumin in the Management of Symptoms of Polycystic Ovary Syndrome. Nutrients. 2022; 14(15):3220. https://doi.org/10.3390/nu14153220
Chicago/Turabian StyleCardinale, Vincenzo, Elisa Lepore, Sabrina Basciani, Salvatore Artale, Maurizio Nordio, Mariano Bizzarri, and Vittorio Unfer. 2022. "Positive Effects of α-Lactalbumin in the Management of Symptoms of Polycystic Ovary Syndrome" Nutrients 14, no. 15: 3220. https://doi.org/10.3390/nu14153220
APA StyleCardinale, V., Lepore, E., Basciani, S., Artale, S., Nordio, M., Bizzarri, M., & Unfer, V. (2022). Positive Effects of α-Lactalbumin in the Management of Symptoms of Polycystic Ovary Syndrome. Nutrients, 14(15), 3220. https://doi.org/10.3390/nu14153220







