The Effect of Probiotics Use on Salivary Cariogenic Bacteria in Orthodontic Patients with Various Caries Risk Status
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Power Analysis and Sample Size Estimation
2.3. Study Design
2.3.1. Bracket and Band Selection
2.3.2. Saliva Sample Collection
2.3.3. Use of Probiotics
2.3.4. Saliva Sampling Method
2.3.5. Incubation Method
2.3.6. Bacteria Colony Formation Density Scoring Method
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Changes in Cariogenic Bacterial Count Scores in Orthodontic Patients over Time
4.2. The Effect of the Use of Probiotics in Orthodontic Patients with Low Caries Risk
4.3. The Effect of the Use of Probiotics in Orthodontic Patients with High Caries Risk
4.4. Probiotics Selection and the Possible Mechanism of Bacterial Ecological Changes
4.5. Weaknesses and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanders, M.E. Probiotics: Definition, sources, selection, and uses. Clin. Infect. Dis. 2008, 46 (Suppl. 2), S58–S61, discussion S144–S151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, G.; Younes, J.A.; Van der Mei, H.C.; Gloor, G.B.; Knight, R.; Busscher, H.J. Microbiota restoration: Natural and supplemented recovery of human microbial communities. Nat. Rev. Microbiol. 2011, 9, 27–38. [Google Scholar] [CrossRef]
- Bustamante, M.; Oomah, B.D.; Mosi-Roa, Y.; Rubilar, M.; Burgos-Diaz, C. Probiotics as an adjunct therapy for the treatment of halitosis, dental caries and periodontitis. Probiotics Antimicrob Proteins 2020, 12, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Laleman, I.; Teughels, W. Probiotics in the dental practice: A review. Quintessence Int. 2015, 46, 255–264. [Google Scholar] [PubMed]
- Karpiński, T.M.; Szkaradkiewicz, A.K. Microbiology of dental caries. J. Biol. Earth Sci. 2013, 3, M21–M24. [Google Scholar]
- Lin, T.H.; Lin, C.H.; Pan, T.M. The implication of probiotics in the prevention of dental caries. Appl. Microbiol. Biotechnol. 2018, 102, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Twetman, S.; Keller, M.K. Probiotics for caries prevention and control. Adv. Dent. Res. 2012, 24, 98–102. [Google Scholar] [PubMed]
- Cagetti, M.G.; Mastroberardino, S.; Milia, E.; Cocco, F.; Lingstrom, P.; Campus, G. The use of probiotic strains in caries prevention: A systematic review. Nutrients 2013, 5, 2530–2550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laleman, I.; Detailleur, V.; Slot, D.E.; Slomka, V.; Quirynen, M.; Teughels, W. Probiotics reduce mutans streptococci counts in humans: A systematic review and meta-analysis. Clin. Oral Investig. 2014, 18, 1539–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadelman, P.; Magno, M.B.; Masterson, D.; da Cruz, A.G.; Maia, L.C. Are dairy products containing probiotics beneficial for oral health? A systematic review and meta-analysis. Clin. Oral Investig. 2018, 22, 2763–2785. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.J.; Shon, B.H. Initial changes of dental plaque, gingivitis and decalcification in korean orthodontic patients with fixed appliance. Korean J. Orthod. 1999, 29, 361–374. [Google Scholar]
- Hadj-Hamou, R.; Senok, A.C.; Athanasiou, A.E.; Kaklamanos, E.G. Do probiotics promote oral health during orthodontic treatment with fixed appliances? A systematic review. BMC Oral Health 2020, 20, 126. [Google Scholar] [CrossRef] [PubMed]
- Gizani, S.; Petsi, G.; Twetman, S.; Caroni, C.; Makou, M.; Papagianoulis, L. Effect of the probiotic bacterium lactobacillus reuteri on white spot lesion development in orthodontic patients. Eur. J. Orthod. 2016, 38, 85–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, G.S.; Cenci, M.S.; Azevedo, M.S.; Epifanio, M.; Jones, M.H. Effect of yogurt containing bifidobacterium animalis subsp. Lactis dn-173010 probiotic on dental plaque and saliva in orthodontic patients. Caries Res. 2014, 48, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Alp, S.; Baka, Z.M. Effects of probiotics on salivary streptecoccus mutans and lactobacillus levels in orthodontic patients. Am. J. Orthod. Dentofac. Orthop. 2018, 154, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Enerback, H.; Lingstrom, P.; Moller, M.; Nylen, C.; Bresin, C.O.; Ros, I.O.; Westerlund, A. Validation of caries risk assessment methods in orthodontic patients. Am. J. Orthod. Dentofac. Orthop. 2020, 158, 92–101.e103. [Google Scholar] [CrossRef] [PubMed]
- Al Mulla, A.H.; Kharsa, S.A.; Kjellberg, H.; Birkhed, D. Caries risk profiles in orthodontic patients at follow-up using cariogram. Angle Orthod. 2009, 79, 323–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolodkin-Gal, I.; Romero, D.; Cao, S.; Clardy, J.; Kolter, R.; Losick, R. D-amino acids trigger biofilm disassembly. Science 2010, 328, 627–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwamoto, A.; Nakamura, T.; Narisawa, N.; Kawasaki, Y.; Abe, S.; Torii, Y.; Senpuku, H.; Takenaga, F. The japanese fermented food natto inhibits sucrose-dependent biofilm formation by cariogenic streptococci. Food Sci. Technol. Res. 2018, 24, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Martin, V.V.; Monique, D.; Laurence, V.N. Disruption potential of a multi-species oral biofilm by probiotics: Preliminary investigation of a new bacillus subtilis strain. J. Probiotics Health 2021, 9, 1–10. [Google Scholar]

| T0 | T1 | T2 | p Value a | Dunn-Bonferroni Post Hoc b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | T0 vs. T1 | T0 vs. T2 | T1 vs. T2 | ||
| MS CRT score c | 2.45 | 1.06 | 3.21 | 1.11 | 3.03 | 0.92 | 0.001 ** | 0.011 * | 1 | 0.109 |
| LB CRT score c | 2.55 | 0.94 | 3.55 | 0.75 | 3.18 | 0.88 | <0.001 ** | 0.001 ** | 0.372 | 0.109 |
| Low Caries Risk Group (n = 11) | High Caries Risk Group (n = 22) | p Value | |
|---|---|---|---|
| Gender | 0.711 | ||
| M | 5 (45.5%) | 13 (59.1%) | |
| F | 6 (54.5%) | 9 (40.9%) | |
| Age | 15.27 ± 3.23 | 15.68 ± 3.14 | 0.576 |
| T0MS | 1.55 ± 0.52 | 2.91 ± 0.97 | <0.001 ** |
| T0SB | 1.55 ± 0.52 | 3.05 ± 0.65 | <0.001 ** |
| T1MS | 2.91 ± 1.22 | 3.36 ± 1.05 | 0.232 |
| T1SB | 3.36 ± 1.03 | 3.64 ± 0.58 | 0.613 |
| T2MS | 2.91 ± 1.14 | 3.09 ± 0.81 | 0.716 |
| T2SB | 2.82 ± 0.98 | 3.36 ± 0.79 | 0.115 |
| Bacteria Count | CRT Score | p Value | Multiple Comparison | |||
|---|---|---|---|---|---|---|
| p Value | ||||||
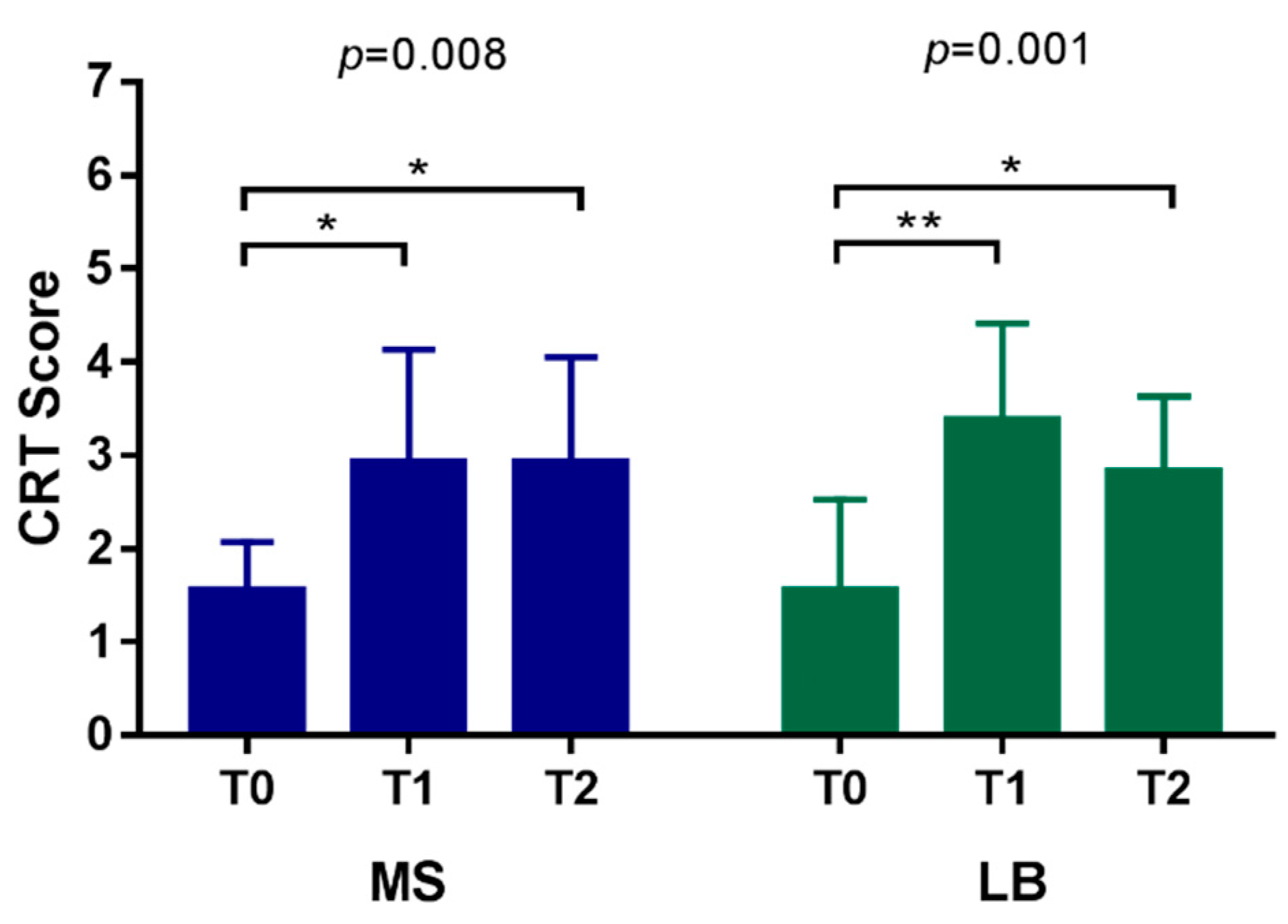
| MS count | T0 | 1.55 ± 0.52 | 0.008 * | T0–T1 | T1–T2 | T0–T2 |
| T1 | 2.91 ± 1.22 | 0.024 * | 1 | 0.020 * | ||
| T2 | 2.91 ± 1.14 | |||||
| LB count | T0 | 1.55 ± 0.52 | 0.001 * | 0.001 * | 0.933 | 0.021 * |
| T1 | 3.36 ± 1.03 | |||||
| T2 | 2.82 ± 0.98 | |||||
| Bacteria Count | CRT Score | p Value | Multiple Comparison | |||
|---|---|---|---|---|---|---|
| T0–T1 | T1–T2 | T0–T2 | ||||
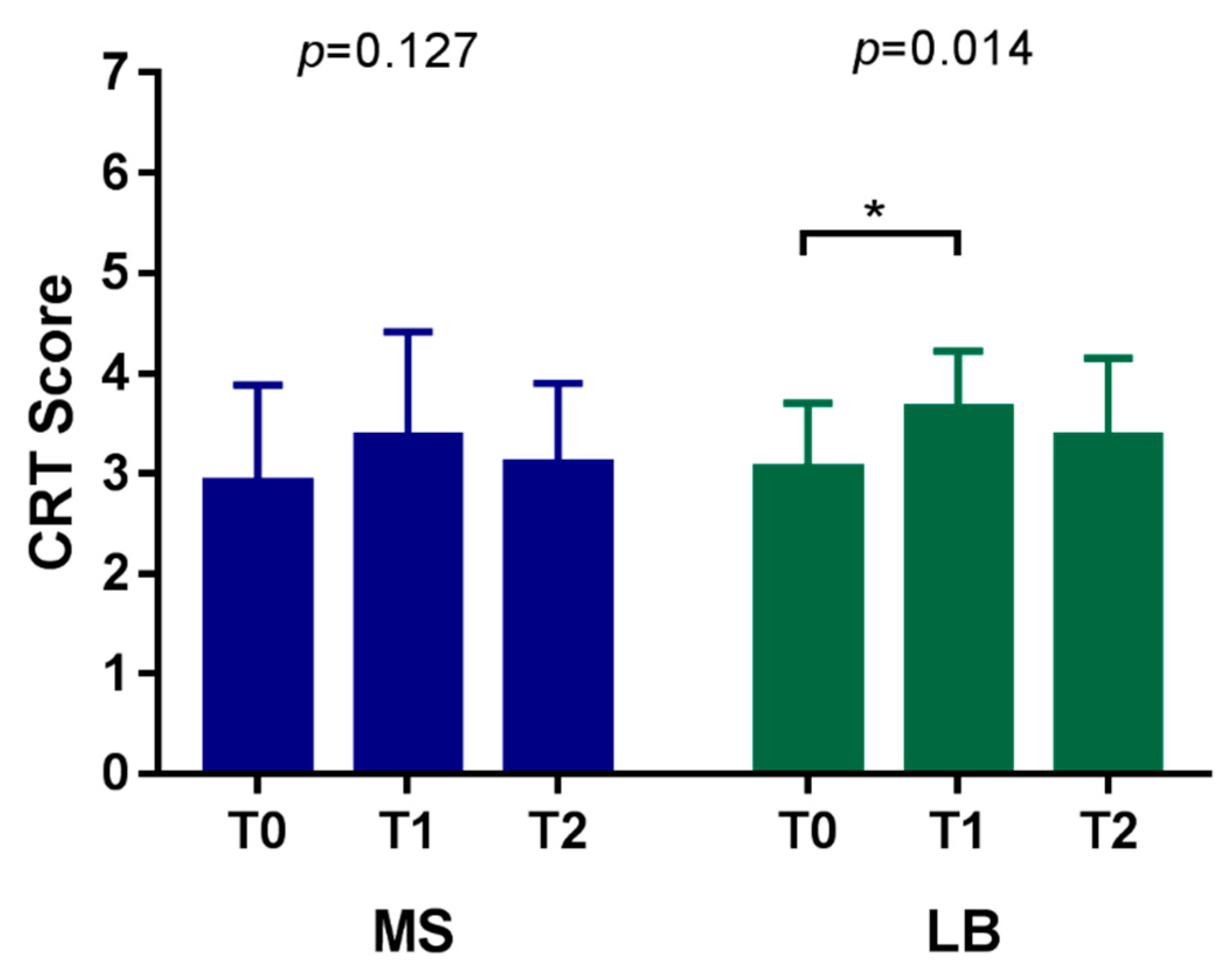
| MS count | T0 | 2.91 ± 0.97 | 0.127 | |||
| T1 | 3.36 ± 1.05 | |||||
| T2 | 3.09 ± 0.81 | |||||
| LB count | T0 | 3.05 ± 0.65 | 0.014 * | 0.011 * | 0.256 | 0.717 |
| T1 | 3.64 ± 0.58 | |||||
| T2 | 3.36 ± 0.79 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-R.; Lai, C.-L.; Chen, J.-P.; Kao, C.-T. The Effect of Probiotics Use on Salivary Cariogenic Bacteria in Orthodontic Patients with Various Caries Risk Status. Nutrients 2022, 14, 3196. https://doi.org/10.3390/nu14153196
Chen L-R, Lai C-L, Chen J-P, Kao C-T. The Effect of Probiotics Use on Salivary Cariogenic Bacteria in Orthodontic Patients with Various Caries Risk Status. Nutrients. 2022; 14(15):3196. https://doi.org/10.3390/nu14153196
Chicago/Turabian StyleChen, Liang-Ru, Chia-Li Lai, Jun-Peng Chen, and Chia-Tze Kao. 2022. "The Effect of Probiotics Use on Salivary Cariogenic Bacteria in Orthodontic Patients with Various Caries Risk Status" Nutrients 14, no. 15: 3196. https://doi.org/10.3390/nu14153196
APA StyleChen, L.-R., Lai, C.-L., Chen, J.-P., & Kao, C.-T. (2022). The Effect of Probiotics Use on Salivary Cariogenic Bacteria in Orthodontic Patients with Various Caries Risk Status. Nutrients, 14(15), 3196. https://doi.org/10.3390/nu14153196






