Evaluation of the Nutritional Status of Gaucher Disease Type I Patients under Enzyme Replacement Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Study Design
2.3. Control Group
2.4. Anthropometry
2.5. Bioelectrical Impedance Analysis
2.6. Resting Energy Expenditure Assessment
2.7. Assessment of the Habitual Physical Activity Level
2.8. Evaluation of Food Habits
2.9. Biochemical Parameters
2.10. Blood Pressure Measurement
2.11. Spleen and Liver Volumes
2.12. Statistical Analysis
3. Results
3.1. Study Participants
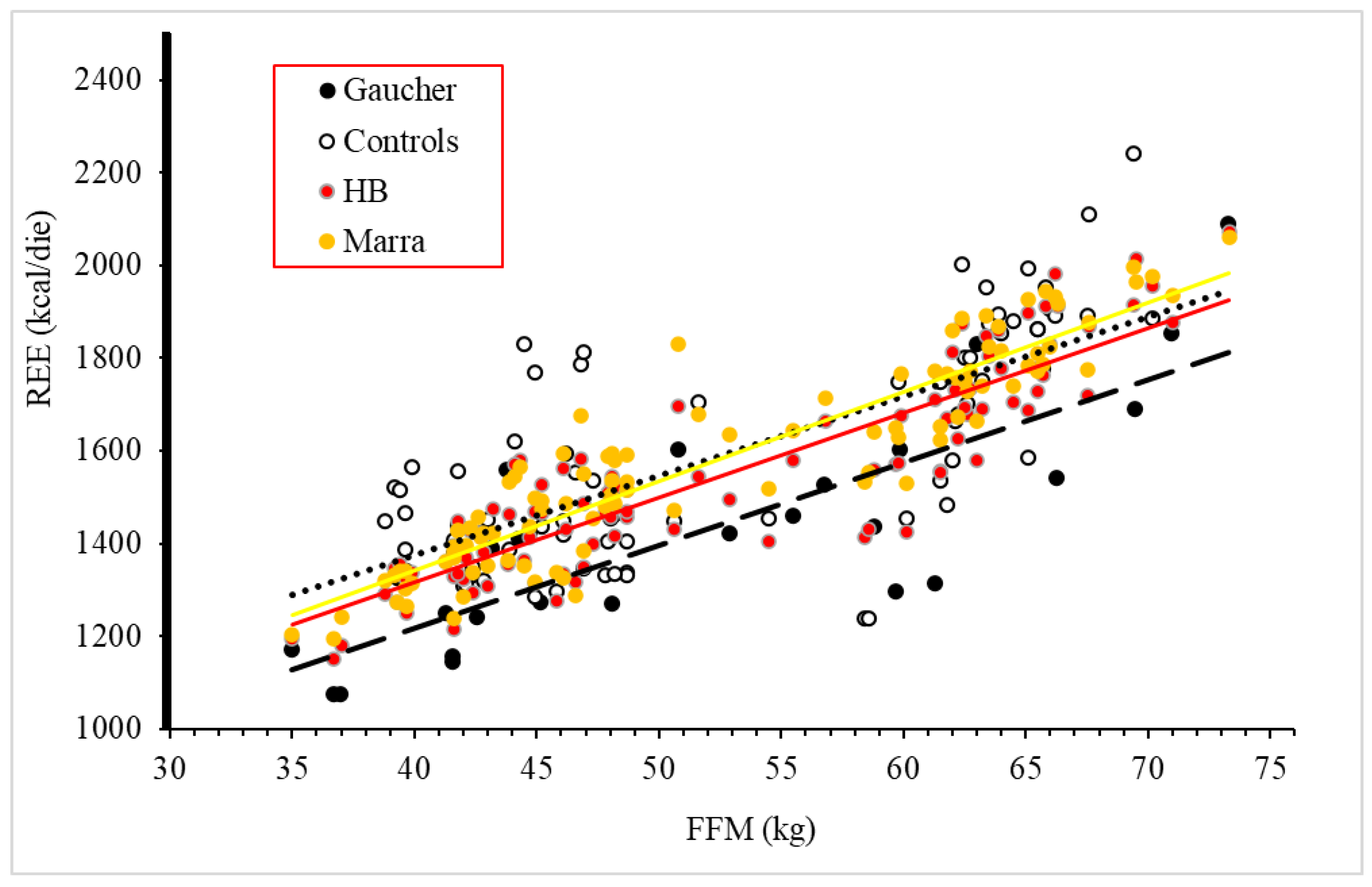
3.2. Body Composition and Resting Energy Expenditure
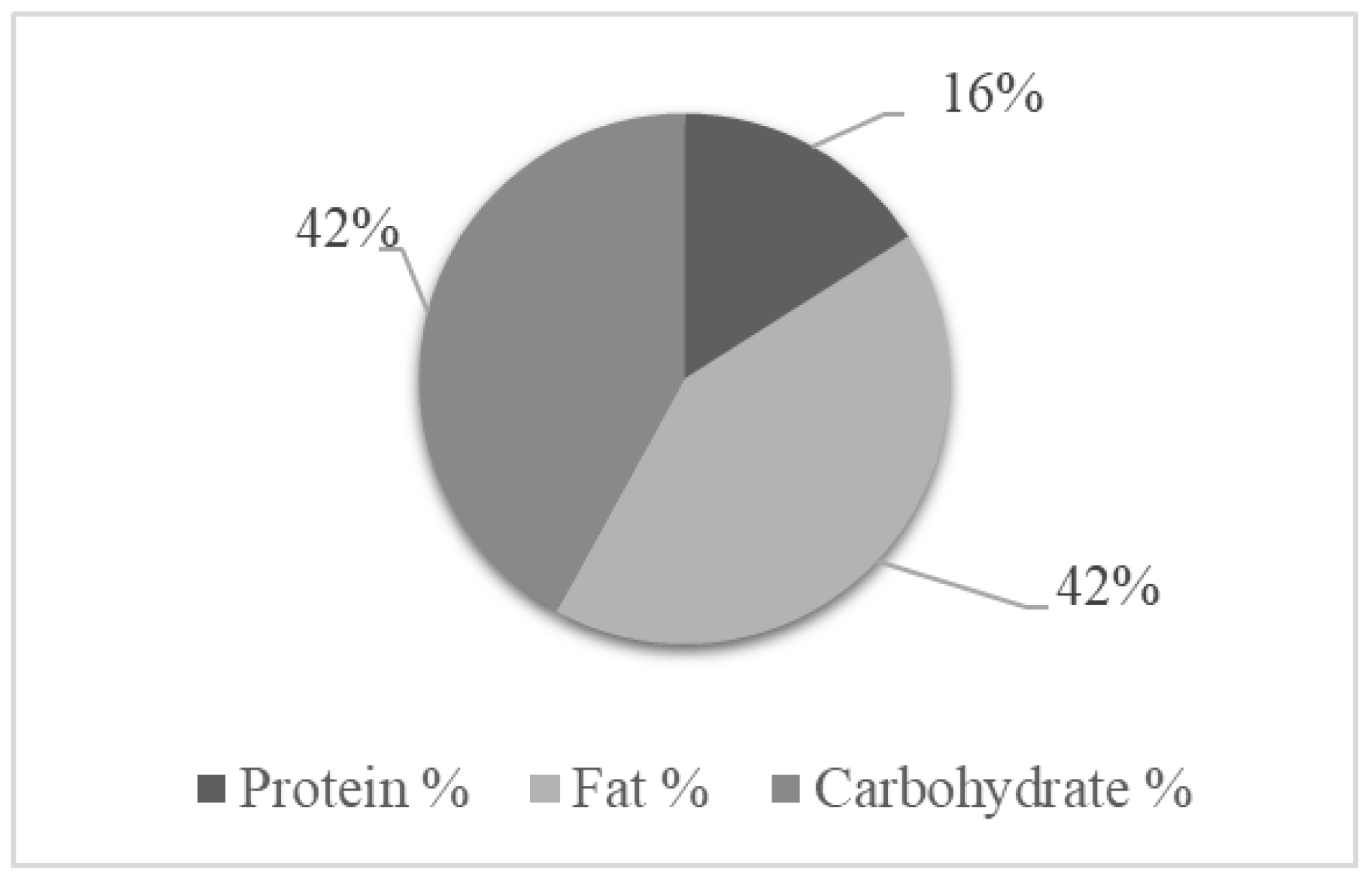
3.3. Food Habits and Physical Activity Level of GD1 Patients
4. Discussion
- GD1 patients on ERT had normal biochemical parameters in 80% of cases, except for HDL-cholesterol, which was lower than the normal cut-off in almost 50% of the population;
- A total of 60% of GD1 patients were overweight or obese;
- GD1 patients consumed a hyper-lipidic diet, and almost half of the population was inactive;
- Body composition did not differ between patients and healthy controls;
- REE measured by indirect calorimetry was significantly lower than predicted by the Harris–Benedict equation. This was confirmed using a specific equation for the REE estimation of Southern Italy residents;
- Measured REE was also reduced if compared to healthy controls;
- REE was directly related to hepatic volume in GD1 patients.
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grabowski, G.A. Phenotype, Diagnosis, and Treatment of Gaucher’s Disease. Lancet 2008, 372, 1263–1271. [Google Scholar] [CrossRef]
- Stirnemann, J.Ô.; Belmatoug, N.; Camou, F.; Serratrice, C.; Froissart, R.; Caillaud, C.; Levade, T.; Astudillo, L.; Serratrice, J.; Brassier, A.; et al. A Review of Gaucher Disease Pathophysiology, Clinical Presentation and Treatments. Int. J. Mol. Sci. 2017, 18, 441. [Google Scholar] [CrossRef] [PubMed]
- Barton, D.J.; Ludman, M.D.; Benkov, K.; Grabowski, G.A.; LeLeiko, N.S. Resting Energy Expenditure in Gaucher’s Disease Type 1: Effect of Gaucher’s Cell Burden on Energy Requirements. Metabolism 1989, 38, 1238–1243. [Google Scholar] [CrossRef]
- Kałużna, M.; Trzeciak, I.; Ziemnicka, K.; Machaczka, M.; Ruchała, M. Endocrine and Metabolic Disorders in Patients with Gaucher Disease Type 1: A Review. Orphanet J. Rare Dis. 2019, 14, 275. [Google Scholar] [CrossRef] [PubMed]
- Corssmit, E.P.; Hollak, C.E.; Endert, E.; Van Oers, M.H.; Sauerwein, H.P.; Romijn, J.A. Increased Basal Glucose Production in Type 1 Gaucher’s Desease. J. Clin. Endocrinol. Metab. 1995, 80, 2653–2657. [Google Scholar]
- Hollak, C.E.M.; Corssmit, E.P.M.; Aerts, J.M.F.G.; Endert, E.; Sauerwein, H.P.; Romijn, J.A.; Van Oers, M.H.J. Differential Effects of Enzyme Supplementation Therapy on Manifestations of Type 1 Gaucher Disease. Am. J. Med. 1997, 103, 185–191. [Google Scholar] [CrossRef][Green Version]
- Langeveld, M.; de Fost, M.; Aerts, J.M.F.G.; Sauerwein, H.P.; Hollak, C.E.M. Overweight, Insulin Resistance and Type II Diabetes in Type I Gaucher Disease Patients in Relation to Enzyme Replacement Therapy. Blood Cells Mol. Dis. 2008, 40, 428–432. [Google Scholar] [CrossRef]
- Doneda, D.; Lopes, A.L.; Oliveira, A.R.; Netto, C.B.; Moulin, C.C.; Schwartz, I.V.D. Gaucher Disease Type I: Assessment of Basal Metabolic Rate in Patients from Southern Brazil. Blood Cells Mol. Dis. 2011, 46, 42–46. [Google Scholar] [CrossRef]
- Buscemi, S.; Rosafio, G.; Vasto, S.; Massenti, F.M.; Grosso, G.; Galvano, F.; Rini, N.; Barile, A.M.; Maniaci, V.; Cosentino, L.; et al. Validation of a Food Frequency Questionnaire for Use in Italian Adults Living in Sicily. Int. J. Food Sci. Nutr. 2015, 66, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Sun, G.; French, C.R.; Martin, G.R.; Younghusband, B.; Green, R.C.; Xie, Y.G.; Mathews, M.; Barron, J.R.; Fitzpatrick, D.G.; Gulliver, W.; et al. Comparison of Multifrequency Bioelectrical Impedance Analysis with Dual-Energy X-Ray Absorptiometry for Assessment of Percentage Body Fat in a Large, Healthy Population. Am. J. Clin. Nutr. 2005, 81, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Elia, M.; Livesey, G. Theory and and Validity of Indirect Calorimetry during Net Lipid Synthesis. Am. J. Clin. Nutr. 1988, 47, 591–607. [Google Scholar] [CrossRef] [PubMed]
- Weir, J.B.D.V. New Methods to Calculate Metabolic Rate with Special Refernce to Protein Metabolism. J. Physiol. 1949, 109, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A.; Benedict, F.G. A biometric study of human basal metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Marra, M.; Sammarco, R.; Cioffi, I.; Morlino, D.; Di Vincenzo, O.; Speranza, E.; Pasanisi, F. New Predictive Equations for Estimating Resting Energy Expenditure in Subjects with Normal Weight and Overweight. Nutrition 2021, 84, 111105. [Google Scholar] [CrossRef]
- Ekelund, U.; Sepp, H.; Brage, S.; Becker, W.; Jakes, R.; Hennings, M.; Wareham, N.J. Criterion-Related Validity of the Last 7-Day, Short Form of the International Physical Activity Questionnaire in Swedish Adults. Public Health Nutr. 2006, 9, 258–265. [Google Scholar] [CrossRef]
- Raskovalova, T.; Deegan, P.B.; Mistry, P.K.; Pavlova, E.; Yang, R.; Zimran, A.; Berger, J.; Bourgne, C.; Pereira, B.; Labarère, J.; et al. Accuracy of Chitotriosidase Activity and CCL18 Concentration in Assessing Type I Gaucher Disease Severity. A Systematic Review with Meta-Analysis of Individual Participant Data. Haematologica 2020, 105, 437–445. [Google Scholar] [CrossRef]
- Barton, N.W.; Brady, R.O.; Dambrosia, J.M.; Bisceglie, A.M.; Doppelt, S.H.; Hill, S.C.; Mankin, H.J.; Murray, G.J.; Parker, R.I.; Argoff, C.E.; et al. Replacement Therapy for Inherited Enzyme Deficiency—Macrophage Targetedglucocerebrosidase for Gaucher’s Disease. N. Engl. J. Med. 1991, 324, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, C. Current Methods of Autopsy Practice. JAMA 1979, 242, 1791–1792. [Google Scholar] [CrossRef]
- Società Italiana di Nutrizione Umana. LARN—Livelli di Assunzione di Riferimento di Nutrienti ed Energia per la popolazione Italiana. IV Revisione; SICS: Milano, Italy, 2014. [Google Scholar]
- Zimmermann, A.; Grigorescu-Sido, P.; Rossmann, H.; Lackner, K.J.; Drugan, C.; Al Khzouz, C.; Bucerzan, S.; Naşcu, I.; Zimmermann, T.; Leucuţa, D.; et al. Dynamic Changes of Lipid Profile in Romanian Patients with Gaucher Disease Type 1 under Enzyme Replacement Therapy: A Prospective Study. J. Inherit. Metab. Dis. 2013, 36, 555–563. [Google Scholar] [CrossRef]
- Donata, M.; Taranto, D.; Giacobbe, C.; Buonaiuto, A.; Calcaterra, I.; Palma, D.; Maione, G.; Iannuzzo, G.; Nicola, M.; Di, D.; et al. A Real-World Experience of Clinical, Biochemical and Genetic Assessment of Patients with Homozygous Familial Hypercholesterolemia. J. Clin. Med. 2020, 9, 219. [Google Scholar]
- Giampaoli, S.; Krogh, V.; Grioni, S.; Palmieri, L.; Gulizia, M.M.; Stamler, J.; Vanuzzo, D.; Gruppo di ricerca dell’Osservatorio epidemiologico cardiovascolare/Health Examination Survey. Eating Behaviours of Italian Adults: Results of the Osservatorio Epidemiologico Cardiovascolare/Health Examination Survey. Epidemiol. Prev. 2015, 39, 373–379. [Google Scholar] [PubMed]
- Koff, R.S.; Schimmel, E.M. Energy Metabolism: Tissue Determinants and Cellular Corollaries; Kinney, J.M., Tucker, H.N., Eds.; Raven Press: New York, NY, USA, 1992. [Google Scholar]
- Donald, A.; Cizer, H.; Finnegan, N.; Collin-Histed, T.; Hughes, D.A.; Davies, E.H. Measuring Disease Activity and Patient Experience Remotely Using Wearable Technology and a Mobile Phone App: Outcomes from a Pilot Study in Gaucher Disease. Orphanet J. Rare Dis. 2019, 14, 212. [Google Scholar] [CrossRef] [PubMed]
| Whole Population (n = 26) | Men (n = 11) | Women (n = 15) | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Age (years) | 50.6 ± 14.8 | 52.6 ± 9.2 | 49.1 ± 18.1 |
| DOT (months) | 189 ± 96 | 160 ± 102 * | 209 ± 89 |
| Body weight (kg) | 74.9 ± 14.3 | 83.8 ± 11.0 * | 68.4 ± 13.1 |
| Height (cm) | 165 ± 10 | 174 ± 6 * | 158 ± 7 |
| BMI (kg/m2) | 27.6 ± 4.3 | 27.8 ± 3.5 | 27.5 ± 4.9 |
| Waist circumference (cm) | 100 ± 10 | 100 ± 8 | 101 ± 12 |
| Hip circumference (cm) | 89.1 ± 10.7 | 97.1 ± 6.9 | 83.3 ± 9.3 |
| Systolic blood pressure (mmHg) | 128 ± 13 | 134 ± 11 | 125 ± 13 |
| Diastolic blood pressure (mmHg) | 80 ± 9 | 84 ± 9 | 76 ± 7 |
| Whole Population (n = 26) | Men (n = 11) | Women (n = 15) | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| FFM (kg) | 51.5 ± 11.5 | 63.2 ± 6.0 * | 42.9 ± 5.0 |
| FM (kg) | 23.5 ± 8.2 | 20.6 ± 6.2 | 25.5 ± 9.1 |
| FM (%) | 31.3 ± 8.3 | 24.2 ± 4.7 * | 36.4 ± 6.4 |
| Phase angle (degrees) | 6.14 ± 0.86 | 6.67 ± 0.67 * | 5.75 ± 0.79 |
| REE (kcal/day) | 1422 ± 251 | 1603 ± 244 * | 1290 ± 161 |
| REE-HB (kcal/day) | 1539 ± 250 | 1746 ± 189 * | 1386 ± 165 |
| REE-Marra (kcal/day) | 1574 ± 252 | 1793 ± 150 * | 1413 ± 179 |
| Male Patients n = 11 | Male Controls n = 33 | p | |
|---|---|---|---|
| Age (years) | 52.6 ± 9.2 | 52.4 ± 8.1 | 0.942 |
| Body weight (kg) | 83.8 ± 11.0 | 82.6 ± 9.1 | 0.708 |
| Height (cm) | 174 ±6 | 172 ± 6 | 0.488 |
| BMI (kg/m2) | 27.8 ±3.6 | 27.8 ± 2.5 | 0.984 |
| FFM (kg) | 63.2 ± 6.0 | 63.5 ± 3.2 | 0.837 |
| FM (kg) | 20.6± 6.2 | 19.1 ± 6.9 | 0.513 |
| FM (%) | 24.2 ± 4.7 | 22.5 ± 6.2 | 0.410 |
| Phase Angle (degrees) | 6.67 ± 0.67 | 6.60± 0.43 | 0.709 |
| REE (kcal/day) | 1603 ± 244 | 1767 ± 226 | 0.047 |
| REE-HB (kcal/day) | 1746 ± 189 | 1724 ± 159 | 0.701 |
| Δ REE vs. HB (Kcal/day) | −143 ± 192 | 43 ± 129 | 0.001 |
| REE-Marra (kcal/day) | 1793 ± 150 | 1773 ± 129 | 0.668 |
| Δ REE vs. Marra (Kcal/die) | −190 ± 181 | 6 ± 143 | 0.001 |
| Female Patients n = 15 | Female Controls n = 45 | p | |
|---|---|---|---|
| Age (years) | 49.1 ± 18.1 | 47.9 ± 9.5 | 0.742 |
| Body weight (kg) | 68.4 ± 13.1 | 70.0 ± 9.6 | 0.608 |
| Height (cm) | 158 ± 7 | 159 ± 6 | 0.620 |
| BMI (kg/m2) | 27.5 ± 4.9 | 28.0 ± 5.1 | 0.746 |
| FFM (kg) | 42.9 ± 5.0 | 44.6 ± 3.4 | 0.135 |
| FM (kg) | 25.5 ± 9.1 | 25.4 ± 7.9 | 0.964 |
| FM (%) | 36.4 ± 6.3 | 35.6 ± 6.8 | 0.664 |
| Phase Angle (degrees) | 5.75 ± 0.79 | 5.74 ± 0.77 | 0.981 |
| REE (kcal/die) | 1290 ± 161 | 1462 ± 142 | 0.000 |
| REE-HB (kcal/day) | 1386 ± 165 | 1410 ± 86 | 0.485 |
| Δ REE vs. HB (Kcal/day) | −97 ± 119 | 52 ± 150 | 0.001 |
| REE-Marra (kcal/day) | 1413 ± 179 | 1439 ± 109 | 0.513 |
| Δ REE vs. Marra (Kcal/die) | −123 ± 126 | 23 ± 160 | 0.002 |
| Whole Population n = 26 | Men n = 11 | Women n = 15 | |
|---|---|---|---|
| Inactive | 42.3% (n = 11) | 36.4 (n = 4) | 46.7% (n = 7) |
| Sufficiently active | 26.9% (n = 7) | 36.4 (n = 4) | 20.0% (n = 3) |
| Active or very active | 30.8% (n = 8) | 27.3 (n = 3) | 31.3% (n = 5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iaccarino Idelson, P.; Speranza, E.; Marra, M.; Pasanisi, F.; Sammarco, R.; Galletti, F.; Strazzullo, P.; Barbato, A. Evaluation of the Nutritional Status of Gaucher Disease Type I Patients under Enzyme Replacement Treatment. Nutrients 2022, 14, 3180. https://doi.org/10.3390/nu14153180
Iaccarino Idelson P, Speranza E, Marra M, Pasanisi F, Sammarco R, Galletti F, Strazzullo P, Barbato A. Evaluation of the Nutritional Status of Gaucher Disease Type I Patients under Enzyme Replacement Treatment. Nutrients. 2022; 14(15):3180. https://doi.org/10.3390/nu14153180
Chicago/Turabian StyleIaccarino Idelson, Paola, Enza Speranza, Maurizio Marra, Fabrizio Pasanisi, Rosa Sammarco, Ferruccio Galletti, Pasquale Strazzullo, and Antonio Barbato. 2022. "Evaluation of the Nutritional Status of Gaucher Disease Type I Patients under Enzyme Replacement Treatment" Nutrients 14, no. 15: 3180. https://doi.org/10.3390/nu14153180
APA StyleIaccarino Idelson, P., Speranza, E., Marra, M., Pasanisi, F., Sammarco, R., Galletti, F., Strazzullo, P., & Barbato, A. (2022). Evaluation of the Nutritional Status of Gaucher Disease Type I Patients under Enzyme Replacement Treatment. Nutrients, 14(15), 3180. https://doi.org/10.3390/nu14153180






