Omega-9 Modifies Viscoelasticity and Augments Bone Strength and Architecture in a High-Fat Diet-Fed Murine Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
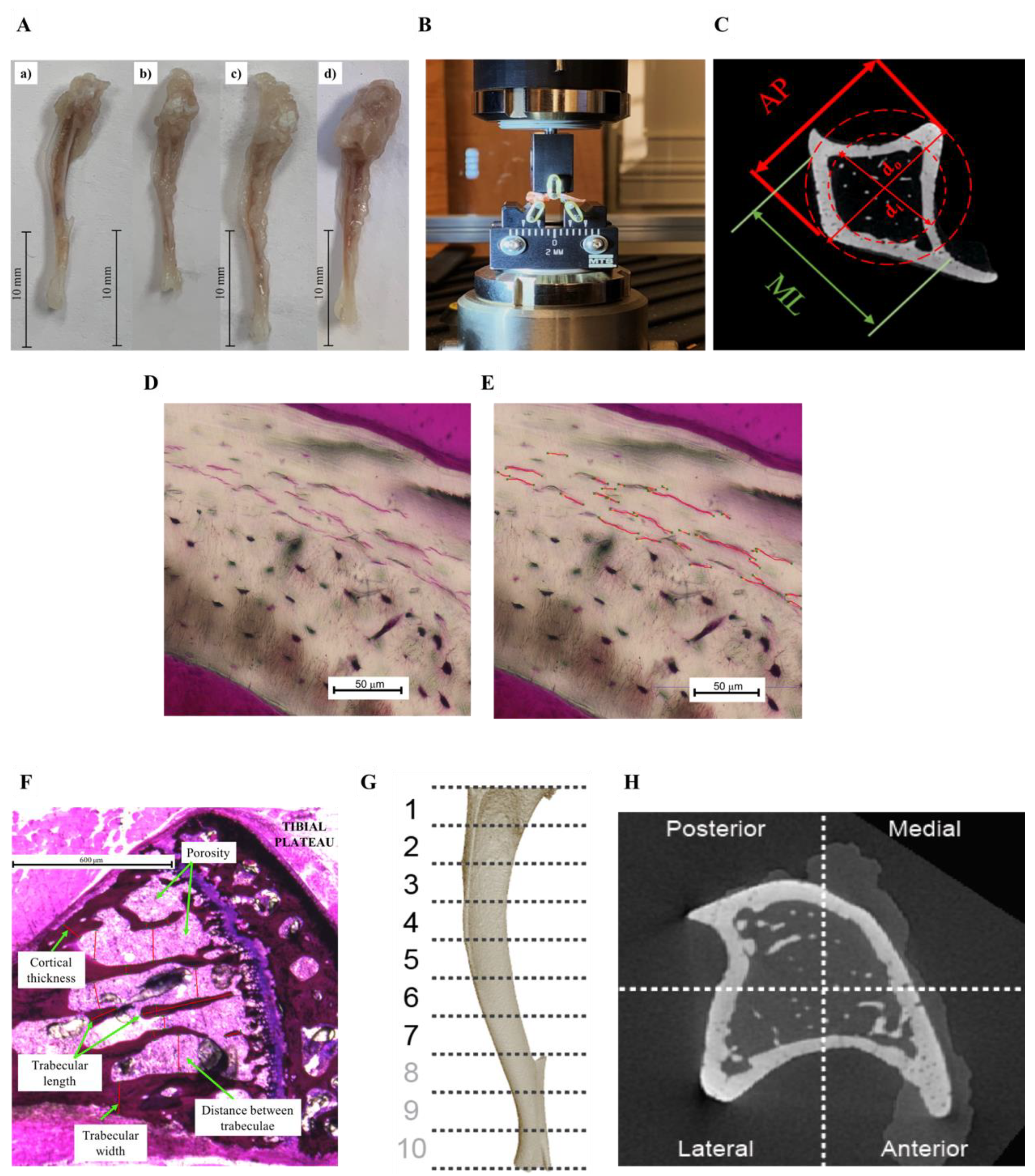
2.2. Dynamic Mechanical Analysis
2.3. Three-Point Bending
2.4. Histological Preparation
2.5. MicroCT
2.6. Statistical Analysis
3. Results
3.1. Food Intake and Bodyweight
3.2. Dynamic Mechanical Analysis
3.3. Three-Point-Bending
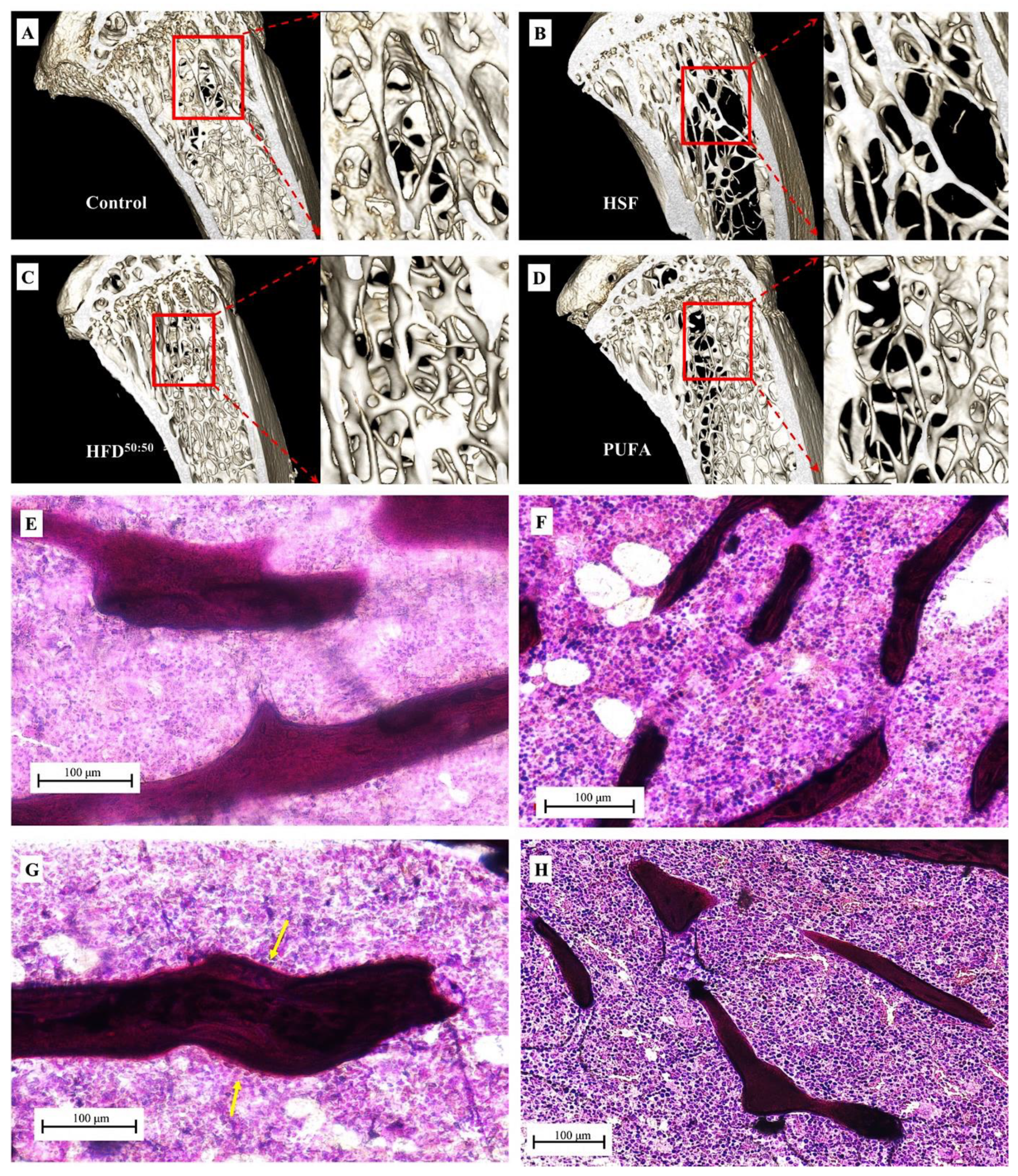
3.4. SEM and Histological Analyses
3.5. Tibial Morphometrics, BMD, BMC and BV/TV%
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wright, N.C.; Looker, A.C.; Saag, K.G.; Curtis, J.R.; Delzell, E.S.; Randall, S.; Dawson-Hughes, B. The Recent Prevalence of Osteoporosis and Low Bone Mass in the United States Based on Bone Mineral Density at the Femoral Neck or Lumbar Spine. J. Bone Min. Res. 2014, 29, 2520–2526. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Campion, G.; Melton, L.J. Hip fractures in the elderly: A world-wide projection. Osteoporos. Int. 1992, 2, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.; Dennison, E.; Cooper, C. Osteoporosis: Impact on health and economics. Nat. Rev. Rheumatol. 2010, 6, 99–105. [Google Scholar] [CrossRef]
- Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr. Cart. 2013, 21, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Wallace, W.A. The Increasing Incidence of Fractures of the Proximal Femur: An Orthopaedic Epidemic. Lancet 1983, 321, 1413–1414. [Google Scholar] [CrossRef]
- Wohl, G.R.; Loehrke, L.; Watkins, B.A.; Zernicke, R.F. Effects of High-Fat Diet on Mature Bone Mineral Content, Structure, and Mechanical Properties. Calcif. Tissue Int. 1998, 63, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Movassagh, E.Z.; Vatanparast, H. Current Evidence on the Association of Dietary Patterns and Bone Health: A Scoping Review. Adv. Nutr. Int. Rev. J. 2017, 8, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.; Mangano, K.M.; McLean, R.R.; Hannan, M.T.; Kiel, D.P. Dietary Approaches for Bone Health: Lessons from the Framingham Osteoporosis Study. Curr. Osteoporos. Rep. 2015, 13, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Langsetmo, L.; Hanley, D.A.; Prior, J.C.; Barr, S.I.; Anastassiades, T.; Towheed, T.; Goltzman, D.; Morin, S.; Poliquin, S.; Kreiger, N. Dietary patterns and incident low-trauma fractures in postmenopausal women and men aged ≥50 y: A population-based cohort study. Am. J. Clin. Nutr. 2011, 93, 192–199. [Google Scholar] [CrossRef]
- Vandevijvere, S.; Chow, C.C.; Hall, K.D.; Umali, E.; Swinburn, B.A. Increased food energy supply as a major driver of the obesity epidemic: A global analysis. Bull. World Health Organ. 2015, 93, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Gonnelli, S.; Caffarelli, C.; Nuti, R. Obesity and fracture risk. Clin. Cases Min. Bone Metab. 2014, 11, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.; Adhikary, S.; Gautam, J.; Maurya, P.; Ahmad, N.; Kushwaha, P.; Khan, M.P.; Kumar, A.; Barthwal, M.; Maurya, R.; et al. Detrimental effects of atherogenic and high fat diet on bone and aortic calcification rescued by an isoflavonoid Caviunin β-d-glucopyranoside. Biomed. Pharm. 2017, 92, 757–771. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, P.K.; Horowitz, M.C.; MacDougald, O.A.; Scheller, E.L.; Rodeheffer, M.S.; Rosen, C.j.; Klibanski, A. Marrow Fat and Bone—New Perspectives. J. Clin. Endocrinol. Metab. 2013, 98, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.V. Marrow Fat and Bone: Review of Clinical Findings. Front. Endocrinol. 2015, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Weiler, H.A.; Kovacs, H.; Nitschmann, E.; Bankovic-Calic, N.; Aukema, H.; Ogborn, M. Feeding flaxseed oil but not secoisolariciresinol diglucoside results in higher bone mass in healthy rats and rats with kidney disease. Prostaglandins Leukot. Essent. Fat. Acids 2007, 76, 269–275. [Google Scholar] [CrossRef]
- Korotkova, M.; Ohlsson, C.; Hanson, L.Å.; Strandvik, B. Dietary n -6: N -3 fatty acid ratio in the perinatal period affects bone parameters in adult female rats. Br. J. Nutr. 2004, 92, 643–648. [Google Scholar] [CrossRef]
- Lau, B.Y.; Fajardo, V.A.; McMeekin, L.; Sacco, S.M.; Ward, W.E.; Roy, B.D.; Peters, S.J.; LeBlanc, P.J. Influence of high-fat diet from differential dietary sources on bone mineral density, bone strength, and bone fatty acid composition in rats. Appl. Physiol. Nutr. Metab. 2010, 35, 598–606. [Google Scholar] [CrossRef]
- Farahnak, Z.; Freundorfer, M.T.; Lavery, P.; Weiler, H.A. Dietary docosahexaenoic acid contributes to increased bone mineral accretion and strength in young female Sprague-Dawley rats. Prostaglandins Leukot. Essent. Fat Acids 2019, 144, 32–39. [Google Scholar] [CrossRef]
- De Abreu Maíra, D.C.; Letícia, R.P.; Rodrigues da Costa, L.; Ferolla da Camara, B.; Carolina, R.P.; D’Avila Pereira, A.; Cavalcante Ribeiro, D.; Moreira da Silva, E.; Soares da Costa, C.A.; Gilson, T.B. Flaxseed (Linum usitatissimum) flour contributes to bone health in adult male rats. Nutrition 2018, 49, 48–50. [Google Scholar] [CrossRef]
- Salari, P.; Rezaie, A.; Larijani, B.; Abdollahi, M. A systematic review of the impact of n-3 fatty acids in bone health and osteoporosis. Med. Sci. Monit. 2008, 14, RA37–RA44. [Google Scholar]
- Farina, E.K.; Kiel, D.P.; Roubenoff, R.; Schaefer, E.J.; Cupples, L.A.; Tucker, K.L. Dietary Intakes of Arachidonic Acid and α-Linolenic Acid Are Associated with Reduced Risk of Hip Fracture in Older Adults. J. Nutr. 2011, 141, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Mangano, K.M.; Sahni, S.; Kerstetter, J.E.; Kenny, A.M.; Hannan, M.T. Polyunsaturated fatty acids and their relation with bone and muscle health in adults. Curr. Osteoporos. Rep. 2013, 11, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, R.; Tuppurainen, M.; Erkkilä, A.T.; Penttinen, P.; Kärkkäinen, M.; Salovaara, K.; Jurvelin, J.S.; Kröger, H. Associations of dietary polyunsaturated fatty acids with bone mineral density in elderly women. Eur. J. Clin. Nutr. 2012, 66, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Farina, E.K.; Kiel, D.P.; Roubenoff, R.; Schaefer, E.J.; Cupples, L.A.; Tucker, K.L. Plasma phosphatidylcholine concentrations of polyunsaturated fatty acids are differentially associated with hip bone mineral density and hip fracture in older adults: The framingham osteoporosis study. J. Bone Min. Res. 2012, 27, 1222–1230. [Google Scholar] [CrossRef]
- Martínez-Ramírez, M.J.; Palma, S.; Martínez-González, M.A.; Delgado-Martinez, A.D.; de la Fuente, C.; Delgado-Rodriguez, M. Dietary fat intake and the risk of osteoporotic fractures in the elderly. Eur. J. Clin. Nutr. 2007, 61, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Orchard, T.S.; Ing, S.W.; Lu, B.; Belury, M.A.; Johnson, K.; Wactawski-Wende, J.; Jackson, R.D. The association of red blood cell n-3 and n-6 fatty acids with bone mineral density and hip fracture risk in the women’s health initiative. J. Bone Min. Res. 2013, 28, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, J.K.; Mozaffarian, D.; Cauley, J.A.; Mukamal, K.J.; Robbins, J.; Siscovick, D.S. Fish consumption, bone mineral density, and risk of hip fracture among older adults: The cardiovascular health study. J. Bone Min. Res. 2010, 25, 1972–1979. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Georgiou, E.; Bassiakos, Y.; Lipworth, L.; Lagiou, P.; Proukakis, C.; Trichopoulos, D. Energy Intake and Monounsaturated Fat in Relation to Bone Mineral Density among Women and Men in Greece. Prev. Med. 1997, 26, 395–400. [Google Scholar] [CrossRef]
- Wang, Y.; Dellatore, P.; Douard, V.; Qin, L.; Watford, M.; Ferraris, R.P.; Lin, T.; Shapses, S.A. High fat diet enriched with saturated, but not monounsaturated fatty acids adversely affects femur, and both diets increase calcium absorption in older female mice. Nutr. Res. 2016, 36, 742–750. [Google Scholar] [CrossRef]
- Varela-López, A.; Ochoa, J.J.; Llamas-Elvira, J.M.; Lopez-Frias, M.; Planells, E.; Speranza, L.; Battino, M.; Quiles, J.L. Loss of Bone Mineral Density Associated with Age in Male Rats Fed on Sunflower Oil Is Avoided by Virgin Olive Oil Intake or Coenzyme Q Supplementation. Int. J. Mol. Sci. 2017, 18, 1397. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, H.; Djafarian, K.; Mofrad, M.D.; Shab-Bidar, S. Dietary fat, saturated fatty acid, and monounsaturated fatty acid intakes and risk of bone fracture: A systematic review and meta-analysis of observational studies. Osteoporos. Int. 2018, 29, 1949–1961. [Google Scholar] [CrossRef]
- Jamsa, T.; Jalovaara, P.; Peng, Z.; Vaananen, H.; Tuukkanen, J. Comparison of three-point bending test and peripheral quantitative computed tomography analysis in the evaluation of the strength of mouse femur and tibia. Bone 1998, 23, 155–161. [Google Scholar] [CrossRef]
- Schriefer, J.L.; Robling, A.G.; Warden, S.J.; Fournier, A.J.; Mason, J.J.; Turner, C.H. A comparison of mechanical properties derived from multiple skeletal sites in mice. J. Biomech. 2005, 38, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Deckard, C.; Walker, A.; Hill, B.J. Using three-point bending to evaluate tibia bone strength in ovariectomized young mice. J. Bio. Phys. 2017, 43, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Boudiffa, M.; Dall’Ara, E.; Bellantuono, I.; Viceconti, M. Development of a protocol to quantify local bone adaptation over space and time: Quantification of reproducibility. J. Biomech. 2016, 49, 2095–2099. [Google Scholar] [CrossRef] [PubMed]
- Zappone, B.; Thurner, P.J.; Adams, J.; Fanter, G.E.; Hansma, P.K. Effect of Ca2+ Ions on the Adhesion and Mechanical Properties of Adsorbed Layers of Human Osteopontin. Biophys. J. 2008, 95, 2939–2950. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salim, M.S.; Salleh, A.F.; Daud, R. Bone Fracture Modeling and Analysis by Three Point Bending: A Review. MATEC Web Conf. 2016, 77, 11004. [Google Scholar] [CrossRef]
- Thurner, P.J.; Lam, S.; Weaver, J.C.; Morse, D.E.; Hansma, P.K. Localization of Phosphorylated Serine, Osteopontin, and Bone Sialoprotein on Bone Fracture Surfaces. J. Adhes. 2009, 85, 526–545. [Google Scholar] [CrossRef]
- McKee, M.D.; Nanci, A. Osteopontin at mineralized tissue interfaces in bone, teeth, and osseointegrated implants: Ultrastructural distribution and implications for mineralized tissue formation, turnover, and repair. Microsc. Res. Tech. 1996, 33, 141–164. [Google Scholar] [CrossRef]
- Thurner, P.J.; Chen, C.G.; Ionova-Martin, S.; Sun, L.; Harman, A.; Porter, A.; Ager, J.W.; Ritchie, R.O.; Alliston, T. Osteopontin deficiency increasese bone fragility but preserves bone mass. Bone 2010, 46, 1564–1573. [Google Scholar] [CrossRef]
- Gautam, J.; Choudhary, D.; Khedgikar, V.; Kushwaha, P.; Singh, R.S.; Singh, D.; Tiwari, S.; Trivedi, R. Micro-architectural changes in cancellous bone differ in female and male C57BL/6 mice with high-fat diet-induced low bone mineral density. Br. J. Nutr. 2014, 111, 1811–1821. [Google Scholar] [CrossRef]
- Tencerova, M.; Figeac, F.; Ditzel, N.; Taipaleenmaki, H.; Nielsen, T.K.; Kassem, M. High-Fat Diet-Induced Obesity Promotes Expansion of Bone Marrow Adipose Tissue and Impairs Skeletal Stem Cell Functions in Mice. J.Bone Miner. Res. 2018, 33, 1154–1165. [Google Scholar] [CrossRef] [PubMed]
- Schapery, R.A. A theory of crack initiation and growth in viscoelastic media II. Approximate methods of analysis. Int. J. Fract. 1975, 11, 369–388. [Google Scholar] [CrossRef]
- Burr, D.B.; Turner, C.H.; Naick, P.; Forwood, M.R.; Ambrosius, W.; Sayeed Hasan, M.; Pidaparti, R. Does microdamage accumulation affect the mechanical properties of bone? J. Biomech. 1998, 31, 337–345. [Google Scholar] [CrossRef]
- Pearson, O.M.; Lieberman, D.E. The aging of Wolff’s “law”: Ontogeny and responses to mechanical loading in cortical bone. Am. J. Phys. Anthr. 2004, 125, 63–99. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, H.H.; Morgan, E.F.; Niebur, G.L.; Morris, G.E.; Wong, E.K.; Keaveny, T.M. Comparison of the elastic and yield properties of human femoral trabecular and cortical bone tissue. J. Biomech. 2004, 37, 27–35. [Google Scholar] [CrossRef]
- Burstein, A.H.; Currey, J.D.; Frankel, V.H.; Reilly, D.T. The ultimate properties of bone tissue: The effects of yielding. J. Biomech. 1972, 5, 35–44. [Google Scholar] [CrossRef]
- Mak, I.L.; Lavery, P.; Agellon, S.; Rauch, F.; Murshed, M.; Weiler, H.A. Arachidonic acid exacerbates diet-induced obesity and reduces bone mineral content without impacting bone strength in growing male rats. J. Nutr. Biochem. 2019, 73, 108226. [Google Scholar] [CrossRef]
- Da Costa, C.A.S.; da Silva, P.C.A.; Ribeiro, D.C.; Pereira, A.; dos Santos, A.; de Abreu, M.D.C.; Pessoa, L.R.; Boueri, B.F.; Pessanha, C.R.; do Nascimento-Saba, C.C.A.; et al. Effects of diet containing flaxseed flour (Linum usitatissimum) on body adiposity and bone health in young male rats. Food Funct. 2016, 7, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, Y.; Wang, H.; Dong, Z.; Wang, Y.; Zhang, M.; Li, J.; Shao, S.; Yu, C.; Huan, Z.; et al. Flaxseed oil ameliorated high-fat-diet-induced bone loss in rats by promoting osteoblastic function in rat primary osteoblasts. Nutr. Metab. 2019, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.; Farrell, V.; Houtkooper, L.; Going, S.; Lohman, T. Associations of Polyunsaturated Fatty Acid Intake with Bone Mineral Density in Postmenopausal Women. J. Osteoporos. 2015, 2015, 737521. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.A.; Barrett-Connor, E.; von Mühlen, D. Ratio of n–6 to n–3 fatty acids and bone mineral density in older adults: The Rancho Bernardo Study. Am. J. Clin. Nutr. 2005, 81, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Maurin, A.C.; Chavassieux, P.M.; Meunier, P.J. Expression of PPARγ and β/δ in Human Primary Osteoblastic Cells: Influence of Polyunsaturated Fatty Acids. Calcif. Tissue Int. 2005, 76, 385–392. [Google Scholar] [CrossRef]
- Lecka-Czernik, B.; Moerman, E.J.; Grant, D.F.; Lehmann, J.M.; Manolaga, S.C.; Jilka, R.L. Divergent Effects of Selective Peroxisome Proliferator-Activated Receptor-γ2 Ligands on Adipocyte Versus Osteoblast Differentiation. Endocrinology 2002, 143, 2376–2384. [Google Scholar] [CrossRef] [PubMed]
- Das, U. Essential fatty acids and osteoporosis. Nutrition 2000, 16, 386–390. [Google Scholar] [CrossRef]
- LeBlanc, C.J.; Horohov, D.W.; Bauer, J.E.; Hosgood, G.; Mauldin, G.E. Effects of dietary supplementation with fish oil on in vivo production of inflammatory mediators in clinically normal dogs. Am. J. Vet. Res. 2008, 69, 486–493. [Google Scholar] [CrossRef]
- Rahman, M.M.; Bhattacharya, A.; Banu, J.; Kang, J.X.; Fernandes, G. Endogenous n-3 fatty acids protect ovariectomy induced bone loss by attenuating osteoclastogenesis. J. Cell Mol. Med. 2009, 13, 1833–1844. [Google Scholar] [CrossRef]
- Watkins, B.A.; Li, Y.; Lippman, H.E.; Feng, S. Modulatory effect of omega-3 polyunsaturated fatty acids on osteoblast function and bone metabolism. Prostaglandins Leukot. Essent. Fat Acids 2003, 68, 387–398. [Google Scholar] [CrossRef]
- Kasonga, A.E.; Kruger, M.C.; Coetzee, M. Free fatty acid receptor 4-β-arrestin 2 pathway mediates the effects of different classes of unsaturated fatty acids in osteoclasts and osteoblasts. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 281–289. [Google Scholar] [CrossRef]
- Sales-Campos, H.; Souza PR de Peghini, B.C.; da Silva, J.S.; Cardoso, C.R. An overview of the modulatory effects of oleic acid in health and disease. Mini Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar] [PubMed]
- Sun, D.; Krishnan, A.; Zaman, K.; Lawrence, R.; Bhattacharya, A.; Fernandes, G. Dietary n-3 Fatty Acids Decrease Osteoclastogenesis and Loss of Bone Mass in Ovariectomized Mice. J. Bone Min. Res. 2003, 18, 1206–1216. [Google Scholar] [CrossRef]
- Van Heerden, B.; Kasonga, A.; Kruger, M.; Caetzee, M. Palmitoleic Acid Inhibits RANKL-Induced Osteoclastogenesis and Bone Resorption by Suppressing NF-κB and MAPK Signalling Pathways. Nutrients 2017, 9, 441. [Google Scholar] [CrossRef] [PubMed]
- Drosatos-Tampakaki, Z.; Drosatos, K.; Siegelin, Y.; Gong, S.; Khan, S.; Van Dyke, T.; Goldberg, I.J.; Schulze, P.C.; Schulze-Spate, U. Palmitic Acid and DGAT1 Deficiency Enhance Osteoclastogenesis, while Oleic Acid-Induced Triglyceride Formation Prevents It. J. Bone Min. Res. 2014, 29, 1183–1195. [Google Scholar] [CrossRef]
- Bala, Y.; Farlay, D.; Boivin, G. Bone mineralization: From tissue to crystal in normal and pathological contexts. Osteoporos. Int. 2013, 24, 2153–2166. [Google Scholar] [CrossRef]
- Farlay, D.; Panczer, G.; Rey, C.; Delmas, P.D.; Boivin, G. Mineral maturity and crystallinity index are distinct characteristics of bone mineral. J. Bone Min. Metab. 2010, 28, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Vashishth, D.; Gibson, G.; Khoury, J.; Schaffer, M.B.; Kimura, J.; Fyhrie, D.P. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone 2001, 28, 195–201. [Google Scholar] [CrossRef]
- Jia, S.; Gong, H.; Cen, H.; Shi, P.; Zhang, R.; Li, Z.; Bi, X. Influence of non-enzymatic glycation on the mechanical properties of cortical bone. J. Mech. Behav. Biomed. Mater. 2021, 119, 104553. [Google Scholar] [CrossRef]
- Sanders, T.A.B. Omega-6 Fatty Acids and Cardiovascular Disease. Circulation 2019, 139, 2437–2439. [Google Scholar] [CrossRef] [PubMed]
- Delgado, G.E.; Krämer, B.K.; Lorkowski, S.; März, W.; von Schacky, C.; Kleber, M.E. Individual omega-9 monounsaturated fatty acids and mortality—The Ludwigshafen Risk and Cardiovascular Health Study. J. Clin. Lipidol. 2017, 11, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids, olive oil and health status: A systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014, 13, 154. [Google Scholar] [CrossRef]
- Kumar, J.B.S.; Sharma, B. A review on neuropharmacological role of erucic acid: An omega-9 fatty acid from edible oils. Nutr. Neurosci. 2022, 25, 1041–1055. [Google Scholar] [CrossRef]
- Molendi-Coste, O.; Legry, V.; Leclercq, I.A. Why and How Meet n-3 PUFA Dietary Recommendations? Gastroenterol. Res. Pract. 2011, 2011, 364040. [Google Scholar] [CrossRef]





| Diet Type | Diet # | Fat ratio: (Unsaturated to Saturated) | Total Fat (kcal%) | ω-3% | ω-6% | ω-9% |
|---|---|---|---|---|---|---|
| Control Diet | D07020902 | 1:1 | 10 | 2.8 | 31.6 | 30.7 |
| High Polyunsaturated Fat Diet | D06062303 | 3.3:1 | 60 | 0.48 | 43.0 | 29.0 |
| High Fat Diet50:50 | D12492 | 1:1 | 60 | 1.25 | 14.15 | 44.2 |
| High Saturated Fat Diet | D06062302 | 1:10 | 60 | 0.48 | 2.95 | 10.0 |
| Control Diet | HFD50:50 | HSF | PUFA | |||||
|---|---|---|---|---|---|---|---|---|
| g | kcal | g | kcal | g | kcal | g | kcal | |
| Protein (% by wt) | 19.2 | 20 | 26.2 | 20 | 26.2 | 20 | 26.2 | 20 |
| Carbohydrate (% by wt) | 67.3 | 70 | 26.3 | 20 | 26.3 | 20 | 26.3 | 20 |
| Fat (% by wt) | 4.3 | 10 | 34.9 | 60 | 34.9 | 60 | 34.9 | 60 |
| Total kcal | 100 | 100 | 100 | 100 | ||||
| kcal/g | 3.85 | 5.24 | 5.24 | 5.24 | ||||
| Ingredients | ||||||||
| Protein Casein, 80 Mesh | 200 | 800 | 200 | 800 | 200 | 800 | 200 | 800 |
| L-Cystine | 3 | 12 | 3 | 12 | 3 | 12 | 3 | 12 |
| Carbohydrate | ||||||||
| Corn Starch | 500 | 2000 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maltodexrin 10 | 100 | 400 | 125 | 500 | 125 | 500 | 125 | 500 |
| Sucrose | 100 | 400 | 68.8 | 275 | 68.8 | 275 | 68.8 | 275 |
| Cellulose, BW200 | 50 | 0 | 50 | 0 | 50 | 0 | 50 | 0 |
| Lipid | ||||||||
| Soybean Oil | 10 | 90 | 25 | 225 | 10 | 90 | 10 | 90 |
| Lard | 5 | 45 | 245 | 2205 | 25 | 225 | 130 | 1170 |
| Coconut-Oil, Hydrogenated | 0 | 0 | 0 | 0 | 235 | 2115 | 0 | 0 |
| Safflower Oil | 0 | 0 | 0 | 0 | 0 | 0 | 130 | 1170 |
| Cocoa Butter | 30 | 270 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mineral Mix S10026 | ||||||||
| Dicalcium phosphate | 13 | 0 | 13 | 0 | 13 | 0 | 13 | 0 |
| Calcium carbonate | 5.5 | 0 | 5.5 | 0 | 5.5 | 0 | 5.5 | 0 |
| Potassium citrate, 1 H2O | 16.5 | 0 | 16.5 | 0 | 16.5 | 0 | 16.5 | 0 |
| Vitamin Mix V10001 * | 10 | 40 | 10 | 40 | 10 | 40 | 10 | 40 |
| Choline Bitartrate | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 |
| FD&C Yellow Dye #5 | 0 | 0 | 0 | 0 | 0.025 | 0 | 0 | 0 |
| FD&C Red Dye #40 | 0 | 0 | 0 | 0 | 0 | 0 | 0.025 | 0 |
| FD&C Blue Dye #1 | 0 | 0 | 0.05 | 0 | 0.025 | 0 | 0.05 | 0 |
| Total | 1055 | 4057 | 773.85 | 4057 | 773.85 | 4057 | 773.85 | 4057 |
| Variables | Control | PUFA | HFD50:50 | HSF |
|---|---|---|---|---|
| Starting body weight (g) | 23.84 ± 0.28 | 26.9 ± 0.45 | 25.26 ± 0.54 | 25.42 ± 0.37 |
| Final body weight (g) | 27.02 ± 0.25 | 41.22 ± 1.42 | 39.66 ± 2.1 | 36.24 ± 0.59 |
| Cumulative gain in body weight (g) | 3.2 ± 0.09 | 14.32 ± 1.39 | 14.42 ± 1.7 | 10.84 ± 0.45 |
| Mean cumulative water consumed (mL) | 191.7 ± 18.8 | 240.4 ± 23.9 | 199.4 ± 8.0 | 185.4 ± 8.58 |
| Mean starting food intake (g) | 21.04 ± 0.75 | 30.78 ± 0.68 | 24.66 ± 0.93 | 20.86 ± 0.76 |
| Mean final food intake (g) | 19.76 ± 0.74 | 34.38 ± 2.87 | 23.46 ± 0.54 | 20.88 ± 0.86 |
| Mean starting energy consumed (kcal) | 81.04 ± 2.89 | 161.14 ± 3.56 | 129.2 ± 4.83 | 109.36 ± 3.98 |
| Mean final energy consumed (kcal) | 76.1 ± 2.84 | 180.08 ± 14.69 | 122.94 ± 2.85 | 109.44 ± 4.46 |
| Mean cumulative food consumed (kcal) | 618.96 ± 15.80 | 1390.24 ± 37.01 | 953.36 ± 17.70 | 900.04 ± 16.76 |
| Variables | Control | PUFA | HFD50:50 | HSF |
|---|---|---|---|---|
| Yield Force (N) | 13.05 ± 2.72 | 11.67 ± 4.94 | 13.43 ± 4.69 | 11.62 ± 2.91 |
| Ultimate Force (N) | 17.22 ± 4.42 | 17.50 ± 4.49 | 18.91 ± 2.02 | 14.95 ± 1.07 |
| Fracture Force (N) | 14.79 ± 5.04 | 14.56 ± 5.91 | 15.54 ± 2.89 | 13.71 ± 1.39 |
| Stiffness (N/mm) | 74.84 ± 27.51 | 81.12 ± 28.29 | 75.63 ± 28.57 | 52.84 ± 19.36 |
| PYD (mm) | 0.50 ± 0.31 | 0.45 ± 0.18 | 0.37 ± 0.15 | 0.33 ± 0.09 |
| Variables | Control | HFD50:50 | HSF | PUFA |
|---|---|---|---|---|
| Tibial morphology | ||||
| Length (mm) | 20.4 ± 0.51 | 22.6 ± 0.51 | 17.6 ± 0.60 | 19.5 ± 1.76 |
| Outer diameter (mm) | 1.45 ± 0.11 | 1.41 ± 0.04 | 1.61 ± 0.08 | 1.45 ± 0.20 |
| Inner diameter (mm) | 0.89 ± 0.07 | 0.91 ± 0.03 | 1.07 ± 0.05 | 0.95 ± 0.16 |
| Moment of inertia (m4) | 1.97 ± 0.6 × 10−13 | 1.65 ± 0.11 × 10−13 | (2.74 ± 0.52) × 10−13 | (2.07 ± 1.44) × 10−13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omer, M.; Ali, H.; Orlovskaya, N.; Ballesteros, A.; Cheong, V.S.; Martyniak, K.; Wei, F.; Collins, B.E.; Yarmolenko, S.N.; Asiatico, J.; et al. Omega-9 Modifies Viscoelasticity and Augments Bone Strength and Architecture in a High-Fat Diet-Fed Murine Model. Nutrients 2022, 14, 3165. https://doi.org/10.3390/nu14153165
Omer M, Ali H, Orlovskaya N, Ballesteros A, Cheong VS, Martyniak K, Wei F, Collins BE, Yarmolenko SN, Asiatico J, et al. Omega-9 Modifies Viscoelasticity and Augments Bone Strength and Architecture in a High-Fat Diet-Fed Murine Model. Nutrients. 2022; 14(15):3165. https://doi.org/10.3390/nu14153165
Chicago/Turabian StyleOmer, Mahmoud, Hessein Ali, Nina Orlovskaya, Amelia Ballesteros, Vee San Cheong, Kari Martyniak, Fei Wei, Boyce E. Collins, Sergey N. Yarmolenko, Jackson Asiatico, and et al. 2022. "Omega-9 Modifies Viscoelasticity and Augments Bone Strength and Architecture in a High-Fat Diet-Fed Murine Model" Nutrients 14, no. 15: 3165. https://doi.org/10.3390/nu14153165
APA StyleOmer, M., Ali, H., Orlovskaya, N., Ballesteros, A., Cheong, V. S., Martyniak, K., Wei, F., Collins, B. E., Yarmolenko, S. N., Asiatico, J., Kinzel, M., Ngo, C., Sankar, J., Calder, A., Gilbertson, T., Meckmongkol, T., Ghosh, R., & Coathup, M. (2022). Omega-9 Modifies Viscoelasticity and Augments Bone Strength and Architecture in a High-Fat Diet-Fed Murine Model. Nutrients, 14(15), 3165. https://doi.org/10.3390/nu14153165








