Abstract
Major Depressive Disorder (MDD) is a growing disabling condition affecting around 280 million people worldwide. This complex entity is the result of the interplay between biological, psychological, and sociocultural factors, and compelling evidence suggests that MDD can be considered a disease that occurs as a consequence of an evolutionary mismatch and unhealthy lifestyle habits. In this context, diet is one of the core pillars of health, influencing multiple biological processes in the brain and the entire body. It seems that there is a bidirectional relationship between MDD and malnutrition, and depressed individuals often lack certain critical nutrients along with an aberrant dietary pattern. Thus, dietary interventions are one of the most promising tools to explore in the field of MDD, as there are a specific group of nutrients (i.e., omega 3, vitamins, polyphenols, and caffeine), foods (fish, nuts, seeds fruits, vegetables, coffee/tea, and fermented products) or dietary supplements (such as S-adenosylmethionine, acetyl carnitine, creatine, amino acids, etc.), which are being currently studied. Likewise, the entire nutritional context and the dietary pattern seem to be another potential area of study, and some strategies such as the Mediterranean diet have demonstrated some relevant benefits in patients with MDD; although, further efforts are still needed. In the present work, we will explore the state-of-the-art diet in the prevention and clinical support of MDD, focusing on the biological properties of its main nutrients, foods, and dietary patterns and their possible implications for these patients.
1. Major Depressive Disorder and Diet: What Is the Relationship?
1.1. A General Perspective of Major Depressive Disorder
Major Depressive Disorder (MDD) is a leading disabling condition highly representative in our society, affecting nearly 280 million people worldwide [1]. Currently, MDD is placed as the third cause of disability globally, just after headache and lower back pain; however, the World Health Organization (WHO) projects that by 2030, MDD will become the first cause of disability worldwide [2,3]. Nowadays, the growing prevalence and annual incidence affected by pandemic times of the coronavirus disease 2019 (COVID-19) has made us reconsider the importance of mental health. In fact, according to current data, there has been a rise of 53.2 million MDD cases globally, representing an increase of 27.6% during the course of this pandemic [4]. This condition affects people from all age ranges, sex, and cultures; although, being aged between 45 and 59, of the female sex, and from a high-income country are associated with a higher risk of suffering from MDD. In fact, prevalence is double in women than in men [5,6]. In addition, it seems that the distribution of MDD can be different across regions. Thus, the lifetime prevalence of MDD can vary between 2 and 21% with the highest rates in some European countries and the lowest in certain Asian countries [7].
The precise causes of this complex malady are not fully understood. It seems that MDD is the result of an interplay between genetic and environmental factors. The estimated role of genetics is about 37%; although, a single gene has not been identified as a causative factor of MDD [8]. Instead, it seems that the inheritance of MDD is polygenic, the exposure to different environmental factors in certain critical periods being equally required [9]. In this sense, early life stress (i.e., child abuse) is one of the most important environmental factors related to the development of MDD, leading to determinant changes in the brain structure and function [10]. Similarly, chronic stress and frequent episodes of acute stress are also related to the onset of MDD [11]. The presence of physical or mental comorbidities together with multiple social factors can be equally prominent contributors to those suffering from MDD. In this sense, ethnic minorities, marital status (separated/divorced), poor education and healthcare access/quality, neighborhood, built environment, intimate partner violence, and lower socioeconomic resources are the most important social determinants related to the onset of MDD [7,12,13].
From a medical perspective, MDD can be considered a quite heterogeneous entity. Following the DSM-5 (Diagnostic and Statistical Manual of Mental Disorders, 5th Edition) criteria, MDD is clinically diagnosed by the presence of at least one of the two main criteria—depressed mood or anhedonia (the inability to feel pleasure or loss of interest) and ≥ 4 secondary symptoms during a minimum period of 2 weeks [14]. Collectively, the secondary symptoms of MDD can be divided into somatic or non-somatic items. The former include sleep disturbances, appetite changes, poor concentration, fatigue, and psychomotor agitation/retardation, whereas non-somatic symptoms are related to psychobehavioral alterations such as feelings of worthless, and suicide thoughts [15] The severity of MDD is generally assessed by the use of the Hamilton Depression Rating Scale (HAM-D), based on the use of specific punctuation marks for different clinical parameters [16]. Thus, a rating scale between 0 and 7 is defined as no depression; 8 and 16 mild depression; 17 and 23 moderate depression; and ≥24 severe depression [17,18]. The clinical management of these patients can be different according to the severity. Hence, for patients with mild depression, physical activity, or non-medical treatments may be encouraged. However, for patients with moderate or severe depression, the use of antidepressants, alone or in combination with psychotherapy and psychoeducation, is generally the most frequent approach [19].
On the other hand, notwithstanding an important percentage of patients responding to the therapy received, approximately 30% of patients suffer from resistance, which is known as treatment-resistant depression, representing an important medical and socioeconomic challenge [20]. In addition, patients with severe depression often require more intervention visits, more months of antidepressant treatment, and more antidepressant trials, but they tend to present worse clinical outcomes [21]. In this context, the socioeconomic burden of MDD is notably high, and not only are direct costs derived from their clinical management, but also indirect costs related to their lack of productivity or work absence must be considered. In the United States alone, the economic burden of MDD was USD 326.2 billion between 2010 and 2018, with a substantial rise in the indirect costs during this period [22].
Therefore, MDD is a challenging and growing public health concern that has a devastating impact on the individuals who suffer from this condition and the entire society. It is imperative to find novel approaches that facilitate the prevention and clinical management of this neuropsychiatric disorder, and nutritional intervention may represent a promising key to achieve these objectives.
1.2. Nutritional Status of the Patient with MDD
Malnutrition in form of undernutrition, overnutrition, or an imbalance of specific nutrients is a global community concern [23]. In general, children suffer more frequently from undernutrition, whereas adults tend to present overnutrition, especially in low- or middle-income countries and the poor population [24]. Likewise, malnutrition is remarkably higher in elder subjects, particularly with specific nutrient deficiencies [25]. It seems that there is a bidirectional link between malnutrition and MDD. On the one hand, malnutrition can drive several biological changes that can lead to the onset and progression of MDD. On the other hand, MDD can drive to eating disorders and lifestyle behaviors that may promote malnutrition [26]. However, there is an important association between malnutrition and MDD that can be observed in these patients.
Nutritional questionnaires such as the Mini Nutritional Assessment (MNA) have been useful to understand the connection between both entities. MNA represents a useful tool for monitoring patients of any age who are at risk of malnutrition. In this sense, rating as “malnourished” or “at risk of malnutrition” is associated with a higher risk of MDD, especially in elder individuals [27,28]. Moreover, some studies have found that patients with MDD are more likely to be at risk of undernutrition rather than overnutrition [29].
Simultaneously, the introduction of anthropometric variables has also aided in further insights being gained into the relationship between malnutrition and MDD. For instance, higher median levels of body weight, waist circumference, hip circumference, and waist-to-hip ratio were found in patients with MDD [30]. In this line, the waist-to-hip ratio appears to be inversely related to suicidality and severity of depression in women with postnatal depression, denoting the relevance of anthropometric measures in the clinical management of MDD [31].
When combining biochemical plus anthropometric variables, some studies have found that women with MDD have higher triglyceride, aspartate aminotransferase (AST), blood urea nitrogen (BUN), and creatinine levels and lower high-density lipoprotein cholesterol (HDL-C), hematocrit, and red blood cell counts; while depressed men were more likely to have higher triglyceride levels, lower hematocrit, and BUN and, in general, they present with less height and weight than their control group [32]. Self-perception can also be different between women and men. Women are more prone to perceive their body as larger than it really is, whereas, in men, the perception is just the opposite, idealizing a larger body [33].
Regarding the lack or excessive intake of specific nutrients, it is common that patients with MDD have an aberrant distribution of macronutrients (carbohydrates, proteins, and fats) and a deficiency of critical micronutrients (vitamins and minerals). In this context, prior works have associated high consumption of carbohydrates and low intake of protein with MDD [34]. Refined carbohydrates are one of the most deleterious nutrients included in westernized diets, being associated with multiple disease conditions [35]. Likewise, a direct relationship between trans unsaturated fatty acids and depression risk has been demonstrated [36], and a high dietary intake of saturated fats is sufficient to promote depressive-like behaviors in animal models [37]. Conversely, low high-quality fat intake, such as omega 3 polyunsaturated fatty acids (ꞷ-3 PUFA), has been associated with a higher risk of MDD [38]. Thus, patients with MDD often present with an insufficient protein and high-quality fat intake and with overconsumption of refined carbohydrates and poor-quality fats. In this situation, deficiencies in multiple vitamins and minerals are importantly linked to MDD. Particularly, a low intake of vitamin D and from the B complex—prominently thiamine (B1), niacin (B3), pyridoxin (B6), folate (B9), and cyanocobalamin (B12)—are observed in patients with MDD [39,40] Regarding minerals, low calcium, magnesium, iron, and zinc consumption has been associated with MDD [41], whereas low potassium, phosphorus, and copper intake can also be related to depressive symptoms for women, but not for men [42].
Collectively, there is compelling evidence that patients with MDD are commonly malnourished, or at least at risk of malnutrition. Thus, as it will be subsequently discussed, patients with MDD can benefit from addressing their nutritional status, as these dietary changes may favorably influence the intricate biology of depression.
1.3. Biology of Depression: Is There a Role for Diet?
The numerous difficulties related to the clinical management of MDD may be a consequence of the complex pathophysiological signature of this neuropsychiatric condition. Traditionally, the most widely known biological mechanism implicated in the pathophysiology of MDD has been aberrant neurotransmitter functioning and, particularly, decreased monoamine levels. Monoamines are represented by serotonin, dopamine, and norepinephrine, acting in critical brain regions and mediating several perceptions and behaviors in the brain [43]. However, currently, it is widely accepted that monoamines only represent a single mechanism of a really intricate picture [44]. Apart from monoamines, abnormal functioning of other neurotransmitters, such as gamma-aminobutyric acid (GABA), glutamate, and acetylcholine, has been reported in patients with MDD [45]. The available literature also considers the relevance of altered neuropeptides in the pathophysiology of MDD, including galanin (GAL), cholecystokinin (CCK), neuropeptide Y (NPY), oxytocin (OXT), vasopressin (VP), neuropeptide S (NPS), and melanin-concentrating hormone (MCH) [46]. Likewise, alterations in the neuropeptide melatonin and circadian disruption are similarly reported in patients with MDD [47], collectively supporting that there are many neurotransmitters and neuropeptides that are not working properly in the brain of depressed patients.
Furthermore, impaired neurogenesis and neuroplasticity have also been observed in the brain of depressed patients, having been proposed as a major pathophysiological signature of MDD [48]. For instance, alterations in neurotrophic factors such as brain-derived neurotrophic factor (BDNF) or glial-derived neurotrophic factor (GDNF) are critically involved in these changes, affecting several cellular processes [49,50]. The aberrant neurogenesis and neuroplasticity are closely linked to the effects of psychological stress in the patient with MDD. In this sense, it is widely accepted that this condition is associated with severe dysfunction of the hypothalamus–pituitary–adrenal (HPA) axis, characterized by altered levels of cortisol and their regulators, cortisol release factor (CRF) and the adrenocorticotropin hormone (ACTH), entailing detrimental consequences for the brain and the entire organism [51].
Moreover, consistent damage to neuronal and glial cells has been described in the brain of patients with MDD, with an enhanced mitochondrial dysfunction, apoptosis, oxidative stress, and excitotoxicity [52,53] All these changes lead to structural, functional, and connectivity abnormalities in specific regions of the central nervous system (CNS), such as the hippocampus, amygdala, dorsomedial thalamus, dorsal and medial prefrontal cortex, the dorsal and ventral anterior cingulate cortex, the orbital frontal cortex, the insula, and the striatum and raphe nucleus [54], which ultimately are responsible for the behavioral and mood dysfunctions related to MDD.
Not only local, but also extra-brain affections are observed in the patient with MDD. The immune system is a pivotal player in the pathophysiology of this condition and an aberrant inflammatory response can be observed either in the brain (neuroinflammation), or at systemic levels [55,56,57,58]. Metabolic and endocrine alterations (insulin resistance, type 2 diabetes, obesity, etc.) are closely related to immune dysfunction and can influence at the onset and development of MDD [59]. Indeed, an exacerbated inflammatory response frequently co-occurs with endocrine, neural, and psychiatric alterations such as in MDD, which is studied in the field of psychoneuroimmunoendocrinology, explaining the biological link between MDD and systemic diseases [60]. Moreover, gut dysbiosis and an altered microbiota–gut–brain (MGB) axis are tightly linked with inflammation and the above-mentioned mechanisms, also representing a potential etiopathogenic mechanism of MDD [61,62]. Thereby, the pathophysiology of MDD is undoubtedly complex, explaining the clinical and translational difficulties of this condition.
Diet has pleiotropic effects in the organism. Depending on the content of specific nutrients and foods, as well as the whole dietary context, they may promote health or facilitate the onset and progression of multiple pathological conditions [35]. In the field of MDD, an umbrella review using 28 meta-analyses has defined the potential of nutrients, foods, beverages, and dietary patterns in the amelioration or progression of MDD; although, the quality of evidence is still moderate or low [63]. This means that further studies are warranted before drawing any definitive conclusion about the potential of diet and MDD. Indeed, some authors have claimed that narrative reviews tend to overestimate the impact of diet as preventive or as adjunctive support in this field [64], and we agree that is critical to be objective and put in context the promising but still inconclusive role of diet in MDD. What the evidence seems to indicate is that dietary interventions can aid in the alleviation of depressive symptoms, but most of these results have been obtained in patients without a clinical diagnosis of depression [65], so once again it should be understood that MDD is a complex and multifactorial disorder in which plenty biological, cultural, and psychosocial factors are interacting, being a huge challenge for healthcare professionals (Figure 1).
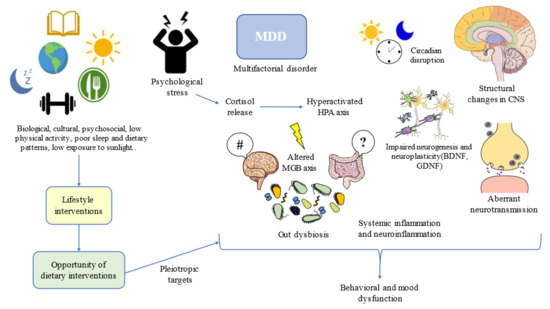
Figure 1.
Lifestyle interventions such as dietary interventions suppose an opportunity for addressing multiple targets in the complex network of depression. MDD: Major Depressive Disorder; HPA: hypothalamic–pituitary–adrenal; CNS: central nervous system; MGB: microbiota–gut–brain; BDNF: brain-derived neurotrophic factor; GDNF: glial-derived neurotrophic factor. #/?: Aberrant gut brain crosstalk due to an impaired MGB axis.
One of the most plausible explanations for the high prevalence of MDD and its relationship with diet can be perceived from an evolutionary context. Following the words of Theodosius Dobzhansky, “nothing in the biological aspects of medicine makes sense except in the light of evolution”. Evolutionary-based approaches have brought great advances in the understanding and clinical management of MDD [66]. MDD can be considered a disease that emerged as the result of low physical activity levels, sleep and circadian disturbances, low exposure to sunlight, social isolation, poor contact with nature, and, of course, due to poor dietary patterns [67]. Thus, lifestyle interventions following this evolutionary background have led to notable improvements in physical and mental health, while aiding to prevent the onset of mood disorders [68]. Moreover, addressing the entire picture from an evolutionary perspective, in combination with antidepressants and/or psychosocial support, along with additional medical therapy, if needed, can bring maximum benefits to patients with MDD (Figure 1).
In this context, the diet has the potential to modulate various pathophysiological mechanisms of MDD, including: (1) Amelioration of immune dysfunction and inflammation; (2) Favorable modulatory effects on gut microbiota and their derived products; (3) Limiting the impact of oxidative stress; (4) Enhancing mitochondrial function and displaying cytoprotective effects; (5) Boosting neurogenesis; (6) Epigenetic reprogramming; (7) Targeting HPA dysfunction; and (8) Influencing neurotransmitter and neuropeptides biosynthesis/degradation [69]. Additionally, diet has the potential to revert or promote specific biological mechanisms implicated in the pathophysiology of MDD in women, interacting with reproductive hormones throughout the menstrual cycle and playing a critical role during different critical stages such as pregnancy or menopause [70].
Hence, once the nutritional status of depressed patients, their background, and the actions of diet in the biology of MDD have been explored, the main nutrients, foods, and dietary habits studied in this field will be subsequently discussed.
2. Translational Opportunities: Clinical Management of MDD through Diet
2.1. Foods and Nutrients of Interest
Many efforts have been placed to find potential foods and dietary supplements with antidepressant effects as well as to prevent the onset of depression. These foods contain a wide variety of nutrients that act synergically to exert their benefits. Many times, these effects are different than those played by the nutrient alone. This concept is defined under the term food matrix, having numerous consequences for the global effect of foods [71]. Dietary supplements consist of an isolated nutrient with some interesting properties and both foods and supplements with potential preventive and supportive antidepressant activity can be defined as nutraceuticals, a growing area of research in the field of MDD [72]. In the field and clinical management of MDD, it is equally important to limit or avoid the consumption of ultra-processed foods, sugar-sweetened drinks, alcohol, as well as red and processed meats consumption, as they increase the risk of suffering from MDD [63]. In this part, we will focus on different food sources and supplements with potential preventive and antidepressant effects.
2.1.1. Fish, Seeds, and Nuts
Compelling evidence is starting to support the benefits of fish intake in the management and prevention of MDD. Indeed, various meta-analyses show that moderate fish consumption was associated with a reduced risk of depression, especially in women ([73,74]. Furthermore, long-term intake of fish was also protective for elder individuals aged > 65 years, thereby supporting the benefits of fish consumption on mental health [75]. Due to their many bioactive components, fish intake is recommended as a key pillar of a healthy diet at least twice per week [76], or 350 g weekly including 200 g, which may be from oily fishes [77]. However, optimizing the most suitable recommendation of fish following preferences and costs is a major objective of current studies [78].
Fish and especially oily fishes as well as fish oil are rich in ω-3 PUFA, particularly docosahexaenoic acid (DHA; 22:6 ω-3) and eicosapentaenoic acid (EPA; 20:5 ω-3), proving multiple antidepressant effects [79]. Despite DHA and EPA sharing multiple biological actions, there are some disparities in their molecular activities explaining their differential role as antidepressants [80]. A meta-analysis of randomized control trials claimed that EPA, but not DHA, was responsible for the most antidepressant effects of ꞷ-3 PUFA [81]. Conversely, in a 5-year follow-up, it was demonstrated that the protective role against depression of high fish intake (≥2 times/week) was strongly influenced by the DHA content; although, other components rather than DHA may also contribute to the antidepressant effects of fish [82,83]. However, ꞷ-3 PUFA are well-known anti-inflammatory mediators, and this effect is importantly achieved by the alteration in epigenetic markers such as DNA methylation. [84]. In a similar manner, compelling evidence supports the benefits of ꞷ-3 PUFA due to its prebiotic effect, critically modulating the composition of the gut microbiota [85]. Other mechanisms involved in their antidepressant action include an increased membrane fluidity, leading to an increase in serotonin transport by endothelial cells, an enhanced dopamine concentration and dopamine 2 receptor in the frontal cortex, and direct interactions with other neuronal receptors and second messengers, exerting pleiotropic effects [86]. Other dietary sources of ꞷ-3 PUFA include seeds and nuts; although, they are frequently found in the form of α-linolenic acid (ALA; 18:3 ω-3), a precursor of EPA and DHA [87]. Because of that, some preclinical and observational studies have demonstrated some evidence about the use of seeds and nuts (especially walnuts) in the prevention and amelioration of depressive symptoms [88,89,90]. Specifically, 30 g/day of mixed nuts (15 g walnuts, 7.5 g hazelnuts, and 7.5 g almonds) comprised in a healthy dietary pattern appears to be an important recommendation to prevent MDD [91] Thus, ꞷ-3 PUFA consumption or supplementation has provided cumulative evidence of antidepressant and preventive effects in MDD; although, further studies are still required.
ꞷ-3 PUFA can present synergic effects with vitamin D in the brain, which is also abundant in seafood and oily fishes, helping to explain the antidepressant role of these foods [92]. Vitamin D is equally found in eggs and other animal products, mushrooms, or certain fortified products [93]. Despite its relevance to health, vitamin D intake is generally low worldwide, and supplementation with vitamin D is common or at least required in many population groups [94]. Specifically, low serum vitamin D levels appear to be inversely related to clinical depression [95]. In this context, notwithstanding some studies have found some favorable antidepressant benefits from vitamin D supplementation, these results are inconsistent with other works that did not report any protective or antidepressant effects of vitamin D [96,97]. The underlying biological mechanisms that help to explain the potential antidepressant role of vitamin D are through: (1) Immune and gut microbiota modulation; (2) Serotonin synthesis; (3) Circadian clock regulation; and (4) Augmented BDNF production [98,99,100]. In addition, sunlight exposure can aid in vitamin D production (but does not replace foods or supplementation with this nutraceutical), exerting synergic antidepressant actions proven in prior studies [101].
Apart from their high content in ω-3 PUFA and vitamin D, fish, seafood, seeds, and nuts are rich in high-quality proteins. A recent study has observed that a diet with high protein contents can interact with the rs7041 polymorphism of the vitamin D-binding protein (VDBP) and decrease the risk of severe depression [102]. Likewise, seafood, seeds, and nuts are also major sources of dietary zinc, along with other high protein foods such as legumes, poultry, and meat, aiding to prevent the onset of MDD [103]. Finally, a study conducted by Cediel Ulloa et al. [104] demonstrated that high prenatal exposure to methylmercury due to high fish consumption was associated with differential DNA methylation at seven years of age at specific CpG sites, with adverse consequences in neurodevelopment and mental health. During pregnancy, it should be important to limit the consumption of fish with high levels of mercury. Further studies are needed to unravel the effect of fish and its different components in the etiopathogenesis of MDD.
2.1.2. Fruits, Vegetables, and Berries
Fruits and vegetables are also a group of foods widely explored in the prevention and therapeutic support of many diseases such as MDD. Current dietary guidelines recommend that these groups of foods may constitute about one-half of the plate at each meal and a total mean intake of 800 g per day [105]. However, there are some subgroups of fruits and vegetables more interesting for cognitive working and mental health, as is the case of berries, citrus, and green leafy vegetables [106]. Meta-regression and analytic data show that a 100 g increased intake either of fruit or vegetables was associated with a 3% reduced risk of depression [107]. Conversely, patients with MDD often eat fewer fruits, vegetables, and legumes than healthy subjects, while consuming more sweets and pastries [108]. The benefits of this group of foods can be attributed to three main components: (a) dietary fiber; (b) vitamins; and (c) polyphenols.
The first component, dietary fiber seems to exert critical actions in the gut microbiota, leading to the production of short-chain fatty acids (SCFAs) and lowering inflammation by modifying both the pH and the permeability of the gut. Importantly, these changes may be associated with a reduction in depressive symptoms [109]. Some previous studies have found that 1 g of fiber per 1000 kcal is sufficient to observe the protective actions of fiber against depression in premenopausal but not postmenopausal women [110]. Further studies are warranted to determine the quantity of fiber intake to show its preventive actions against depression.
Fruits and vegetables are critical sources of vitamins, especially for the antioxidant vitamins C (ascorbic acid) and E (Tocopherol) along with those from the B complex, with the exception of vitamin B12, which is found in animal-based products and fortified cereals or nutritional yeast [111]. Indeed, prior works have denoted that a daily intake above 400 g of fruits or vegetables ensures high blood concentrations of these vitamins [112]. Similarly, vegetables (prominently dark green leafy vegetables) are a critical source of vitamin K1 (Phylloquinone) and colorful fruits and vegetables are rich in provitamin A (beta carotenoids), a precursor of vitamin A in the body [113]. However, fruits and vegetables lack significant quantities of vitamin D, which may be obtained from animal-based foods, fungi, or some algae [114].
Vitamins are major mediators of mental health, likewise, influencing gut microbiota and SCFAs production [115]. Thus, some studies have observed potential preventive or antidepressant effects derived from vitamins. For instance, some researchers have found slight benefits from supplementation with B6 and B12 alone or integrated within a vitamin B complex [116,117]; although, other data suggest that vitamins from the B complex may provide some benefits for stress but not for depressive symptoms [118]. However, it is common that patients with MDD present low B1, B2, B3, B6, B9, and B12 vitamin levels, and, in turn, these deficits can promote the pro-inflammatory status of the immune system, participating in the pathophysiological changes related to MDD [119]. Furthermore, there is some initial evidence supporting supplementation with vitamin A and E as antidepressants, especially due to their antioxidant and anti-inflammatory effects [72], and the use of vitamin C as an adjuvant with antidepressants has obtained controversial results, with benefits in children in combination with fluoxetine, but not in adults [120].
Vitamin K has presented potential preventive actions, having been demonstrated that people with a high intake of this vitamin (>232 μg/day) had significantly lower odds of developing depressive symptoms at baseline, and at each per 100 μg/day, the odds of this condition decrease by 12% [121]. Vitamin supplementation may be interesting for individuals with some type of nutritional deficit or who have a poor nutritional status. However, due to the presence of multiple vitamins and other critical nutrients in fruits and vegetables, we think that for the general population, it is much more interesting to encourage the consumption of the recommended fruit and vegetable intake per day instead of focusing on supplementation.
The last group of nutrients reviewed in this section are polyphenols. Polyphenols are organic compounds abundantly found in plants playing critical anti-inflammatory and antioxidant properties with a promising, protective, and therapeutic role in many diseases [122]. Common polyphenols in the diet include flavonols (quercetin in onions, apples, and tea), hydroxycinnamates (coffee, many fruits) flavanols (cocoa, tea, apples, and broad beans), flavanones (hesperidin in citrus fruit), and anthocyanins (berries) [123]. The relevance of many of these polyphenols is being studied as promising agents in the field of MDD. Curcumin is one of the best-studied polyphenols in this sense, and there are some clinical trials providing evidence that 1000 mg/day of curcumin may be used as adjunctive therapy for patients with MDD; although, these benefits were not observed at lower doses (500 mg/day) [124]. However, despite there being some interesting results on the effects of curcumin as an antidepressant, further studies should be conducted to better assess this relationship, particularly in Western countries [125].
Resveratrol, a well-known polyphenol predominant in red grapes, also has cumulative evidence of antidepressant effects in preclinical models at doses between 10 and 80 mg/kg/day; although, higher doses had the most significant benefits [126]. Similarly, Wang et al. [127] used an extract from Concord grape juice, grape seed extract, and trans-resveratrol and observed that two components, dihydrocaffeic acid (DHCA) and malvidin-3′-O-glucoside (Mal-gluc), exerted antidepressant effects in mice. Interestingly, they reported how both components modulate the epigenome of their model. DHCA reduces pro-inflammatory interleukin 6 (IL-6) by inhibiting DNA methylation at the CpG-rich IL-6 sequences introns 1 and 3, whereas Mal-gluc promoted histone acetylation of the regulatory sequences of the Rac1 gene, thereby modulating synaptic plasticity.
Quercetin, a type of flavonol that has also been tested as a potential antidepressant in vivo, ameliorates lipopolysaccharide (LPS)-induced depressive rats, leading to the upregulation of BDNF, as well as other molecular markers [128].
Anthocyanins, mainly found in berries, are also being investigated in mice models as prominent antidepressants, upregulating monoamines and neurotrophic factors such as brain-derived neurotrophic factor (BDNF), which plays a critical role in MDD [129]. These are only a few examples of how polyphenols may aid in the clinical management of MDD.
More interestingly, there are more than 8000 recognized polyphenols and the function and mechanisms of many of them have not been fully elucidated [130]. Polyphenols are perhaps one of the most interesting compounds to be explored and plant-derived foods are particularly rich in these types of nutrients. Thus, an adequate dietary intervention should include fruits and vegetables to take advantage of the multiple benefits of polyphenols, vitamins, fiber, and other compounds present in these foods.
2.1.3. Coffee and Tea: The Role of Caffeine and Specific Bioactive Compounds
Coffee and tea, apart from water, are the most consumed beverages worldwide, constituting the top sources of caffeine and antioxidant polyphenols in the American diet [131]. The polyphenols found in tea are named catechins, and the most important are epigallocatechin-3-gallate (EGCG), epicatechin-3-gallate (ECG), epigallocatechin (EGC), and epicatechin (EC) [132]. Other components of note in tea are theaflavins, flavonol glycosides, L-theanine, caffeine, theobromine, and volatile organic substances [133]. Among the most important components in coffee, the role of certain polyphenols should be highlighted, especially chlorogenic acids (in green beans) and caffeic acid (in roasted coffee beans), alkaloids (caffeine and trigonelline), and diterpenes (cafestol and kahweol) [134]. Due to their wide variety of beneficial components, either tea or coffee are considered critical beverages to include in the diet, and more positive effects can be reported when including both drinks [135]. Regarding daily recommendations, a prior meta-analysis found a nonlinear J-shaped relation between coffee consumption and risk of depression with a peak of protective effect for 400 mL/day [136]. Likewise, more than three cups of tea/week also seemed to exert relevant antidepressant effects [137].
Among their actions in the organism, coffee and tea seem to exert an important neuroprotective role, lowering the risk of suffering from cognitive decline and brain disorders [138,139]. Indeed, there is compelling evidence that the high consumption of coffee and tea provided reduced the risk of suffering from MDD [140]. Caffeine is one of the major determinants of these neuroprotective and antidepressant actions. In more detail, a nonlinear response between caffeine consumption and depression was found, showing their most benefits above 68 mg/day and below 509 mg/day [141]. Moreover, caffeine has also been studied as adjunctive therapy with antidepressants, showing some promising results in preclinical and clinical models [142,143]. Among the multiple mechanisms of action of caffeine, it seems that its consumption affects the epigenetic status of immune cells and also of neurons and glial cells [144,145]. It is still unknown if these epigenetic effects are related to the non-selective adenosine antagonist action of caffeine; however, this interplay appears to be promising for the treatment of motivational and dopaminergic dysfunction in depression [146]. Nevertheless, excessive consumption of coffee and caffeine has also been noted for some serious adverse effects such as anxiety, headache, increased blood pressure, nausea, and restlessness [147], as well as negatively influencing the development of the brain at early stages [148]. More studies are needed to evaluate the therapeutic safety and efficacy of caffeine in the field of MDD.
Polyphenols contained in coffee and tea can also be of great aid to the multiple benefits of these beverages. For instance, EGCG has proven some critical antidepressant effects in preclinical models, leading to an augmented BDNF, serotonin, and reduced stress hormones [124,149]. However, the major benefits observed in green tea are due to the interaction of EGCG with other catechins, teasaponin, L-theanine, and theaflavins, as they may also favorably regulate the dopaminergic system and attenuate inflammation through the downregulation of NF-κB signaling [150]. Caffeic acid also provides significant antidepressant effects in vivo, especially when combined with antidepressants [151]. Chlorogenic acid found in green coffee appears to be a major inhibitor of monoamine oxidase A (MAO-A), upregulating serotonin levels and providing antidepressant actions [152].
Future studies can be directed to describe the therapeutic and preventive mechanisms of tea and coffee consumption, with a special focus on their epigenetic mechanisms. However, the evidence appears to support that both coffee and tea and some of their components may exert antidepressant benefits. It is likely that patients with MDD may benefit from including regular doses of tea and coffee, including both drinks daily and in the morning. However, it should be equally important to consider the individual effects of coffee and tea, without the heavy drinking of these beverages. More data are required to find a proper dose of coffee and tea to prevent or support the clinical management of MDD.
2.1.4. Psychobiotics: Relevance of Probiotic Food in MDD
Psychobiotics are a group of probiotics with favorable effects on the CNS function and behaviors mediated by the MGB axis, acting through immune, humoral, neural, and metabolic pathways [153]. Thus, psychobiotics can directly influence many of the pathophysiological mechanisms involved in MDD, modulating the HPA axis, inflammation, neurotransmitter production (monoamines, GABA, glutamate, and acetylcholine), BDNF levels, as well as host metabolism and systemic actions via the vagus nerve or through critical microbial metabolites such as SCFAs [154]. Psychobiotics are mainly represented by lactic acid bacteria (LAB), bacteria from the order of Lactobacillales and Bifidobacteria [155], which they can be found in supplements—using single or multispecies formulas—or included in fermented foods and beverages such as in dairy products (yogurt and kefir), soy derivates (i.e., tempeh), and other products [156]. In this section, we will explore some of the most relevant psychobiotic potential of fermented food and beverages.
On the one hand, yogurt and kefir are two groups of fermented dairy products with many benefits for human health [157]; yogurt is particularly rich in Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus [158]. Sometimes they can also have added LAB species such as Bifidobacterium animalis subsp. lactis, boosting their global benefits [159] Kefir is a fermented milk that comes from kefir grains, which include a specific and complex mixture of lactic acid and acetic acid bacteria that appears in symbiosis with lactose-fermenting and nonfermenting yeast [160]. Interestingly, kefir without bacteria or yeast in its composition seems to critically limit the benefits from this product [161]. Prior works have demonstrated that 3 weeks with daily consumption of probiotic yogurt improved the mood of those whose mood was initially poor [162]. Mohammadi et al. [163] observed that whereas probiotic yogurt displayed significant benefits on mental health by positively modulating the HPA axis, conventional yogurt did not have any benefit, suggesting the need of improving the probiotic formula of certain yogurts.
Likewise, a cohort of 14,539 men and women during more than 9 years from the SUN (Seguimiento Universidad de Navarra) project observed that whole-fat yogurt, but not low fat, was related to a decreased risk of depression in women, once again supporting the relevance of the entire food matrix in the benefits of a food product [164]. Moreover, the combination of yogurt plus exercise in healthy individuals led to greater increases in serotonin levels and reductions in triglyceride and high-sensitivity C-reactive protein (CRP) levels, relative to those observed for yogurt or exercise alone, demonstrating the synergic role of different lifestyle factors in the prevention of depression and inflammatory conditions [165].
Simultaneously, kefir has demonstrated a major modulatory role in the MGB axis, influencing host behavior, serotonin synthesis, immune system composition, and GABA levels linked to an increased prevalence of Lactobacillus reuteri [166]. Apart from their microbial composition, kefir peptides appear to be a promising antidepressant tested in vivo, activating the BDNF/phosphorylated tropomyosin receptor kinase B (TrkB) signaling pathway [167]. Further clinical studies are needed to clarify the benefits of yogurt and kefir in patients with MDD due to their promising actions.
Soy-based fermented products are mainly represented by a wide variety of traditional Asian foods such as doenjang (soybean paste or Japanese miso), ganjang (soy sauce), tempeh, or natto. Soybean contains a broad spectrum of bioactive compounds such as isoflavones, soyasaponins, lignans, cinnamic acid derivatives, terpenes, and sterols, along with antinutrients such as phytates, trypsin inhibitors, and lectins [168]. Through the fermentation process mediated by LAB, most of these antinutrients are reduced and novel compounds are generated, which, together with direct effects in the MGB axis, aid to explain the global benefits of these products [169]. Observational studies have proven that traditional Japanese dietary practices, with a high adherence to fermented soy products, are associated with lower rates of depressive symptoms [170]. In the same manner, different studies have demonstrated the efficacy of using fermented soy-based products in the clinical management of depression and cognitive impairment, with which it appears commonly associated [171]. In this line, Hwang et al. [172] conducted a 12-week intervention of 800 mg/day of a mixture of fermented soybean powder and Lactobacillus plantarum C29 (1.25 × 1010 CFU/g). Interestingly, they reported that the intervention group reported greater improvements in cognitive performance, being associated with higher BDNF levels. Likewise, a study using soy-based milk by Lactobacillus brevis FPA 3709 (1 × 106 CFU/mL) demonstrated antidepressant efficacy in Sprague-Dawley (SD) rats [173]. This effect was achieved due to the increase in GABA levels, demonstrating similar effects to the administration of the antidepressant fluoxetine. Further studies can be directed to explore further combinations and applications of soy-derived psychobiotics in the field of MDD.
Finally, other psychobiotics have been explored to aid in the clinical management or to prevent the onset of neuropsychiatric maladies. Although less studied, the role of certain psychobiotic beverages such as water kefir or kombucha is starting to be unraveled. Water kefir is a sparkling, acidic beverage produced by fermenting a sucrose solution, to which dried fruits and water kefir grains are added. Presumably, the benefits of this beverage are similar to those reported by milk kefir, being a healthy vegan alternative, but further knowledge on the understanding of the biological dynamics of water kefir fermentation is required [174]. Regarding kombucha, this beverage is formed by the tea fermentation of Camellia sinensis and by a biofilm of cellulose, containing a symbiotic culture of bacteria and yeast (SCOBY) [175]. In preclinical studies, kombucha has proven antioxidant and antiaging properties, antimicrobial activity, antihypertensive, anti-inflammatory, neuroprotective, and many other benefits ([176]. In this sense, the administration of kombucha appears to mitigate the inflammatory response mediated by LPS, reducing the levels of tumor necrosis factor-α (TNF-α) and interleukins IL-1β and IL-6, restoring the levels of T cells and macrophages, and inhibiting NF-κB signaling [177]. Patients with MDD can exhibit high levels of LPS in their bloodstream (endotoxemia), associated with systemic inflammation and neuroinflammation [56,178]. The anti-inflammatory effect of kombucha should be further studied in patients with MDD, especially regarding endotoxemia and its subsequent immune response. Moreover, in a rat model, kombucha seemed to increase the integrity of the blood–brain barrier (BBB) and decrease the brain and serum levels of the malondialdehyde (MDA), a marker of oxidative stress [179]. Interestingly, both an enhanced BBB permeability and changes in MDA levels have been observed in patients with MDD [180,181]. However, despite the promising role of kombucha obtained in preclinical studies, the available clinical evidence is still insufficient and future studies should be conducted on human beings to extrapolate the benefits reported previously mentioned [182]. Another potential example of the benefits of psychobiotics is the consumption of fermented Laminaria japonica. In a study conducted by Reid et al. [183], subjects received 1.5 g/day of fermented Laminaria japonica for 6 weeks, displaying enhanced GABA and antioxidant activity, with neuroprotective effects.
Collectively, the role of psychobiotic foods in the clinical management and prevention of depression remains to be deeply studied. However, there are some promising results obtained in this field; preclinical and future studies should mostly be directed to unravel potential applications, doses, and recommendations.
2.1.5. Dietary Supplements
Apart from food, there have been some dietary supplements explored in the context of MDD that can also work as nutraceuticals. The dietary supplements with the most evidence proven as antidepressant adjuvants are S-adenosylmethionine (SAMe), 5-methyltetrahydrofolate, ω-3 PUFA, and vitamin D [184]. Likewise, there are meta-analyses supporting the relevance of acetyl carnitine as an antidepressant in comparison to a placebo, with few adverse effects [185]. Creatine and some amino acids have also provided some clinical effects as antidepressants, in trace and ultra-trace elements; although, the level of evidence is still quite low [72]. Not only psychobiotics, but also prebiotics and postbiotics are being deeply investigated, due to the critical role of the MGB axis in MDD [62]. However, the debate about nutraceutical supplement use sometimes resides in their inconclusive evidence due to the lack of long-term studies, given the fact that their effectiveness becomes more obvious over a longer period than pharmacological drugs. In favor of bioactive components, nutraceuticals, or supplements, there is a rising research interest in them nowadays due to their having minimal side effects compared to drugs. In this sense, we encourage further studies evaluating the long-term effects of these components.
Collectively, isolated nutraceuticals present mixed evidence, and it seems that in cases of certain nutritional deficits, the benefits are greater. In this sense, we want to remark on another critical point, some studies have failed to find any significant benefit from using multi-nutrient or nutraceutical combinations in patients with MDD [186,187]. These observations could be due to the fact that despite the benefits from foods residing on particular nutrients and components of them, the concept of the food matrix is essential to understand how these compounds exert their effects in the organism. This should be taken into consideration for future nutraceuticals design, either using foods with their complete matrix or somehow emulating their matrix in designed products.
Likewise, the use of certain medicinal plants has also been explored in the field of MDD. Particularly, extracts from Hypericum perforatum (St John’s wort) are widely used for the treatment of depression of varying severity. Indeed, for patients with mild-to-moderate depression, St John’s wort has comparable efficacy and safety when compared to antidepressants, causing fewer adverse events [188]. However, the level of evidence on its long-term efficacy is limited, as most studies only ranged from 4 to 12 weeks. In addition, it is also unclear if this plant would be beneficial for patients with severe depression, high suicidality, or suicide risk [189]. Hypericum perforatum extracts have a plethora of bioactive compounds such as naphthodianthrones, phloroglucinol derivatives, flavonoids, bioflavonoids, proanthocyanidins, and chlorogenic acid; although, two molecules, hypericin and hyperforin, appear to be the most important antidepressant components [190]. In this sense, the following biological mechanisms have been previously described: (a) Inhibition of MAOs; (b) Inhibition of synaptosomal reuptake of amines; (c) Modulation of monoamine transporters; and (d) Regulatory actions on serotonin receptors [191]. All in all, Hypericum perforatum leads to profound changes in neurotransmitter levels, including monoamines, GABA, Glutamate, and acetylcholine [190]. However, as previously described, MDD entails a plethora of brain and systemic alterations involved in its pathogenesis, thereby explaining the limited benefits of this product in patients with severe depression. Not only Hypericum perforatum, but also other herbal products such as Ginkgo biloba leaves, ginseng, and so on can be used for alleviating depressive symptoms, acting through different biological mechanisms [192]. Future studies should be conducted on this promising line of research, considering their clinical applications in patients with MDD.
Finally, the use of dietary supplements is common in patients with MDD, especially in women; and frequently not just single, but different supplements have proven effective [193]. It is important to highlight that, unlike foods or drugs, dietary supplements and nutraceuticals do not need to be registered or approved by the US Food and Drug Administration (FDA) prior to production or sales. This could be associated with some important concerns. On the one hand, the quality and composition of the commercial finished products are poorer than the regulated ones [194]. On the other hand, although rare, this can bring severe consequences for some individuals. For instance, serotonin syndrome is a potentially life-threatening condition precipitated by the use of serotonergic drugs. These effects can be due to therapeutic medication use, interactions between medications or recreational drugs, or intentional overdose [195]. Indeed, there are some case reports in which the interaction between drugs and dietary supplements was associated with the co-occurrence of serotonin and acute compartment syndrome, demonstrating the remarkable issue of studying these interactions [196]. In addition, some deleterious interactions between nutraceuticals/dietary supplements with drugs in the field of MDD have been previously defined [197]. In this sense, it is critical to give more effort to the prevention of adverse outcomes derived from the use of dietary supplements and their interactions. In the last years, some countries have started detecting, monitoring, or reporting adverse events derived from dietary supplements in an activity designed as Nutrivigilance. Clinicians should be conscious about the use of dietary supplements by patients with MDD and obtain enough information before recommending some nutraceuticals/dietary supplements, taking into consideration possible or reported interactions. Overall, Nutrivigilance is a novel field that remains to be deeply explored and some caution may be required. In this line, we recommend focusing on ensuring the intake of beneficial foods and nutrients rather than supplements (if no severe deficits are reported), in order to maximize the benefits of these components
2.2. Dietary Strategies to Implement in Patients with MDD
Not only specific nutrients and foods, but also the entire dietary context are important approaches to consider in the clinical management and prevention of MDD. Indeed, unhealthy dietary patterns clearly influence the development and aggravation of MDD. For instance, it is well-known that excessive ultra-processed food consumption in westernized and proinflammatory diets is associated with pro-depressant effects [198,199]. On the other hand, different dietary approaches have been emerging since the last century in order to improve depressive symptoms. Some of the best-known strategies studied to support the prevention and treatment of MDD include the Mediterranean diet (MDiet), low-carbohydrate diet (LCD), and plant-based diet (vegetarianism and veganism). Here, it must be understood that the type of dietary strategy is not the most important point to consider, but the adherence to a healthy dietary pattern and the inclusion of a wide variety of food and nutrients is what really matters.
The MDiet is a type of dietary pattern mainly characterized by a richness of highly complex carbohydrates in fiber (cereals, legumes, vegetables, and fruits), polyunsaturated fatty acids with antiatherogenic and anti-inflammatory properties (i.e., fish, extra virgin olive oil, and nuts), and a plethora of bioactive compounds with antioxidative properties such as flavonoids, phytosterols, terpenes, and polyphenols [35]. This strategy has been widely explored in the prevention and management of non-communicable diseases (NCDs), especially those associated with aging [200] In this sense, some authors have described the relevance of the MDiet and some of their most representative elements on a differential epigenetic profile, which may explain the benefits of this diet in different disorders [201]. MDD is an intricate and multifactorial disorder and different results have been obtained regarding the inclusion of the MDiet. Some of the most important studies regarding the role of the MDiet in the prevention of different NCDs are PREDIMED (Prevención con Dieta Mediterránea) trials. One study described that moderate fish and long-chain ꞷ-3 PUFA consumption integrated in an MDiet was associated with lower odds of depression in a U-shaped way [202]. Likewise, one systematic review and meta-analysis found that adherence to the MDiet was prominently related to a reduced incidence of depression [203]. More detailly, another meta-analysis also found benefits from high and moderate MDiet adherence with a reduced risk of depression. Nevertheless, the positive association of moderate adherence seemed to fade with age [204]. Despite these conclusions, there are also other systematic reviews and meta-analyses that have failed to find significant associations between high adherence to the MDiet and the risk of depression [203,205], suggesting promising but still inconsistent evidence of the benefits of the MDiet in the prevention of depression. In the context of clinical management of patients with MDD, the SMILES (Supporting the Modification of lifestyle in Lowered Emotional States) trial should be highlighted. A total of 67 individuals were studied, 33 with a modified MDiet intervention and 34 without any nutritional but social support. Of them, 55 were also receiving some type of therapy, pharmacology, psychotherapy, or a combination of both. A total of 31 of the dietary support group and 25 of the controls completed the study at 12 weeks and after further analysis, the study concluded that this type of nutritional intervention helped in the clinical management of MDD, measured with the Montgomery–Åsberg Depression Rating Scale (MADRS) [206]. The dietary protocol for these patients is described in [207]. Extra virgin olive oil (EVOO), one of the most representative elements of the MDiet has recently proven antidepressant effects in patients with severe MDD, but not in those with mild to moderate [208]. All in all, the growing number of studies focusing on the preventive and therapeutic support of the MDiet has opened an interesting field of research. Further efforts are warranted to unravel the mechanisms and optimum protocols to obtain the greatest benefits from this type of dietary intervention.
LCDs such as the ketogenic or Atkins diet are mainly characterized by a reduced carbohydrate and high fat intake. Both aim to induce nutritional ketosis, and previous studies have found some evidence from the use of these strategies in the prevention and treatment of some neurological disorders such as epilepsy or Alzheimer’s disease [209]. Ketone bodies are not only a fat-derived energy supply, but they also exert important epigenetic functions, especially through histone post-translational modifications [210]. To date, there is some evidence of the antidepressant actions of ketone bodies in preclinical studies, case reports, and case series, but no clinical trials have been conducted in this field [211]. The benefits seem to be related to their regulatory role on different neurotransmitter levels, mitigating oxidative stress, insulin dysfunction, and inflammation, while favoring mitochondrial function and neurotrophic factors [211]. A negative point of following an LCD, however, may be related to negative long-term effects, as some authors have hypothesized that LCDs can induce metabolic depression, linked to reduced glycogen stores, and increased feelings of fatigue [212]. Likewise, combining this kind of strategy with intermittent fasting can exert synergic effects in the amelioration or prevention of depressive symptoms, sharing some similarities with an evolutionary or Paleolithic lifestyle; although, there are no clinical studies conducted with intermittent fasting in psychiatric populations yet [213,214]. Further efforts are required in this area before establishing the adequacy of this dietary context in MDD.
Plant-based diets also have different dietary patterns that have gained popularity in the last years, mainly aiming to reduce the environmental footprint of the diet and promote human health and animal welfare [215]. In general terms, plant-based diets are prominently represented by non-animal products; although, there is a broad spectrum of possibilities: including low meat but all food items (omnivore), all except meat (pesco-vegetarian), all except meat and fish (ovo-lacto-vegetarian), or plant-based items (vegan). Despite some evidence arising regarding the actions of a plant-based diet in the brain, the benefits and underlying mechanisms remain to be explored [216]. In this line, a possible epigenetic role of different bioactive components found in plants, especially by modulating DNA methylation, has been described [217]. There is some evidence that vegetarians/vegans may be at a higher risk of suffering from depression [218,219]. It is inconclusive if these associations can be mediated by a proper diet or if the vegans/vegetarians’ dietary pattern is a result of an increased susceptibility to depression. A potential explanation for this fact is that the vegetarian/vegan diet may not be properly balanced, being associated with the lack of some critical dietary components [220]. However, some authors argue that when well-designed and supplemented with vitamin B12, plant-based diets could bring potential benefits for overall health [221]. Once again, the quality of the diet may be a determinant factor. A cross-sectional study provided evidence that high-quality plant-based products (avoiding ultra-processed products and ensuring an adequate intake of different nutrients) can be protective for vegans/vegetarians without depression; although, individuals affected by depression did not show that benefit [222]. Similarly, avoiding the consumption of animal-based products such as meat, eggs, or dairy products did not show an improvement in mental health or MDD, according to some studies and meta-analyses [223,224]. This is attributed to the fact that these natural foods are rich in the different above-mentioned nutrients with potential benefits for the body [225]; although, vegetarianism/veganism if properly balanced, is also a laudable choice. Overall, it must be added that the actual studies in this area are quite heterogeneous. Currently, for patients with MDD, it seems that limiting or avoiding animal products may not be the appropriate approach. However, further research is needed, especially on well-designed and balanced vegetarian/vegan diets, before any final conclusions can be drawn.
Collectively, different studies regarding the implementation of a dietary pattern to prevent and ameliorate depression have been conducted. The MDiet has been the most widely studied strategy, providing many promising results; although, the level of evidence of its benefits is still insufficient. There are few studies on LCDs in depression; although, they may have some short-term benefits from depression due to the multiple regulatory benefits of ketone bodies. Finally, it seems that there is a negative association between a plant-based diet and depression; however, there is great heterogeneity in the current literature. Moreover, it is not well-understood if vegetarianism/veganism can be a consequence of, or contributes to, some pathophysiological mechanisms. The most important points to consider are both the adherence to a healthy dietary pattern and, more prominently, the inclusion of high-quality foods, avoiding ultra-processed products, and ensuring a complete nutritional content, which will bring the most benefits for the prevention and, in an adequate context, for the clinical management of MDD.
3. Conclusions
MDD is a complex neuropsychiatric disorder with many biological, psychosocial, and cultural factors implicated. A mismatch of our evolutive design—for instance, following unhealthy dietary patterns with a low presence of certain critical nutrients—can contribute to the onset and development of this complex disorder. In this context, dietary interventions can potentially aid to prevent and improve the clinical management of patients with MDD, as summarized in Table 1. There are foods of special interest such as fish, nuts, seeds, EVOO, fruits, vegetables, coffee, and tea, which have been widely explored in the field of MDD. These foods are especially rich in critical nutrients such as ꞷ-3 PUFA, dietary fiber, vitamins, minerals, polyphenols, and various bioactive compounds with direct and indirect implications on the different pathophysiological mechanisms of MDD. For instance, by restoring neurotransmitter levels, targeting the HPA axis, attenuating the inflammatory response, or modulating the MGB axis, among others. Most of these foods and nutrients are encompassed under the MDiet pattern, and growing evidence supports the protective and adjuvant role of this diet in many NCDs, including in certain neuropsychiatric maladies. Other groups of foods or dietary strategies, such as psychobiotics found in fermented products, certain dietary supplements, or ketogenic and plant-based diets, are being currently investigated in the field of MDD. However, it should be noticed that nutrition is a complex research area in which most studies are preclinical or observational, and the greatest benefits from this lifestyle habit are observed over the long term (Figure 2). As the great physician, Hippocrates said, “Illnesses do not come upon us out of the blue. They are developed from small daily sins against Nature. When enough sins have accumulated, illnesses will suddenly appear.” Nevertheless, due to the promising role of diet in the prevention and management of MDD, we encourage further studies in this growing area in combination with other lifestyle medicine approaches, aiding to prevent and revert these daily “sins”.

Table 1.
Main nutrients and dietary recommendations with potential antidepressant effects.
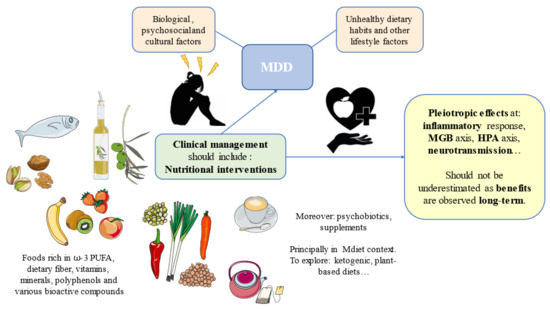
Figure 2.
Summary of the main components of promising nutritional interventions and their pleiotropic effects in the pathophysiology of depression. MDD: Major Depressive Disorder; HPA: hypothalamic–pituitary–adrenal; MGB: microbiota–gut–brain; ꞷ-3 PUFA: omega 3 polyunsaturated fatty acids.
Author Contributions
Conceptualization, M.A.O., Ó.F.-M., C.G.-M., M.A.A.-M., M.A.D.M.; methodology, M.A.O., Ó.F.-M., C.G.-M., M.A.A.-M., M.A.D.M.; software, M.A.O., Ó.F.-M., C.G.-M., M.A.A.-M., M.A.D.M.; validation, M.A.D.M.; formal analysis, M.A.O., Ó.F.-M., C.G.-M., M.A.A.-M., M.A.D.M.; investigation, M.A.O., Ó.F.-M., C.G.-M., M.A.A.-M., M.A.D.M.; writing—original draft preparation, M.A.O., Ó.F.-M., C.G.-M., M.A.A.-M., G.L., J.M., M.L.-V., L.G.-R., R.M., R.R.-J., J.Q., M.A.D.M.; writing—review and editing, M.A.O., Ó.F.-M., C.G.-M., M.A.A.-M., G.L., J.M., M.L.-V., L.G.-R., R.M., R.R.-J., J.Q., M.A.D.M..; visualization, M.A.O., Ó.F.-M., C.G.-M., M.A.A.-M., M.A.D.M.; project administration, M.A.D.M.; funding acquisition, M.A.D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially supported by grants from the Fondo de Investigación de la Seguridad Social, Instituto de Salud Carlos III (PI18/01726 and PI19/00766), Spain, Programa de Actividades de I+D de la Comunidad de Madrid en Biomedicina (B2017/BMD3804 and B2020/MITICAD-CM), and HALEKULANI S.L.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ACTH | adrenocorticotropin hormone |
| ALA | α-linolenic acid |
| AST | aspartate aminotransferase |
| BBB | blood–brain barrier |
| BDNF | brain-derived neurotrophic factor |
| BUN | blood urea nitrogen |
| CCK | cholecystokinin |
| CNS | central nervous system |
| COVID-19 | coronavirus disease 2019 |
| CRF | cortisol release factor |
| CRP | C-reactive protein |
| DHA | docosahexaenoic acid |
| DHCA | dihydrocaffeic acid |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders, 5th Edition |
| EC | epicatechin |
| ECG | epigallocatechin |
| EGCG | epigallocatechin-3-gallate |
| ELS | Early Life Stress |
| EPA | eicosapentaenoic acid |
| EVOO | extra virgin olive oil |
| GABA | gamma-aminobutyric acid |
| GAL | galanin |
| GDNF | glial-derived neurotrophic factor |
| GHDx | Global Health Data Exchange |
| HAM-D | Hamilton Depression Rating Scale |
| HDL-C | high-density lipoprotein cholesterol |
| HPA axis | hypothalamus–pituitary–adrenal |
| IL-1β | interleukin 1β |
| IL-6 | interleukin 6 |
| LAB | lactic acid bacteria |
| LCD | low-carbohydrate diet |
| LPS | lipopolysaccharide |
| MADRS | Montgomery-Åsberg Depression Rating Scale |
| Mal-gluc | malvidin-3′-O-glucoside |
| MAO-A | monoamine oxidase |
| MCH | melanin-concentrating hormone |
| MDA | malondialdehyde |
| MDD | Major Depressive Disorder |
| MDiet | Mediterranean Diet |
| MGB axis | microbiota–gut–brain axis |
| MNA | Mini Nutritional Assessment |
| NCDs | non-communicable diseases |
| NPS | neuropeptide S |
| NPY | neuropeptide Y |
| OXT | oxytocin |
| PREDIMED trials | Prevención con Dieta Mediterránea trials |
| SAMe | S-adenosylmethionine |
| SCFAs | short-chain fatty acids |
| SCOBY | symbiotic culture of bacteria and yeast |
| SD | Sprague-Dawley |
| SMILES trial | Supporting the Modification of lifestyle in Lowered Emotional States trial |
| SUN project | Seguimiento Universidad de Navarra project |
| TNF-α | tumor necrosis factor-α |
| TrkB | tropomyosin receptor kinase B |
| VDBP | vitamin D-binding protein |
| VP | vasopressin |
| WHO | World Health Organization |
| ω-3 PUFA | omega 3 polyunsaturated fatty acids |
References
- World Health Organization. Depression. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 20 July 2022).
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Ortega, M.A.; Alvarez-Mon, M.A.; García-Montero, C.; Fraile-Martinez, O.; Lahera, G.; Monserrat, J.; Muñoz-Merida, L.; Mora, F.; Rodríguez-Jiménez, R.; Fernandez-Rojo, S.; et al. MicroRNAs as Critical Biomarkers of Major Depressive Disorder: A Comprehensive Perspective. Biomedicines 2021, 9, 1659. [Google Scholar] [CrossRef] [PubMed]
- Santomauro, D.F.; Herrera, A.M.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Abbafati, C.; Adolph, C.; Amlag, J.O.; Aravkin, A.Y.; et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef]
- de la Torre, J.A.; Vilagut, G.; Ronaldson, A.; Dregan, A.; Ricci-Cabello, I.; Hatch, S.L.; Serrano-Blanco, A.; Valderas, J.M.; Hotopf, M.; Alonso, J. Prevalence and age patterns of depression in the United Kingdom. A population-based study. J. Affect. Disord. 2021, 279, 164–172. [Google Scholar] [CrossRef]
- Kessler, R.C.; Bromet, E.J. The Epidemiology of Depression Across Cultures. Annu. Rev. Public Health 2013, 34, 119–138. [Google Scholar] [CrossRef]
- Gutiérrez-Rojas, L.; Porras-Segovia, A.; Dunne, H.; Andrade-González, N.; Cervilla, J.A. Prevalence and correlates of major depressive disorder: A systematic review. Rev. Bras. Psiquiatr. 2020, 42, 657. [Google Scholar] [CrossRef]
- Mullins, N.; Lewis, C. Genetics of Depression: Progress at Last. Curr. Psychiatry Rep. 2017, 19, 43. [Google Scholar] [CrossRef]
- Saveanu, R.V.; Nemeroff, C.B. Etiology of Depression: Genetic and Environmental Factors. Psychiatr. Clin. North Am. 2012, 35, 51–71. [Google Scholar] [CrossRef]
- Saleh, A.; Potter, G.G.; McQuoid, D.R.; Boyd, B.; Turner, R.; MacFall, J.R.; Taylor, W.D. Effects of early life stress on depression, cognitive performance and brain morphology. Psychol. Med. 2017, 47, 171. [Google Scholar] [CrossRef]
- Hammen, C.; Kim, E.Y.; Eberhart, N.K.; Brennan, P.A. Chronic and acute stress and the prediction of major depression in women. Depress. Anxiety 2009, 26, 718–723. [Google Scholar] [CrossRef]
- Yelton, B.; Friedman, D.B.; Noblet, S.; Lohman, M.C.; Arent, M.A.; Macauda, M.M.; Sakhuja, M.; Leith, K.H. Social Determinants of Health and Depression among African American Adults: A Scoping Review of Current Research. Int. J. Environ. Res. Public Health 2022, 19, 1498. [Google Scholar] [CrossRef]
- Assari, S. Social Determinants of Depression: The Intersections of Race, Gender, and Socioeconomic Status. Brain Sci. 2017, 7, 156. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, TX, USA, 2013. [Google Scholar]
- Tolentino, J.C.; Schmidt, S.L. DSM-5 Criteria and Depression Severity: Implications for Clinical Practice. Front. Psychiatry 2018, 9, 450. [Google Scholar] [CrossRef]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef]
- Carrozzino, D.; Patierno, C.; Fava, G.A.; Guidi, J. The Hamilton Rating Scales for Depression: A Critical Review of Clinimetric Properties of Different Versions. Psychother. Psychosom. 2020, 89, 133–150. [Google Scholar] [CrossRef]
- Zimmerman, M.; Martinez, J.H.; Young, D.; Chelminski, I.; Dalrymple, K. Severity classification on the Hamilton depression rating scale. J. Affect. Disord. 2013, 150, 384–388. [Google Scholar] [CrossRef]
- Grover, S.; Gautam, S.; Jain, A.; Gautam, M.; Vahia, V.N. Clinical Practice Guidelines for the management of Depression. Indian J. Psychiatry 2017, 59, S34–S50. [Google Scholar] [CrossRef]
- Voineskos, D.; Daskalakis, Z.J.; Blumberger, D.M. Management of Treatment-Resistant Depression: Challenges and Strategies. Neuropsychiatr. Dis. Treat. 2020, 16, 221. [Google Scholar] [CrossRef]
- Katon, W.; Unützer, J.; Russo, J. Major depression: The importance of clinical characteristics and treatment response to prognosis. Depress. Anxiety 2010, 27, 19–26. [Google Scholar] [CrossRef]
- Greenberg, P.E.; Fournier, A.-A.; Sisitsky, T.; Simes, M.; Berman, R.; Koenigsberg, S.H.; Kessler, R.C. The Economic Burden of Adults with Major Depressive Disorder in the United States (2010 and 2018). Pharmacoeconomics 2021, 39, 653–665. [Google Scholar] [CrossRef]
- Steiber, A.; Hegazi, R.; Herrera, M.; Zamor, M.L.; Chimanya, K.; Pekcan, A.G.; Redondo-Samin, D.C.D.; Correia, M.I.T.; Ojwang, A.A. Spotlight on Global Malnutrition: A Continuing Challenge in the 21st Century. J. Acad. Nutr. Diet. 2015, 115, 1335–1341. [Google Scholar] [CrossRef]
- Perez-Escamilla, R.; Bermudez, O.; Buccini, G.S.; Kumanyika, S.; Lutter, C.; Monsivais, P.; Victora, C. Nutrition disparities and the global burden of malnutrition. BMJ 2018, 361, k2252. [Google Scholar] [CrossRef]
- Norman, K.; Haß, U.; Pirlich, M. Malnutrition in Older Adults—Recent Advances and Remaining Challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, Ó.; García-Montero, C.; Alvarez-Mon, M.A.; Lahera, G.; Monserrat, J.; Llavero-Valero, M.; Mora, F.; Rodríguez-Jiménez, R.; Fernandez-Rojo, S.; et al. Nutrition, Epigenetics, and Major Depressive Disorder: Understanding the Connection. Front. Nutr. 2022, 9, 867150. [Google Scholar] [CrossRef]
- Alam, M.R.; Karmokar, S.; Reza, S.; Kabir, R.; Ghosh, S.; Al Mamun, A. Geriatric malnutrition and depression: Evidence from elderly home care population in Bangladesh. Prev. Med. Rep. 2021, 23, 101478. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Alva, M.C.; Irigoyen-Camacho, M.E.; Cabrer-Rosales, M.F.; Lazarevich, I.; Arrieta-Cruz, I.; Gutiérrez-Juárez, R.; Zepeda-Zepeda, M.A. Prevalence of Malnutrition and Depression in Older Adults Living in Nursing Homes in Mexico City. Nutrients 2020, 12, 2429. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.C.; Chou, Y.-T.; Chang, T.-L. Usefulness of the Mini Nutritional Assessment (MNA) in predicting the nutritional status of people with mental disorders in Taiwan. J. Clin. Nurs. 2011, 20, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Kaner, G.; Soylu, M.; Yüksel, N.; Inanç, N.; Ongan, D.; Başmısırlı, E. Evaluation of Nutritional Status of Patients with Depression. BioMed Res. Int. 2015, 2015, 521481. [Google Scholar] [CrossRef] [PubMed]
- Nachane, H.B.; Nayak, A.S. Maternal anthropometric determinants as risk markers of suicidality and severity of illness in women with postnatal depression. J. Postgrad. Med. 2020, 66, 11–16. [Google Scholar] [CrossRef]
- Lee, B.J. Association of depressive disorder with biochemical and anthropometric indices in adult men and women. Sci. Rep. 2021, 11, 13596. [Google Scholar] [CrossRef]
- Silva, D.; Ferriani, L.; Viana, M.C. Depression, anthropometric parameters, and body image in adults: A systematic review. Rev. Assoc. Med. Bras. 2019, 65, 731–738. [Google Scholar] [CrossRef]
- Oh, J.; Yun, K.; Chae, J.-H.; Kim, T.-S. Association Between Macronutrients Intake and Depression in the United States and South Korea. Front. Psychiatry 2020, 11, 207. [Google Scholar] [CrossRef]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.; Pekarek, L.; Castellanos, A.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota–Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Sánchez-Villegas, A.; Verberne, L.; De Irala, J.; Ruíz-Canela, M.; Toledo, E.; Serra-Majem, L.; Martínez-González, M.A. Dietary Fat Intake and the Risk of Depression: The SUN Project. PLoS ONE 2011, 6, e16268. [Google Scholar] [CrossRef]
- Vagena, E.; Ryu, J.K.; Baeza-Raja, B.; Walsh, N.M.; Syme, C.; Day, J.P.; Houslay, M.D.; Baillie, G.S. A high-fat diet promotes depression-like behavior in mice by suppressing hypothalamic PKA signaling. Transl. Psychiatry 2019, 9, 141. [Google Scholar] [CrossRef]
- Ciesielski, T.H.; Williams, S.M. Low Omega-3 intake is associated with high rates of depression and preterm birth on the country level. Sci. Rep. 2020, 10, 19749. [Google Scholar] [CrossRef]
- Cuomo, A.; Giordano, N.; Goracci, A.; Fagiolini, A. Depression and Vitamin D Deficiency: Causality, Assessment, and Clinical Practice Implications. Neuropsychiatry 2017, 7, 606–614. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Stojanovska, L.; Apostolopoulos, V. The Effects of Vitamin B in Depression. Curr. Med. Chem. 2016, 23, 4317–4337. [Google Scholar] [CrossRef]
- Miki, T.; Kochi, T.; Eguchi, M.; Kuwahara, K.; Tsuruoka, H.; Kurotani, K.; Ito, R.; Akter, S.; Kashino, I.; Pham, N.M.; et al. Dietary intake of minerals in relation to depressive symptoms in Japanese employees: The Furukawa Nutrition and Health Study. Nutrition 2015, 31, 686–690. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Miyagi, S.; Tsujiguchi, H.; Kambayashi, Y.; Hara, A.; Nakamura, H.; Suzuki, K.; Yamada, Y.; Shimizu, Y.; Nakamura, H. Association between Lower Intake of Minerals and Depressive Symptoms among Elderly Japanese Women but Not Men: Findings from Shika Study. Nutrients 2019, 11, 389. [Google Scholar] [CrossRef]
- Azizi, S.A. Monoamines: Dopamine, Norepinephrine, and Serotonin, Beyond Modulation, “Switches” That Alter the State of Target Networks. Neuroscientist 2022, 28, 121–143. [Google Scholar] [CrossRef]
- Hindmarch, I. Beyond the monoamine hypothesis: Mechanisms, molecules and methods. Eur. Psychiatry 2002, 17, 294–299. [Google Scholar] [CrossRef]
- Pytka, K.; Dziubina, A.; Młyniec, K.; Dziedziczak, A.; Żmudzka, E.; Furgała, A.; Olczyk, A.; Sapa, J.; Filipek, B. The role of glutamatergic, GABA-ergic, and cholinergic receptors in depression and antidepressant-like effect. Pharmacol. Rep. 2016, 68, 443–450. [Google Scholar] [CrossRef]
- Rana, T.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Abdeen, A.; Ibrahim, S.F.; Mani, V.; Iqbal, M.S.; Bhatia, S.; et al. Exploring the role of neuropeptides in depression and anxiety. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 114, 110478. [Google Scholar] [CrossRef]
- Salva, M.A.Q.; Hartley, S.; Barbot, F.; Alvarez, J.C.; Lofaso, F.; Guilleminault, C. Circadian Rhythms, Melatonin and Depression. Curr. Pharm. Des. 2011, 17, 1459–1470. [Google Scholar] [CrossRef]
- Bourin, M. Neurogenesis and Neuroplasticity in Major Depression: Its Therapeutic Implication. Adv. Exp. Med. Biol. 2021, 1305, 157–173. [Google Scholar] [CrossRef]
- Castrén, E.; Monteggia, L.M. Brain-Derived Neurotrophic Factor Signaling in Depression and Antidepressant Action. Biol. Psychiatry 2021, 90, 128–136. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Wang, X.; Wang, Y.; Li, C.; Zhu, X. Alterations of DNA Methylation at GDNF Gene Promoter in the Ventral Tegmental Area of Adult Depression-Like Rats Induced by Maternal Deprivation. Front. Psychiatry 2019, 10, 732. [Google Scholar] [CrossRef]
- Keller, J.; Gomez, R.; Williams, G.; Lembke, A.; Lazzeroni, L.; Murphy, G.M., Jr.; Schatzberg, A.F. HPA axis in major depression: Cortisol, clinical symptomatology and genetic variation predict cognition. Mol. Psychiatry 2017, 22, 527. [Google Scholar] [CrossRef]
- Rajkowska, G.; Miguel-Hidalgo, J. Gliogenesis and Glial Pathology in Depression. CNS Neurol. Disord. Drug Targets 2007, 6, 219–233. [Google Scholar] [CrossRef]
- Duman, R.S. Neuronal damage and protection in the pathophysiology and treatment of psychiatric illness: Stress and depression. Dialog. Clin. Neurosci. 2009, 11, 239–255. [Google Scholar] [CrossRef]
- Pandya, M.; Altinay, M.; Malone, D.A.; Anand, A. Where in the Brain Is Depression? Curr. Psychiatry Rep. 2012, 14, 634–642. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22. [Google Scholar] [CrossRef]
- Alvarez-Mon, M.A.; Gómez, A.M.; Orozco, A.; Lahera, G.; Sosa, M.D.; Diaz, D.; Auba, E.; Albillos, A.; Monserrat, J.; Alvarez-Mon, M. Abnormal Distribution and Function of Circulating Monocytes and Enhanced Bacterial Translocation in Major Depressive Disorder. Front. Psychiatry 2019, 10, 812. [Google Scholar] [CrossRef]
- Alvarez-Mon, M.; Gómez-Lahoz, A.; Orozco, A.; Lahera, G.; Diaz, D.; Ortega, M.; Albillos, A.; Quintero, J.; Aubá, E.; Monserrat, J.; et al. Expansion of CD4 T Lymphocytes Expressing Interleukin 17 and Tumor Necrosis Factor in Patients with Major Depressive Disorder. J. Pers. Med. 2021, 11, 220. [Google Scholar] [CrossRef]
- Alvarez-Mon, M.A.; Gomez-Lahoz, A.M.; Orozco, A.; Lahera, G.; Sosa-Reina, M.D.; Diaz, D.; Albillos, A.; Quintero, J.; Molero, P.; Monserrat, J.; et al. Blunted Expansion of Regulatory T Lymphocytes Is Associated With Increased Bacterial Translocation in Patients With Major Depressive Disorder. Front. Psychiatry 2021, 11, 1530. [Google Scholar] [CrossRef]
- Kahl, K.G. Metabolic alterations in patients with depression and their relationship to the etiology of depressive disorders. Ann. Gen. Psychiatry 2010, 9, S34. [Google Scholar] [CrossRef][Green Version]
- Francisco, V.; Gualillo, O.; van den Bossche, J.; Ortega, M.A.; García-Montero, C.; Fraile-Martinez, O.; Angel Alvarez-Mon, M.; Maria Gómez-Lahoz, A.; Lahera, G.; Monserrat, J.; et al. Immune-Mediated Diseases from the Point of View of Psychoneuroimmunoendocrinology. Biology 2022, 11, 973. [Google Scholar] [CrossRef]
- Cepeda, M.S.; Katz, E.G.; Blacketer, C. Microbiome-Gut-Brain Axis: Probiotics and Their Association With Depression. J. Neuropsychiatry Clin. Neurosci. 2016, 29, 39–44. [Google Scholar] [CrossRef]
- Ortega, M.A.; Alvarez-Mon, M.A.; García-Montero, C.; Fraile-Martinez, O.; Guijarro, L.G.; Lahera, G.; Monserrat, J.; Valls, P.; Mora, F.; Rodríguez-Jiménez, R.; et al. Gut Microbiota Metabolites in Major Depressive Disorder—Deep Insights into Their Pathophysiological Role and Potential Translational Applications. Metabolites 2022, 12, 50. [Google Scholar] [CrossRef]
- Xu, Y.; Zeng, L.; Zou, K.; Shan, S.; Wang, X.; Xiong, J.; Zhao, L.; Zhang, L.; Cheng, G. Role of dietary factors in the prevention and treatment for depression: An umbrella review of meta-analyses of prospective studies. Transl. Psychiatry 2021, 11, 478. [Google Scholar] [CrossRef] [PubMed]
- Thomas-Odenthal, F.; Molero, P.; Van Der Does, W.; Molendijk, M. Impact of review method on the conclusions of clinical reviews: A systematic review on dietary interventions in depression as a case in point. PLoS ONE 2020, 15, e0238131. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Marx, W.; Dash, S.; Carney, R.; Teasdale, S.; Solmi, M.; Stubbs, B.; Schuch, F.; Carvalho, A.F.; Jacka, F.; et al. The Effects of Dietary Improvement on Symptoms of Depression and Anxiety: A Meta-Analysis of Randomized Controlled Trials. Psychosom. Med. 2019, 81, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Hollon, S.D.; Andrews, P.W.; Thomson, J.A. Cognitive Behavior Therapy for Depression From an Evolutionary Perspective. Front. Psychiatry 2021, 12, 667592. [Google Scholar] [CrossRef]
- Hidaka, B.H. Depression as a disease of modernity: Explanations for increasing prevalence. J. Affect. Disord. 2012, 140, 205–214. [Google Scholar] [CrossRef]
- Durisko, Z.; Mulsant, B.H.; Mckenzie, K.; Andrews, P.W. Using Evolutionary Theory to Guide Mental Health Research. Can. J. Psychiatry 2016, 61, 159–165. [Google Scholar] [CrossRef]
- Marx, W.; Lane, M.; Hockey, M.; Aslam, H.; Berk, M.; Walder, K.; Borsini, A.; Firth, J.; Pariante, C.M.; Berding, K.; et al. Diet and depression: Exploring the biological mechanisms of action. Mol. Psychiatry 2020, 26, 134–150. [Google Scholar] [CrossRef]
- García-Montero, C.; Ortega, M.A.; Alvarez-Mon, M.A.; Fraile-Martinez, O.; Romero-Bazán, A.; Lahera, G.; Montes-Rodríguez, J.M.; Molina-Ruiz, R.M.; Mora, F.; Rodriguez-Jimenez, R.; et al. The Problem of Malnutrition Associated with Major Depressive Disorder from a Sex-Gender Perspective. Nutrients 2022, 14, 1107. [Google Scholar] [CrossRef]
- Aguilera, J.M. The food matrix: Implications in processing, nutrition and health. Crit. Rev. Food Sci. Nutr. 2018, 59, 3612–3629. [Google Scholar] [CrossRef]
- Alvarez-Mon, M.A.; Ortega, M.A.; García-Montero, C.; Fraile-Martinez, O.; Monserrat, J.; Lahera, G.; Mora, F.; Rodriguez-Quiroga, A.; Fernandez-Rojo, S.; Quintero, J.; et al. Exploring the Role of Nutraceuticals in Major Depressive Disorder (MDD): Rationale, State of the Art and Future Prospects. Pharmaceuticals 2021, 14, 821. [Google Scholar] [CrossRef]
- Li, F.; Liu, X.; Zhang, D. Fish consumption and risk of depression: A meta-analysis. J. Epidemiol. Community Health 2016, 70, 299–304. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, Y.; Je, Y. Fish consumption and risk of depression: Epidemiological evidence from prospective studies. Asia-Pac. Psychiatry 2018, 10, e12335. [Google Scholar] [CrossRef]
- Bountziouka, V.; Polychronopoulos, E.; Zeimbekis, A.; Papavenetiou, E.; Ladoukaki, E.; Papairakleous, N.; Gotsis, E.; Metallinos, G.; Lionis, C.; Panagiotakos, D. Long-Term Fish Intake Is Associated With Less Severe Depressive Symptoms Among Elderly Men and Women: The MEDIS (MEDiterranean ISlands Elderly) Epidemiological Study. J. Aging Health 2009, 21, 864–880. [Google Scholar] [CrossRef]
- Chen, J.; Jayachandran, M.; Bai, W.; Xu, B. A critical review on the health benefits of fish consumption and its bioactive constituents. Food Chem. 2022, 369, 130874. [Google Scholar] [CrossRef]
- Persson, M.; Fagt, S.; Nauta, M.J. Personalised fish intake recommendations: The effect of background exposure on optimisation. Br. J. Nutr. 2018, 120, 946–957. [Google Scholar] [CrossRef]
- Persson, M.; Fagt, S.; Nauta, M.J. Optimising healthy and safe fish intake recommendations: A trade-off between personal preference and cost. Br. J. Nutr. 2019, 122, 206–219. [Google Scholar] [CrossRef]
- Sikka, P.; Behl, T.; Sharma, S.; Sehgal, A.; Bhatia, S.; Al-Harrasi, A.; Singh, S.; Sharma, N.; Aleya, L. Exploring the therapeutic potential of omega-3 fatty acids in depression. Environ. Sci. Pollut. Res. 2021, 28, 43021–43034. [Google Scholar] [CrossRef]
- Kalkman, H.; Hersberger, M.; Walitza, S.; Berger, G. Disentangling the Molecular Mechanisms of the Antidepressant Activity of Omega-3 Polyunsaturated Fatty Acid: A Comprehensive Review of the Literature. Int. J. Mol. Sci. 2021, 22, 4393. [Google Scholar] [CrossRef]
- Martins, J.G. EPA but not DHA Appears to be Responsible for the Efficacy of Omega-3 Long Chain Polyunsaturated Fatty Acid Supplementation in Depression: Evidence from a Meta-Analysis of Randomized Controlled Trials. J. Am. Coll. Nutr. 2009, 28, 525–542. [Google Scholar] [CrossRef]
- Reeves, J.L.; Otahal, P.; Magnussen, C.G.; Dwyer, T.; Kangas, A.J.; Soininen, P.; Ala-Korpela, M.; Venn, A.J.; Smith, K.J. DHA mediates the protective effect of fish consumption on new episodes of depression among women. Br. J. Nutr. 2017, 118, 743–749. [Google Scholar] [CrossRef]
- Smith, K.J.; Sanderson, K.; McNaughton, S.; Gall, S.; Dwyer, T.; Venn, A.J. Longitudinal Associations Between Fish Consumption and Depression in Young Adults. Am. J. Epidemiol. 2014, 179, 1228–1235. [Google Scholar] [CrossRef]
- Hussey, B.; Lindley, M.R.; Mastana, S.S. Omega 3 fatty acids, inflammation and DNA methylation: An overview. Clin. Lipidol. 2017, 12, 24–32. [Google Scholar] [CrossRef]
- Vijay, A.; Astbury, S.; Le Roy, C.; Spector, T.D.; Valdes, A.M. The prebiotic effects of omega-3 fatty acid supplementation: A six-week randomised intervention trial. Gut Microbes 2021, 13, 1–11. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Jain, A.; Aggarwal, K.; Zhang, P. Omega-3 Fatty Acids and Cardiovascular Disease. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 441–445. [Google Scholar]
- Lu, X.; Ce, Q.; Jin, L.; Zheng, J.; Sun, M.; Tang, X.; Li, D.; Sun, J. Deoiled sunflower seeds ameliorate depression by promoting the production of monoamine neurotransmitters and inhibiting oxidative stress. Food Funct. 2021, 12, 573–586. [Google Scholar] [CrossRef]
- Bikri, S.; Aboussaleh, Y.; Berrani, A.; Louragli, I.; Hafid, A.; Chakib, S.; Ahami, A. Effects of date seeds administration on anxiety and depressive symptoms in streptozotocin-induced diabetic rats: Biochemical and behavioral evidences. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 1031–1040. [Google Scholar] [CrossRef]
- Arab, L.; Guo, R.; Elashoff, D. Lower Depression Scores among Walnut Consumers in NHANES. Nutrients 2019, 11, 275. [Google Scholar] [CrossRef]
- Opie, R.S.; Itsiopoulos, C.; Parletta, N.; Sanchez-Villegas, A.; Akbaraly, T.N.; Ruusunen, A.; Jacka, F.N. Dietary recommendations for the prevention of depression. Nutr. Neurosci. 2017, 20, 161–171. [Google Scholar] [CrossRef]
- Patrick, R.P.; Ames, B.N. Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: Relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J. 2015, 29, 2207–2222. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Walther, B. Natural Vitamin D Content in Animal Products. Adv. Nutr. 2013, 4, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Hossein-Nezhad, A.; Holick, M.F. Vitamin D for Health: A Global Perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Kar, S.; Suthar, N.; Nebhinani, N. Vitamin D and Depression: A Critical Appraisal of the Evidence and Future Directions. Indian J. Psychol. Med. 2020, 42, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Guzek, D.; Kołota, A.; Lachowicz, K.; Skolmowska, D.; Stachoń, M.; Głąbska, D. Association between Vitamin D Supplementation and Mental Health in Healthy Adults: A Systematic Review. J. Clin. Med. 2021, 10, 5156. [Google Scholar] [CrossRef]
- Tomé, A.L.; Cebriá, M.J.R.; González-Teruel, A.; Carbonell-Asíns, J.A.; Nicolás, C.C.; Hernández-Viadel, M. Efficacy of vitamin D in the treatment of depression: A systematic review and meta-analysis. Actas. Esp. Psiquiatr. 2021, 49, 12–23. [Google Scholar]
- Fakhoury, H.M.A.; Kvietys, P.R.; AlKattan, W.; Al Anouti, F.A.; Elahi, M.A.; Karras, S.N.; Grant, W.B. Vitamin D and intestinal homeostasis: Barrier, microbiota, and immune modulation. J. Steroid Biochem. Mol. Biol. 2020, 200, 105663. [Google Scholar] [CrossRef]
- Patrick, R.P.; Ames, B.N. Vitamin D hormone regulates serotonin synthesis. Part 1: Relevance for autism. FASEB J. 2014, 28, 2398–2413. [Google Scholar] [CrossRef]
- Singh, P.; Rawat, A.; Alwakeel, M.; Sharif, E.; Al Khodor, S. The potential role of vitamin D supplementation as a gut microbiota modifier in healthy individuals. Sci. Rep. 2020, 10, 21641. [Google Scholar] [CrossRef]
- Knippenberg, S.; Damoiseaux, J.; Bol, Y.; Hupperts, R.; Taylor, B.V.; Ponsonby, A.-L.; Dwyer, T.; Simpson, S.; Van Der Mei, I.A.F.; Simpson, J.S. Higher levels of reported sun exposure, and not vitamin D status, are associated with less depressive symptoms and fatigue in multiple sclerosis. Acta Neurol. Scand. 2014, 129, 123–131. [Google Scholar] [CrossRef]
- Pooyan, S.; Rahimi, M.H.; Mollahosseini, M.; Khorrami-Nezhad, L.; Nasir, Y.; Maghbooli, Z.; Mirzaei, K. A High-Protein/Low-Fat Diet May Interact with Vitamin D-Binding Protein Gene Variants to Moderate the Risk of Depression in Apparently Healthy Adults. Lifestyle Genom. 2018, 11, 64–72. [Google Scholar] [CrossRef]
- Vashum, K.P.; McEvoy, M.; Milton, A.H.; McElduff, P.; Hure, A.; Byles, J.; Attia, J. Dietary zinc is associated with a lower incidence of depression: Findings from two Australian cohorts. J. Affect. Disord. 2014, 166, 249–257. [Google Scholar] [CrossRef]
- Ulloa, A.C.; Gliga, A.; Love, T.M.; Pineda, D.; Mruzek, D.W.; Watson, G.E.; Davidson, P.W.; Shamlaye, C.F.; Strain, J.; Myers, G.J.; et al. Prenatal methylmercury exposure and DNA methylation in seven-year-old children in the Seychelles Child Development Study. Environ. Int. 2021, 147, 106321. [Google Scholar] [CrossRef]
- Wallace, T.C.; Bailey, R.L.; Blumberg, J.B.; Burton-Freeman, B.; Chen, C.-Y.O.; Crowe-White, K.M.; Drewnowski, A.; Hooshmand, S.; Johnson, E.; Lewis, R.; et al. Fruits, vegetables, and health: A comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit. Rev. Food Sci. Nutr. 2020, 60, 2174–2211. [Google Scholar] [CrossRef]
- Głąbska, D.; Guzek, D.; Groele, B.; Gutkowska, K. Fruit and Vegetable Intake and Mental Health in Adults: A Systematic Review. Nutrients 2020, 12, 115. [Google Scholar] [CrossRef]
- Saghafian, F.; Malmir, H.; Saneei, P.; Milajerdi, A.; Larijani, B.; Esmaillzadeh, A. Fruit and vegetable consumption and risk of depression: Accumulative evidence from an updated systematic review and meta-analysis of epidemiological studies. Br. J. Nutr. 2018, 119, 1087–1101. [Google Scholar] [CrossRef]
- Grases, G.; Colom, M.A.; Sanchis, P.; Grases, F. Possible relation between consumption of different food groups and depression. BMC Psychol. 2019, 7, 14. [Google Scholar] [CrossRef]
- Swann, O.G.; Kilpatrick, M.; Breslin, M.; Oddy, W.H. Dietary fiber and its associations with depression and inflammation. Nutr. Rev. 2020, 78, 394–411. [Google Scholar] [CrossRef]
- Kim, Y.; Hong, M.; Kim, S.; Shin, W.-Y.; Kim, J.-H. Inverse association between dietary fiber intake and depression in premenopausal women: A nationwide population-based survey. Menopause 2020, 28, 150–156. [Google Scholar] [CrossRef]
- Obeid, R.; Heil, S.G.; Verhoeven, M.M.A.; van den Heuvel, E.G.H.M.; de Groot, L.; Eussen, S.J.P.M. Vitamin B12 Intake From Animal Foods, Biomarkers, and Health Aspects. Front. Nutr. 2019, 6, 93. [Google Scholar] [CrossRef]
- Mielgo-Ayuso, J.; Valtueña, J.; Huybrechts, I.; Breidenassel, C.; Cuenca-García, M.; De Henauw, S.; Stehle, P.; Kafatos, A.; Kersting, M.; Widhalm, K.; et al. Fruit and vegetables consumption is associated with higher vitamin intake and blood vitamin status among European adolescents. Eur. J. Clin. Nutr. 2017, 71, 458–467. [Google Scholar] [CrossRef]
- Carazo, A.; Macáková, K.; Matoušová, K.; Krčmová, L.; Protti, M.; Mladěnka, P. Vitamin A Update: Forms, Sources, Kinetics, Detection, Function, Deficiency, Therapeutic Use and Toxicity. Nutrients 2021, 13, 1703. [Google Scholar] [CrossRef]
- Jäpelt, R.B.; Jakobsen, J. Vitamin D in plants: A review of occurrence, analysis, and biosynthesis. Front. Plant Sci. 2013, 4, 136. [Google Scholar] [CrossRef]
- Pham, V.T.; Dold, S.; Rehman, A.; Bird, J.K.; Steinert, R.E. Vitamins, the gut microbiome and gastrointestinal health in humans. Nutr. Res. 2021, 95, 35–53. [Google Scholar] [CrossRef]
- Gougeon, L.; Payette, H.; Morais, J.A.; Gaudreau, P.; Shatenstein, B.; Gray-Donald, K. Intakes of folate, vitamin B6 and B12 and risk of depression in community-dwelling older adults: The Quebec Longitudinal Study on Nutrition and Aging. Eur. J. Clin. Nutr. 2016, 70, 380–385. [Google Scholar] [CrossRef]
- Lewis, J.E.; Tiozzo, E.; Melillo, A.B.; Leonard, S.; Chen, L.; Mendez, A.; Woolger, J.M.; Konefal, J. The Effect of Methylated Vitamin B Complex on Depressive and Anxiety Symptoms and Quality of Life in Adults with Depression. ISRN Psychiatry 2013, 2013, 621453. [Google Scholar] [CrossRef]
- Markun, S.; Gravestock, I.; Jäger, L.; Rosemann, T.; Pichierri, G.; Burgstaller, J. Effects of Vitamin B12 Supplementation on Cognitive Function, Depressive Symptoms, and Fatigue: A Systematic Review, Meta-Analysis, and Meta-Regression. Nutrients 2021, 13, 923. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Stojanovska, L.; Prakash, M.; Apostolopoulos, V. The effects of vitamin B on the immune/cytokine network and their involvement in depression. Maturitas 2017, 96, 58–71. [Google Scholar] [CrossRef]
- Amr, M.; El-Mogy, A.; Shams, T.; Vieira, K.; Lakhan, S.E. Efficacy of vitamin C as an adjunct to fluoxetine therapy in pediatric major depressive disorder: A randomized, double-blind, placebo-controlled pilot study. Nutr. J. 2013, 12, 31. [Google Scholar] [CrossRef]
- Bolzetta, F.; Veronese, N.; Stubbs, B.; Noale, M.; Vaona, A.; Demurtas, J.; Celotto, S.; Cacco, C.; Cester, A.; Caruso, M.G.; et al. The Relationship between Dietary Vitamin K and Depressive Symptoms in Late Adulthood: A Cross-Sectional Analysis from a Large Cohort Study. Nutrients 2019, 11, 787. [Google Scholar] [CrossRef]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Trebatická, J.; Ďuračková, Z. Psychiatric Disorders and Polyphenols: Can They Be Helpful in Therapy? Oxidative Med. Cell. Longev. 2015, 2015, 248529. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, L.; Vozza, L.; Gabbiadini, A.; Vanella, A.; Concas, I.; Tinacci, S.; Petralia, A.; Signorelli, M.; Aguglia, E. Curcumin for depression: A meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 60, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Beidler, J.; Hong, M.Y. Resveratrol and Depression in Animal Models: A Systematic Review of the Biological Mechanisms. Molecules 2018, 23, 2197. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hodes, G.; Zhang, H.; Zhang, S.; Zhao, W.; Golden, S.A.; Bi, W.; Menard, C.; Kana, V.; Leboeuf, M.; et al. Epigenetic modulation of inflammation and synaptic plasticity promotes resilience against stress in mice. Nat. Commun. 2018, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Ke, F.; Li, H.R.; Chen, X.X.; Gao, X.R.; Huang, L.L.; Du, A.Q.; Jiang, C.; Li, H.; Ge, J.F. Quercetin Alleviates LPS-Induced Depression-like Behavior in Rats via Regulating BDNF-Related Imbalance of Copine 6 and TREM1/2 in the Hippocampus and PFC. Front. Pharmacol. 2020, 10, 1544. [Google Scholar] [CrossRef]
- Fang, J.-L.; Luo, Y.; Jin, S.-H.; Yuan, K.; Guo, Y. Ameliorative effect of anthocyanin on depression mice by increasing monoamine neurotransmitter and up-regulating BDNF expression. J. Funct. Foods 2020, 66, 103757. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Bhatti, S.K.; O’Keefe, J.; Lavie, C.J. Coffee and tea. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 688–697. [Google Scholar] [CrossRef]
- LIczbiński, P.; Bukowska, B. Tea and coffee polyphenols and their biological properties based on the latest in vitro investigations. Ind. Crop. Prod. 2022, 175, 114265. [Google Scholar] [CrossRef]
- Samanta, S. Potential Bioactive Components and Health Promotional Benefits of Tea (Camellia sinensis). J. Am. Nutr. Assoc. 2022, 41, 65–93. [Google Scholar] [CrossRef]
- Socała, K.; Szopa, A.; Serefko, A.; Poleszak, E.; Wlaź, P. Neuroprotective Effects of Coffee Bioactive Compounds: A Review. Int. J. Mol. Sci. 2020, 22, 107. [Google Scholar] [CrossRef]
- Brandt, P.A.V.D. Coffee or Tea? A prospective cohort study on the associations of coffee and tea intake with overall and cause-specific mortality in men versus women. Eur. J. Epidemiol. 2018, 33, 183–200. [Google Scholar] [CrossRef]
- Grosso, G.; Micek, A.; Castellano, S.; Pajak, A.; Galvano, F. Coffee, tea, caffeine and risk of depression: A systematic review and dose-response meta-analysis of observational studies. Mol. Nutr. Food Res. 2016, 60, 223–234. [Google Scholar] [CrossRef]
- Tsai, A.C.; Chi, S.-H.; Wang, J.-Y. Cross-sectional and longitudinal associations of lifestyle factors with depressive symptoms in ≥53-year old Taiwanese—Results of an 8-year cohort study. Prev. Med. 2013, 57, 92–97. [Google Scholar] [CrossRef]
- Wasim, S.; Kukkar, V.; Awad, V.M.; Sakhamuru, S.; Malik, B.H. Neuroprotective and Neurodegenerative Aspects of Coffee and Its Active Ingredients in View of Scientific Literature. Cureus 2020, 12, e9578. [Google Scholar] [CrossRef]
- Bastianetto, S.; Yao, Z.-X.; Papadopoulos, V.; Quirion, R. Neuroprotective effects of green and black teas and their catechin gallate esters against β-amyloid-induced toxicity. Eur. J. Neurosci. 2006, 23, 55–64. [Google Scholar] [CrossRef]
- Kang, D.; Kim, Y.; Je, Y. Non-alcoholic beverage consumption and risk of depression: Epidemiological evidence from observational studies. Eur. J. Clin. Nutr. 2018, 72, 1506–1516. [Google Scholar] [CrossRef]
- Wang, L.; Shen, X.; Wu, Y.; Zhang, D. Coffee and caffeine consumption and depression: A meta-analysis of observational studies. Aust. N. Z. J. Psychiatry 2016, 50, 228–242. [Google Scholar] [CrossRef]
- Liu, Q.-S.; Deng, R.; Fan, Y.; Li, K.; Meng, F.; Li, X.; Liu, R. Low dose of caffeine enhances the efficacy of antidepressants in major depressive disorder and the underlying neural substrates. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Szopa, A.; Poleszak, E.; Wyska, E.; Serefko, A.; Wośko, S.; Wlaź, A.; Pieróg, M.; Wróbel, A.; Wlaź, P. Caffeine enhances the antidepressant-like activity of common antidepressant drugs in the forced swim test in mice. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 211–221. [Google Scholar] [CrossRef]
- Chuang, Y.-H.; Quach, A.; Absher, D.; Assimes, T.; Horvath, S.; Ritz, B. Coffee consumption is associated with DNA methylation levels of human blood. Eur. J. Hum. Genet. 2017, 25, 608. [Google Scholar] [CrossRef]
- Marques, S.; Batalha, V.L.; Lopes, L.V.; Outeiro, T.F. Modulating Alzheimer’s Disease Through Caffeine: A Putative Link to Epigenetics. J. Alzheimer’s Dis. 2011, 24, 161–171. [Google Scholar] [CrossRef]
- Lopez-Cruz, L.; Salamone, J.D.; Correa, M. Caffeine and Selective Adenosine Receptor Antagonists as New Therapeutic Tools for the Motivational Symptoms of Depression. Front. Pharmacol. 2018, 9, 526. [Google Scholar] [CrossRef]
- Tenore, G.; Daglia, M.; Orlando, V.; D’Urso, E.; Saadat, S.H.; Novellino, E.; Nabavi, S. Coffee and Depression: A Short Review of Literature. Curr. Pharm. Des. 2015, 21, 5034–5040. [Google Scholar] [CrossRef]
- Islam, M.T.; Tabrez, S.; Jabir, N.R.; Ali, M.; Kamal, M.A.; da Silva Araújo, L.; de Oliveira Santos, J.V.; Oliveira Ferreira da Mata, A.M.; Sousa de Aguiar, R.P.; De Carvalho Melo Cavalcante, A.A. An Insight into the Therapeutic Potential of Major Coffee Components. Curr. Drug Metab. 2018, 19, 544–556. [Google Scholar] [CrossRef]
- Li, G.; Yang, J.; Wang, X.; Zhou, C.; Zheng, X.; Lin, W. Effects of EGCG on depression-related behavior and serotonin concentration in a rat model of chronic unpredictable mild stress. Food Funct. 2020, 11, 8780–8787. [Google Scholar] [CrossRef]
- Rothenberg, D.O.; Zhang, L. Mechanisms Underlying the Anti-Depressive Effects of Regular Tea Consumption. Nutrients 2019, 11, 1361. [Google Scholar] [CrossRef]
- Monteiro, Á.B.; Rodrigues, C.K.D.S.; Nascimento, E.P.D.; Sales, V.D.S.; Delmondes, G.D.A.; da Costa, M.H.N.; de Oliveira, V.A.P.; de Morais, L.P.; Boligon, A.A.; Barbosa, R.; et al. Anxiolytic and antidepressant-like effects of Annona coriacea (Mart.) and caffeic acid in mice. Food Chem. Toxicol. 2020, 136, 111049. [Google Scholar] [CrossRef]
- Grzelczyk, J.; Budryn, G.; Peña-García, J.; Szwajgier, D.; Gałązka-Czarnecka, I.; Oracz, J.; Pérez-Sánchez, H. Evaluation of the inhibition of monoamine oxidase A by bioactive coffee compounds protecting serotonin degradation. Food Chem. 2021, 348, 129108. [Google Scholar] [CrossRef]
- Cheng, L.-H.; Liu, Y.-W.; Wu, C.-C.; Wang, S.; Tsai, Y.-C. Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders. J. Food Drug Anal. 2019, 27, 632–648. [Google Scholar] [CrossRef] [PubMed]
- Del Toro-Barbosa, M.; Hurtado-Romero, A.; Garcia-Amezquita, L.E.; García-Cayuela, T. Psychobiotics: Mechanisms of Action, Evaluation Methods and Effectiveness in Applications with Food Products. Nutrients 2020, 12, 3896. [Google Scholar] [CrossRef] [PubMed]
- Saez-Lara, M.J.; Gomez-Llorente, C.; Plaza-Diaz, J.; Gil, A. The Role of Probiotic Lactic Acid Bacteria and Bifidobacteria in the Prevention and Treatment of Inflammatory Bowel Disease and Other Related Diseases: A Systematic Review of Randomized Human Clinical Trials. BioMed. Res. Int. 2015, 2015, 505878. [Google Scholar] [CrossRef] [PubMed]
- Casertano, M.; Fogliano, V.; Ercolini, D. Psychobiotics, gut microbiota and fermented foods can help preserving mental health. Food Res. Int. 2022, 152, 110892. [Google Scholar] [CrossRef] [PubMed]
- Kok, C.R.; Hutkins, R. Yogurt and other fermented foods as sources of health-promoting bacteria. Nutr. Rev. 2018, 76, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, S. Yogurt Production. Methods Mol. Biol. 2019, 1887, 45–54. [Google Scholar] [CrossRef]
- Le Roy, C.I.; Kurilshikov, A.; Leeming, E.R.; Visconti, A.; Bowyer, R.C.E.; Menni, C.; Falchi, M.; Koutnikova, H.; Veiga, P.; Zhernakova, A.; et al. Yoghurt consumption is associated with changes in the composition of the human gut microbiome and metabolome. BMC Microbiol. 2022, 22, 39. [Google Scholar] [CrossRef]
- Rosa, D.D.; Dias, M.M.S.; Grześkowiak, Ł.M.; Reis, S.A.; Conceição, L.L.; Peluzio, M.D.C.G. Milk Kefir: Nutritional, Microbiological and Health Benefits. Nutr. Res. Rev. 2017, 30, 82–96. [Google Scholar] [CrossRef]
- Bourrie, B.C.T.; Ju, T.; Fouhse, J.M.; Forgie, A.J.; Sergi, C.; Cotter, P.D.; Willing, B.P. Kefir microbial composition is a deciding factor in the physiological impact of kefir in a mouse model of obesity. Br. J. Nutr. 2020, 125, 129–138. [Google Scholar] [CrossRef]
- Benton, D.; Williams, C.; Brown, A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur. J. Clin. Nutr. 2007, 61, 355–361. [Google Scholar] [CrossRef]
- Mohammadi, A.A.; Jazayeri, S.; Khosravi-Darani, K.; Solati, Z.; Mohammadpour, N.; Asemi, Z.; Adab, Z.; Djalali, M.; Tehrani-Doost, M.; Hosseini, M.; et al. The effects of probiotics on mental health and hypothalamic–pituitary–adrenal axis: A randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr. Neurosci. 2016, 19, 387–395. [Google Scholar] [CrossRef]
- Pérez-Cornago, A.; Sánchez-Villegas, A.; Bes-Rastrollo, M.; Gea, A.; Molero, P.; Lahortiga-Ramos, F.; Martínez-González, M.A. Intake of High-Fat Yogurt, but Not of Low-Fat Yogurt or Prebiotics, Is Related to Lower Risk of Depression in Women of the SUN Cohort Study. J. Nutr. 2016, 146, 1731–1739. [Google Scholar] [CrossRef]
- Kim, H.-K.; Kim, S.-H.; Jang, C.-S.; Kim, S.-I.; Kweon, C.-O.; Kim, B.-W.; Ryu, J.-K. The combined effects of yogurt and exercise in healthy adults: Implications for biomarkers of depression and cardiovascular diseases. Food Sci. Nutr. 2018, 6, 1968–1974. [Google Scholar] [CrossRef]
- van de Wouw, M.; Walsh, A.M.; Crispie, F.; Van Leuven, L.; Lyte, J.M.; Boehme, M.; Clarke, G.; Dinan, T.G.; Cotter, P.D.; Cryan, J.F. Distinct actions of the fermented beverage kefir on host behaviour, immunity and microbiome gut-brain modules in the mouse. Microbiome 2020, 8, 67. [Google Scholar] [CrossRef]
- Chen, H.-L.; Lan, Y.-W.; Tu, M.-Y.; Tung, Y.-T.; Chan, M.N.-Y.; Wu, H.-S.; Yen, C.-C.; Chen, C.-M. Kefir peptides exhibit antidepressant-like activity in mice through the BDNF/TrkB pathway. J. Dairy Sci. 2021, 104, 6415–6430. [Google Scholar] [CrossRef]
- Jang, C.; Oh, J.; Lim, J.; Kim, H.; Kim, J.-S. Fermented Soy Products: Beneficial Potential in Neurodegenerative Diseases. Foods 2021, 10, 636. [Google Scholar] [CrossRef]
- Cao, Z.-H.; Green-Johnson, J.M.; Buckley, N.D.; Lin, Q.-Y. Bioactivity of soy-based fermented foods: A review. Biotechnol. Adv. 2019, 37, 223–238. [Google Scholar] [CrossRef]
- Selhub, E.M.; Logan, A.C.; Bested, A.C. Fermented foods, microbiota, and mental health: Ancient practice meets nutritional psychiatry. J. Physiol. Anthr. 2014, 33, 2. [Google Scholar] [CrossRef]
- Perini, G.; Ramusino, M.C.; Sinforiani, E.; Bernini, S.; Petrachi, R.; Costa, A. Cognitive impairment in depression: Recent advances and novel treatments. Neuropsychiatr. Dis. Treat. 2019, 15, 1249. [Google Scholar] [CrossRef]
- Hwang, Y.-H.; Park, S.; Paik, J.-W.; Chae, S.-W.; Kim, D.-H.; Jeong, D.-G.; Ha, E.; Kim, M.; Hong, G.; Park, S.-H.; et al. Efficacy and Safety of Lactobacillus Plantarum C29-Fermented Soybean (DW2009) in Individuals with Mild Cognitive Impairment: A 12-Week, Multi-Center, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2019, 11, 305. [Google Scholar] [CrossRef]
- Ko, C.Y.; Lin, H.-T.V.; Tsai, G.J. Gamma-aminobutyric acid production in black soybean milk by Lactobacillus brevis FPA 3709 and the antidepressant effect of the fermented product on a forced swimming rat model. Process Biochem. 2013, 48, 559–568. [Google Scholar] [CrossRef]
- Lynch, K.M.; Wilkinson, S.; Daenen, L.; Arendt, E.K. An update on water kefir: Microbiology, composition and production. Int. J. Food Microbiol. 2021, 345, 109128. [Google Scholar] [CrossRef]
- de Miranda, J.F.; Ruiz, L.F.; Silva, C.B.; Uekane, T.M.; Silva, K.A.; Gonzalez, A.G.M.; Fernandes, F.F.; Lima, A.R. Kombucha: A review of substrates, regulations, composition, and biological properties. J. Food Sci. 2022, 87, 503–527. [Google Scholar] [CrossRef]
- Abaci, N.; Deniz, F.S.S.; Orhan, I.E. Kombucha—An ancient fermented beverage with desired bioactivities: A narrowed review. Food Chem. X 2022, 14, 100302. [Google Scholar] [CrossRef]
- Wang, P.; Feng, Z.; Sang, X.; Chen, W.; Zhang, X.; Xiao, J.; Chen, Y.; Chen, Q.; Yang, M.; Su, J. Kombucha ameliorates LPS-induced sepsis in a mouse model. Food Funct. 2021, 12, 10263–10280. [Google Scholar] [CrossRef]
- O’Connor, J.C.; Lawson, M.A.; André, C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 2009, 14, 511–522. [Google Scholar] [CrossRef]
- Kabiri, N.; Setorki, M. Protective effect of Kombucha tea on brain damage induced by transient cerebral ischemia and reperfusion in rat. Bangladesh J. Pharmacol. 2016, 11, 675–683. [Google Scholar] [CrossRef]
- Alvarez-Mon, M.A.; Ortega, M.A.; García-Montero, C.; Fraile-Martinez, O.; Lahera, G.; Monserrat, J.; Gomez-Lahoz, A.M.; Molero, P.; Gutierrez-Rojas, L.; Rodriguez-Jimenez, R.; et al. Differential malondialdehyde (MDA) detection in plasma samples of patients with major depressive disorder (MDD): A potential biomarker. J. Int. Med. Res. 2022, 50, 30006052210949. [Google Scholar] [CrossRef]
- Dudek, K.A.; Dion-Albert, L.; Lebel, M.; LeClair, K.; Labrecque, S.; Tuck, E.; Perez, C.F.; Golden, S.A.; Tamminga, C.; Turecki, G.; et al. Molecular Adaptations of the Blood–Brain Barrier Promote Stress Resilience vs. Depression. Proc. Natl. Acad. Sci. USA 2020, 117, 3326–3336. [Google Scholar] [CrossRef]
- Kapp, J.M.; Sumner, W. Kombucha: A systematic review of the empirical evidence of human health benefit. Ann. Epidemiol. 2019, 30, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.N.S.; Ryu, J.-K.; Kim, Y.; Jeon, B.H. The Effects of Fermented Laminaria japonica on Short-Term Working Memory and Physical Fitness in the Elderly. Evid. Based Complement. Altern. Med. 2018, 2018, 8109621. [Google Scholar] [CrossRef]
- Sarris, J.; Murphy, J.; Mischoulon, D.; Papakostas, G.I.; Fava, M.; Berk, M.; Ng, C.H. Adjunctive Nutraceuticals for Depression: A Systematic Review and Meta-Analyses. Am. J. Psychiatry 2016, 173, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Stubbs, B.; Solmi, M.; Ajnakina, O.; Carvalho, A.F.; Maggi, S. Acetyl-l-Carnitine Supplementation and the Treatment of Depressive Symptoms: A Systematic Review and Meta-Analysis. Psychosom. Med. 2018, 80, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Byrne, G.J.; Stough, C.; Bousman, C.; Mischoulon, D.; Murphy, J.; Macdonald, P.; Adams, L.; Nazareth, S.; Oliver, G.; et al. Nutraceuticals for major depressive disorder- more is not merrier: An 8-week double-blind, randomised, controlled trial. J. Affect. Disord. 2019, 245, 1007–1015. [Google Scholar] [CrossRef]
- Bot, M.; Brouwer, I.; Roca, M.; Kohls, E.; Penninx, B.W.J.H.; Watkins, E.; van Grootheest, G.; Cabout, M.; Hegerl, U.; Gili, M.; et al. Effect of Multinutrient Supplementation and Food-Related Behavioral Activation Therapy on Prevention of Major Depressive Disorder Among Overweight or Obese Adults with Subsyndromal Depressive Symptoms. JAMA 2019, 321, 858–868. [Google Scholar] [CrossRef]
- Haller, H.; Anheyer, D.; Cramer, H.; Dobos, G. Complementary therapies for clinical depression: An overview of systematic reviews. BMJ Open 2019, 9, e028527. [Google Scholar] [CrossRef]
- Ng, Q.X.; Venkatanarayanan, N.; Ho, C.Y.X. Clinical use of Hypericum perforatum (St John’s wort) in depression: A meta-analysis. J. Affect. Disord. 2017, 210, 211–221. [Google Scholar] [CrossRef]
- Kholghi, G.; Arjmandi-Rad, S.; Zarrindast, M.-R.; Vaseghi, S. St. John’s wort (Hypericum perforatum) and depression: What happens to the neurotransmitter systems? Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 629–642. [Google Scholar] [CrossRef]
- Zirak, N.; Shafiee, M.; Soltani, G.; Mirzaei, M.; Sahebkar, A. Hypericum perforatum in the treatment of psychiatric and neurodegenerative disorders: Current evidence and potential mechanisms of action. J. Cell. Physiol. 2019, 234, 8496–8508. [Google Scholar] [CrossRef]
- Liu, L.; Liu, C.; Wang, Y.; Wang, P.; Li, Y.; Li, B. Herbal Medicine for Anxiety, Depression and Insomnia. Curr. Neuropharmacol. 2015, 13, 481. [Google Scholar] [CrossRef]
- Silvers, K.M.; Woolley, C.C.; Hedderley, D. Dietary Supplement Use in People Being Treated for Depression. Asia Pac. J. Clin. Nutr. 2006, 15, 30–34. [Google Scholar]
- Booker, A.; Agapouda, A.; Frommenwiler, D.A.; Scotti, F.; Reich, E.; Heinrich, M. St John’s wort (Hypericum perforatum) products—An assessment of their authenticity and quality. Phytomedicine 2018, 40, 158–164. [Google Scholar] [CrossRef]
- Simon, L.V.; Keenaghan, M. Serotonin Syndrome. Pain Med. Essent. Rev. 2022, 11, 201–202. [Google Scholar]
- Patel, Y.A.; Marzella, N. Dietary Supplement-Drug Interaction-Induced Serotonin Syndrome Progressing to Acute Compartment Syndrome. Am. J. Case Rep. 2017, 18, 926. [Google Scholar] [CrossRef]
- Ronis, M.J.; Pedersen, K.B.; Watt, J. Adverse Effects of Nutraceuticals and Dietary Supplements. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 583–601. [Google Scholar] [CrossRef]
- Li, Y.; Lv, M.-R.; Wei, Y.-J.; Sun, L.; Zhang, J.-X.; Zhang, H.-G.; Li, B. Dietary patterns and depression risk: A meta-analysis. Psychiatry Res. 2017, 253, 373–382. [Google Scholar] [CrossRef]
- Tolkien, K.; Bradburn, S.; Murgatroyd, C. An anti-inflammatory diet as a potential intervention for depressive disorders: A systematic review and meta-analysis. Clin. Nutr. 2019, 38, 2045–2052. [Google Scholar] [CrossRef]
- Dominguez, L.; Di Bella, G.; Veronese, N.; Barbagallo, M. Impact of Mediterranean Diet on Chronic Non-Communicable Diseases and Longevity. Nutrients 2021, 13, 2028. [Google Scholar] [CrossRef]
- Arpón, A.; Milagro, F.I.; Razquin, C.; Corella, D.; Estruch, R.; Fitó, M.; Marti, A.; Martínez-González, M.A.; Ros, E.; Salas-Salvadó, J.; et al. Impact of Consuming Extra-Virgin Olive Oil or Nuts within a Mediterranean Diet on DNA Methylation in Peripheral White Blood Cells within the PREDIMED-Navarra Randomized Controlled Trial: A Role for Dietary Lipids. Nutrients 2017, 10, 15. [Google Scholar] [CrossRef]
- Sánchez-Villegas, A.; Álvarez-Pérez, J.; Toledo, E.; Salas-Salvadó, J.; Ortega-Azorín, C.; Zomeño, M.D.; Vioque, J.; Martínez, J.A.; Romaguera, D.; Pérez-López, J.; et al. Seafood Consumption, Omega-3 Fatty Acids Intake, and Life-Time Prevalence of Depression in the PREDIMED-Plus Trial. Nutrients 2018, 10, 2000. [Google Scholar] [CrossRef]
- Shafiei, F.; Moghaddam, A.S.; Larijani, B.; Esmaillzadeh, A. Adherence to the Mediterranean diet and risk of depression: A systematic review and updated meta-analysis of observational studies. Nutr. Rev. 2019, 77, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Psaltopoulou, T.; Sergentanis, T.N.; Panagiotakos, D.B.; Sergentanis, I.N.; Kosti, R.; Scarmeas, N. Mediterranean diet, stroke, cognitive impairment, and depression: A meta-analysis. Ann. Neurol. 2013, 74, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Hershey, M.S.; Sanchez-Villegas, A.; Sotos-Prieto, M.; Fernandez-Montero, A.; Pano, O.; Lahortiga-Ramos, F.; Martínez-González, M.; Ruiz-Canela, M. The Mediterranean Lifestyle and the Risk of Depression in Middle-Aged Adults. J. Nutr. 2022, 152, 227–234. [Google Scholar] [CrossRef]
- Jacka, F.N.; O’Neil, A.; Opie, R.; Itsiopoulos, C.; Cotton, S.; Mohebbi, M.; Castle, D.; Dash, S.; Mihalopoulos, C.; Chatterton, M.L.; et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 2017, 15, 23. [Google Scholar] [CrossRef]
- Opie, R.; O’Neil, A.; Jacka, F.N.; Pizzinga, J.; Itsiopoulos, C. A modified Mediterranean dietary intervention for adults with major depression: Dietary protocol and feasibility data from the SMILES trial. Nutr. Neurosci. 2018, 21, 487–501. [Google Scholar] [CrossRef]
- Foshati, S.; Ghanizadeh, A.; Akhlaghi, M. Extra-Virgin Olive Oil Improves Depression Symptoms Without Affecting Salivary Cortisol and Brain-Derived Neurotrophic Factor in Patients with Major Depression: A Double-Blind Randomized Controlled Trial. J. Acad. Nutr. Diet. 2022, 122, 284–297.e1. [Google Scholar] [CrossRef] [PubMed]
- Oh, R.; Gilani, B.; Uppaluri, K.R. Low Carbohydrate Diet. In StatPearls; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Dąbek, A.; Wojtala, M.; Pirola, L.; Balcerczyk, A. Modulation of Cellular Biochemistry, Epigenetics and Metabolomics by Ketone Bodies. Implications of the Ketogenic Diet in the Physiology of the Organism and Pathological States. Nutrients 2020, 12, 788. [Google Scholar] [CrossRef] [PubMed]
- Brietzke, E.; Mansur, R.B.; Subramaniapillai, M.; Balanzá-Martínez, V.; Vinberg, M.; González-Pinto, A.; Rosenblat, J.D.; Ho, R.; McIntyre, R.S. Ketogenic diet as a metabolic therapy for mood disorders: Evidence and developments. Neurosci. Biobehav. Rev. 2018, 94, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Winwood-Smith, H.S.; Franklin, C.E.; White, C.R. Low-carbohydrate diet induces metabolic depression: A possible mechanism to conserve glycogen. Am. J. Physiol. Integr. Comp. Physiol. 2017, 313, R347–R356. [Google Scholar] [CrossRef]
- Igwe, O.; Sone, M.; Matveychuk, D.; Baker, G.B.; Dursun, S.M. A review of effects of calorie restriction and fasting with potential relevance to depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 111, 110206. [Google Scholar] [CrossRef]
- Berthelot, E.; Etchecopar-Etchart, D.; Thellier, D.; Lancon, C.; Boyer, L.; Fond, G. Fasting Interventions for Stress, Anxiety and Depressive Symptoms: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3947. [Google Scholar] [CrossRef]
- Alcorta, A.; Porta, A.; Tárrega, A.; Alvarez, M.D.; Pilar Vaquero, M. Foods for Plant-Based Diets: Challenges and Innovations. Foods 2021, 10, 293. [Google Scholar] [CrossRef]
- Medawar, E.; Huhn, S.; Villringer, A.; Witte, A.V. The effects of plant-based diets on the body and the brain: A systematic review. Transl. Psychiatry 2019, 9, 226. [Google Scholar] [CrossRef]
- Tiffon, C. The Impact of Nutrition and Environmental Epigenetics on Human Health and Disease. Int. J. Mol. Sci. 2018, 19, 3425. [Google Scholar] [CrossRef]
- Iguacel, I.; Huybrechts, I.; Moreno, L.A.; Michels, N. Vegetarianism and veganism compared with mental health and cognitive outcomes: A systematic review and meta-analysis. Nutr. Rev. 2021, 79, 361–381. [Google Scholar] [CrossRef]
- Ocklenburg, S.; Borawski, J. Vegetarian diet and depression scores: A meta-analysis. J. Affect. Disord. 2021, 294, 813–815. [Google Scholar] [CrossRef]
- Guzek, D.; Gła¸bska, D.; Groele, B.; Gutkowska, K. Fruit and Vegetable Dietary Patterns and Mental Health in Women: A Systematic Review. Nutr. Rev. 2022, 80, 1357–1370. [Google Scholar] [CrossRef]
- Barnard, N.D.; Leroy, F. Children and adults should avoid consuming animal products to reduce risk for chronic disease: YES. Am. J. Clin. Nutr. 2020, 112, 926–930. [Google Scholar] [CrossRef]
- Lee, M.F.; Eather, R.; Best, T. Plant-based dietary quality and depressive symptoms in Australian vegans and vegetarians: A cross-sectional study. BMJ Nutr. Prev. Health 2021, 4, e000332. [Google Scholar] [CrossRef]
- Sangsefidi, Z.S.; Mirzaei, M.; Hosseinzadeh, M. The relation between dietary intakes and psychological disorders in Iranian adults: A population-based study. BMC Psychiatry 2020, 20, 257. [Google Scholar] [CrossRef]
- Dobersek, U.; Wy, G.; Adkins, J.; Altmeyer, S.; Krout, K.; Lavie, C.J.; Archer, E. Meat and mental health: A systematic review of meat abstention and depression, anxiety, and related phenomena. Crit. Rev. Food Sci. Nutr. 2021, 61, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Fogelholm, M.; Jalo, E.; Poppitt, S.D.; Silvestre, M.P.; Møller, G.; Huttunen-Lenz, M.; Stratton, G.; Sundvall, J.; Macdonald, I.A.; et al. Animal-based food choice and associations with long-term weight maintenance and metabolic health after a large and rapid weight loss: The PREVIEW study. Clin. Nutr. 2022, 41, 817–828. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).