Food Additives Associated with Gut Microbiota Alterations in Inflammatory Bowel Disease: Friends or Enemies?
Abstract
:1. Introduction
2. Artificial Sweeteners
2.1. Aspartame (E 951)
2.2. Acesulfame K (E 950)
| Food Additives | Study | Model | Sample and Gut Microbiota Alterations | Inflammatory Effects |
|---|---|---|---|---|
| Artificial sweeteners | ||||
| Ace-K | Hanawa et al., 2019 [25] | Mice | (F) Increase: Desulfovibrio in genus. | Induced the expression of inflammatory cytokines. |
| Sucralose | Wang et al., 2018 [8] | Rats with TNBS-induced colitis | (F) Increase: Proteobacteria and Bacteroidetes; Decrease: Firmicutes and Actinomycetes. | Exacerbated colitis, aggravated changes in colon length, MPO, TNF-α and IL-1β in gut tissue. |
| Sucralose | Li et al., 2020 [28] | Mice with DSS/AOM-induced colon cancer | (F) Increase: F. Actinomycetes, P. stomatis, C. symbiosum, P. anaerobius Decrease: Proteobacteria | Aggravation of colorectal tumors; induction of inflammatory cytokines and pathways (TNF-α, IL-1β, IL-6, IL-10). |
| Splenda | Rodriguez-Palacios et al., 2018 [29] | Ileitis-prone SAMP mice | (F) Increase: Proteobacteria and E. coli with increased bacterial infiltration into the lamina propria; malX gene–carrying bacterial | Increase MPO activity; no impact on the severity of ileitis. |
| Saccharin | Sünderhauf et al, 2020 [30] | Mice with DSS-induced colitis | (F) Influenced on β-diversity Increase: Bacteroidetes and Proteobacteria phylum Decrease: S. aureus, K. pneumonia and P. aeruginosa | Improved intestinal inflammation with less weight loss, lower DAI and histological score. |
| Sugar alcohols | ||||
| Lactitol | Wang et al., 2019 [31] | Mice with DSS-induced colitis | (F) Altered the α-diversity; increase: Akkermansia | Improved inflammation in acute colitis mice. |
| Coating and thickening agents | ||||
| MDX | Zangara et al., 2021 [32] | IL10 KO and NOD2 KO mice | (F) Decrease in α-diversity; altered β-diversity | Accelerated the onset of colitis; elevated intestinal infiltration of CD3+ cells and intestinal pathology; reduced mucin granule content. |
| MDX | Thymann et al., 2009 [33] | Pigs with NEC | (IC) Lower the bacterial diversity Increase: Pseudomonas spp., Streptococcus spp., Leuconostoc spp. Decrease: Weissella app | Induced higher incidence of NEC; reduced villus height. |
| MDX | Kourtney et al., 2009 [34] | Mice with Salmonella gastroenteritis | Enhances mucosal Salmonella colonization in vivo | Wrecked the intestinal antimicrobial barrier in vivo. Suppressed NAPDH oxidase expression; reduced recruitment of NADPH oxidase to Salmonella-containing vesicles, resulting in persistence of Salmonella in vesicles. |
| MDX | Kourtney et al., 2009 [35] | AIEC isolated from patients | MDX enhanced AIEC specific biofilm formation | Induced type I pili expression; increased bacterial adhesion to intestinal epithelial. |
| Emulsifiers | ||||
| P80 | Hirotaka et al., 2019 [36] | Mice with indomethacin-induced colitis | (IC) Decreased the α-diversity in the small intestine Increased: Gammaproteobacteria and P. mirabilis | Exacerbated colitis; increased the interleukin-1β expression. Antibiotic pretreatment abolished this effect. |
| P80 | Roberts et al., 2010 [37] | E coli isolates from patients | - | Increased the translocation of E coli across M epithelial cells. |
| CMC | Zangara et al., 2021 [32] | IL10 KO mice and NOD2 KO mice | (F) Flagella expression by microbes was elevated | Accelerated the onset of colitis; elevated intestinal infiltration of CD3+ cells and intestinal pathology; reduced mucin granule content. |
| CMC | Swidsinski et al., 2011 [38] | IL10 KO mice | (Intestinal mucosa) Bacterial overgrowth | Distention of spaces between villi, with bacteria filling these spaces, adherence of bacteria to the mucosa and migration of bacteria to the bottom of the crypts. |
| CMC and P80 | Chassaing et al., 2017 [39] | M-SHIME; ASF and GF mice | In vitro: influenced on diversity and composition (F) Increase in inflammation-related bacteria, decreased health-associated bacteria | Promoted low-grade gut inflammation. |
| CMC and P80 | Chassaing et al., 2015 [11] | Wildtype, IL10 KO and TLR5 KO mice; | (F) Induced a reduction in microbial diversity Increase: Verrucomicrobia phylum, A. muciniphila, Proteobacteria | Induced low-grade intestinal inflammation and promoted robust colitis. |
| CMC and P80 | Viennois et al., 2020 [10] | IL10 KO and ASF/GF mice; DSS-induced colitis | - | Induced chronic intestinal inflammation and metabolism dysregulations, especially in IL10 KO. |
| Carrageenan | Li et al., 2014 [40] | GF mice | GF mice inoculated with B. xylanisolvens 38F6A4 or E. coli 38F6C1 | Increased intestinal permeability and was related to the onset of colitis. |
| Carrageenan | Shang et al., 2017 [41] | Mice | (CC) Decrease: A.muciniphila | Induced low-grade colitis. |
| Carrageenan | Ye et al., 2020 [42] | Mice with HFD induced-colitis | (F) Increase: A. finegoldii and B. acidifaciens | Aggravated intestinal inflammation in colitis mice. |
| Carrageenan | Wu et al., 2017 [43] | Mice with Citrobacter freundii DBS100-induced colitis | - | Aggravated intestinal inflammation in colitis mice. |
| Carrageenan | End et al., 2009 [44] | Mice with DSS-induced colitis | Inhibits the bacterial aggregating function of DMBT1 | Disrupts the mucosal protection provided by DMBT1. |
| Carrageenan | Munyaka et al., 2016 [45] | Mice inoculated with AIEC | (IC) Decreased bacterial richness and composition Increase: Proteobacteria and Deferribacteres Decrease: Firmicutes, Actinobacteria, Bacteroidetes | Induced colitis in mice. |
| Carrageenan | Onderdonk et al., 1978 [46] | Guinea pigs | - | Induced the cecal ulcerations; no effect on GF pigs. |
| Carrageenan | Onderdonk et al., 1983 [47] | Guinea pigs; GF mice | - | Inoculated with B. vulgatus developed cecal ulcerations. |
| GML | Mo et al., 2019 [48] | Mice | (F) Increase: Barnesiella; Clostridium XIVa, Oscillibacter, Parasutterella | Maintained intestine barrier; promoted anti-inflammatory environment. |
| GML | Zhao et al., 2020 [49] | Mice with HFD | (F) Increase: Bifidobacterium pseudolongum | Ameliorated the metabolic disorders and gut inflammation. |
| GML | Zhao et al., 2019 [50] | Mice with HFD | (F) GML ameliorates gut microbiota dysbiosis Increase: B. uniformis, Akkermansia, Bifidobacterium, Lactobacillus Decrease: E. coli, Lactococcus, Flexispira | Ameliorates metabolic disorders and reduced serum TNF-α. |
| GML | Mo et al., 2021 [51] | Mice with DSS-induced colitis | (F) Increase: Lactobacillus and Bifidobacterium Decrease: Helicobacter ganmani | Improved colitis in mice. |
| Food colorants | ||||
| TiO2 | Cao et al., 2020 [52] | Mice with HFD | (F)Increase: Firmicutes Decrease: Bacteroidetes, Bifidobacterium, Lactobacillus | Induced strong colonic inflammation, especially in obese mice. |
| TiO2 | Zhu et al., 2021 [53] | Mice with HFD | (F) Increase: Firmicutes; Decrease: Bacteroidete | Escalated the low-grade inflammation induced by HFD through gut microbiome; disrupted mucus layer. |
| TiO2 | Yan et al., 2020 [54] | Mice | (CC) Decrease: Akkermansia, Barnesiella, Bacteroides Increase: Barnesiella | Caused intestinal inflammation; reduced intestinal mucus barrier. |
| TiO2 | Kurtz et al., 2020 [55] | Mice | (CC) Affected the colonization of mucosa-associated bacteria | Elicits an inflammatory response in ileum. |
| TiO2 | Chen et al., 2019 [56] | Rats | (F) Increase: L. gasseri, Turicibacter, L. NK4A136 group Decrease: Veillonella | Induced inflammatory infiltration and mitochondrial abnormalities. |
| TiO2 | Pinget et al., 2019 [57] | Mice | (F) Promoted biofilm formation by E. faecalis or E. coli | Wrecked the gut barrier and induced gut inflammation. |
| TiO2 | Mu et al., 2019 [58] | Mice with DSS-induced colitis | (F) Affected the diversity Decrease: Bifidobacterium, Lactobacillus | Induced intestinal inflammation; aggravated colitis. |
| TiO2 | Chen et al., 2017 [59] | Mice with DSS-induced colitis | (F) No influence | No influence. |
| Azo dyes | He et al., 2021 [60] | GF, Rag1-/-and R23FR mice | (F) No influence on bacterial composition. | Red 40 and ANSA-Na promoted colitis. |
| Azo dyes | Wu et al., 2021 [61] | Crucian carp | (IC) Increase: Bdellovibrio Shewanella Decrease: Roseomonas, Rhodococcu, Bacillus, Bacteroides, Clostridium | Induced the oxidative stress; elicited a tendency to gut inflammation. |
| Food preservatives | ||||
| Mixture | Hrncirova et al., 2019 [62] | Wildtype, NOD2 KO mice | (F) Increase: Proteobacteria phylum Decrease: Clostridiales order | Dysbiosis was induced, especially in the NOD2 KO mice. |
| Sulfite | Schooth et al., 2020 [63] | P. mirabilis, M. morganii, E. fergusonii, K. pneumoniae | Reduced the growth rate of all strains. | Influenced the growth kinetics of Crohn’s disease pathobionts, which may initiate and promote disease. |
| TCS | Yang et al., 2018 [64] | Mice with DSS-in duced colitis; IL10 KO mice | (F) Lower the α- and β-diversity Increase: Firmicutes Decrease: Bacteroidetes, Actinomycetes, Cyanobacteria | Induced low-grade colonic inflammation, increased colitis, and exacerbated colitis-associated colon cancer in mice |
| Food Additives | Study | Sample | Metabolite Alterations | ||
|---|---|---|---|---|---|
| Increase | Decrease | ||||
| Artificial sweeteners | Aspartame | Gerasimidis et al., 2021 [17] | F | Total SCFAs, acetic acid, propionic acid, caprylic acid | Valeric acid, caproic acid; BCFAs (such as isobutyric acid, isovaleric acid) |
| Palmnäs et al., 2014 [18] | S | Propionate, acetate and butyrate | - | ||
| Jodi et al., 2020 [19] | CC | Propionate, butyrate and isobutyrate | - | ||
| Sucralose | Uebanso et al., 2017 [22] | CC | The CA/CDCA ratio | - | |
| V amanu et al., 2019 [65] | F | Ammonium, formic acid, phenyllactic acid, HO-phenyllactic acid; butyric acid | Benzoic acid | ||
| Saccharin | V amanu et al., 2019 [65] | F | Ammonium, formic acid, phenyllactic acid, HO-phenyllactic acid; acetic and butyric acid | Benzoic acid, propionic acid | |
| Suez et al., 2014 [66] | F | Propionate and acetate | - | ||
| Bian et al., 2017 [67] | F | Daidzein, dihydrodaidzein and O-desmethylangolensin; quinolinic acid | Equol, linoleoyl, ethanolamide, N, N-Dimethylsphingosine | ||
| Neotame | Liang et al., 2018 [68] | F | Cholesterol, campesterol and stigmastanol | Malic acid, mannose-6-phosphate, 5-aminovaleric acid and glyceric acid; 1, 3-dipalmitate, 1-monopalmitin, linoleic acid and stearic acid | |
| Cyclamate | V amanu et al., 2019 [65] | F | Formic aid, phenyllactic acid, HO-phenyllactic acid; acetic acid | Benzoic acid, propionic acid | |
| Splenda | Karley et al., 2019 [69] | F | Butyric and pentanoic acid | - | |
| Sugar alcohols | Isomalt | Gostner et al., 2016 [70] | F | No influence on SCFAs, lactate, bile acids and neutral sterols. | |
| Lactitol | Chu et al., 2019 [71] | F | No influence on SCFAs. | ||
| Ballongue et al., 2016 [72] | F | Acetic acid, lactic acids | Propionic, butyric and valeric acids | ||
| Finney et al., 2007 [73] | F | Propionic and butyric acids | Acetic acid, lactic acids | ||
| Peuranen et al., 2004 [74] | F | Butyrate | - | ||
| Pinna et al., 2014 [75] | IC | Putrescine | The acetic acid to propionic acid ratio | ||
| Coating and thickening agents | MDX | Gerasimidis et al., 2020 [17] | F | Total SCFAs, propionic acid; caprylic acid | Valeric acid, caproic acid; isobutyric and isovaleric acid |
| Thymann et al., 2009 [33] | IC | Formic acid, acetic acid, butyric acid | Lactic acid, succinic acid | ||
| Kong et al., 2020 [76] | F | Total SCFAs, acetate, butyrate and valerate | - | ||
| Emulsifiers | P80 | Chassaing et al., 2015 [11] | F | Flagellin | - |
| CMC | Chassaing et al., 2017 [39] | F | Butyrate; LCA, HDCA/UDCA, αMCA, GLCA, TCDCA, TDCA, THDCA/TUDCA, TCA | - | |
| Chassaing et al., 2015 [11] | F | Butyrate, heptanoate; αMCA | - | ||
| Gerasimidis et al., 2020 [17] | F | - | Isovaleric acid | ||
| Carrageenan | Gerasimidis et al., 2020 [17] | F | No influence on SCFAs or BCFAs. | ||
| Munyaka et al., 2016 [45] | CC | - | Butyric and acetic acid | ||
| Food colorants | TiO2 | Cao et al., 2020 [52] | CC | - | Butyric and propionic acid; acetic and isovaleric acids in obese mice |
| Chen et al., 2019 [56] | F | N-acetylhistamine, caprolactam and glycerophosphocholine | 4-methyl-5-thiazoleethanol, L-histidine and L-ornithine | ||
| Pinget et al., 2019 [57] | S | - | SCFAs | ||
| Waller et al., 2017 [77] | CC | - | pH level | ||
| Agans et al., 2019 [78] | F | No influence on SCFAs. | |||
| Dudefoi et al., 2017 [79] | F | No influence on overall fatty acid compositions. | |||
| Gerasimidis et al., 2021 [17] | M | No influence on SCFAs or BCFAs. | |||
| Azo dyes | Polic et al., 2018 [80] | M | - | Acetate, butyrate and propionate | |
| Chen et al., 2009 [81] | M | Metabolites of Sudan III and IV, aniline and o-toluidine (2-methylaniline) were carcinogenic aromatic amines | |||
| Pan el al, 2012 [82] | M | 1-Amino-2-naphthol, a common metabolite of the dyes, was capable of inhibiting growth of most of the tested bacteria | |||
| Preservatives | Benzoic acid | Torrallardona et al., 2007 [83] | U | Hippuric acid | - |
| Kluge et al., 2005 [84] | IC | - | Acetic acid | ||
| Diao et al., 2013 [85] | CC | Propionic acid and total volatile fatty acid | NH3–N | ||
| Diao et al., 2014 [86] | CC | Butyric acid | - | ||
| Ag NPs | Cueva et al., 2019 [87] | F | Ammonium | - | |
| Antioxidant | Rosemary extract | Romo-Vaquero et al., 2014 [88] | F | SCFAs (acetic, propionic and butyric acid) in obese mice | SCFAs in lean mice |
2.3. Sucralose (E 950)
2.4. Saccharin (E 954)
2.5. Neotame (E 961)
2.6. Cyclamate (E 952)
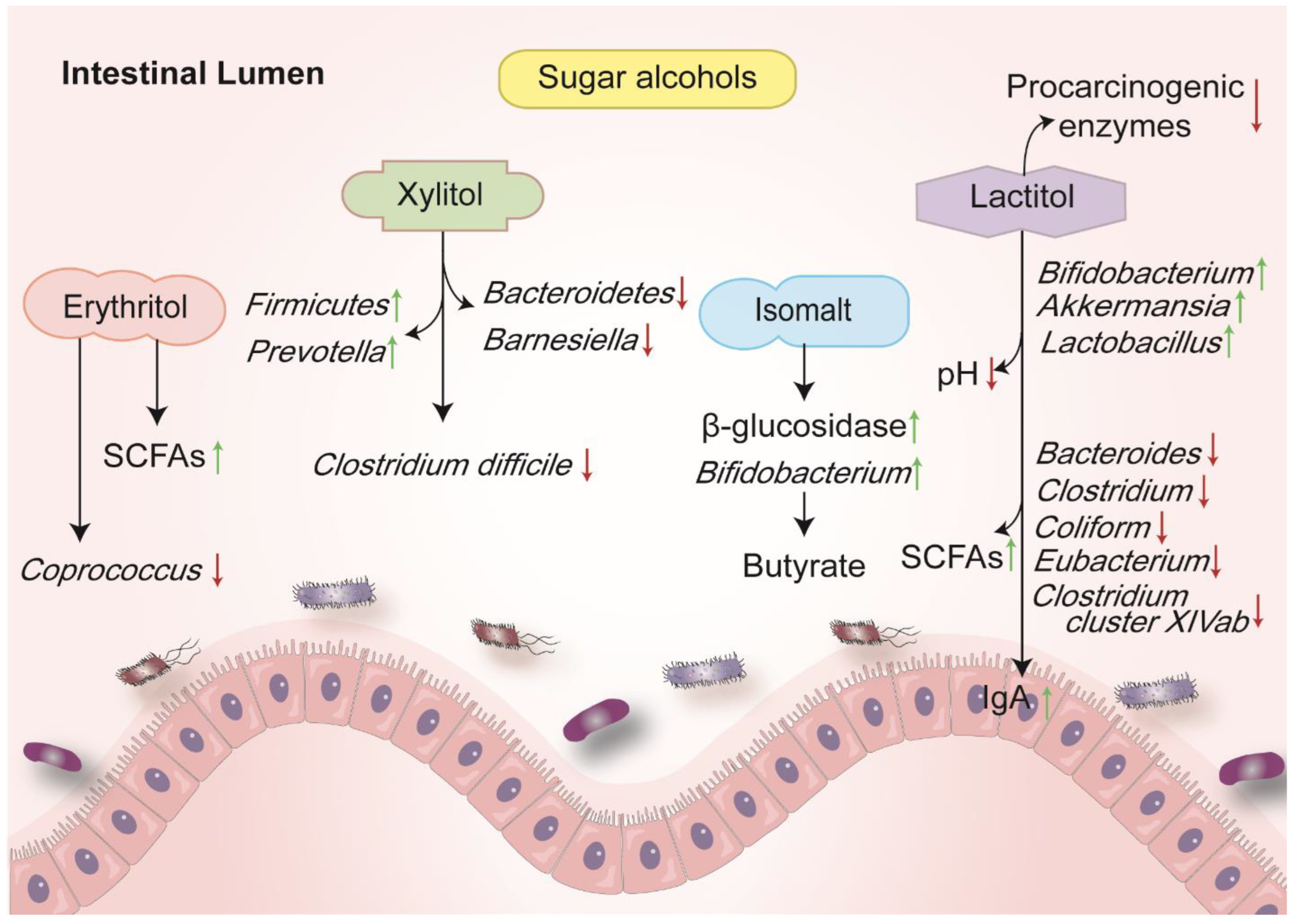
3. Sugar Alcohols
3.1. Erythritol (E 968)
3.2. Isomalt (E 953)
3.3. Xylitol (E 967)
3.4. Lactitol (E 966)
4. Coating and Thickening Agents
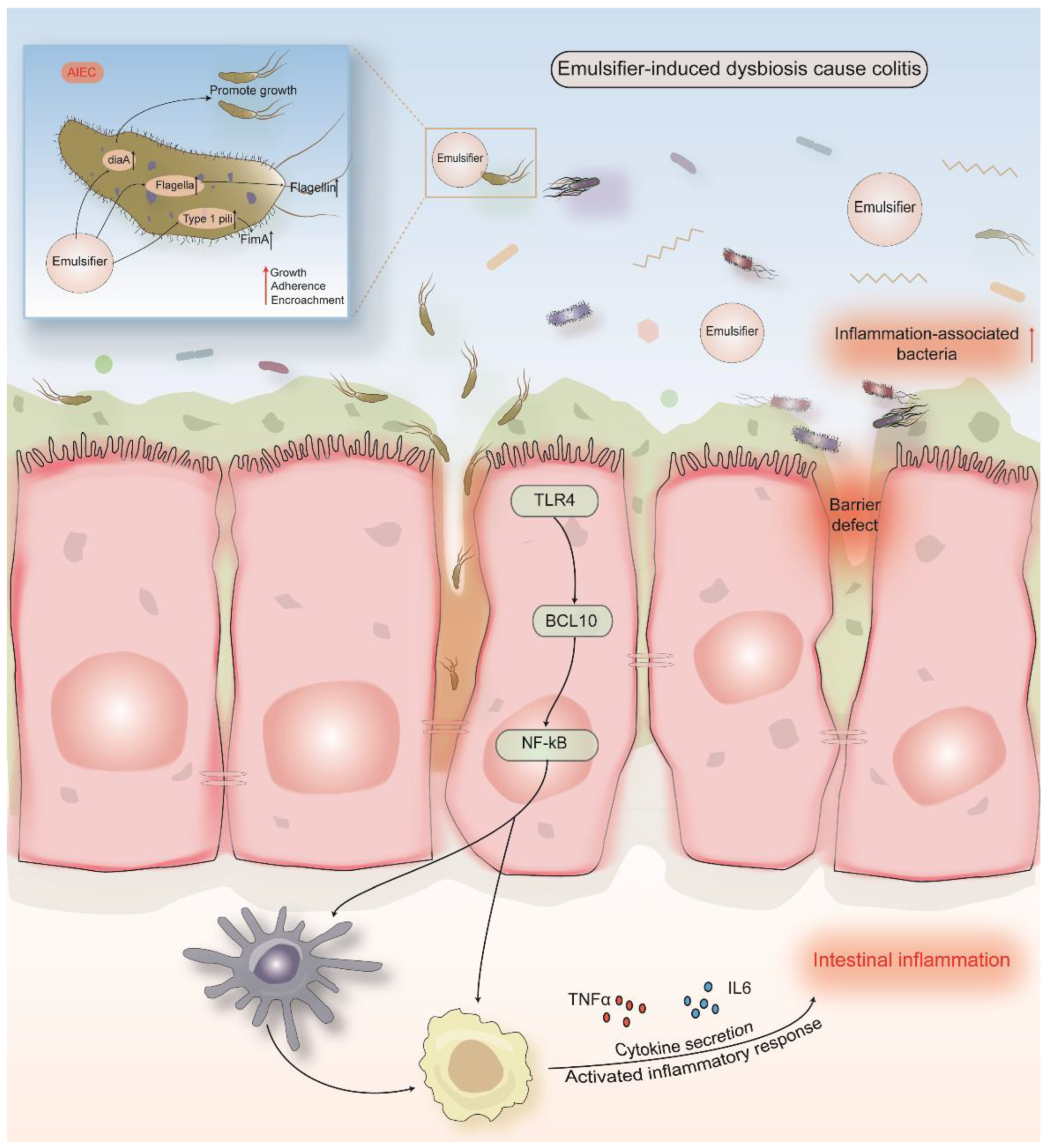
5. Emulsifiers
5.1. Carboxymethylcellulose (E 466) and Polysorbate 80 (E 433)
5.2. Carrageenan (E 407)
5.3. Glycerol Monolaurate
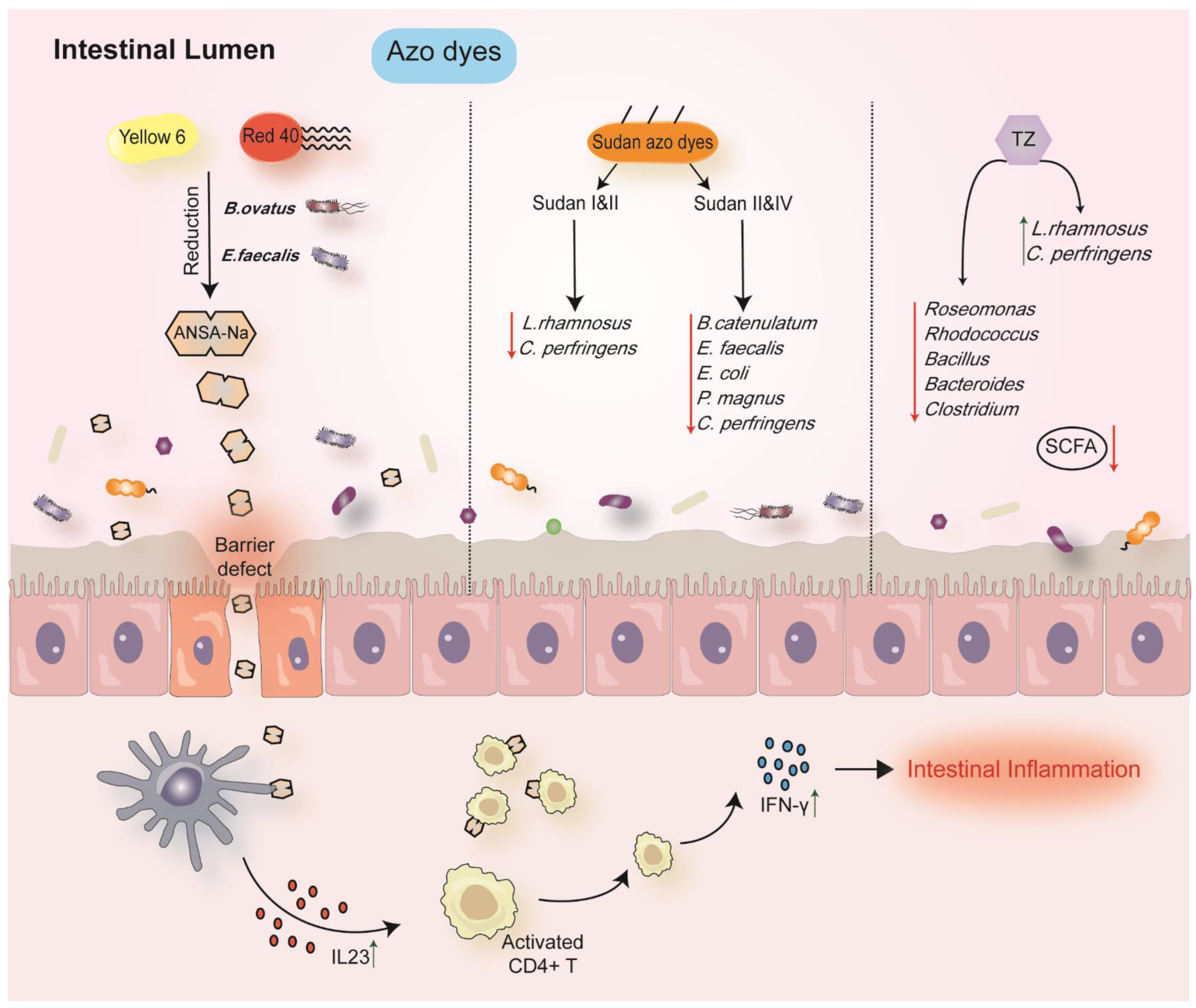
6. Food Colorants
6.1. Titanium Dioxide (E 171)
6.2. Azo Dyes
7. Preservatives
7.1. Benzoic Acid and Sodium Benzoate (E210-213)
7.2. Potassium Sorbate (E 202)
7.3. Sulfites (E 211)
7.4. Ag NPs (E 174)
7.5. Other Preservatives
8. Antioxidants
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Statement of Significance
References
- Hodson, R. Inflammatory bowel disease. Nature 2016, 540, S97. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef] [PubMed]
- Owczarek, D.; Rodacki, T.; Domagała-Rodacka, R.; Cibor, D.; Mach, T. Diet and nutritional factors in inflammatory bowel diseases. World J. Gastroenterol. 2016, 22, 895–905. [Google Scholar] [CrossRef]
- Campmans-Kuijpers, M.J.E.; Dijkstra, G. Food and Food Groups in Inflammatory Bowel Disease (IBD): The Design of the Groningen Anti-Inflammatory Diet (GrAID). Nutrients 2021, 13, 1067. [Google Scholar] [CrossRef] [PubMed]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berglund, F. Food Additives. In Toxicological Aspects of Food Safety; Springer: Berlin/Heidelberg, Germany, 1978; Volume 1, pp. 33–46. [Google Scholar]
- Food Ingredients and Colors. Available online: https://www.fda.gov/food/food-ingredients-packaging/overview-food-ingredients-additives-colors (accessed on 26 February 2022).
- Wang, X.; Guo, J.; Liu, Y.; Yu, H.; Qin, X. Sucralose Increased Susceptibility to Colitis in Rats. Inflamm. Bowel Dis. 2019, 25, e3–e4. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019, 4, 293–305. [Google Scholar] [CrossRef]
- Viennois, E.; Bretin, A.; Dubé, P.E.; Maue, A.C.; Dauriat, C.J.G.; Barnich, N.; Gewirtz, A.T.; Chassaing, B. Dietary Emulsifiers Directly Impact Adherent-Invasive E. coli Gene Expression to Drive Chronic Intestinal Inflammation. Cell Rep. 2020, 33, 108229. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef] [Green Version]
- The European Parliament and the Council of the European Union. Regulation (EC) No 1333/2008 of the European Parliament and of the Council. Off. J. Eur. Union 2008, 81, 16–33. [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Re-evaluation of fatty acids (E 570) as a food additive. EFSA J. 2017, 15, e04785. [Google Scholar] [PubMed] [Green Version]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of aspartame (E 951) as a food additive. EFSA J. 2013, 11, 3496. [Google Scholar]
- Frankenfeld, C.L.; Sikaroodi, M.; Lamb, E.; Shoemaker, S.; Gillevet, P.M. High-intensity sweetener consumption and gut microbiome content and predicted gene function in a cross-sectional study of adults in the United States. Ann. Epidemiol. 2015, 25, 736–742.e4. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.Y.; Friel, J.; Mackay, D. The Effects of Non-Nutritive Artificial Sweeteners, Aspartame and Sucralose, on the Gut Microbiome in Healthy Adults: Secondary Outcomes of a Randomized Double-Blinded Crossover Clinical Trial. Nutrients 2020, 12, 3408. [Google Scholar] [CrossRef] [PubMed]
- Gerasimidis, K.; Bryden, K.; Chen, X.; Papachristou, E.; Verney, A.; Roig, M.; Hansen, R.; Nichols, B.; Papadopoulou, R.; Parrett, A. The impact of food additives, artificial sweeteners and domestic hygiene products on the human gut microbiome and its fibre fermentation capacity. Eur. J. Nutr. 2020, 59, 3213–3230. [Google Scholar] [CrossRef] [Green Version]
- Palmnäs, M.S.; Cowan, T.E.; Bomhof, M.R.; Su, J.; Reimer, R.A.; Vogel, H.J.; Hittel, D.S.; Shearer, J. Low-dose aspartame consumption differentially affects gut microbiota-host metabolic interactions in the diet-induced obese rat. PLoS ONE 2014, 9, e109841. [Google Scholar] [CrossRef]
- Nettleton, J.E.; Cho, N.A.; Klancic, T.; Nicolucci, A.C.; Shearer, J.; Borgland, S.L.; Johnston, L.A.; Ramay, H.R.; Noye Tuplin, E.; Chleilat, F.; et al. Maternal low-dose aspartame and stevia consumption with an obesogenic diet alters metabolism, gut microbiota and mesolimbic reward system in rat dams and their offspring. Gut 2020, 69, 1807–1817. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, R.; Shehreen, S.; Shahriar, S.; Rahman, M.S.; Akhteruzzaman, S.; Sajib, A.A. Non-Caloric Artificial Sweeteners Modulate the Expression of Key Metabolic Genes in the Omnipresent Gut Microbe Escherichia coli. J. Mol. Microbiol. Biotechnol. 2019, 29, 43–56. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Safety of the proposed extension of use of acesulfame K (E 950) in foods for special medical purposes in young children. EFSA J. 2016, 14, 4437. [Google Scholar]
- Uebanso, T.; Ohnishi, A.; Kitayama, R.; Yoshimoto, A.; Nakahashi, M.; Shimohata, T.; Mawatari, K.; Takahashi, A. Effects of Low-Dose Non-Caloric Sweetener Consumption on Gut Microbiota in Mice. Nutrients 2017, 9, 560. [Google Scholar] [CrossRef] [Green Version]
- Bian, X.; Chi, L.; Gao, B.; Tu, P.; Ru, H.; Lu, K. The artificial sweetener acesulfame potassium affects the gut microbiome and body weight gain in CD-1 mice. PLoS ONE 2017, 12, e0178426. [Google Scholar] [CrossRef]
- Olivier-Van Stichelen, S.; Rother, K.I.; Hanover, J.A. Maternal Exposure to Non-nutritive Sweeteners Impacts Progeny’s Metabolism and Microbiome. Front. Microbiol. 2019, 10, 1360. [Google Scholar] [CrossRef] [Green Version]
- Hanawa, Y.; Higashiyama, M.; Sugihara, N.; Wada, A.; Inaba, K.; Horiuchi, K.; Furuhashi, H.; Kurihara, C.; Okada, Y.; Shibuya, N.; et al. Su1775—Artificial Sweetener Acesulfame Potassium Enhanced Lymphocyte Migration to Intestinal Microvessels by Enhancing Expression of Adhesion Molecules Through Dysbiosis. Gastroenterology 2019, 156, S-606. [Google Scholar] [CrossRef]
- Wang, Q.P.; Browman, D.; Herzog, H.; Neely, G.G. Non-nutritive sweeteners possess a bacteriostatic effect and alter gut microbiota in mice. PLoS ONE 2018, 13, e0199080. [Google Scholar] [CrossRef]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Wang, Y.; Li, X.; Liu, X.; Guo, M.; Tan, Y.; Qin, X.; Wang, X.; Jiang, M. Sucralose Promotes Colitis-Associated Colorectal Cancer Risk in a Murine Model along with Changes in Microbiota. Front. Oncol. 2020, 10, 710. [Google Scholar] [CrossRef]
- Rodriguez-Palacios, A.; Harding, A.; Menghini, P.; Himmelman, C.; Retuerto, M.; Nickerson, K.P.; Lam, M.; Croniger, C.M.; McLean, M.H.; Durum, S.K.; et al. The Artificial Sweetener Splenda Promotes Gut Proteobacteria, Dysbiosis, and Myeloperoxidase Reactivity in Crohn’s Disease-Like Ileitis. Inflamm. Bowel Dis. 2018, 24, 1005–1020. [Google Scholar] [CrossRef]
- Sünderhauf, A.; Pagel, R.; Künstner, A.; Wagner, A.E.; Rupp, J.; Ibrahim, S.M.; Derer, S.; Sina, C. Saccharin Supplementation Inhibits Bacterial Growth and Reduces Experimental Colitis in Mice. Nutrients 2020, 12, 1122. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.N.; Meng, X.C.; Dong, Y.F.; Zhao, X.H.; Qian, J.M.; Wang, H.Y.; Li, J.N. Effects of probiotics and prebiotics on intestinal microbiota in mice with acute colitis based on 16S rRNA gene sequencing. Chin. Med. J. 2019, 132, 1833–1842. [Google Scholar] [CrossRef]
- Zangara, M.; Sangwan, N.; Mcdonald, C.J.G. Common Food Additives Accelerate Onset of Inflammatory Bowel Disease in Mice by Altering Microbiome Composition and Host-Microbe Interaction. Gastroenterology 2021, 160, S53. [Google Scholar] [CrossRef]
- Thymann, T.; Møller, H.K.; Stoll, B.; Støy, A.C.; Buddington, R.K.; Bering, S.B.; Jensen, B.B.; Olutoye, O.O.; Siggers, R.H.; Mølbak, L.; et al. Carbohydrate maldigestion induces necrotizing enterocolitis in preterm pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G1115–G1125. [Google Scholar] [CrossRef]
- Nickerson, K.P.; Homer, C.R.; Kessler, S.P.; Dixon, L.J.; Kabi, A.; Gordon, I.O.; Johnson, E.E.; de la Motte, C.A.; McDonald, C. The dietary polysaccharide maltodextrin promotes Salmonella survival and mucosal colonization in mice. PLoS ONE 2014, 9, e101789. [Google Scholar] [CrossRef] [Green Version]
- Nickerson, K.P.; McDonald, C. Crohn’s disease-associated adherent-invasive Escherichia coli adhesion is enhanced by exposure to the ubiquitous dietary polysaccharide maltodextrin. PLoS ONE 2012, 7, e52132. [Google Scholar] [CrossRef] [Green Version]
- Furuhashi, H.; Higashiyama, M.; Okada, Y.; Kurihara, C.; Wada, A.; Horiuchi, K.; Hanawa, Y.; Mizoguchi, A.; Nishii, S.; Inaba, K.; et al. Dietary emulsifier polysorbate-80-induced small-intestinal vulnerability to indomethacin-induced lesions via dysbiosis. J. Gastroenterol. Hepatol. 2020, 35, 110–117. [Google Scholar] [CrossRef]
- Roberts, C.L.; Keita, A.V.; Duncan, S.H.; O’Kennedy, N.; Söderholm, J.D.; Rhodes, J.M.; Campbell, B.J. Translocation of Crohn’s disease Escherichia coli across M-cells: Contrasting effects of soluble plant fibres and emulsifiers. Gut 2010, 59, 1331–1339. [Google Scholar] [CrossRef] [Green Version]
- Swidsinski, A.; Ung, V.; Sydora, B.C.; Loening-Baucke, V.; Doerffel, Y.; Verstraelen, H.; Fedorak, R.N. Bacterial overgrowth and inflammation of small intestine after carboxymethylcellulose ingestion in genetically susceptible mice. Inflamm. Bowel Dis. 2009, 15, 359–364. [Google Scholar] [CrossRef]
- Chassaing, B.; Van de Wiele, T.; De Bodt, J.; Marzorati, M.; Gewirtz, A.T. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut 2017, 66, 1414–1427. [Google Scholar] [CrossRef]
- Li, M. The Degradation and Utilization Study of Agarose, κ-Carrageenan and Their Oligosaccharides by Human Gut Microbiota. Ph.D. Thesis, Ocean University of China, Qingdao, China, 2014. [Google Scholar]
- Shang, Q.; Sun, W.; Shan, X.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Carrageenan-induced colitis is associated with decreased population of anti-inflammatory bacterium, Akkermansia muciniphila, in the gut microbiota of C57BL/6J mice. Toxicol. Lett. 2017, 279, 87–95. [Google Scholar] [CrossRef]
- Mi, Y.; Chin, Y.X.; Cao, W.X.; Chang, Y.G.; Lim, P.E.; Xue, C.H.; Tang, Q.J. Native κ-carrageenan induced-colitis is related to host intestinal microecology. Int. J. Biol. Macromol. 2020, 147, 284–294. [Google Scholar] [CrossRef]
- Wu, W.; Zhen, Z.; Niu, T.; Zhu, X.; Gao, Y.; Yan, J.; Chen, Y.; Yan, X.; Chen, H. κ-Carrageenan Enhances Lipopolysaccharide-Induced Interleukin-8 Secretion by Stimulating the Bcl10-NF-κB Pathway in HT-29 Cells and Aggravates C. freundii-Induced Inflammation in Mice. Mediat. Inflamm. 2017, 2017, 8634865. [Google Scholar] [CrossRef] [Green Version]
- End, C.; Bikker, F.; Renner, M.; Bergmann, G.; Lyer, S.; Blaich, S.; Hudler, M.; Helmke, B.; Gassler, N.; Autschbach, F.; et al. DMBT1 functions as pattern-recognition molecule for poly-sulfated and poly-phosphorylated ligands. Eur. J. Immunol. 2009, 39, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Munyaka, P.M.; Sepehri, S.; Ghia, J.E.; Khafipour, E. Carrageenan Gum and Adherent Invasive Escherichia coli in a Piglet Model of Inflammatory Bowel Disease: Impact on Intestinal Mucosa-associated Microbiota. Front. Microbiol. 2016, 7, 462. [Google Scholar] [CrossRef] [PubMed]
- Onderdonk, A.B.; Hermos, J.A.; Dzink, J.L.; Bartlett, J.G. Protective effect of metronidazole in experimental ulcerative colitis. Gastroenterology 1978, 74, 521–526. [Google Scholar] [CrossRef]
- Onderdonk, A.B.; Cisneros, R.L.; Bronson, R.T. Enhancement of experimental ulcerative colitis by immunization with Bacteroides vulgatus. Infect. Immun. 1983, 42, 783–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, Q.; Fu, A.; Deng, L.; Zhao, M.; Li, Y.; Zhang, H.; Feng, F. High-dose Glycerol Monolaurate Up-Regulated Beneficial Indigenous Microbiota without Inducing Metabolic Dysfunction and Systemic Inflammation: New Insights into Its Antimicrobial Potential. Nutrients 2019, 11, 1981. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Jiang, Z.; Cai, H.; Li, Y.; Mo, Q.; Deng, L.; Zhong, H.; Liu, T.; Zhang, H.; Kang, J.X.; et al. Modulation of the Gut Microbiota during High-Dose Glycerol Monolaurate-Mediated Amelioration of Obesity in Mice Fed a High-Fat Diet. mBio 2020, 11, e00190-20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; Cai, H.; Jiang, Z.; Li, Y.; Zhong, H.; Zhang, H.; Feng, F. Glycerol-Monolaurate-Mediated Attenuation of Metabolic Syndrome is Associated with the Modulation of Gut Microbiota in High-Fat-Diet-Fed Mice. Mol. Nutr. Food Res. 2019, 63, e1801417. [Google Scholar] [CrossRef]
- Mo, Q.; Liu, T.; Fu, A.; Ruan, S.; Zhong, H.; Tang, J.; Zhao, M.; Li, Y.; Zhu, S.; Cai, H.; et al. Novel Gut Microbiota Patterns Involved in the Attenuation of Dextran Sodium Sulfate-Induced Mouse Colitis Mediated by Glycerol Monolaurate via Inducing Anti-inflammatory Responses. mBio 2021, 12, e0214821. [Google Scholar] [CrossRef]
- Cao, X.; Han, Y.; Gu, M.; Du, H.; Song, M.; Zhu, X.; Ma, G.; Pan, C.; Wang, W.; Zhao, E.; et al. Foodborne Titanium Dioxide Nanoparticles Induce Stronger Adverse Effects in Obese Mice than Non-Obese Mice: Gut Microbiota Dysbiosis, Colonic Inflammation, and Proteome Alterations. Small 2020, 16, e2001858. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, L.; Liu, Z.; Zhou, Q.; Zhu, Y.; Zhao, Y.; Yang, X. Long-term exposure to titanium dioxide nanoparticles promotes diet-induced obesity through exacerbating intestinal mucus layer damage and microbiota dysbiosis. Nano Res. 2021, 14, 1512–1522. [Google Scholar] [CrossRef]
- Yan, J.; Wang, D.; Li, K.; Chen, Q.; Lai, W.; Tian, L.; Lin, B.; Tan, Y.; Liu, X.; Xi, Z. Toxic effects of the food additives titanium dioxide and silica on the murine intestinal tract: Mechanisms related to intestinal barrier dysfunction involved by gut microbiota. Environ. Toxicol. Pharmacol. 2020, 80, 103485. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, C.C.; Mitchell, S.; Nielsen, K.; Crawford, K.D.; Mueller-Spitz, S.R. Acute high-dose titanium dioxide nanoparticle exposure alters gastrointestinal homeostasis in mice. J. Appl. Toxicol. JAT 2020, 40, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, S.; Zhou, D.; Zhou, S.; Jia, G. Effects of oral exposure to titanium dioxide nanoparticles on gut microbiota and gut-associated metabolism in vivo. Nanoscale 2019, 11, 22398–22412. [Google Scholar] [CrossRef] [PubMed]
- Pinget, G.; Tan, J.; Janac, B.; Kaakoush, N.O.; Angelatos, A.S.; O’Sullivan, J.; Koay, Y.C.; Sierro, F.; Davis, J.; Divakarla, S.K.; et al. Impact of the Food Additive Titanium Dioxide (E171) on Gut Microbiota-Host Interaction. Front. Nutr. 2019, 6, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, W.; Wang, Y.; Huang, C.; Fu, Y.; Li, J.; Wang, H.; Jia, X.; Ba, Q. Effect of Long-Term Intake of Dietary Titanium Dioxide Nanoparticles on Intestine Inflammation in Mice. J. Agric. Food Chem. 2019, 67, 9382–9389. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, R.; Wang, B.; Cai, C.; Zheng, L.; Wang, H.; Wang, M.; Yang, H.; Zhou, X.; Chai, Z.; et al. The effects of orally administered Ag, TiO2 and SiO2 nanoparticles on gut microbiota composition and colitis induction in mice. NanoImpact 2017, 8, 80–88. [Google Scholar] [CrossRef]
- He, Z.; Chen, L.; Catalan-Dibene, J.; Bongers, G.; Faith, J.J.; Suebsuwong, C.; DeVita, R.J.; Shen, Z.; Fox, J.G.; Lafaille, J.J.; et al. Food colorants metabolized by commensal bacteria promote colitis in mice with dysregulated expression of interleukin-23. Cell Metab. 2021, 33, 1358–1371.e5. [Google Scholar] [CrossRef]
- Wu, L.; Xu, Y.; Lv, X.; Chang, X.; Ma, X.; Tian, X.; Shi, X.; Li, X.; Kong, X. Impacts of an azo food dye tartrazine uptake on intestinal barrier, oxidative stress, inflammatory response and intestinal microbiome in crucian carp (Carassius auratus). Ecotoxicol. Environ. Saf. 2021, 223, 112551. [Google Scholar] [CrossRef]
- Hrncirova, L.; Machova, V.; Trckova, E.; Krejsek, J.; Hrncir, T. Food Preservatives Induce Proteobacteria Dysbiosis in Human-Microbiota Associated Nod2-Deficient Mice. Microorganisms 2019, 7, 383. [Google Scholar] [CrossRef] [Green Version]
- Schooth, L.F.; Loayza, J.J.J.; Teh, J.J.; Zhang, J.; Zhang, F.; Liu, Q.; Hamilton, A.L.; Wilson-O’Brien, A.; Trakman, G.L.; Lin, W.Y.; et al. 4 Crohn’s Disease Pathobiont Enterobacteriaceae Growth Is Modulated by Food Additives and Oxygen Availability: Factors in Pathophysiology and Recurrence. The Enigma Study. Gastroenterology 2020, 158, S-2. [Google Scholar] [CrossRef]
- Yang, H.; Wang, W.; Romano, K.A.; Gu, M.; Sanidad, K.Z.; Kim, D.; Yang, J.; Schmidt, B.; Panigrahy, D.; Pei, R.; et al. A common antimicrobial additive increases colonic inflammation and colitis-associated colon tumorigenesis in mice. Sci. Transl. Med. 2018, 10, eaan4116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vamanu, E.; Pelinescu, D.; Gatea, F.; Sârbu, I. Altered in Vitro Metabolomic Response of the Human Microbiota to Sweeteners. Genes 2019, 10, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Bian, X.; Tu, P.; Chi, L.; Gao, B.; Ru, H.; Lu, K. Saccharin induced liver inflammation in mice by altering the gut microbiota and its metabolic functions. Food Chem. Toxicol. 2017, 107, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.; Bian, X.; Gao, B.; Tu, P.; Lai, Y.; Ru, H.; Lu, K. Effects of the Artificial Sweetener Neotame on the Gut Microbiome and Fecal Metabolites in Mice. Molecules 2018, 23, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahalak, K.K.; Firrman, J.; Tomasula, P.M.; Nuñez, A.; Lee, J.J.; Bittinger, K.; Rinaldi, W.; Liu, L.S. Impact of Steviol Glycosides and Erythritol on the Human and Cebus apella Gut Microbiome. J. Agric. Food Chem. 2020, 68, 13093–13101. [Google Scholar] [CrossRef] [PubMed]
- Gostner, A.; Blaut, M.; Schäffer, V.; Kozianowski, G.; Theis, S.; Klingeberg, M.; Dombrowski, Y.; Martin, D.; Ehrhardt, S.; Taras, D.; et al. Effect of isomalt consumption on faecal microflora and colonic metabolism in healthy volunteers. Br. J. Nutr. 2006, 95, 40–50. [Google Scholar] [CrossRef]
- Chu, J.R.; Kang, S.Y.; Kim, S.E.; Lee, S.J.; Lee, Y.C.; Sung, M.K. Prebiotic UG1601 mitigates constipation-related events in association with gut microbiota: A randomized placebo-controlled intervention study. World J. Gastroenterol. 2019, 25, 6129–6144. [Google Scholar] [CrossRef]
- Ballongue, J.; Schumann, C.; Quignon, P. Effects of lactulose and lactitol on colonic microflora and enzymatic activity. Scand. J. Gastroenterol. Suppl. 1997, 222, 41–44. [Google Scholar] [CrossRef]
- Finney, M.; Smullen, J.; Foster, H.A.; Brokx, S.; Storey, D.M. Effects of low doses of lactitol on faecal microflora, pH, short chain fatty acids and gastrointestinal symptomology. Eur. J. Nutr. 2007, 46, 307–314. [Google Scholar] [CrossRef]
- Peuranen, S.; Tiihonen, K.; Apajalahti, J.; Kettunen, A.; Saarinen, M.; Rautonen, N. Combination of polydextrose and lactitol affects microbial ecosystem and immune responses in rat gastrointestinal tract. Br. J. Nutr. 2004, 91, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Pinna, C.; Stefanelli, C.; Biagi, G. In vitro effect of dietary protein level and nondigestible oligosaccharides on feline fecal microbiota. J. Anim. Sci. 2014, 92, 5593–5602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, H.; Yu, L.; Gu, Z.; Li, C.; Ban, X.; Cheng, L.; Hong, Y.; Li, Z. Novel Short-Clustered Maltodextrin as a Dietary Starch Substitute Attenuates Metabolic Dysregulation and Restructures Gut Microbiota in db/db Mice. J. Agric. Food Chem. 2020, 68, 12400–12412. [Google Scholar] [CrossRef]
- Waller, T.; Chen, C.; Walker, S.L. Food and Industrial Grade Titanium Dioxide Impacts Gut Microbiota. Environ. Eng. Sci. 2017, 34, 537–550. [Google Scholar] [CrossRef]
- Agans, R.T.; Gordon, A.; Hussain, S.; Paliy, O. Titanium Dioxide Nanoparticles Elicit Lower Direct Inhibitory Effect on Human Gut Microbiota Than Silver Nanoparticles. Toxicol. Sci. 2019, 172, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Dudefoi, W.; Moniz, K.; Allen-Vercoe, E.; Ropers, M.H.; Walker, V.K. Impact of food grade and nano-TiO2 particles on a human intestinal community. Food Chem. Toxicol. 2017, 106, 242–249. [Google Scholar] [CrossRef]
- Polic, I.I. Evaluation of the Impact of Azo Dyes on the Metabolism of Stabilized Fecal Communities and In Vitro Cell Culture. Master’s Thesis, The University of Guelph, Guelph, ON, Canada.
- Chen, H.; Xu, H.; Heinze, T.M.; Cerniglia, C.E. Decolorization of water and oil-soluble azo dyes by Lactobacillus acidophilus and Lactobacillus fermentum. J. Ind. Microbiol. Biotechnol. 2009, 36, 1459–1466. [Google Scholar] [CrossRef]
- Pan, H.; Feng, J.; He, G.X.; Cerniglia, C.E.; Chen, H. Evaluation of impact of exposure of Sudan azo dyes and their metabolites on human intestinal bacteria. Anaerobe 2012, 18, 445–453. [Google Scholar] [CrossRef] [Green Version]
- Torrallardona, D.; Badiola, I.; Broz, J. Effects of benzoic acid on performance and ecology of gastrointestinal microbiota in weanling piglets. Livest. Sci. 2007, 108, 210–213. [Google Scholar] [CrossRef]
- Kluge, H.; Broz, J.; Eder, K. Effect of benzoic acid on growth performance, nutrient digestibility, nitrogen balance, gastrointestinal microflora and parameters of microbial metabolism in piglets. J. Anim. Physiol. Anim. Nutr. 2006, 90, 316–324. [Google Scholar] [CrossRef]
- Diao, H.; Zheng, P.; Yu, B.; He, J.; Mao, X.B.; Yu, J.; Chen, D.W. Effects of dietary supplementation with benzoic acid on intestinal morphological structure and microflora in weaned piglets. Livest. Sci. 2014, 167, 249–256. [Google Scholar] [CrossRef]
- Diao, H.; Zheng, P.; Yu, B.; He, J.; Mao, X.; Yu, J.; Chen, D. Effects of benzoic Acid and thymol on growth performance and gut characteristics of weaned piglets. Asian-Australas. J. Anim. Sci. 2015, 28, 827–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cc, A.; Gs, A.; At, A.; Bm, A.; Jc, B.; Bb, A.; Ma, A.J.F.; Toxicology, C. Gastrointestinal digestion of food-use silver nanoparticles in the dynamic SIMulator of the GastroIntestinal tract (simgi ®). Impact on human gut microbiota. Food Chem. Toxicol. 2019, 132, 110657. [Google Scholar]
- Romo-Vaquero, M.; Selma, M.V.; Larrosa, M.; Obiol, M.; García-Villalba, R.; González-Barrio, R.; Issaly, N.; Flanagan, J.; Roller, M.; Tomás-Barberán, F.A.; et al. A rosemary extract rich in carnosic acid selectively modulates caecum microbiota and inhibits β-glucosidase activity, altering fiber and short chain fatty acids fecal excretion in lean and obese female rats. PLoS ONE 2014, 9, e94687. [Google Scholar]
- Thomson, P.; Santibañez, R.; Aguirre, C.; Galgani, J.E.; Garrido, D. Short-term impact of sucralose consumption on the metabolic response and gut microbiome of healthy adults. Br. J. Nutr. 2019, 122, 856–862. [Google Scholar] [CrossRef] [Green Version]
- Méndez-García, L.A.; Bueno-Hernández, N.; Cid-Soto, M.A.; De León, K.L.; Mendoza-Martínez, V.M.; Espinosa-Flores, A.J.; Carrero-Aguirre, M.; Esquivel-Velázquez, M.; León-Hernández, M.; Viurcos-Sanabria, R.; et al. Ten-Week Sucralose Consumption Induces Gut Dysbiosis and Altered Glucose and Insulin Levels in Healthy Young Adults. Microorganisms 2022, 10, 434. [Google Scholar] [CrossRef]
- Bian, X.; Chi, L.; Gao, B.; Tu, P.; Ru, H.; Lu, K. Gut Microbiome Response to Sucralose and Its Potential Role in Inducing Liver Inflammation in Mice. Front. Physiol. 2017, 8, 487. [Google Scholar] [CrossRef] [Green Version]
- Loayza, J.; Berendsen, E.; Teh, J.-J.; Hoedt, E.C.; Zhang, J.; Liu, Q.; Hamilton, A.L.; Wilson-O’Brien, A.; Trakman, G.L.; Lin, W.Y.; et al. Su1994—The Common Food Additives Sodium Sulfite and Polysorbate 80 Have a Profound Inhibatory Effect on the Commensal, Anti-Inflammatory Bacterium Faecalibacterium prausnitzii. The Enigma Study. Gastroenterology 2019, 156, S-684–S-685. [Google Scholar] [CrossRef]
- Rettig, S.; Tenewitz, J.; Ahearn, G.; Coughlin, C.J.F.J. Sucralose causes a concentration dependent metabolic inhibition of the gut flora Bacteroides, B. fragilis and B. uniformis not observed in the Firmicutes, E. faecalis and C. sordellii (1118.1). FASEB J. 2014, 28, 1118.1. [Google Scholar] [CrossRef]
- Serrano, J.; Smith, K.R.; Crouch, A.L.; Sharma, V.; Yi, F.; Vargova, V.; LaMoia, T.E.; Dupont, L.M.; Serna, V.; Tang, F.; et al. High-dose saccharin supplementation does not induce gut microbiota changes or glucose intolerance in healthy humans and mice. Microbiome 2021, 9, 11. [Google Scholar] [CrossRef]
- Xu, D.; Wu, X.; Yu, T.; Xu, H.; Zhou, S.Y.; Li, J.Y.; Chung, O.J.G. 1099—Artificial Sweetener (Saccharin) Modulates Visceral Nociception by Lipopolysaccharide-Mediated Gut Barrier Dysfunction and Intestinal Inflammation. AGA J. 2018, 154, S-217. [Google Scholar] [CrossRef]
- Anderson, R.L.; Kirkland, J.J. The effect of sodium saccharin in the diet on caecal microflora. Food Cosmet. Toxicol. 1980, 18, 353–355. [Google Scholar] [CrossRef]
- Bilan, M.V.; Lieshchova, M.A.; Tishkina, N.M.; Brygadyrenko, V.V. Combined effect of glyphosate, saccharin and sodium benzoate on the gut microbiota of rats. Regul. Mech. Biosyst. 2019, 10, 228–232. [Google Scholar] [CrossRef]
- Daly, K.; Darby, A.C.; Hall, N.; Nau, A.; Bravo, D.; Shirazi-Beechey, S.P. Dietary supplementation with lactose or artificial sweetener enhances swine gut Lactobacillus population abundance. Br. J. Nutr. 2014, 111, S30-5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daly, K.; Darby, A.C.; Hall, N.; Wilkinson, M.C.; Pongchaikul, P.; Bravo, D.; Shirazi-Beechey, S.P. Bacterial sensing underlies artificial sweetener-induced growth of gut Lactobacillus. Environ. Microbiol. 2016, 18, 2159–2171. [Google Scholar] [CrossRef]
- Li, Z.; Gao, J.; Guo, Y.; Cui, Y.; Wang, Y.; Duan, W.; Wu, Z. Enhancement of antibiotic resistance dissemination by artificial sweetener acesulfame potassium: Insights from cell membrane, enzyme, energy supply and transcriptomics. J. Hazard. Mater. 2022, 422, 126942. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Y.; Henderson, I.R.; Guo, J. Artificial sweeteners stimulate horizontal transfer of extracellular antibiotic resistance genes through natural transformation. ISME J. 2022, 16, 543–554. [Google Scholar] [CrossRef]
- Yu, Z.; Guo, J. Non-caloric artificial sweeteners exhibit antimicrobial activity against bacteria and promote bacterial evolution of antibiotic tolerance. J. Hazard. Mater. 2022, 433, 128840. [Google Scholar] [CrossRef]
- Markus, V.; Share, O.; Shagan, M.; Halpern, B.; Bar, T.; Kramarsky-Winter, E.; Teralı, K.; Özer, N.; Marks, R.S.; Kushmaro, A.; et al. Inhibitory Effects of Artificial Sweeteners on Bacterial Quorum Sensing. Int. J. Mol. Sci. 2021, 22, 9863. [Google Scholar] [CrossRef]
- Lobach, A.R.; Roberts, A.; Rowland, I.R. Assessing the in vivo data on low/no-calorie sweeteners and the gut microbiota. Food Chem. Toxicol. 2019, 124, 385–399. [Google Scholar] [CrossRef]
- Pfeffer, M.; Ziesenitz, S.C.; Siebert, G. Acesulfame K, cyclamate and saccharin inhibit the anaerobic fermentation of glucose by intestinal bacteria. Z. Ernahr. 1985, 24, 231–235. [Google Scholar] [CrossRef]
- Bernt, W.O.; Borzelleca, J.F.; Flamm, G.; Munro, I.C. Erythritol: A review of biological and toxicological studies. Regul. Toxicol. Pharmacol. RTP 1996, 24, S191-7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrigoni, E.; Brouns, F.; Amadò, R. Human gut microbiota does not ferment erythritol. Br. J. Nutr. 2005, 94, 643–646. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Kwon, E.Y.; Choi, M.S. Anti-Diabetic Effects of Allulose in Diet-Induced Obese Mice via Regulation of mRNA Expression and Alteration of the Microbiome Composition. Nutrients 2020, 12, 2113. [Google Scholar] [CrossRef] [PubMed]
- Kawano, R.; Okamura, T.; Hashimoto, Y.; Majima, S.; Senmaru, T.; Ushigome, E.; Asano, M.; Yamazaki, M.; Takakuwa, H.; Sasano, R.; et al. Erythritol Ameliorates Small Intestinal Inflammation Induced by High-Fat Diets and Improves Glucose Tolerance. Int. J. Mol. Sci. 2021, 22, 5558. [Google Scholar] [CrossRef] [PubMed]
- Caballero, B.; Finglas, P.; Toldrá, F. (Eds.) Encyclopedia of Food and Health; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Jiang, L.; Xie, M.; Chen, G.; Qiao, J.; Zhang, H.; Zeng, X. Phenolics and Carbohydrates in Buckwheat Honey Regulate the Human Intestinal Microbiota. Evid.-Based Complement. Altern. Med. 2020, 2020, 6432942. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.; Milgrom, P. Xylitol and dental caries: An overview for clinicians. J. Calif. Dent. Assoc. 2003, 31, 205–209. [Google Scholar]
- Campus, G.; Cagetti, M.G.; Sacco, G.; Solinas, G.; Mastroberardino, S.; Lingström, P. Six months of daily high-dose xylitol in high-risk schoolchildren: A randomized clinical trial on plaque pH and salivary mutans streptococci. Caries Res. 2009, 43, 455–461. [Google Scholar] [CrossRef]
- Uebanso, T.; Kano, S.; Yoshimoto, A.; Naito, C.; Shimohata, T.; Mawatari, K.; Takahashi, A. Effects of Consuming Xylitol on Gut Microbiota and Lipid Metabolism in Mice. Nutrients 2017, 9, 756. [Google Scholar] [CrossRef] [Green Version]
- Tamura, M.; Hoshi, C.; Hori, S. Xylitol affects the intestinal microbiota and metabolism of daidzein in adult male mice. Int. J. Mol. Sci. 2013, 14, 23993–24007. [Google Scholar] [CrossRef] [Green Version]
- Salminen, S.; Salminen, E.; Koivistoinen, P.; Bridges, J.; Marks, V. Gut microflora interactions with xylitol in the mouse, rat and man. Food Chem. Toxicol. 1985, 23, 985–990. [Google Scholar] [CrossRef]
- Söderling, E.M.; Ekman, T.C.; Taipale, T.J. Growth inhibition of Streptococcus mutans with low xylitol concentrations. Curr. Microbiol. 2008, 56, 382–385. [Google Scholar] [CrossRef]
- Schauber, J.; Weiler, F.; Gostner, A.; Melcher, R.; Kudlich, T.; Lührs, H.; Scheppach, W. Human rectal mucosal gene expression after consumption of digestible and non-digestible carbohydrates. Mol. Nutr. Food Res. 2006, 50, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Chen, L.; Pan, X.; Yao, Y.; Zhang, H.; Zhu, X.; Lou, X.; Zhu, C.; Wang, J.; Li, L.; et al. Lactitol Supplementation Modulates Intestinal Microbiome in Liver Cirrhotic Patients. Front. Med. 2021, 8, 762930. [Google Scholar] [CrossRef]
- Björklund, M.; Ouwehand, A.C.; Forssten, S.D.; Nikkilä, J.; Tiihonen, K.; Rautonen, N.; Lahtinen, S.J. Gut microbiota of healthy elderly NSAID users is selectively modified with the administration of Lactobacillus acidophilus NCFM and lactitol. Age 2012, 34, 987–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouwehand, A.C.; Tiihonen, K.; Saarinen, M.; Putaala, H.; Rautonen, N. Influence of a combination of Lactobacillus acidophilus NCFM and lactitol on healthy elderly: Intestinal and immune parameters. Br. J. Nutr. 2009, 101, 367–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.Q.; Zhang, X.M.; Wu, X.; Lan, Y.; Xu, L.; Meng, X.C.; Li, J.N. Beneficial effects of lactitol on the composition of gut microbiota in constipated patients. J. Dig. Dis. 2020, 21, 445–453. [Google Scholar] [CrossRef]
- Chen, C.; Li, L.; Wu, Z.; Chen, H.; Fu, S. Effects of lactitol on intestinal microflora and plasma endotoxin in patients with chronic viral hepatitis. J. Infect. 2007, 54, 98–102. [Google Scholar] [CrossRef]
- Naimi, S.; Viennois, E.; Gewirtz, A.T.; Chassaing, B. Direct impact of commonly used dietary emulsifiers on human gut microbiota. Microbiome 2021, 9, 66. [Google Scholar] [CrossRef]
- Calgaro, M.; Pandolfo, M.; Salvetti, E.; Marotta, A.; Larini, I.; Pane, M.; Amoruso, A.; Del Casale, A.; Vitulo, N.; Fiorio, M.; et al. Metabarcoding analysis of gut microbiota of healthy individuals reveals impact of probiotic and maltodextrin consumption. Benef. Microbes 2021, 12, 121–136. [Google Scholar] [CrossRef]
- Laudisi, F.; Di Fusco, D.; Dinallo, V.; Stolfi, C.; Di Grazia, A.; Marafini, I.; Colantoni, A.; Ortenzi, A.; Alteri, C.; Guerrieri, F.; et al. The Food Additive Maltodextrin Promotes Endoplasmic Reticulum Stress-Driven Mucus Depletion and Exacerbates Intestinal Inflammation. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 457–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, N.; Tanabe, H.; Yamamoto, T. Isomaltodextrin, a highly branched α-glucan, increases rat colonic H2 production as well as indigestible dextrin. Biosci. Biotechnol. Biochem. 2016, 80, 554–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beards, E.; Tuohy, K.; Gibson, G. A human volunteer study to assess the impact of confectionery sweeteners on the gut microbiota composition. Br. J. Nutr. 2010, 104, 701–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, S.; Sandall, A.; Smith, L.; Rossi, M.; Whelan, K. Food additive emulsifiers: A review of their role in foods, legislation and classifications, presence in food supply, dietary exposure, and safety assessment. Nutr. Rev. 2021, 79, 726–741. [Google Scholar] [CrossRef]
- Garzón, R.; Hernando, I.; Llorca, E.; Rosell, C.M. Understanding the effect of emulsifiers on bread aeration during breadmaking. J. Sci. Food Agric. 2018, 98, 5494–5502. [Google Scholar] [CrossRef] [Green Version]
- Partridge, D.; Lloyd, K.A.; Rhodes, J.M.; Walker, A.W.; Johnstone, A.M.; Campbell, B.J. Food additives: Assessing the impact of exposure to permitted emulsifiers on bowel and metabolic health—Introducing the FADiets study. Nutr. Bull. 2019, 44, 329–349. [Google Scholar] [CrossRef] [Green Version]
- Rather, P.N. Swarmer cell differentiation in Proteus mirabilis. Environ. Microbiol. 2005, 7, 1065–1073. [Google Scholar] [CrossRef]
- Allison, C.; Lai, H.C.; Hughes, C. Co-ordinate expression of virulence genes during swarm-cell differentiation and population migration of Proteus mirabilis. Mol. Microbiol. 1992, 6, 1583–1591. [Google Scholar]
- Chassaing, B.; Compher, C.; Bonhomme, B.; Liu, Q.; Tian, Y.; Walters, W.; Nessel, L.; Delaroque, C.; Hao, F.; Gershuni, V.; et al. Randomized Controlled-Feeding Study of Dietary Emulsifier Carboxymethylcellulose Reveals Detrimental Impacts on the Gut Microbiota and Metabolome. Gastroenterology 2022, 162, 743–756. [Google Scholar] [CrossRef]
- Lock, J.Y.; Carlson, T.L.; Wang, C.M.; Chen, A.; Carrier, R.L. Acute Exposure to Commonly Ingested Emulsifiers Alters Intestinal Mucus Structure and Transport Properties. Sci. Rep. 2018, 8, 10008. [Google Scholar] [CrossRef]
- Martino, J.V.; Van Limbergen, J.; Cahill, L.E. The Role of Carrageenan and Carboxymethylcellulose in the Development of Intestinal Inflammation. Front. Pediatr. 2017, 5, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicklin, S.; Miller, K. Intestinal uptake and immunological effects of carrageenan--current concepts. Food Addit. Contam. 1989, 6, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.; Macfarlane, G.T. Digestive fates of soluble polysaccharides from marine macroalgae: Involvement of the colonic microflora and physiological consequences for the host. J. Appl. Bacteriol. 1996, 80, 349–369. [Google Scholar] [CrossRef]
- Ariffin, S.H.; Yeen, W.W.; Abidin, I.Z.; Abdul Wahab, R.M.; Ariffin, Z.Z.; Senafi, S. Cytotoxicity effect of degraded and undegraded kappa and iota carrageenan in human intestine and liver cell lines. BMC Complement. Altern. Med. 2014, 14, 508. [Google Scholar]
- Bhattacharyya, S.; Shumard, T.; Xie, H.; Dodda, A.; Varady, K.A.; Feferman, L.; Halline, A.G.; Goldstein, J.L.; Hanauer, S.B.; Tobacman, J.K. A randomized trial of the effects of the no-carrageenan diet on ulcerative colitis disease activity. Nutr. Healthy Aging 2017, 4, 181–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallett, A.K.; Rowland, I.R.; Bearne, C.A.; Nicklin, S. Influence of dietary carrageenans on microbial biotransformation activities in the cecum of rodents and on gastrointestinal immune status in the rat. Toxicol. Appl. Pharmacol. 1985, 78, 377–385. [Google Scholar] [CrossRef]
- du Preez, R.; Paul, N.; Mouatt, P.; Majzoub, M.E.; Thomas, T.; Panchal, S.K.; Brown, L. Carrageenans from the Red Seaweed Sarconema filiforme Attenuate Symptoms of Diet-Induced Metabolic Syndrome in Rats. Mar. Drugs 2020, 18, 97. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Li, Q.; Dai, X. Carrageenan Oligosaccharides Extend Life Span and Health Span in Male Drosophila melanogaster by Modulating Antioxidant Activity, Immunity, and Gut Microbiota. J. Med. Food 2021, 24, 101–109. [Google Scholar] [CrossRef]
- Zhang, M.S.; Sandouk, A.; Houtman, J.C. Glycerol Monolaurate (GML) inhibits human T cell signaling and function by disrupting lipid dynamics. Sci. Rep. 2016, 6, 30225. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Zhao, M.; Zhang, H.; Li, Y.; Liu, M.; Feng, F. Antimicrobial Emulsifier-Glycerol Monolaurate Induces Metabolic Syndrome, Gut Microbiota Dysbiosis, and Systemic Low-Grade Inflammation in Low-Fat Diet Fed Mice. Mol. Nutr. Food Res. 2018, 62, 1700547. [Google Scholar] [CrossRef]
- Sakhr, K.; El Khatib, S. Physiochemical properties and medicinal, nutritional and industrial applications of Lebanese Sumac (Syrian Sumac—Rhus coriaria): A review. Heliyon 2020, 6, e03207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkler, H.C.; Notter, T.; Meyer, U.; Naegeli, H. Critical review of the safety assessment of titanium dioxide additives in food. J. Nanobiotechnol. 2018, 16, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreau, F.; Tisseyre, C.; Ménard, S.; Ferrand, A.; Carriere, M. Titanium dioxide particles from the diet: Involvement in the genesis of inflammatory bowel diseases and colorectal cancer. Part. Fibre Toxicol. 2021, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Aquilina, G.; Castle, L.; Engel, K.-H.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gundert-Remy, U.; Gürtler, R.; Husøy, T.; et al. Safety assessment of titanium dioxide (E171) as a food additive. EFSA J. 2021, 19, e06585. [Google Scholar]
- Baranowska-Wójcik, E.; Gustaw, K.; Szwajgier, D.; Oleszczuk, P.; Pawlikowska-Pawlęga, B.; Pawelec, J.; Kapral-Piotrowska, J. Four Types of TiO2 Reduced the Growth of Selected Lactic Acid Bacteria Strains. Foods 2021, 10, 939. [Google Scholar] [CrossRef]
- Liu, L.Y.; Sun, L.; Zhong, Z.T.; Zhu, J.; Song, H.Y. Effects of titanium dioxide nanoparticles on intestinal commensal bacteria. Nucl. Sci. Tech. 2016, 27, 5. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, X.; Cheng, S.; Fan, J.; Qin, X.; Wang, T.; Zhang, Y.; Zhang, J.; Qiu, Y.; Qiu, J.; et al. Titanium dioxide nanoparticles via oral exposure leads to adverse disturbance of gut microecology and locomotor activity in adult mice. Arch. Toxicol. 2020, 94, 1173–1190. [Google Scholar] [CrossRef]
- Ubeda, C.; Bucci, V.; Caballero, S.; Djukovic, A.; Toussaint, N.C.; Equinda, M.; Lipuma, L.; Ling, L.; Gobourne, A.; No, D.; et al. Intestinal microbiota containing Barnesiella species cures vancomycin-resistant Enterococcus faecium colonization. Infect. Immun. 2013, 81, 965–973. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.; Ahn, H.; Park, H. A review on the role of gut microbiota in immune checkpoint blockade therapy for cancer. Mamm. Genome 2021, 32, 223–231. [Google Scholar] [CrossRef]
- Butler, K.S.; Casey, B.J.; Garborcauskas, G.V.; Dair, B.J.; Elespuru, R.K. Assessment of titanium dioxide nanoparticle effects in bacteria: Association, uptake, mutagenicity, co-mutagenicity and DNA repair inhibition. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2014, 768, 14–22. [Google Scholar] [CrossRef]
- Carroll, I.M.; Andrus, J.M.; Bruno-Bárcena, J.M.; Klaenhammer, T.R.; Hassan, H.M.; Threadgill, D.S. Anti-inflammatory properties of Lactobacillus gasseri expressing manganese superoxide dismutase using the interleukin 10-deficient mouse model of colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G729–G738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Zhou, D.; Han, S.; Zhou, S.; Jia, G. Hepatotoxicity and the role of the gut-liver axis in rats after oral administration of titanium dioxide nanoparticles. Part. Fibre Toxicol. 2019, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, Y.; Hu, C.; Lam, P.K.S.; Lam, J.C.W.; Zhou, B. Dysbiosis of gut microbiota by chronic coexposure to titanium dioxide nanoparticles and bisphenol A: Implications for host health in zebrafish. Environ. Pollut. 2018, 234, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, S.; Lei, R.; Gu, W.; Qin, Y.; Ma, S.; Chen, K.; Chang, Y.; Bai, X.; Xia, S.; et al. Oral administration of rutile and anatase TiO2 nanoparticles shifts mouse gut microbiota structure. Nanoscale 2018, 10, 7736–7745. [Google Scholar] [CrossRef]
- Butler, M.; Boyle, J.J.; Powell, J.J.; Playford, R.J.; Ghosh, S. Dietary microparticles implicated in Crohn’s disease can impair macrophage phagocytic activity and act as adjuvants in the presence of bacterial stimuli. Inflamm. Res. 2007, 56, 353–361. [Google Scholar] [CrossRef]
- Ogugbue, C.J.; Morad, N.; Sawidis, T.; Oranusi, N.A. Decolorization and partial mineralization of a polyazo dye by Bacillus firmus immobilized within tubular polymeric gel. 3 Biotech 2012, 2, 67–78. [Google Scholar] [CrossRef] [Green Version]
- Sperandio, F.F.; Huang, Y.Y.; Hamblin, M.R. Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent Pat. Anti-Infect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef] [Green Version]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef] [Green Version]
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. In Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins; International Agency for Research on Cance: Lyon, France, 2010.
- McCann, D.; Barrett, A.; Cooper, A.; Crumpler, D.; Dalen, L.; Grimshaw, K.; Kitchin, E.; Lok, K.; Porteous, L.; Prince, E.; et al. Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: A randomised, double-blinded, placebo-controlled trial. Lancet 2007, 370, 1560–1567. [Google Scholar] [CrossRef]
- Pogoda, J.M.; Preston-Martin, S.; Howe, G.; Lubin, F.; Mueller, B.A.; Holly, E.A.; Filippini, G.; Peris-Bonet, R.; McCredie, M.R.; Cordier, S.; et al. An international case-control study of maternal diet during pregnancy and childhood brain tumor risk: A histology-specific analysis by food group. Ann. Epidemiol. 2009, 19, 148–160. [Google Scholar] [CrossRef] [Green Version]
- Tonacchera, M.; Pinchera, A.; Dimida, A.; Ferrarini, E.; Agretti, P.; Vitti, P.; Santini, F.; Crump, K.; Gibbs, J. Relative potencies and additivity of perchlorate, thiocyanate, nitrate, and iodide on the inhibition of radioactive iodide uptake by the human sodium iodide symporter. Thyroid 2004, 14, 1012–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, X.; Yang, Q.; Chen, D.; Yu, B.; He, J. Benzoic Acid Used as Food and Feed Additives Can Regulate Gut Functions. BioMed Res. Int. 2019, 2019, 5721585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guggenbuhl, P.; Séon, A.; Quintana, A.P.; Nunes, C.S. Effects of dietary supplementation with benzoic acid (VevoVitall®) on the zootechnical performance, the gastrointestinal microflora and the ileal digestibility of the young pig. Livest. Sci. 2007, 108, 218–221. [Google Scholar] [CrossRef]
- Silva Júnior, C.D.; Martins, C.C.S.; Dias, F.T.F.; Sitanaka, N.Y.; Ferracioli, L.B.; Moraes, J.E.; Pizzolante, C.C.; Budiño, F.E.L.; Pereira, R.; Tizioto, P.; et al. The use of an alternative feed additive, containing benzoic acid, thymol, eugenol, and piperine, improved growth performance, nutrient and energy digestibility, and gut health in weaned piglets. J. Anim. Sci. 2020, 98, skaa119. [Google Scholar] [CrossRef]
- Hrncirova, L.; Hudcovic, T.; Sukova, E.; Machova, V.; Trckova, E.; Krejsek, J.; Hrncir, T. Human gut microbes are susceptible to antimicrobial food additives in vitro. Folia Microbiol. 2019, 64, 497–508. [Google Scholar] [CrossRef]
- Peng, Q.; Chang, H.; Wang, R.; You, Z.; Jiang, S.; Ma, C.; Huo, D.; Zhu, X.; Zhang, J. Potassium sorbate suppresses intestinal microbial activity and triggers immune regulation in zebrafish (Danio rerio). Food Funct. 2019, 10, 7164–7173. [Google Scholar] [CrossRef] [PubMed]
- Irwin, S.V.; Fisher, P.; Graham, E.; Malek, A.; Robidoux, A. Sulfites inhibit the growth of four species of beneficial gut bacteria at concentrations regarded as safe for food. PLoS ONE 2017, 12, e0186629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coll Ferrer, M.C.; Dastgheyb, S.; Hickok, N.J.; Eckmann, D.M.; Composto, R.J. Designing nanogel carriers for antibacterial applications. Acta Biomater. 2014, 10, 2105–2111. [Google Scholar] [CrossRef] [Green Version]
- Salem, H.M.; Ismael, E.; Shaalan, M. Evaluation of the Effects of Silver Nanoparticles Against Experimentally Induced Necrotic Enteritis in Broiler Chickens. Int. J. Nanomed. 2021, 16, 6783–6796. [Google Scholar] [CrossRef]
- Das, P.; McDonald, J.A.K.; Petrof, E.O.; Allen-Vercoe, E.; Walker, V.K. Nanosilver-Mediated Change in Human Intestinal Microbiota. J. Nanomed. Nanotechnol. 2014, 5, 235. [Google Scholar]
- van den Brule, S.; Ambroise, J.; Lecloux, H.; Levard, C.; Soulas, R.; De Temmerman, P.J.; Palmai-Pallag, M.; Marbaix, E.; Lison, D. Dietary silver nanoparticles can disturb the gut microbiota in mice. Part. Fibre Toxicol. 2016, 13, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, K.; Milner, J.; Boudreau, M.D.; Gokulan, K.; Cerniglia, C.E.; Khare, S. Effects of subchronic exposure of silver nanoparticles on intestinal microbiota and gut-associated immune responses in the ileum of Sprague-Dawley rats. Nanotoxicology 2015, 9, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Javurek, A.B.; Suresh, D.; Spollen, W.G.; Hart, M.L.; Hansen, S.A.; Ellersieck, M.R.; Bivens, N.J.; Givan, S.A.; Upendran, A.; Kannan, R.; et al. Gut Dysbiosis and Neurobehavioral Alterations in Rats Exposed to Silver Nanoparticles. Sci. Rep. 2017, 7, 2822. [Google Scholar] [CrossRef] [PubMed]
- Wilding, L.A.; Bassis, C.M.; Walacavage, K.; Hashway, S.; Leroueil, P.R.; Morishita, M.; Maynard, A.D.; Philbert, M.A.; Bergin, I.L. Repeated dose (28-day) administration of silver nanoparticles of varied size and coating does not significantly alter the indigenous murine gut microbiome. Nanotoxicology 2016, 10, 513–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Song, L.; Lei, Y.; Jia, P.; Lu, C.; Wu, J.; Xi, C.; Strauss, P.R.; Pei, D.-S. Sex dependent effects of silver nanoparticles on the zebrafish gut microbiota. Environ. Sci. Nano 2018, 5, 740–751. [Google Scholar] [CrossRef]
- Tian, X.; Jiang, X.; Welch, C.; Croley, T.R.; Wong, T.Y.; Chen, C.; Fan, S.; Chong, Y.; Li, R.; Ge, C.; et al. Bactericidal Effects of Silver Nanoparticles on Lactobacilli and the Underlying Mechanism. ACS Appl. Mater. Interfaces 2018, 10, 8443–8450. [Google Scholar] [CrossRef]
- You, X.; Einson, J.E.; Lopez-Pena, C.L.; Song, M.; Xiao, H.; McClements, D.J.; Sela, D.A. Food-grade cationic antimicrobial ε-polylysine transiently alters the gut microbial community and predicted metagenome function in CD-1 mice. NPJ Sci. Food 2017, 1, 8. [Google Scholar] [CrossRef] [Green Version]
- Brindisi, M.; Bouzidi, C.; Frattaruolo, L.; Loizzo, M.R.; Tundis, R.; Dugay, A.; Deguin, B.; Cappello, A.R.; Cappello, M.S. Chemical Profile, Antioxidant, Anti-Inflammatory, and Anti-Cancer Effects of Italian Salvia rosmarinus Spenn. Methanol Leaves Extracts. Antioxidants 2020, 9, 826. [Google Scholar] [CrossRef]
- Yang, M.; Yin, Y.; Wang, F.; Bao, X.; Long, L.; Tan, B.; Yin, Y.; Chen, J. Effects of dietary rosemary extract supplementation on growth performance, nutrient digestibility, antioxidant capacity, intestinal morphology, and microbiota of weaning pigs. J. Anim. Sci. 2021, 99, skab237. [Google Scholar] [CrossRef]
- Ou, J.; Huang, J.; Zhao, D.; Du, B.; Wang, M. Protective effect of rosmarinic acid and carnosic acid against streptozotocin-induced oxidation, glycation, inflammation and microbiota imbalance in diabetic rats. Food Funct. 2018, 9, 851–860. [Google Scholar] [CrossRef]
- Guo, Y.; Xie, J.; Li, X.; Yuan, Y.; Zhang, L.; Hu, W.; Luo, H.; Yu, H.; Zhang, R. Antidepressant Effects of Rosemary Extracts Associate With Anti-inflammatory Effect and Rebalance of Gut Microbiota. Front. Pharmacol. 2018, 9, 1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Reference | [17] | [124] | [32] | [33] | [34] | [76] |
|---|---|---|---|---|---|---|
| α-diversity | ||||||
| Richness | - | ↑ | ↓ | - | - | - |
| Diversity | - | - | ↓ | - | - | - |
| β-diversity | - | S | S | - | - | - |
| Genus | ||||||
| Bifidobacterium | ↑ | ↑ | ||||
| Bacteroides | ↓ | |||||
| Mucispirillum | ↑ | |||||
| Desulfovibrio | ↓ | |||||
| Lactobacillus | ↑ | |||||
| Enterococcus | ↓ | |||||
| Faecalibacterium | ↑ | |||||
| Akkermansia | ↑ | |||||
| Roseburia | ↑ | |||||
| Streptococcus | ↑ | ↓ | ||||
| Alistipes | ↓ | |||||
| Leuconostoc | ↑ | |||||
| Pseudomonas | ↑ | |||||
| Weissella | ↓ | |||||
| Oscillibacter | ||||||
| Species | ||||||
| Escherichia coli | ↑ | |||||
| Blautia coccoides | ↑ |
| Reference | [17] | [58] | [151] | [78] | [79] | [52] | [53] | [54] | [59] | [152] | [56] | [57] | [155] | [55] | [159] | [77] | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ↑ | ↓ | |||||||||||||||||
| α-diversity | ||||||||||||||||||
| Richness | - | N | - | ↓ | - | - | - | ↓ | - | ↓ | N | N | ↓ | - | N | - | 0 | 4 |
| Diversity | - | N | - | N | - | - | - | ↓ | - | ↓ | N | N | - | - | N | - | 0 | 2 |
| β-diversity | S | - | - | N | - | - | - | S | S | S | N | N | S | - | N | - | S = 5 | |
| Phylum | ||||||||||||||||||
| Bacteroidetes | ↑ | ↓ | ↓ | ↓ | ↑ | ↑ | 3 | 3 | ||||||||||
| Verrucomicrobia | ↓ | ↑ | 1 | 1 | ||||||||||||||
| Firmicutes | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | 6 | 0 | ||||||||||
| Proteobacteria | ↑ | ↓ | 1 | 1 | ||||||||||||||
| Actinomycetes | ↑ | ↑ | 2 | 0 | ||||||||||||||
| Cyanobacteria | ↑ | 1 | ||||||||||||||||
| Deferribacteres | ↑ | 1 | ||||||||||||||||
| Genus | ||||||||||||||||||
| Bifidobacterium | ↓ | ↓ | 0 | 2 | ||||||||||||||
| Bacteroides | ↓ | ↑ | 1 | 1 | ||||||||||||||
| Parabacteroides | ↑ | 1 | 0 | |||||||||||||||
| Lactobacillu | ↓ | ↑ | ↓ | ↑ | 2 | 2 | ||||||||||||
| Prevotella | ↓ | 0 | 1 | |||||||||||||||
| Turicibacter | ↑ | 1 | 0 | |||||||||||||||
| Akkermansia | ↓ | 0 | 1 | |||||||||||||||
| Adlercreutzia | ↓ | 0 | 1 | |||||||||||||||
| Barnesiella | ↓ | 0 | 1 | |||||||||||||||
| Rhodococcus | ↑ | 1 | 0 | |||||||||||||||
| Lawsonia | ↑ | 1 | 0 | |||||||||||||||
| Allobaculum | ↑ | 1 | 0 | |||||||||||||||
| Enterobacteria | ↑ | 1 | 0 | |||||||||||||||
| Acetobacteria | ↑ | 1 | 0 | |||||||||||||||
| Species | ||||||||||||||||||
| Clostridium leptum | ↓ | 0 | 1 | |||||||||||||||
| Clostridium cocleatum | ↑ | 1 | 0 | |||||||||||||||
| Bacteroides ovatus | ↓ | 0 | 1 | |||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Zhan, S.; Tian, Z.; Li, N.; Li, T.; Wu, D.; Zeng, Z.; Zhuang, X. Food Additives Associated with Gut Microbiota Alterations in Inflammatory Bowel Disease: Friends or Enemies? Nutrients 2022, 14, 3049. https://doi.org/10.3390/nu14153049
Liu C, Zhan S, Tian Z, Li N, Li T, Wu D, Zeng Z, Zhuang X. Food Additives Associated with Gut Microbiota Alterations in Inflammatory Bowel Disease: Friends or Enemies? Nutrients. 2022; 14(15):3049. https://doi.org/10.3390/nu14153049
Chicago/Turabian StyleLiu, Caiguang, Shukai Zhan, Zhenyi Tian, Na Li, Tong Li, Dongxuan Wu, Zhirong Zeng, and Xiaojun Zhuang. 2022. "Food Additives Associated with Gut Microbiota Alterations in Inflammatory Bowel Disease: Friends or Enemies?" Nutrients 14, no. 15: 3049. https://doi.org/10.3390/nu14153049
APA StyleLiu, C., Zhan, S., Tian, Z., Li, N., Li, T., Wu, D., Zeng, Z., & Zhuang, X. (2022). Food Additives Associated with Gut Microbiota Alterations in Inflammatory Bowel Disease: Friends or Enemies? Nutrients, 14(15), 3049. https://doi.org/10.3390/nu14153049






