Physical Complaints Decrease after Following a Few-Foods Diet in Children with ADHD
Abstract
:1. Introduction
2. Materials and Methods
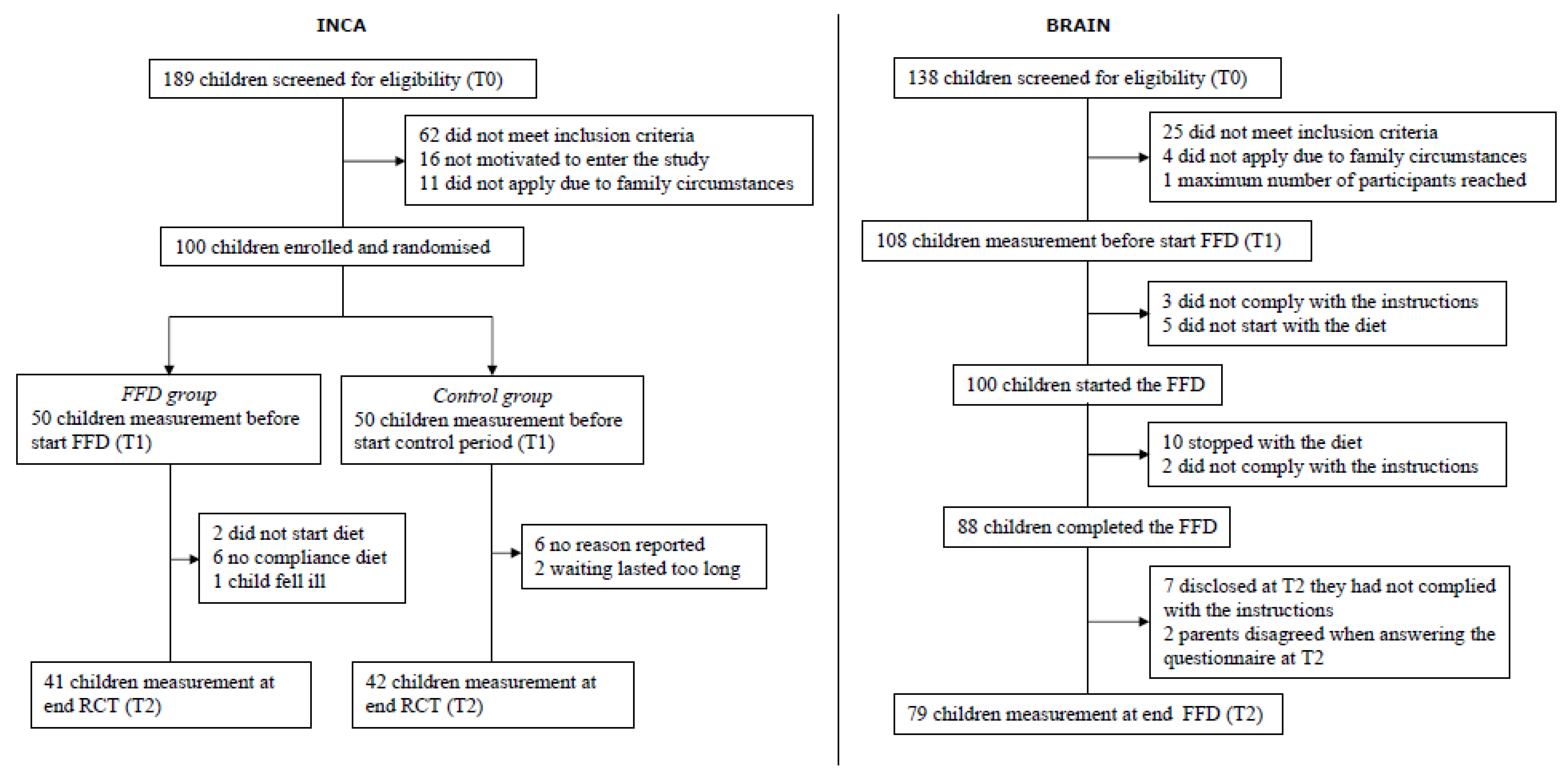
2.1. Subjects and Design
2.2. Interventions
2.3. Questionnaires
2.4. Statistical Analysis
3. Results
3.1. Physical Complaints at Tend versus Tstart in the INCA RCT
3.2. Physical Complaints at Tend versus Tstart in the Open-Label BRAIN Study and the INCA FFD Group
3.3. Reduction in Physical Complaints in ARS Responders and Non-Responders after the FFD
3.4. Additional Complaints and the mBSFS-C in the BRAIN Study
3.5. Physical Complaints and ARS Response in Children with Allergies or Atopic Constitution
4. Discussion
5. Strengths/Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Polanczyk, G.V.; Willcutt, E.G.; Salum, G.A.; Kieling, C.; Rohde, L.A. ADHD prevalence estimates across three decades: An updated systematic review and meta-regression analysis. Int. J. Epidemiol. 2014, 43, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, R.; Balducci, J.; Poppi, C.; Arcolin, E.; Cutino, A.; Ferri, P.; D’Amico, R.; Filippini, T. Children and adolescents with ADHD followed up to adulthood: A systematic review of long-term outcomes. Acta Neuropsychiatr. 2021, 33, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Faraone, S.V.; Asherson, P.; Banaschewski, T.; Biederman, J.; Buitelaar, J.K.; Ramos-Quiroga, J.A.; Rohde, L.A.; Sonuga-Barke, E.J.; Tannock, R.; Franke, B. Attention-deficit/hyperactivity disorder. Nat. Rev. Dis. Primers 2015, 1, 15020. [Google Scholar] [CrossRef]
- Tistarelli, N.; Fagnani, C.; Troianiello, M.; Stazi, M.A.; Adriani, W. The nature and nurture of ADHD and its comorbidities: A narrative review on twin studies. Neurosci. Biobehav. Rev. 2020, 109, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Momen, N.C.; Plana-Ripoll, O.; Agerbo, E.; Benros, M.E.; Børglum, A.D.; Christensen, M.K.; Dalsgaard, S.; Degenhardt, L.; de Jonge, P.; Debost, J.P.G.; et al. Association between Mental Disorders and Subsequent Medical Conditions. N. Engl. J. Med. 2020, 382, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- Galéra, C.; Cortese, S.; Orri, M.; Collet, O.; van der Waerden, J.; Melchior, M.; Boivin, M.; Tremblay, R.E.; Côté, S.M. Medical conditions and Attention-Deficit/Hyperactivity Disorder symptoms from early childhood to adolescence. Mol. Psychiatry 2022, 27, 976–984. [Google Scholar] [CrossRef]
- Laugesen, B.; Lauritsen, M.B.; Færk, E.; Mohr-Jensen, C. Medical disorders in a Danish cohort of children with attention-deficit hyperactivity disorder. Eur. Child Adolesc. Psychiatry 2022, 31, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Pelsser, L.M.; Frankena, K.; Toorman, J.; Rodrigues Pereira, R. Diet and ADHD, Reviewing the Evidence: A Systematic Review of Meta-Analyses of Double-Blind Placebo-Controlled Trials Evaluating the Efficacy of Diet Interventions on the Behavior of Children with ADHD. PLoS ONE 2017, 12, e0169277. [Google Scholar] [CrossRef]
- Dölp, A.; Schneider-Momm, K.; Heiser, P.; Clement, C.; Rauh, R.; Clement, H.W.; Schulz, E.; Fleischhaker, C. Oligoantigenic Diet Improves Children’s ADHD Rating Scale Scores Reliably in Added Video-Rating. Front. Psychiatry 2020, 11, 730. [Google Scholar] [CrossRef]
- Pelsser, L.; Frankena, K.; Toorman, J.; Rodrigues Pereira, R. Retrospective Outcome Monitoring of ADHD and Nutrition (ROMAN): The Effectiveness of the Few-Foods Diet in General Practice. Front. Psychiatry 2020, 11, 96. [Google Scholar] [CrossRef]
- Yorgidis, E.; Beiner, L.; Blazynski, N.; Schneider-Momm, K.; Clement, H.W.; Rauh, R.; Schulz, E.; Clement, C.; Fleischhaker, C. Individual Behavioral Reactions in the Context of Food Sensitivities in Children with Attention-Deficit/Hyperactivity Disorder before and after an Oligoantigenic Diet. Nutrients 2021, 13, 2598. [Google Scholar] [CrossRef] [PubMed]
- Hontelez, S.; Stobernack, T.; Pelsser, L.M.; van Baarlen, P.; Frankena, K.; Groefsema, M.M.; Kleerebezem, M.; Rodrigues Pereira, R.; Postma, E.M.; Smeets, P.A.M.; et al. Correlation between brain function and ADHD symptom changes in children with ADHD following a few-foods diet: An open-label intervention trial. Sci. Rep. 2021, 11, 22205. [Google Scholar] [CrossRef] [PubMed]
- Pelsser, L.M.; Frankena, K.; Toorman, J.; Savelkoul, H.F.; Dubois, A.E.; Pereira, R.R.; Haagen, T.A.; Rommelse, N.N.; Buitelaar, J.K. Effects of a restricted elimination diet on the behaviour of children with attention-deficit hyperactivity disorder (INCA study): A randomised controlled trial. Lancet 2011, 377, 494–503. [Google Scholar] [CrossRef]
- Egger, J.; Carter, C.M.; Graham, P.J.; Gumley, D.; Soothill, J.F. Controlled trial of oligoantigenic treatment in the hyperkinetic syndrome. Lancet 1985, 1, 540–545. [Google Scholar] [CrossRef]
- Carter, C.M.; Urbanowicz, M.; Hemsley, R.; Mantilla, L.; Strobel, S.; Graham, P.J.; Taylor, E. Effects of a few food diet in attention deficit disorder. Arch. Dis. Child. 1993, 69, 564–568. [Google Scholar] [CrossRef] [Green Version]
- Pelsser, L.M.; Frankena, K.; Buitelaar, J.K.; Rommelse, N.N. Effects of food on physical and sleep complaints in children with ADHD: A randomised controlled pilot study. Eur. J. Pediatr. 2010, 169, 1129–1138. [Google Scholar] [CrossRef] [Green Version]
- Stobernack, T.; de Vries, S.P.W.; Rodrigues Pereira, R.; Pelsser, L.M.; Ter Braak, C.J.F.; Aarts, E.; van Baarlen, P.; Kleerebezem, M.; Frankena, K.; Hontelez, S. Biomarker Research in ADHD: The Impact of Nutrition (BRAIN)—Study protocol of an open-label trial to investigate the mechanisms underlying the effects of a few-foods diet on ADHD symptoms in children. BMJ Open 2019, 9, e029422. [Google Scholar] [CrossRef]
- DuPaul, G.J.; Reid, R.; Anastopoulos, A.D.; Lambert, M.C.; Watkins, M.W.; Power, T.J. Parent and teacher ratings of attention-deficit/hyperactivity disorder symptoms: Factor structure and normative data. Psychol. Assess. 2016, 28, 214–225. [Google Scholar] [CrossRef] [Green Version]
- von Gontard, A.; Equit, M. Comorbidity of ADHD and incontinence in children. Eur. Child Adolesc. Psychiatry 2015, 24, 127–140. [Google Scholar] [CrossRef]
- Lane, M.M.; Czyzewski, D.I.; Chumpitazi, B.P.; Shulman, R.J. Reliability and validity of a modified Bristol Stool Form Scale for children. J. Pediatr. 2011, 159, 437–441. [Google Scholar] [CrossRef] [Green Version]
- Chinn, S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat. Med. 2000, 19, 3127–3131. [Google Scholar] [CrossRef]
- Dunst, C.J.; Hamby, D.W. Guide for calculating and interpreting effect sizes and confidence intervals in intellectual and developmental disability research studies. J. Intellect. Dev. Disabil. 2012, 37, 89–99. [Google Scholar] [CrossRef]
- Rubin, M. When to adjust alpha during multiple testing: A consideration of disjunction, conjunction, and individual testing. Synthese 2021, 199, 10969–11000. [Google Scholar] [CrossRef]
- Davis, S.L.; Johnson, A.H.; Lynch, T.; Gray, L.; Pryor, E.R.; Azuero, A.; Soistmann, H.C.; Phillips, S.R.; Rice, M. Inclusion of Effect Size Measures and Clinical Relevance in Research Papers. Nurs. Res. 2021, 70, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Aarts, S.; van den Akker, M.; Winkens, B. The importance of effect sizes. Eur. J. Gen. Pract. 2014, 20, 61–64. [Google Scholar] [CrossRef]
- Nordahl-Hansen, A.; Øien, R.A.; Volkmar, F.; Shic, F.; Cicchetti, D.V. Enhancing the understanding of clinically meaningful results: A clinical research perspective. Psychiatry Res. 2018, 270, 801–806. [Google Scholar] [CrossRef]
- Kozlowska, K.; Scher, S.; Helgeland, H. The Autonomic Nervous System and Functional Somatic Symptoms. In Functional Somatic Symptoms in Children and Adolescents: A Stress-System Approach to Assessment and Treatment; Springer International Publishing: Cham, Switzerland, 2020; pp. 119–136. [Google Scholar]
- Ihekweazu, F.D.; Versalovic, J. Development of the Pediatric Gut Microbiome: Impact on Health and Disease. Am. J. Med. Sci. 2018, 356, 413–423. [Google Scholar] [CrossRef] [Green Version]
- Hadizadeh, F.; Walter, S.; Belheouane, M.; Bonfiglio, F.; Heinsen, F.-A.; Andreasson, A.; Agreus, L.; Engstrand, L.; Baines, J.F.; Rafter, J.; et al. Stool frequency is associated with gut microbiota composition. Gut 2017, 66, 559–560. [Google Scholar] [CrossRef]
- Kwon, H.J.; Lim, J.H.; Kang, D.; Lim, S.; Park, S.J.; Kim, J.H. Is stool frequency associated with the richness and community composition of gut microbiota? Intest. Res. 2019, 17, 419–426. [Google Scholar] [CrossRef]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [Green Version]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Saffouri, G.B.; Shields-Cutler, R.R.; Chen, J.; Yang, Y.; Lekatz, H.R.; Hale, V.L.; Cho, J.M.; Battaglioli, E.J.; Bhattarai, Y.; Thompson, K.J.; et al. Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders. Nat. Commun. 2019, 10, 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rask, C.U.; Olsen, E.M.; Elberling, H.; Christensen, M.F.; Ornbøl, E.; Fink, P.; Thomsen, P.H.; Skovgaard, A.M. Functional somatic symptoms and associated impairment in 5–7-year-old children: The Copenhagen Child Cohort 2000. Eur. J. Epidemiol. 2009, 24, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Devanarayana, N.M.; Rajindrajith, S. Irritable bowel syndrome in children: Current knowledge, challenges and opportunities. World J. Gastroenterol. 2018, 24, 2211–2235. [Google Scholar] [CrossRef] [PubMed]
- Schans, J.v.d.; Çiçek, R.; de Vries, T.W.; Hak, E.; Hoekstra, P.J. Association of atopic diseases and attention-deficit/hyperactivity disorder: A systematic review and meta-analyses. Neurosci. Biobehav. Rev. 2017, 74, 139–148. [Google Scholar] [CrossRef]
- Xu, G.; Liu, B.; Yang, W.; Snetselaar, L.G.; Chen, M.; Bao, W.; Strathearn, L. Association of Food Allergy, Respiratory Allergy, and Skin Allergy with Attention Deficit/Hyperactivity Disorder among Children. Nutrients 2022, 14, 474. [Google Scholar] [CrossRef]
- Akmatov, M.K.; Ermakova, T.; Bätzing, J. Psychiatric and Nonpsychiatric Comorbidities Among Children With ADHD: An Exploratory Analysis of Nationwide Claims Data in Germany. J. Atten. Disord. 2019, 25, 874–884. [Google Scholar] [CrossRef]
- Du Rietz, E.; Brikell, I.; Butwicka, A.; Leone, M.; Chang, Z.; Cortese, S.; D’Onofrio, B.M.; Hartman, C.A.; Lichtenstein, P.; Faraone, S.V.; et al. Mapping phenotypic and aetiological associations between ADHD and physical conditions in adulthood in Sweden: A genetically informed register study. Lancet Psychiatry 2021, 8, 774–783. [Google Scholar] [CrossRef]
- The MTA Cooperative Group. A 14-Month Randomized Clinical Trial of Treatment Strategies for Attention-Deficit/Hyperactivity Disorder. Arch. Gen. Psychiatry 1999, 56, 1073–1086. [Google Scholar] [CrossRef]
- Walker, L.S.; Beck, J.E.; Garber, J.; Lambert, W. Children’s Somatization Inventory: Psychometric properties of the revised form (CSI-24). J. Pediatr. Psychol. 2009, 34, 430–440. [Google Scholar] [CrossRef] [Green Version]

| Nr 2 | Complaint |
|---|---|
| 1 | Headache |
| 2 | Abdominal pain |
| 3 | Growing pain |
| 4 | Unusual thirst |
| 5 | Unusual perspiration (at night or daytime) |
| 6 | Often warm |
| 7 | Eczema |
| 8 | Asthma |
| 9 | Persisting cold (rhinitis) |
| 10 | Blotches in the face |
| 11 | Red edged mouth |
| 12 | Red ears |
| 13 | Bags under eyes |
| 14 | Often tired |
| 15 | Diarrhoea |
| 16 | Constipation |
| 17 | Flatulence |
| 18 | Nausea/vomiting |
| 19 | Problems sleeping in |
| 20 | Problems sleeping on |
| 21 | Nocturnal enuresis |
| 22 | Daytime urinary incontinence |
| 23 | Faecal incontinence |
| INCA | BRAIN | |||||
|---|---|---|---|---|---|---|
| FFD n = 41 | Control n = 42 | p-Value FFD vs. Control | Total FFD + Control, n = 83 | FFD n = 79 | p-Value INCA (n = 83) vs. BRAIN (n = 79) | |
| Boys | 35 (85%) | 38 (90%) | 0.52 1 | 73 (88%) | 79 (100%) | 0.0015 1 |
| Mean age (SD) | 6.83 (1.34) | 7.14 (1.19) | 0.28 2 | 6.99 (1.27) | 9.21 (0.85) | <0.0001 2 |
| Atopic constitution family 3 | ||||||
| Non-atopic | 20 (49%) | 18 (43%) | 0.66 1 | 38 (46%) | 28 (35%) | 0.20 1 |
| Atopic | 20 (49%) | 24 (57%) | 44 (53%) | 51 (65%) | ||
| Unknown | 1 (2%) | 0 (0%) | 1 (1%) | 0 (0%) | ||
| Allergy research in child 4 | ||||||
| No research | 30 (73%) | 29 (69%) | 0.89 1 | 59 (71%) | 68 (86%) | 0.054 1 |
| Research negative | 7 (17%) | 7 (17%) | 14 (17%) | 8 (10%) | ||
| Research positive | 4 (10%) | 6 (14%) | 10 (12%) | 3 (4%) | ||
| ADHD type and symptom severity | ||||||
| ADHD combined type | 34 (83%) | 38 (90%) | 0.43 1 | 72 (87%) | 70 (89%) | 0.93 1 |
| ADHD inattentive type | 2 (5%) | 2 (5%) | 4 (5%) | 4 (5%) | ||
| ADHD hyperactive-impulsive type | 5 (12%) | 2 (5%) | 7 (8%) | 5 (6%) | ||
| Mean number of ADHD symptoms (SD) (minimum (0)–maximum (18)) | 14.68 (2.11) (10–18) | 15.98 (1.77) (12–18) | 0.00492 | 15.34 (2.04) (10–18) | 15.96 (2.07) (9–18) | 0.02012 |
| Mean ARS score (SD) (minimum (0)–maximum (54)) | 45.22 (4.78) (35–54) | 47.86 (3.67) (41–54) | 0.01672 | 46.55 (4.43) (35–54) | 46.19 (5.76) (28–54) | 0.78 2 |
| Total number of physical complaints 5 (mean; minimum-maximum) | 117 (2.85; 0–8) | 159 (3.79; 0–9) | 276 (3.33; 0–9) | 233 (2.95; 0–9) | ||
| FFD, n = 41 | Control, n = 42 | FFD vs. Control | Association of Treatment (FFD vs. Control) with Complaint at Tend | |||||
|---|---|---|---|---|---|---|---|---|
| Complaint | Tstart n (%) | Tend n (%) | Tstart n (%) | Tend n (%) | Tstart p-Value 2 | Odds Ratio (95% CI) 3 | p-Value | Cohen’s d |
| Headache | 6 (14.6) | 4 (9.8) | 8 (19.0) | 3 (7.1) | 0.77 | 1.89 (0.24; 17.52) | 0.76 | 0.35 |
| Abdominal pain | 11 (26.8) | 5 (12.2) | 16 (38.1) | 11 (26.2) | 0.35 | 0.45 (0.09; 1.94) | 0.36 | −0.44 |
| Growing pain | 4 (9.8) | 1 (2.4) | 2 (4.8) | 1 (2.4) | 0.43 | 0.41 (0.003; 52.13) | 1.00 | −0.49 |
| Unusual thirst | 15 (36.6) | 2 (4.9) | 17 (40.5) | 13 (31.0) | 0.82 | 0.05 (0.004; 0.38) | 0.0009 | −1.65 |
| Unusual perspiration (at night or daytime) | 9 (22.0) | 1 (2.4) | 18 (42.9) | 14 (33.3) | 0.06 | 0.06 (0.001; 0.50) | 0.0036 | −1.55 |
| Often warm | 23 (56.1) | 7 (17.1) | 19 (45.2) | 15 (35.7) | 0.38 | 0.16 (0.03; 0.66) | 0.0075 | −1.01 |
| Eczema | 4 (9.8) | 1 (2.4) | 4 (9.5) | 4 (9.5) | 1.00 | 0.15 (0.00; 1.26) | 0.07 | −1.05 |
| Asthma | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.4) | NA 4 | 1.02 (0.00; 19.46) | 0.51 | 0.01 |
| Persisting cold (rhinitis) | 3 (7.3) | 0 (0.0) | 7 (16.7) | 7 (16.7) | 0.31 | 0.04 (0.00; 0.38) | 0.0083 | −1.78 |
| Blotches in the face | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA | NA | NA | NA |
| Red edged mouth | 0 (0.0) | 1 (2.4) | 1 (2.4) | 1 (2.4) | 1.00 | 1.00 (0.05; infinite) | 0.50 | 0.00 |
| Red ears | 0 (0.0) | 2 (4.9) | 2 (4.8) | 2 (4.8) | 0.49 | 2.40 (0.28; infinite) | 0.25 | 0.48 |
| Bags under eyes | 4 (9.8) | 1 (2.4) | 11 (26.2) | 10 (23.8) | 0.09 | 0.05 (<0.001; 1.15) | 0.07 | −1.65 |
| Often tired | 10 (24.4) | 12 (29.3) | 13 (31.0) | 13 (31.0) | 0.63 | 1.24 (0.31; 5.47) | 0.98 | 0.12 |
| Diarrhoea | 2 (4.9) | 0 (0.0) | 6 (14.3) | 6 (14.3) | 0.26 | 0.08 (0.00; 0.79) | 0.0357 | −1.39 |
| Constipation | 0 (0.0) | 1 (2.4) | 1 (2.4) | 1 (2.4) | 1.00 | 1.00 (0.05; infinite) | 0.50 | 0.00 |
| Flatulence | 7 (17.1) | 1 (2.4) | 7 (16.7) | 7 (16.7) | 1.00 | 0.04 (<0.001; 0.65) | 0.0151 | −1.78 |
| Nausea/vomiting | 0 (0.0) | 1 (2.4) | 3 (7.1) | 3 (7.1) | 0.24 | 0.95 (0.05; infinite) | 0.51 | −0.03 |
| Problems sleeping in | 10 (24.4) | 4 (9.8) | 17 40.5) | 9 (21.4) | 0.16 | 0.60 (0.09; 3.71) | 0.80 | −0.28 |
| Problems sleeping on | 7 (17.1) | 2 (4.9) | 3 (7.1) | 3 (7.1) | 0.19 | 0.34 (0.02; 4.03) | 0.59 | −0.60 |
| Nocturnal enuresis | 2 (4.9) | 0 (0.0) | 4 (9.5) | 5 (11.9) | 0.68 | 0.10 (0.00; 0.79) | 0.0329 | −1.27 |
| Total number of physical complaints (average; minimum-maximum) | 117 (2.9; 0–8) | 46 (1.1; 0–6) | 159 (3.8; 0–9) | 129 (3.1; 0–9) | 0.47 | 0.44 (0.31; 0.62) 5 | <0.00015 | NA |
| BRAIN (n = 79) (All Participating Children) | INCA (n = 41) (FFD Group Only) | Association of Study (BRAIN vs. INCA) with Complaint at Tend | INCA + BRAIN (n = 120) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Complaint | Tstart n (%) | Tend n (%) | p-Value 2 | Tstart n (%) | Tend n (%) | p-Value 2 | Odds Ratio | p-Value 3 | Tstart n (%) | Tend n (%) | p-Value 2 |
| Headache | 6 (7.6) | 8 (10.1) | 0.77 | 6 (14.6) | 4 (9.8) | 0.69 | 1.19 (0.29; 5.97) | 1.00 | 12 (10.0) | 12 (10.0) | 1.00 |
| Abdominal pain | 20 (25.3) | 15 (19.0) | 0.29 | 11 (26.8) | 5 (12.2) | 0.11 | 1.77 (0.53; 6.96) | 0.46 | 31 (25.8) | 20 (16.7) | 0.0463 |
| Growing pain | 6 (7.6) | 1 (1.3) | 0.13 | 4 (9.8) | 1 (2.4) | 0.25 | 0.58 (0.01; 46.05) | 1.00 | 10 (8.3) | 2 (1.7) | 0.0215 |
| Unusual thirst | 19 (24.1) | 10 (12.7) | 0.06 | 15 (36.6) | 2 (4.9) | 0.0002 | 3.7 (0.69; 38.36) | 0.17 | 34 (28.3) | 12 (10.0) | <0.0001 |
| Unusual perspiration (at night or daytime) | 29 (36.7) | 11 (13.9) | <0.0001 | 9 (22.0) | 1 (2.4) | 0.0078 | 5.01 (0.63; 230.91) | 0.19 | 38 (31.7) | 12 (10.0) | <0.0001 |
| Often warm | 41 (51.9) | 15 (19.0) | <0.0001 | 23 (56.1) | 7 (17.1) | <0.0001 | 1.22 (0.40; 4.05) | 0.91 | 64 (53.3) | 22 (18.3) | <0.0001 |
| Eczema | 1 (1.3) | 0 (0.0) | 1.00 | 4 (9.8) | 1 (2.4) | 0.25 | 4 (0.00; 76.00) | 0.80 | 5 (4.2) | 1 (0.8) | 0.13 |
| Asthma | 0 (0.0) | 0 (0.0) | NA | 0 (0.0) | 0 (0.0) | NA | NA | NA | 0 (0.0) | 0 (0.0) | NA |
| Persisting cold (rhinitis) | 6 (7.6) | 2 (2.5) | 0.13 | 3 (7.3) | 0 (0.0) | 0.25 | 1.35 (0.14; infinite) | 0.42 | 9 (7.5) | 2 (1.7) | 0.0156 |
| Blotches in the face | 1 (1.3) | 3 (3.8) | 0.50 | 0 (0.0) | 0 (0.0) | NA | 1.28 (0.15; infinite) | 0.43 | 1 (0.8) | 3 (2.5) | 0.50 |
| Red edged mouth | 4 (5.1) | 5 (6.3) | 1.00 | 0 (0.0) | 1 (2.4) | 1.00 | 2.24 (0.21; 113.7) | 0.84 | 4 (3.3) | 6 (5.0) | 0.73 |
| Red ears | 2 (2.5) | 2 (2.5) | 1.00 | 0 (0.0) | 2 (4.9) | 0.50 | 0.26 (0.004; 5.12) | 0.55 | 2 (1.7) | 4 (3.3) | 0.63 |
| Bags under eyes | 10 (12.7) | 11 (13.9) | 1.00 | 4 (9.8) | 1 (2.4) | 0.25 | 6.38 (0.82; 293.48) | 0.09 | 14 (11.7) | 12 (10.0) | 0.80 |
| Often tired | 16 (20.3) | 26 (32.9) | 0.07 | 10 (24.4) | 12 (29.3) | 0.75 | 1.23 (0.50; 3.14) | 0.78 | 26 (21.7) | 38 (31.7) | 0.06 |
| Diarrhoea | 5 (6.3) | 2 (2.5) | 0.38 | 2 (4.9) | 0 (0.0) | 0.50 | 1.12 (0.13; infinite) | 0.47 | 7 (5.8) | 2 (1.7) | 0.13 |
| Constipation | 1 (1.3) | 2 (2.5) | 1.00 | 0 (0.0) | 1 (2.4) | 1.00 | 0.52 (0.007; 41.79) | 1.00 | 1 (0.8) | 3 (2.5) | 0.50 |
| Flatulence | 22 (27.8) | 3 (3.8) | <0.0001 | 7 (17.1) | 1 (2.4) | 0.0313 | 1.14 (0.08; 64.77) | 1.00 | 29 (24.2) | 4 (3.3) | <0.0001 |
| Nausea/vomiting | 2 (2.5) | 2 (2.5) | 1.00 | 0 (0.0) | 1 (2.4) | 1.00 | 1.07 (0.06; 64.48) | 1.00 | 2 (1.7) | 3 (2.5) | 1.00 |
| Problems sleeping in | 23 (29.1) | 13 (16.5) | 0.0476 | 10 (24.4) | 4 (9.8) | 0.0313 | 1.73 (0.47; 7.97) | 0.55 | 33 (27.5) | 17 (14.2) | 0.0030 |
| Problems sleeping on | 11 (13.9) | 4 (5.1) | 0.0391 | 7 (17.1) | 2 (4.9) | 0.18 | 1.14 (0.15; 13.59) | 1.00 | 18 (15.0) | 6 (5.0) | 0.0075 |
| Nocturnal enuresis | 8 (10.1) | 3 (3.8) | 0.06 | 2 (4.9) | 0 (0.0) | 0.50 | 1.13 (0.12; infinite) | 0.47 | 10 (8.3) | 3 (2.5) | 0.0156 |
| Total number of physical complaints (average; minimum-maximum) | 233 (2.95; 0–9) | 138 (1.74; 0–8) | NV | 117 (2.85; 0–8) | 46 (1.12; 0–6) | NV | 1.60 4 (1.04; 2.47) | 0.0338 4 | 350 (2.92; 0–9) | 184 (1.53; 0–8) | NV |
| Daytime urinary incontinence 5 | 7 (8.9) | 5 (6.3) | 0.69 | ||||||||
| Faecal incontinence 5 | 5 (6.3) | 0 (0.0) | 0.06 | ||||||||
| Physical Complaints | INCA RCT | 2010 RCT |
|---|---|---|
| Headache | 0 | 0 |
| Abdominal pain | 0 | + |
| Growing pain | 0 | 0 |
| Unusual thirst | + | + |
| Unusual perspiration (at night or daytime) | + | + |
| Often warm | + | + |
| Eczema | + | + |
| Asthma | 0 | NA |
| Persisting cold (rhinitis) | + | NA |
| Blotches in the face | NA | + |
| Red edged mouth | 0 | 0 |
| Red ears | 0 | + |
| Bags under eyes | + | 0 |
| Often tired | 0 | + |
| Diarrhoea | + | + |
| Constipation | 0 | NA |
| Flatulence | + | + |
| Nausea/vomiting | 0 | NA |
| Problems sleeping in | 0 | + |
| Problems sleeping on | + | + |
| Nocturnal enuresis | + | NA |
| Total number of physical complaints (average; minimum-maximum) | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelsser, L.; Stobernack, T.; Frankena, K. Physical Complaints Decrease after Following a Few-Foods Diet in Children with ADHD. Nutrients 2022, 14, 3036. https://doi.org/10.3390/nu14153036
Pelsser L, Stobernack T, Frankena K. Physical Complaints Decrease after Following a Few-Foods Diet in Children with ADHD. Nutrients. 2022; 14(15):3036. https://doi.org/10.3390/nu14153036
Chicago/Turabian StylePelsser, Lidy, Tim Stobernack, and Klaas Frankena. 2022. "Physical Complaints Decrease after Following a Few-Foods Diet in Children with ADHD" Nutrients 14, no. 15: 3036. https://doi.org/10.3390/nu14153036
APA StylePelsser, L., Stobernack, T., & Frankena, K. (2022). Physical Complaints Decrease after Following a Few-Foods Diet in Children with ADHD. Nutrients, 14(15), 3036. https://doi.org/10.3390/nu14153036






