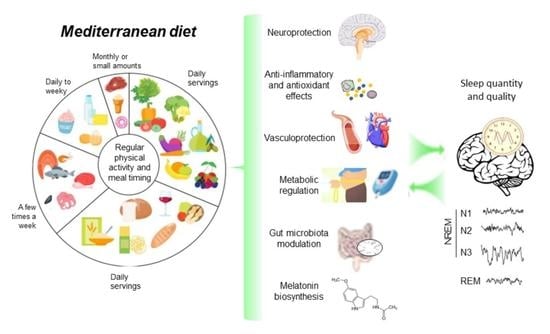
Mediterranean Diet on Sleep: A Health Alliance
Abstract
:1. Introduction
2. Literature Search Methods
3. Main Findings
4. Potential Mechanisms Underlying the Mediterranean Diet Effects on Sleep
4.1. Metabolic and Vascular Improvements
4.2. Blunting of Inflammation and Oxidative Stress
4.3. Neuroprotection
4.4. Melatonin Biosynthesis
4.5. Microbiota Modulation
5. Future Perspective and Conclusions
- large prospective cohort studies are needed to assess the preventive effect of the Mediterranean diet on sleep and identify the potential qualitative and quantitative contribution of typical foods and nutrients;
- clinical intervention studies with the Mediterranean diet as exposure and sleep as outcome in large samples are needed to confirm associations and provide causality;
- preclinical studies in animal models could provide insights into mechanisms and pathways mediating the benefit of the Mediterranean diet on sleep features;
- objective neurophysiological tools for sleep assessment (actigraphy, polysomnography) should be used in the scientific studies;
- the effects of meal timing and frequency, and the influence of individuals’ chronotype in the relation of the Mediterranean diet and sleep should be investigated;
- more studies on both genders and in different age groups, including during pregnancy, are needed to substantiate the influence of the Mediterranean diet on sleep as a life-long exposome;
- the influence of risk factors, such as overweight/obesity, on the relation between the Mediterranean diet and sleep requires investigation;
- the role of gut microbiota in the Mediterranean diet–brain–sleep axis should be further studied, using strategies such as fecal microbiota transplantation, in order to assess whether and how the modulation of gut bacteria ecology by the Mediterranean diet could mediate the effects on sleep, potentially providing new therapeutic pathways and biomarkers.
- public health policy and health promotion programs should include focused attention on diet, especially the Mediterranean diet, and sleep.
- knowledge and eventually education about the diet–sleep relation should be improved among healthcare professionals including nutrition professionals, in order to implement the discussion of this topic with patients during routine healthcare practices in promoting the adoption of healthy and protective lifestyles.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santos, L. The impact of nutrition and lifestyle modification on health. Eur. J. Intern. Med. 2021, 97, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Koehler, K.; Drenowatz, C. Integrated Role of Nutrition and Physical Activity for Lifelong Health. Nutrients 2019, 11, 1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [Green Version]
- English, L.K.; Ard, J.D.; Bailey, R.L.; Bates, M.; Bazzano, L.A.; Boushey, C.J.; Brown, C.; Butera, G.; Callahan, E.H.; de Jesus, J.; et al. Evaluation of Dietary Patterns and All-Cause Mortality: A Systematic Review. JAMA Netw. Open 2021, 4, e2122277. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Appel, L.J.; Vadiveloo, M.; Hu, F.B.; Kris-Etherton, P.M.; Rebholz, C.M.; Sacks, F.M.; Thorndike, A.N.; Van Horn, L.; Wylie-Rosett, J.; et al. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e472–e487. [Google Scholar] [CrossRef]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2018, 72, 30–43. [Google Scholar] [CrossRef]
- Guasch-Ferre, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. Br. J. Pharmacol. 2020, 177, 1241–1257. [Google Scholar] [CrossRef] [Green Version]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [Green Version]
- Trichopoulou, A.; Martinez-Gonzalez, M.A.; Tong, T.Y.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Lorgeril, M. Definitions and potential health benefits of the Mediterranean diet: Views from experts around the world. BMC Med. 2014, 12, 112. [Google Scholar] [CrossRef] [Green Version]
- Luyster, F.S.; Strollo, P.J., Jr.; Zee, P.C.; Walsh, J.K.; Boards of Directors of the American Academy of Sleep Medicine; Sleep Research Society. Sleep: A health imperative. Sleep 2012, 35, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Grigg-Damberger, M.M. The AASM Scoring Manual four years later. J. Clin. Sleep Med. 2012, 8, 323–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grandner, M.A. Sleep, Health, and Society. Sleep Med. Clin. 2017, 12, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Consensus Conference Panel; Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; et al. Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society on the Recommended Amount of Sleep for a Healthy Adult: Methodology and Discussion. J. Clin. Sleep Med. 2015, 11, 931–952. [Google Scholar] [CrossRef] [PubMed]
- Consensus Conference Panel; Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; et al. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. J. Clin. Sleep Med. 2015, 11, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Cunningham, T.J.; Croft, J.B. Trends in Self-Reported Sleep Duration among US Adults from 1985 to 2012. Sleep 2015, 38, 829–832. [Google Scholar] [CrossRef]
- Matricciani, L.; Olds, T.; Petkov, J. In search of lost sleep: Secular trends in the sleep time of school-aged children and adolescents. Sleep Med. Rev. 2012, 16, 203–211. [Google Scholar] [CrossRef]
- Wheaton, A.G.; Jones, S.E.; Cooper, A.C.; Croft, J.B. Short Sleep Duration Among Middle School and High School Students—United States, 2015. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Zomers, M.L.; Hulsegge, G.; van Oostrom, S.H.; Proper, K.I.; Verschuren, W.M.M.; Picavet, H.S.J. Characterizing Adult Sleep Behavior Over 20 Years-The Population-Based Doetinchem Cohort Study. Sleep 2017, 40, zsx085. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; Miller, M.A. Sleep and Cardio-Metabolic Disease. Curr. Cardiol. Rep. 2017, 19, 110. [Google Scholar] [CrossRef] [Green Version]
- Smagula, S.F.; Stone, K.L.; Redline, S.; Ancoli-Israel, S.; Barrett-Connor, E.; Lane, N.E.; Orwoll, E.S.; Cauley, J.A.; Osteoporotic Fractures in Men (MrOS) Research Group. Actigraphy- and Polysomnography-Measured Sleep Disturbances, Inflammation, and Mortality Among Older Men. Psychosom. Med. 2016, 78, 686–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Leeuwen, W.M.; Lehto, M.; Karisola, P.; Lindholm, H.; Luukkonen, R.; Sallinen, M.; Harma, M.; Porkka-Heiskanen, T.; Alenius, H. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS ONE 2009, 4, e4589. [Google Scholar] [CrossRef] [Green Version]
- Vgontzas, A.N.; Liao, D.; Bixler, E.O.; Chrousos, G.P.; Vela-Bueno, A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep 2009, 32, 491–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garbarino, S.; Lanteri, P.; Bragazzi, N.L.; Magnavita, N.; Scoditti, E. Role of sleep deprivation in immune-related disease risk and outcomes. Commun. Biol. 2021, 4, 1304. [Google Scholar] [CrossRef] [PubMed]
- Aldabal, L.; Bahammam, A.S. Metabolic, endocrine, and immune consequences of sleep deprivation. Open Respir. Med. J. 2011, 5, 31–43. [Google Scholar] [CrossRef] [Green Version]
- Irwin, M.R. Sleep and inflammation: Partners in sickness and in health. Nat. Rev. Immunol. 2019, 19, 702–715. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Grandner, M.A.; Brown, D.; Conroy, M.B.; Jean-Louis, G.; Coons, M.; Bhatt, D.L.; American Heart Association Obesity, Behavior Change, Diabetes; Nutrition Committees of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; et al. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e367–e386. [Google Scholar] [CrossRef] [Green Version]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Back, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Hoevenaar-Blom, M.P.; Spijkerman, A.M.; Kromhout, D.; Verschuren, W.M. Sufficient sleep duration contributes to lower cardiovascular disease risk in addition to four traditional lifestyle factors: The MORGEN study. Eur. J. Prev. Cardiol. 2014, 21, 1367–1375. [Google Scholar] [CrossRef]
- Theorell-Haglow, J.; Lemming, E.W.; Michaelsson, K.; Elmstahl, S.; Lind, L.; Lindberg, E. Sleep duration is associated with healthy diet scores and meal patterns: Results from the population-based EpiHealth study. J. Clin. Sleep Med. 2020, 16, 9–18. [Google Scholar] [CrossRef]
- Zuraikat, F.M.; Makarem, N.; Liao, M.; St-Onge, M.P.; Aggarwal, B. Measures of Poor Sleep Quality Are Associated with Higher Energy Intake and Poor Diet Quality in a Diverse Sample of Women from the Go Red for Women Strategically Focused Research Network. J. Am. Heart Assoc. 2020, 9, e014587. [Google Scholar] [CrossRef] [PubMed]
- Zuraikat, F.M.; Wood, R.A.; Barragan, R.; St-Onge, M.P. Sleep and Diet: Mounting Evidence of a Cyclical Relationship. Annu. Rev. Nutr. 2021, 41, 309–332. [Google Scholar] [CrossRef] [PubMed]
- Binks, H.; Vincent, G.E.; Gupta, C.; Irwin, C.; Khalesi, S. Effects of Diet on Sleep: A Narrative Review. Nutrients 2020, 12, 936. [Google Scholar] [CrossRef] [Green Version]
- Godos, J.; Grosso, G.; Castellano, S.; Galvano, F.; Caraci, F.; Ferri, R. Association between diet and sleep quality: A systematic review. Sleep Med. Rev. 2021, 57, 101430. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.C.; Irwin, C.; Vincent, G.E.; Khalesi, S. The Relationship Between Diet and Sleep in Older Adults: A Narrative Review. Curr. Nutr. Rep. 2021, 10, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Peuhkuri, K.; Sihvola, N.; Korpela, R. Diet promotes sleep duration and quality. Nutr. Res. 2012, 32, 309–319. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Crawford, A.; Aggarwal, B. Plant-based diets: Reducing cardiovascular risk by improving sleep quality? Curr. Sleep Med. Rep. 2018, 4, 74–78. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Mikic, A.; Pietrolungo, C.E. Effects of Diet on Sleep Quality. Adv. Nutr. 2016, 7, 938–949. [Google Scholar] [CrossRef]
- Sutanto, C.N.; Wang, M.X.; Tan, D.; Kim, J.E. Association of Sleep Quality and Macronutrient Distribution: A Systematic Review and Meta-Regression. Nutrients 2020, 12, 126. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Cao, Y.; Taylor, A.W.; Wittert, G.; Adams, R.; Shi, Z. Dietary patterns and sleep parameters in a cohort of community dwelling Australian men. Asia Pac. J. Clin. Nutr. 2017, 26, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Jansen, E.C.; Baylin, A.; Cantoral, A.; Tellez Rojo, M.M.; Burgess, H.J.; O’Brien, L.M.; Torres Olascoaga, L.; Peterson, K.E. Dietary Patterns in Relation to Prospective Sleep Duration and Timing among Mexico City Adolescents. Nutrients 2020, 12, 2305. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.A.; Mirzababaei, A.; Shiraseb, F.; Clark, C.C.T.; Mirzaei, K. The association between modified Nordic diet with sleep quality and circadian rhythm in overweight and obese woman: A cross-sectional study. Eat Weight Disord. 2021, 27, 1835–1845. [Google Scholar] [CrossRef]
- Rostami, H.; Khayyatzadeh, S.S.; Tavakoli, H.; Bagherniya, M.; Mirmousavi, S.J.; Farahmand, S.K.; Tayefi, M.; Ferns, G.A.; Ghayour-Mobarhan, M. The relationship between adherence to a Dietary Approach to Stop Hypertension (DASH) dietary pattern and insomnia. BMC Psychiatry 2019, 19, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adelantado-Renau, M.; Beltran-Valls, M.R.; Esteban-Cornejo, I.; Martinez-Vizcaino, V.; Santaliestra-Pasias, A.M.; Moliner-Urdiales, D. The influence of adherence to the Mediterranean diet on academic performance is mediated by sleep quality in adolescents. Acta Paediatr. 2019, 108, 339–346. [Google Scholar] [CrossRef]
- Arriscado, D.; Knox, E.; Zabala, M.; Zurita-Ortega, F.; Dalmau, J.M.; Muros, J.J. Different healthy habits between northern and southern Spanish school children. Z. Gesundh. 2017, 25, 653–660. [Google Scholar] [CrossRef] [Green Version]
- Campanini, M.Z.; Guallar-Castillon, P.; Rodriguez-Artalejo, F.; Lopez-Garcia, E. Mediterranean Diet and Changes in Sleep Duration and Indicators of Sleep Quality in Older Adults. Sleep 2017, 40, zsw083. [Google Scholar] [CrossRef]
- Castro-Diehl, C.; Wood, A.C.; Redline, S.; Reid, M.; Johnson, D.A.; Maras, J.E.; Jacobs, D.R., Jr.; Shea, S.; Crawford, A.; St-Onge, M.P. Mediterranean diet pattern and sleep duration and insomnia symptoms in the Multi-Ethnic Study of Atherosclerosis. Sleep 2018, 41, zsy158. [Google Scholar] [CrossRef]
- Ferranti, R.; Marventano, S.; Castellano, S.; Giogianni, G.; Nolfo, F.; Rametta, S.; Matalone, M.; Mistretta, A. Sleep quality and duration is related with diet and obesity in young adolescent living in Sicily, Southern Italy. Sleep Sci. 2016, 9, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Flor-Alemany, M.; Nestares, T.; Alemany-Arrebola, I.; Marin-Jimenez, N.; Borges-Cosic, M.; Aparicio, V.A. Influence of Dietary Habits and Mediterranean Diet Adherence on Sleep Quality during Pregnancy. The GESTAFIT Project. Nutrients 2020, 12, 3569. [Google Scholar] [CrossRef]
- Godos, J.; Ferri, R.; Caraci, F.; Cosentino, F.I.I.; Castellano, S.; Galvano, F.; Grosso, G. Adherence to the Mediterranean Diet is Associated with Better Sleep Quality in Italian Adults. Nutrients 2019, 11, 976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, K.; Jansen, E.C.; Campos, H.; Baylin, A. Associations between sleep duration and Mediterranean diet score in Costa Rican adults. Appetite 2022, 170, 105881. [Google Scholar] [CrossRef] [PubMed]
- Jaussent, I.; Dauvilliers, Y.; Ancelin, M.L.; Dartigues, J.F.; Tavernier, B.; Touchon, J.; Ritchie, K.; Besset, A. Insomnia symptoms in older adults: Associated factors and gender differences. Am. J. Geriatr. Psychiatry 2011, 19, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Mamalaki, E.; Anastasiou, C.A.; Ntanasi, E.; Tsapanou, A.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.M.; Sakka, P.; Scarmeas, N.; Yannakoulia, M. Associations between the mediterranean diet and sleep in older adults: Results from the hellenic longitudinal investigation of aging and diet study. Geriatr. Gerontol. Int. 2018, 18, 1543–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muscogiuri, G.; Barrea, L.; Aprano, S.; Framondi, L.; Di Matteo, R.; Laudisio, D.; Pugliese, G.; Savastano, S.; Colao, A.; OPERA PREVENTION Project. Sleep Quality in Obesity: Does Adherence to the Mediterranean Diet Matter? Nutrients 2020, 12, 1364. [Google Scholar] [CrossRef] [PubMed]
- Naja, F.; Hasan, H.; Khadem, S.H.; Buanq, M.A.; Al-Mulla, H.K.; Aljassmi, A.K.; Faris, M.E. Adherence to the Mediterranean Diet and Its Association with Sleep Quality and Chronotype Among Youth: A Cross-Sectional Study. Front. Nutr. 2021, 8, 805955. [Google Scholar] [CrossRef]
- O’Connor, L.E.; Biberstine, S.L.; Paddon-Jones, D.; Schwichtenberg, A.J.; Campbell, W.W. Adopting a Mediterranean-Style Eating Pattern with Different Amounts of Lean Unprocessed Red Meat Does Not Influence Short-Term Subjective Personal Well-Being in Adults with Overweight or Obesity. J. Nutr. 2018, 148, 1917–1923. [Google Scholar] [CrossRef]
- Rosi, A.; Giopp, F.; Milioli, G.; Melegari, G.; Goldoni, M.; Parrino, L.; Scazzina, F. Weight Status, Adherence to the Mediterranean Diet, Physical Activity Level, and Sleep Behavior of Italian Junior High School Adolescents. Nutrients 2020, 12, 478. [Google Scholar] [CrossRef] [Green Version]
- Van Egmond, L.; Tan, X.; Sjogren, P.; Cederholm, T.; Benedict, C. Association between Healthy Dietary Patterns and Self-Reported Sleep Disturbances in Older Men: The ULSAM Study. Nutrients 2019, 11, 1029. [Google Scholar] [CrossRef] [Green Version]
- Zaidalkilani, A.T.; Alhaj, O.A.; Serag El-Dine, M.F.; Fekih-Romdhane, F.; AlRasheed, M.M.; Jahrami, H.A.; Bragazzi, N.L. Arab Women Adherence to the Mediterranean Diet and Insomnia. Medicina 2021, 58, 17. [Google Scholar] [CrossRef]
- Zuraikat, F.M.; Makarem, N.; St-Onge, M.P.; Xi, H.; Akkapeddi, A.; Aggarwal, B. A Mediterranean Dietary Pattern Predicts Better Sleep Quality in US Women from the American Heart Association Go Red for Women Strategically Focused Research Network. Nutrients 2020, 12, 2830. [Google Scholar] [CrossRef] [PubMed]
- Hutchins-Wiese, H.L.; Bales, C.W.; Porter Starr, K.N. Mediterranean diet scoring systems: Understanding the evolution and applications for Mediterranean and non-Mediterranean countries. Br. J. Nutr. 2021, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroder, H.; Fito, M.; Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Lamuela-Raventos, R.; Ros, E.; Salaverria, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [Green Version]
- Sofi, F.; Dinu, M.; Pagliai, G.; Marcucci, R.; Casini, A. Validation of a literature-based adherence score to Mediterranean diet: The MEDI-LITE score. Int. J. Food Sci. Nutr. 2017, 68, 757–762. [Google Scholar] [CrossRef]
- Pengo, M.F.; Won, C.H.; Bourjeily, G. Sleep in Women Across the Life Span. Chest 2018, 154, 196–206. [Google Scholar] [CrossRef]
- Okun, M.L.; Schetter, C.D.; Glynn, L.M. Poor sleep quality is associated with preterm birth. Sleep 2011, 34, 1493–1498. [Google Scholar] [CrossRef]
- Sedov, I.D.; Cameron, E.E.; Madigan, S.; Tomfohr-Madsen, L.M. Sleep quality during pregnancy: A meta-analysis. Sleep Med. Rev. 2018, 38, 168–176. [Google Scholar] [CrossRef]
- Benetou, V.; Kanellopoulou, A.; Kanavou, E.; Fotiou, A.; Stavrou, M.; Richardson, C.; Orfanos, P.; Kokkevi, A. Diet-Related Behaviors and Diet Quality among School-Aged Adolescents Living in Greece. Nutrients 2020, 12, 3804. [Google Scholar] [CrossRef]
- Kechribari, I.; Kontogianni, M.D.; Georgoulis, M.; Lamprou, K.; Critselis, E.; Vagiakis, E.; Yiannakouris, N. Association of adherence to the Mediterranean diet and physical activity habits with the presence of insomnia in patients with obstructive sleep apnea. Sleep Breath. 2021, 26, 89–97. [Google Scholar] [CrossRef]
- Bertisch, S.M.; Pollock, B.D.; Mittleman, M.A.; Buysse, D.J.; Bazzano, L.A.; Gottlieb, D.J.; Redline, S. Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: Sleep Heart Health Study. Sleep 2018, 41, zsy047. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E. The role of the Mediterranean diet on weight loss and obesity-related diseases. Rev. Endocr. Metab. Disord. 2020, 21, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, K.; Saneei, P.; Hajhashemy, Z.; Esmaillzadeh, A. Adherence to the Mediterranean Diet, Five-Year Weight Change, and Risk of Overweight and Obesity: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. 2021, 13, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Mancini, J.G.; Filion, K.B.; Atallah, R.; Eisenberg, M.J. Systematic Review of the Mediterranean Diet for Long-Term Weight Loss. Am. J. Med. 2016, 129, 407–415.e404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katagiri, R.; Asakura, K.; Kobayashi, S.; Suga, H.; Sasaki, S. Low intake of vegetables, high intake of confectionary, and unhealthy eating habits are associated with poor sleep quality among middle-aged female Japanese workers. J. Occup. Health 2014, 56, 359–368. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Roberts, A.; Shechter, A.; Choudhury, A.R. Fiber and Saturated Fat Are Associated with Sleep Arousals and Slow Wave Sleep. J. Clin. Sleep Med. 2016, 12, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Kurotani, K.; Kochi, T.; Nanri, A.; Eguchi, M.; Kuwahara, K.; Tsuruoka, H.; Akter, S.; Ito, R.; Pham, N.M.; Kabe, I.; et al. Dietary patterns and sleep symptoms in Japanese workers: The Furukawa Nutrition and Health Study. Sleep Med. 2015, 16, 298–304. [Google Scholar] [CrossRef]
- Noorwali, E.; Hardie, L.; Cade, J. Bridging the Reciprocal Gap between Sleep and Fruit and Vegetable Consumption: A Review of the Evidence, Potential Mechanisms, Implications, and Directions for Future Work. Nutrients 2019, 11, 1382. [Google Scholar] [CrossRef] [Green Version]
- Hysing, M.; Kvestad, I.; Kjellevold, M.; Kolden Midtbo, L.; Graff, I.E.; Lie, O.; Hurum, H.; Stormark, K.M.; Oyen, J. Fatty Fish Intake and the Effect on Mental Health and Sleep in Preschool Children in FINS-KIDS, a Randomized Controlled Trial. Nutrients 2018, 10, 1478. [Google Scholar] [CrossRef] [Green Version]
- Del Brutto, O.H.; Mera, R.M.; Ha, J.E.; Gillman, J.; Zambrano, M.; Castillo, P.R. Dietary fish intake and sleep quality: A population-based study. Sleep Med. 2016, 17, 126–128. [Google Scholar] [CrossRef]
- Hansen, A.L.; Dahl, L.; Olson, G.; Thornton, D.; Graff, I.E.; Froyland, L.; Thayer, J.F.; Pallesen, S. Fish consumption, sleep, daily functioning, and heart rate variability. J. Clin. Sleep Med. 2014, 10, 567–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montgomery, P.; Burton, J.R.; Sewell, R.P.; Spreckelsen, T.F.; Richardson, A.J. Fatty acids and sleep in UK children: Subjective and pilot objective sleep results from the DOLAB study—A randomized controlled trial. J. Sleep Res. 2014, 23, 364–388. [Google Scholar] [CrossRef]
- Jansen, E.C.; Conroy, D.A.; Burgess, H.J.; O’Brien, L.M.; Cantoral, A.; Tellez-Rojo, M.M.; Peterson, K.E.; Baylin, A. Plasma DHA Is Related to Sleep Timing and Duration in a Cohort of Mexican Adolescents. J. Nutr. 2020, 150, 592–598. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomas-Barberan, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [Green Version]
- Tresserra-Rimbau, A.; Medina-Remon, A.; Perez-Jimenez, J.; Martinez-Gonzalez, M.A.; Covas, M.I.; Corella, D.; Salas-Salvado, J.; Gomez-Gracia, E.; Lapetra, J.; Aros, F.; et al. Dietary intake and major food sources of polyphenols in a Spanish population at high cardiovascular risk: The PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 953–959. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Cao, Y.; Taylor, A.W.; Zhen, S.; Adams, R.; Appleton, S.; Shi, Z. Soy Isoflavone Intake and Sleep Parameters over 5 Years among Chinese Adults: Longitudinal Analysis from the Jiangsu Nutrition Study. J. Acad. Nutr. Diet. 2017, 117, 536–544.e532. [Google Scholar] [CrossRef]
- Cui, Y.; Niu, K.; Huang, C.; Momma, H.; Guan, L.; Kobayashi, Y.; Guo, H.; Chujo, M.; Otomo, A.; Nagatomi, R. Relationship between daily isoflavone intake and sleep in Japanese adults: A cross-sectional study. Nutr. J. 2015, 14, 127. [Google Scholar] [CrossRef] [Green Version]
- Godos, J.; Ferri, R.; Castellano, S.; Angelino, D.; Mena, P.; Del Rio, D.; Caraci, F.; Galvano, F.; Grosso, G. Specific Dietary (Poly)phenols Are Associated with Sleep Quality in a Cohort of Italian Adults. Nutrients 2020, 12, 1226. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Simonetta, I.; Daidone, M.; Mogavero, A.; Ortello, A.; Pinto, A. Metabolic and Vascular Effect of the Mediterranean Diet. Int. J. Mol. Sci. 2019, 20, 4716. [Google Scholar] [CrossRef] [Green Version]
- Esposito, K.; Maiorino, M.I.; Ceriello, A.; Giugliano, D. Prevention and control of type 2 diabetes by Mediterranean diet: A systematic review. Diabetes Res. Clin. Pract. 2010, 89, 97–102. [Google Scholar] [CrossRef]
- Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Ruiz-Gutierrez, V.; Covas, M.I.; Fiol, M.; Gomez-Gracia, E.; Lopez-Sabater, M.C.; Vinyoles, E.; et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef]
- Storniolo, C.E.; Casillas, R.; Bullo, M.; Castaner, O.; Ros, E.; Saez, G.T.; Toledo, E.; Estruch, R.; Ruiz-Gutierrez, V.; Fito, M.; et al. A Mediterranean diet supplemented with extra virgin olive oil or nuts improves endothelial markers involved in blood pressure control in hypertensive women. Eur. J. Nutr. 2017, 56, 89–97. [Google Scholar] [CrossRef]
- Carroll, C.M.; Macauley, S.L. The Interaction Between Sleep and Metabolism in Alzheimer’s Disease: Cause or Consequence of Disease? Front. Aging Neurosci. 2019, 11, 258. [Google Scholar] [CrossRef]
- MacIntosh, B.J.; Shirzadi, Z.; Atwi, S.; Detre, J.A.; Dolui, S.; Bryan, R.N.; Launer, L.J.; Swardfager, W. Metabolic and vascular risk factors are associated with reduced cerebral blood flow and poorer midlife memory performance. Hum. Brain Mapp. 2020, 41, 855–864. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.X.; Zhang, T.; Hou, X.H.; Ou, Y.N.; Hu, H.; Wang, Z.T.; Guo, Y.; Xu, W.; Tan, L.; Yu, J.T.; et al. Associations of Vascular Risk with Cognition, Brain Glucose Metabolism, and Clinical Progression in Cognitively Intact Elders. J. Alzheimers Dis. 2021, 80, 321–330. [Google Scholar] [CrossRef]
- Boccellino, M.; D’Angelo, S. Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities. Int. J. Mol. Sci. 2020, 21, 5642. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Bixler, E.O.; Chrousos, G.P. Sleep apnea is a manifestation of the metabolic syndrome. Sleep Med. Rev. 2005, 9, 211–224. [Google Scholar] [CrossRef]
- Jordan, A.S.; McSharry, D.G.; Malhotra, A. Adult obstructive sleep apnoea. Lancet 2014, 383, 736–747. [Google Scholar] [CrossRef] [Green Version]
- Papandreou, C.; Schiza, S.E.; Bouloukaki, I.; Hatzis, C.M.; Kafatos, A.G.; Siafakas, N.M.; Tzanakis, N.E. Effect of Mediterranean diet versus prudent diet combined with physical activity on OSAS: A randomised trial. Eur. Respir. J. 2012, 39, 1398–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgoulis, M.; Yiannakouris, N.; Tenta, R.; Fragopoulou, E.; Kechribari, I.; Lamprou, K.; Perraki, E.; Vagiakis, E.; Kontogianni, M.D. A weight-loss Mediterranean diet/lifestyle intervention ameliorates inflammation and oxidative stress in patients with obstructive sleep apnea: Results of the “MIMOSA” randomized clinical trial. Eur. J. Nutr. 2021, 60, 3799–3810. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Mediterranean dietary pattern, inflammation and endothelial function: A systematic review and meta-analysis of intervention trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 929–939. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Christoph, M.; Hoffmann, G. Effects of Olive Oil on Markers of Inflammation and Endothelial Function—A Systematic Review and Meta-Analysis. Nutrients 2015, 7, 7651–7675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scoditti, E.; Capurso, C.; Capurso, A.; Massaro, M. Vascular effects of the Mediterranean diet-part II: Role of omega-3 fatty acids and olive oil polyphenols. Vasc. Pharmacol. 2014, 63, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Gardener, H.; Wright, C.B.; Rundek, T.; Sacco, R.L. Brain health and shared risk factors for dementia and stroke. Nat. Rev. Neurol. 2015, 11, 651–657. [Google Scholar] [CrossRef]
- Klohs, J. An Integrated View on Vascular Dysfunction in Alzheimer’s Disease. Neurodegener. Dis. 2019, 19, 109–127. [Google Scholar] [CrossRef]
- Naiberg, M.R.; Newton, D.F.; Goldstein, B.I. Flow-Mediated Dilation and Neurocognition: Systematic Review and Future Directions. Psychosom. Med. 2016, 78, 192–207. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef] [Green Version]
- Cherubini, J.M.; Cheng, J.L.; Williams, J.S.; MacDonald, M.J. Sleep deprivation and endothelial function: Reconciling seminal evidence with recent perspectives. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H29–H35. [Google Scholar] [CrossRef]
- Quick, S.; Moss, J.; Rajani, R.M.; Williams, A. A Vessel for Change: Endothelial Dysfunction in Cerebral Small Vessel Disease. Trends Neurosci. 2021, 44, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Faraguna, U.; Vyazovskiy, V.V.; Nelson, A.B.; Tononi, G.; Cirelli, C. A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J. Neurosci. 2008, 28, 4088–4095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giese, M.; Unternahrer, E.; Huttig, H.; Beck, J.; Brand, S.; Calabrese, P.; Holsboer-Trachsler, E.; Eckert, A. BDNF: An indicator of insomnia? Mol. Psychiatry 2014, 19, 151–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marie, C.; Pedard, M.; Quirie, A.; Tessier, A.; Garnier, P.; Totoson, P.; Demougeot, C. Brain-derived neurotrophic factor secreted by the cerebral endothelium: A new actor of brain function? J. Cereb. Blood Flow Metab. 2018, 38, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Villegas, A.; Galbete, C.; Martinez-Gonzalez, M.A.; Martinez, J.A.; Razquin, C.; Salas-Salvado, J.; Estruch, R.; Buil-Cosiales, P.; Marti, A. The effect of the Mediterranean diet on plasma brain-derived neurotrophic factor (BDNF) levels: The PREDIMED-NAVARRA randomized trial. Nutr. Neurosci. 2011, 14, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef]
- Agostinho, P.; Cunha, R.A.; Oliveira, C. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Curr. Pharm. Des. 2010, 16, 2766–2778. [Google Scholar] [CrossRef]
- Leonard, B.E. Inflammation and depression: A causal or coincidental link to the pathophysiology? Acta Neuropsychiatr. 2018, 30, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Clark, I.A.; Vissel, B. Inflammation-sleep interface in brain disease: TNF, insulin, orexin. J. Neuroinflamm. 2014, 11, 51. [Google Scholar] [CrossRef] [Green Version]
- Kanagasabai, T.; Ardern, C.I. Contribution of Inflammation, Oxidative Stress, and Antioxidants to the Relationship between Sleep Duration and Cardiometabolic Health. Sleep 2015, 38, 1905–1912. [Google Scholar] [CrossRef]
- Pourreza, S.; Khademi, Z.; Mirzababaei, A.; Yekaninejad, M.S.; Sadeghniiat-Haghighi, K.; Naghshi, S.; Mirzaei, K. Association of plant-based diet index with inflammatory markers and sleep quality in overweight and obese female adults: A cross-sectional study. Int. J. Clin. Pract. 2021, 75, e14429. [Google Scholar] [CrossRef]
- Kanagasabai, T.; Ardern, C.I. Inflammation, Oxidative Stress, and Antioxidants Contribute to Selected Sleep Quality and Cardiometabolic Health Relationships: A Cross-Sectional Study. Mediat. Inflamm. 2015, 2015, 824589. [Google Scholar] [CrossRef] [Green Version]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hebert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [Green Version]
- Godos, J.; Ferri, R.; Caraci, F.; Cosentino, F.I.I.; Castellano, S.; Shivappa, N.; Hebert, J.R.; Galvano, F.; Grosso, G. Dietary Inflammatory Index and Sleep Quality in Southern Italian Adults. Nutrients 2019, 11, 1324. [Google Scholar] [CrossRef] [Green Version]
- Bazyar, H.; Zare Javid, A.; Bavi Behbahani, H.; Shivappa, N.; Hebert, J.R.; Khodaramhpour, S.; Khaje Zadeh, S.; Aghamohammadi, V. The association between dietary inflammatory index with sleep quality and obesity amongst iranian female students: A cross-sectional study. Int. J. Clin. Pract. 2021, 75, e14061. [Google Scholar] [CrossRef]
- Masaad, A.A.; Yusuf, A.M.; Shakir, A.Z.; Khan, M.S.; Khaleel, S.; Cheikh Ismail, L.; Faris, M.A.E.; Jahrami, H.A.; Shivappa, N.; Hebert, J.R.; et al. Sleep quality and Dietary Inflammatory Index among university students: A cross-sectional study. Sleep Breath. 2021, 25, 2221–2229. [Google Scholar] [CrossRef]
- Lopes, T.V.C.; Borba, M.E.S.; Lopes, R.V.C.; Fisberg, R.M.; Paim, S.L.; Teodoro, V.V.; Zimberg, I.Z.; Araujo, L.B.; Shivappa, N.; Hebert, J.R.; et al. Association between inflammatory potential of the diet and sleep parameters in sleep apnea patients. Nutrition 2019, 66, 5–10. [Google Scholar] [CrossRef]
- Kase, B.E.; Liu, J.; Wirth, M.D.; Shivappa, N.; Hebert, J.R. Associations between dietary inflammatory index and sleep problems among adults in the United States, NHANES 2005–2016. Sleep Health 2021, 7, 273–280. [Google Scholar] [CrossRef]
- Wirth, M.D.; Jessup, A.; Turner-McGrievy, G.; Shivappa, N.; Hurley, T.G.; Hebert, J.R. Changes in dietary inflammatory potential predict changes in sleep quality metrics, but not sleep duration. Sleep 2020, 43, zsaa093. [Google Scholar] [CrossRef]
- Gordon-Dseagu, V.L.Z.; Derkach, A.; Xiao, Q.; Williams, I.; Sampson, J.; Stolzenberg-Solomon, R.Z. The association of sleep with metabolic pathways and metabolites: Evidence from the Dietary Approaches to Stop Hypertension (DASH)-sodium feeding study. Metabolomics 2019, 15, 48. [Google Scholar] [CrossRef]
- Abshirini, M.; Siassi, F.; Koohdani, F.; Qorbani, M.; Khosravi, S.; Hedayati, M.; Aslani, Z.; Soleymani, M.; Sotoudeh, G. Dietary total antioxidant capacity is inversely related to menopausal symptoms: A cross-sectional study among Iranian postmenopausal women. Nutrition 2018, 55–56, 161–167. [Google Scholar] [CrossRef]
- Daneshzad, E.; Keshavarz, S.A.; Qorbani, M.; Larijani, B.; Azadbakht, L. Dietary total antioxidant capacity and its association with sleep, stress, anxiety, and depression score: A cross-sectional study among diabetic women. Clin. Nutr. ESPEN 2020, 37, 187–194. [Google Scholar] [CrossRef]
- Billingsley, H.E.; Carbone, S. The antioxidant potential of the Mediterranean diet in patients at high cardiovascular risk: An in-depth review of the PREDIMED. Nutr. Diabetes 2018, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Gantenbein, K.V.; Kanaka-Gantenbein, C. Mediterranean Diet as an Antioxidant: The Impact on Metabolic Health and Overall Wellbeing. Nutrients 2021, 13, 1951. [Google Scholar] [CrossRef]
- Pitsavos, C.; Panagiotakos, D.B.; Tzima, N.; Chrysohoou, C.; Economou, M.; Zampelas, A.; Stefanadis, C. Adherence to the Mediterranean diet is associated with total antioxidant capacity in healthy adults: The ATTICA study. Am. J. Clin. Nutr. 2005, 82, 694–699. [Google Scholar] [CrossRef]
- Casas, R.; Urpi-Sarda, M.; Sacanella, E.; Arranz, S.; Corella, D.; Castaner, O.; Lamuela-Raventos, R.M.; Salas-Salvado, J.; Lapetra, J.; Portillo, M.P.; et al. Anti-Inflammatory Effects of the Mediterranean Diet in the Early and Late Stages of Atheroma Plaque Development. Mediat. Inflamm. 2017, 2017, 3674390. [Google Scholar] [CrossRef]
- Centritto, F.; Iacoviello, L.; di Giuseppe, R.; De Curtis, A.; Costanzo, S.; Zito, F.; Grioni, S.; Sieri, S.; Donati, M.B.; de Gaetano, G.; et al. Dietary patterns, cardiovascular risk factors and C-reactive protein in a healthy Italian population. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 697–706. [Google Scholar] [CrossRef]
- Esposito, K.; Marfella, R.; Ciotola, M.; Di Palo, C.; Giugliano, F.; Giugliano, G.; D’Armiento, M.; D’Andrea, F.; Giugliano, D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA 2004, 292, 1440–1446. [Google Scholar] [CrossRef] [Green Version]
- Estruch, R. Anti-inflammatory effects of the Mediterranean diet: The experience of the PREDIMED study. Proc. Nutr. Soc. 2010, 69, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Castaner, O.; Covas, M.I.; Khymenets, O.; Nyyssonen, K.; Konstantinidou, V.; Zunft, H.F.; de la Torre, R.; Munoz-Aguayo, D.; Vila, J.; Fito, M. Protection of LDL from oxidation by olive oil polyphenols is associated with a downregulation of CD40-ligand expression and its downstream products in vivo in humans. Am. J. Clin. Nutr. 2012, 95, 1238–1244. [Google Scholar] [CrossRef] [Green Version]
- Pounis, G.; Bonaccio, M.; Di Castelnuovo, A.; Costanzo, S.; de Curtis, A.; Persichillo, M.; Sieri, S.; Donati, M.B.; Cerletti, C.; de Gaetano, G.; et al. Polyphenol intake is associated with low-grade inflammation, using a novel data analysis from the Moli-sani study. Thromb. Haemost. 2016, 115, 344–352. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Arranz, S.; Lamuela-Raventos, R.M.; Estruch, R. Effects of wine, alcohol and polyphenols on cardiovascular disease risk factors: Evidences from human studies. Alcohol Alcohol. 2013, 48, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Kelley, D.S.; Siegel, D.; Fedor, D.M.; Adkins, Y.; Mackey, B.E. DHA supplementation decreases serum C-reactive protein and other markers of inflammation in hypertriglyceridemic men. J. Nutr. 2009, 139, 495–501. [Google Scholar] [CrossRef] [Green Version]
- Landberg, R.; Sun, Q.; Rimm, E.B.; Cassidy, A.; Scalbert, A.; Mantzoros, C.S.; Hu, F.B.; van Dam, R.M. Selected dietary flavonoids are associated with markers of inflammation and endothelial dysfunction in U.S. women. J. Nutr. 2011, 141, 618–625. [Google Scholar] [CrossRef]
- Medina-Remon, A.; Casas, R.; Tressserra-Rimbau, A.; Ros, E.; Martinez-Gonzalez, M.A.; Fito, M.; Corella, D.; Salas-Salvado, J.; Lamuela-Raventos, R.M.; Estruch, R.; et al. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: A substudy of the PREDIMED trial. Br. J. Clin. Pharmacol. 2017, 83, 114–128. [Google Scholar] [CrossRef] [Green Version]
- Urpi-Sarda, M.; Casas, R.; Chiva-Blanch, G.; Romero-Mamani, E.S.; Valderas-Martinez, P.; Salas-Salvado, J.; Covas, M.I.; Toledo, E.; Andres-Lacueva, C.; Llorach, R.; et al. The Mediterranean diet pattern and its main components are associated with lower plasma concentrations of tumor necrosis factor receptor 60 in patients at high risk for cardiovascular disease. J. Nutr. 2012, 142, 1019–1025. [Google Scholar] [CrossRef] [Green Version]
- Carluccio, M.A.; Martinelli, R.; Massaro, M.; Calabriso, N.; Scoditti, E.; Maffia, M.; Verri, T.; Gatta, V.; De Caterina, R. Nutrigenomic Effect of Hydroxytyrosol in Vascular Endothelial Cells: A Transcriptomic Profile Analysis. Nutrients 2021, 13, 3990. [Google Scholar] [CrossRef]
- Konstantinidou, V.; Covas, M.I.; Sola, R.; Fito, M. Up-to date knowledge on the in vivo transcriptomic effect of the Mediterranean diet in humans. Mol. Nutr. Food Res. 2013, 57, 772–783. [Google Scholar] [CrossRef]
- Scoditti, E.; Nestola, A.; Massaro, M.; Calabriso, N.; Storelli, C.; De Caterina, R.; Carluccio, M.A. Hydroxytyrosol suppresses MMP-9 and COX-2 activity and expression in activated human monocytes via PKCalpha and PKCbeta1 inhibition. Atherosclerosis 2014, 232, 17–24. [Google Scholar] [CrossRef]
- Garcia-Casares, N.; Gallego Fuentes, P.; Barbancho, M.A.; Lopez-Gigosos, R.; Garcia-Rodriguez, A.; Gutierrez-Bedmar, M. Alzheimer’s Disease, Mild Cognitive Impairment and Mediterranean Diet. A Systematic Review and Dose-Response Meta-Analysis. J. Clin. Med. 2021, 10, 4642. [Google Scholar] [CrossRef]
- Gardener, H.; Caunca, M.R. Mediterranean Diet in Preventing Neurodegenerative Diseases. Curr. Nutr. Rep. 2018, 7, 10–20. [Google Scholar] [CrossRef]
- Siervo, M.; Shannon, O.M.; Llewellyn, D.J.; Stephan, B.C.; Fontana, L. Mediterranean diet and cognitive function: From methodology to mechanisms of action. Free Radic. Biol. Med. 2021, 176, 105–117. [Google Scholar] [CrossRef]
- Lassale, C.; Batty, G.D.; Baghdadli, A.; Jacka, F.; Sanchez-Villegas, A.; Kivimaki, M.; Akbaraly, T. Healthy dietary indices and risk of depressive outcomes: A systematic review and meta-analysis of observational studies. Mol. Psychiatry 2019, 24, 965–986. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, O.; Keshteli, A.H.; Afshar, H.; Esmaillzadeh, A.; Adibi, P. Adherence to Mediterranean dietary pattern is inversely associated with depression, anxiety and psychological distress. Nutr. Neurosci. 2021, 24, 248–259. [Google Scholar] [CrossRef]
- Fang, H.; Tu, S.; Sheng, J.; Shao, A. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. J. Cell. Mol. Med. 2019, 23, 2324–2332. [Google Scholar] [CrossRef] [Green Version]
- Peterson, M.J.; Benca, R.M. Sleep in mood disorders. Psychiatr. Clin. N. Am. 2006, 29, 1009–1032. [Google Scholar] [CrossRef]
- Tahmasian, M.; Samea, F.; Khazaie, H.; Zarei, M.; Kharabian Masouleh, S.; Hoffstaedter, F.; Camilleri, J.; Kochunov, P.; Yeo, B.T.T.; Eickhoff, S.B.; et al. The interrelation of sleep and mental and physical health is anchored in grey-matter neuroanatomy and under genetic control. Commun. Biol. 2020, 3, 171. [Google Scholar] [CrossRef]
- Luchtman, D.W.; Song, C. Cognitive enhancement by omega-3 fatty acids from child-hood to old age: Findings from animal and clinical studies. Neuropharmacology 2013, 64, 550–565. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; Cerezo, A.B.; de Pablos, R.M.; Krisa, S.; Richard, T.; Garcia-Parrilla, M.C.; Troncoso, A.M. Phenolic Compounds Characteristic of the Mediterranean Diet in Mitigating Microglia-Mediated Neuroinflammation. Front. Cell. Neurosci. 2018, 12, 373. [Google Scholar] [CrossRef]
- Ruxton, C.H.; Calder, P.C.; Reed, S.C.; Simpson, M.J. The impact of long-chain n-3 polyunsaturated fatty acids on human health. Nutr. Res. Rev. 2005, 18, 113–129. [Google Scholar] [CrossRef] [Green Version]
- Janssen, C.I.; Kiliaan, A.J. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: The influence of LCPUFA on neural development, aging, and neurodegeneration. Prog. Lipid Res. 2014, 53, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Horman, T.; Fernandes, M.F.; Tache, M.C.; Hucik, B.; Mutch, D.M.; Leri, F. Dietary n-6/n-3 Ratio Influences Brain Fatty Acid Composition in Adult Rats. Nutrients 2020, 12, 1847. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. (Maywood) 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 fatty acids, inflammation and immunity: New mechanisms to explain old actions. Proc. Nutr. Soc. 2013, 72, 326–336. [Google Scholar] [CrossRef] [Green Version]
- Joffre, C.; Rey, C.; Laye, S. N-3 Polyunsaturated Fatty Acids and the Resolution of Neuroinflammation. Front. Pharmacol. 2019, 10, 1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalon, S.; Vancassel, S.; Zimmer, L.; Guilloteau, D.; Durand, G. Polyunsaturated fatty acids and cerebral function: Focus on monoaminergic neurotransmission. Lipids 2001, 36, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Delion, S.; Chalon, S.; Guilloteau, D.; Besnard, J.C.; Durand, G. alpha-Linolenic acid dietary deficiency alters age-related changes of dopaminergic and serotoninergic neurotransmission in the rat frontal cortex. J. Neurochem. 1996, 66, 1582–1591. [Google Scholar] [CrossRef]
- Grosso, G.; Galvano, F.; Marventano, S.; Malaguarnera, M.; Bucolo, C.; Drago, F.; Caraci, F. Omega-3 fatty acids and depression: Scientific evidence and biological mechanisms. Oxid. Med. Cell. Longev. 2014, 2014, 313570. [Google Scholar] [CrossRef] [Green Version]
- Vines, A.; Delattre, A.M.; Lima, M.M.; Rodrigues, L.S.; Suchecki, D.; Machado, R.B.; Tufik, S.; Pereira, S.I.; Zanata, S.M.; Ferraz, A.C. The role of 5-HT(1)A receptors in fish oil-mediated increased BDNF expression in the rat hippocampus and cortex: A possible antidepressant mechanism. Neuropharmacology 2012, 62, 184–191. [Google Scholar] [CrossRef]
- Zhang, W.; Li, P.; Hu, X.; Zhang, F.; Chen, J.; Gao, Y. Omega-3 polyunsaturated fatty acids in the brain: Metabolism and neuroprotection. Front. Biosci. (Landmark Ed.) 2011, 16, 2653–2670. [Google Scholar] [CrossRef] [Green Version]
- Zimmer, L.; Vancassel, S.; Cantagrel, S.; Breton, P.; Delamanche, S.; Guilloteau, D.; Durand, G.; Chalon, S. The dopamine mesocorticolimbic pathway is affected by deficiency in n-3 polyunsaturated fatty acids. Am. J. Clin. Nutr. 2002, 75, 662–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutuli, D.; Landolfo, E.; Decandia, D.; Nobili, A.; Viscomi, M.T.; La Barbera, L.; Sacchetti, S.; De Bartolo, P.; Curci, A.; D’Amelio, M.; et al. Neuroprotective Role of Dietary Supplementation with Omega-3 Fatty Acids in the Presence of Basal Forebrain Cholinergic Neurons Degeneration in Aged Mice. Int. J. Mol. Sci. 2020, 21, 1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Titova, O.E.; Sjogren, P.; Brooks, S.J.; Kullberg, J.; Ax, E.; Kilander, L.; Riserus, U.; Cederholm, T.; Larsson, E.M.; Johansson, L.; et al. Dietary intake of eicosapentaenoic and docosahexaenoic acids is linked to gray matter volume and cognitive function in elderly. Age (Dordr) 2013, 35, 1495–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araya-Quintanilla, F.; Gutierrez-Espinoza, H.; Sanchez-Montoya, U.; Munoz-Yanez, M.J.; Baeza-Vergara, A.; Petersen-Yanjari, M.; Fernandez-Lecaros, L. Effectiveness of omega-3 fatty acid supplementation in patients with Alzheimer disease: A systematic review and meta-analysis. Neurologia (Engl. Ed.) 2020, 35, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Parletta, N.; Zarnowiecki, D.; Cho, J.; Wilson, A.; Bogomolova, S.; Villani, A.; Itsiopoulos, C.; Niyonsenga, T.; Blunden, S.; Meyer, B.; et al. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: A randomized controlled trial (HELFIMED). Nutr. Neurosci. 2019, 22, 474–487. [Google Scholar] [CrossRef] [Green Version]
- Patan, M.J.; Kennedy, D.O.; Husberg, C.; Hustvedt, S.O.; Calder, P.C.; Khan, J.; Forster, J.; Jackson, P.A. Supplementation with oil rich in eicosapentaenoic acid, but not in docosahexaenoic acid, improves global cognitive function in healthy, young adults: Results from randomized controlled trials. Am. J. Clin. Nutr. 2021, 114, 914–924. [Google Scholar] [CrossRef]
- Figueira, I.; Garcia, G.; Pimpao, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavares, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci. Rep. 2017, 7, 11456. [Google Scholar] [CrossRef]
- Frolinger, T.; Sims, S.; Smith, C.; Wang, J.; Cheng, H.; Faith, J.; Ho, L.; Hao, K.; Pasinetti, G.M. The gut microbiota composition affects dietary polyphenols-mediated cognitive resilience in mice by modulating the bioavailability of phenolic acids. Sci. Rep. 2019, 9, 3546. [Google Scholar] [CrossRef] [Green Version]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S.; et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef]
- Youdim, K.A.; Qaiser, M.Z.; Begley, D.J.; Rice-Evans, C.A.; Abbott, N.J. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic. Biol. Med. 2004, 36, 592–604. [Google Scholar] [CrossRef]
- Quincozes-Santos, A.; Gottfried, C. Resveratrol modulates astroglial functions: Neuroprotective hypothesis. Ann. N. Y. Acad. Sci. 2011, 1215, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E. Neuroinflammation and Neurodegeneration: The Promising Protective Role of the Citrus Flavanone Hesperetin. Nutrients 2020, 12, 2336. [Google Scholar] [CrossRef] [PubMed]
- Valls-Pedret, C.; Lamuela-Raventos, R.M.; Medina-Remon, A.; Quintana, M.; Corella, D.; Pinto, X.; Martinez-Gonzalez, M.A.; Estruch, R.; Ros, E. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J. Alzheimers Dis. 2012, 29, 773–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batarseh, Y.S.; Mohamed, L.A.; Al Rihani, S.B.; Mousa, Y.M.; Siddique, A.B.; El Sayed, K.A.; Kaddoumi, A. Oleocanthal ameliorates amyloid-beta oligomers’ toxicity on astrocytes and neuronal cells: In vitro studies. Neuroscience 2017, 352, 204–215. [Google Scholar] [CrossRef]
- Crespo, M.C.; Tome-Carneiro, J.; Pintado, C.; Davalos, A.; Visioli, F.; Burgos-Ramos, E. Hydroxytyrosol restores proper insulin signaling in an astrocytic model of Alzheimer’s disease. Biofactors 2017, 43, 540–548. [Google Scholar] [CrossRef]
- Feng, X.; Liang, N.; Zhu, D.; Gao, Q.; Peng, L.; Dong, H.; Yue, Q.; Liu, H.; Bao, L.; Zhang, J.; et al. Resveratrol inhibits beta-amyloid-induced neuronal apoptosis through regulation of SIRT1-ROCK1 signaling pathway. PLoS ONE 2013, 8, e59888. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Cui, Y.; Gao, J.L.; Li, R.; Jiang, X.H.; Tian, Y.X.; Wang, K.J.; Li, M.H.; Zhang, H.A.; Cui, J.Z. Neuroprotective effects of resveratrol against traumatic brain injury in rats: Involvement of synaptic proteins and neuronal autophagy. Mol. Med. Rep. 2016, 13, 5248–5254. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, T.; Ikram, M.; Ullah, R.; Rehman, S.U.; Kim, M.O. Hesperetin, a Citrus Flavonoid, Attenuates LPS-Induced Neuroinflammation, Apoptosis and Memory Impairments by Modulating TLR4/NF-kappaB Signaling. Nutrients 2019, 11, 648. [Google Scholar] [CrossRef] [Green Version]
- Rehman, S.U.; Ali, T.; Alam, S.I.; Ullah, R.; Zeb, A.; Lee, K.W.; Rutten, B.P.F.; Kim, M.O. Ferulic Acid Rescues LPS-Induced Neurotoxicity via Modulation of the TLR4 Receptor in the Mouse Hippocampus. Mol. Neurobiol. 2019, 56, 2774–2790. [Google Scholar] [CrossRef]
- Arus, B.A.; Souza, D.G.; Bellaver, B.; Souza, D.O.; Goncalves, C.A.; Quincozes-Santos, A.; Bobermin, L.D. Resveratrol modulates GSH system in C6 astroglial cells through heme oxygenase 1 pathway. Mol. Cell. Biochem. 2017, 428, 67–77. [Google Scholar] [CrossRef]
- Yao, Y.; Li, J.; Niu, Y.; Yu, J.Q.; Yan, L.; Miao, Z.H.; Zhao, X.X.; Li, Y.J.; Yao, W.X.; Zheng, P.; et al. Resveratrol inhibits oligomeric Abeta-induced microglial activation via NADPH oxidase. Mol. Med. Rep. 2015, 12, 6133–6139. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Muhammad, T.; Rehman, S.U.; Khan, A.; Jo, M.G.; Ali, T.; Kim, M.O. Hesperetin Confers Neuroprotection by Regulating Nrf2/TLR4/NF-kappaB Signaling in an Abeta Mouse Model. Mol. Neurobiol. 2019, 56, 6293–6309. [Google Scholar] [CrossRef] [PubMed]
- Gravesteijn, E.; Mensink, R.P.; Plat, J. Effects of nutritional interventions on BDNF concentrations in humans: A systematic review. Nutr. Neurosci. 2021, 25, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kurauchi, Y.; Hisatsune, A.; Isohama, Y.; Mishima, S.; Katsuki, H. Caffeic acid phenethyl ester protects nigral dopaminergic neurons via dual mechanisms involving haem oxygenase-1 and brain-derived neurotrophic factor. Br. J. Pharmacol. 2012, 166, 1151–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, G.P.; Cavegn, N.; Nix, A.; do Nascimento Bevilaqua, M.C.; Stangl, D.; Zainuddin, M.S.; Nardi, A.E.; Gardino, P.F.; Thuret, S. The role of dietary polyphenols on adult hippocampal neurogenesis: Molecular mechanisms and behavioural effects on depression and anxiety. Oxid. Med. Cell. Longev. 2012, 2012, 541971. [Google Scholar] [CrossRef]
- Hsieh, C.P.; Chang, W.T.; Chen, L.; Chen, H.H.; Chan, M.H. Differential inhibitory effects of resveratrol on excitotoxicity and synaptic plasticity: Involvement of NMDA receptor subtypes. Nutr. Neurosci. 2021, 24, 443–458. [Google Scholar] [CrossRef]
- Abraham, J.; Johnson, R.W. Consuming a diet supplemented with resveratrol reduced infection-related neuroinflammation and deficits in working memory in aged mice. Rejuvenation Res. 2009, 12, 445–453. [Google Scholar] [CrossRef] [Green Version]
- Tu, Y.; Cheng, S.X.; Sun, H.T.; Ma, T.Z.; Zhang, S. Ferulic acid potentiates pentobarbital-induced sleep via the serotonergic system. Neurosci. Lett. 2012, 525, 95–99. [Google Scholar] [CrossRef]
- Fernandez, S.P.; Wasowski, C.; Paladini, A.C.; Marder, M. Synergistic interaction between hesperidin, a natural flavonoid, and diazepam. Eur. J. Pharmacol. 2005, 512, 189–198. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, C.S.; Hu, Z.; Han, J.Y.; Kim, S.K.; Yoo, S.K.; Yeo, Y.M.; Chong, M.S.; Lee, K.; Hong, J.T.; et al. Enhancement of pentobarbital-induced sleep by apigenin through chloride ion channel activation. Arch. Pharm. Res. 2012, 35, 367–373. [Google Scholar] [CrossRef]
- Park, K.S.; Han, J.Y.; Moon, D.C.; Hong, J.T.; Oh, K.W. (-)-epigallocatechin-3-O-gallate augments pentobarbital-induced sleeping behaviors through Cl- channel activation. J. Med. Food 2011, 14, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xie, J.; Mu, W.; Ruan, X.; Zhang, J.; Yao, L.; Diao, Z.; Wu, M.; Li, Y.; Ren, W.; et al. Tea polyphenols protect learning and memory in sleep-deprived mice by promoting AMPA receptor internalization. NeuroReport 2020, 31, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Frolinger, T.; Smith, C.; Cobo, C.F.; Sims, S.; Brathwaite, J.; de Boer, S.; Huang, J.; Pasinetti, G.M. Dietary polyphenols promote resilience against sleep deprivation-induced cognitive impairment by activating protein translation. FASEB J. 2018, 32, 5390–5404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.; Wang, J.; Bi, W.; Ferruzzi, M.; Yemul, S.; Freire, D.; Mazzola, P.; Ho, L.; Dubner, L.; Pasinetti, G.M. Novel application of brain-targeting polyphenol compounds in sleep deprivation-induced cognitive dysfunction. Neurochem. Int. 2015, 89, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Masters, A.; Pandi-Perumal, S.R.; Seixas, A.; Girardin, J.L.; McFarlane, S.I. Melatonin, the Hormone of Darkness: From Sleep Promotion to Ebola Treatment. Brain Disord. Ther. 2014, 4, 1000151. [Google Scholar] [CrossRef] [Green Version]
- Iriti, M.; Varoni, E.M.; Vitalini, S. Melatonin in traditional Mediterranean diets. J. Pineal Res. 2010, 49, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Yu, E.; Ruiz-Canela, M.; Guasch-Ferre, M.; Zheng, Y.; Toledo, E.; Clish, C.B.; Salas-Salvado, J.; Liang, L.; Wang, D.D.; Corella, D.; et al. Increases in Plasma Tryptophan Are Inversely Associated with Incident Cardiovascular Disease in the Prevencion con Dieta Mediterranea (PREDIMED) Study. J. Nutr. 2017, 147, 314–322. [Google Scholar] [CrossRef] [Green Version]
- Razquin, C.; Ruiz-Canela, M.; Toledo, E.; Hernandez-Alonso, P.; Clish, C.B.; Guasch-Ferre, M.; Li, J.; Wittenbecher, C.; Dennis, C.; Alonso-Gomez, A.; et al. Metabolomics of the tryptophan-kynurenine degradation pathway and risk of atrial fibrillation and heart failure: Potential modification effect of Mediterranean diet. Am. J. Clin. Nutr. 2021, 114, 1646–1654. [Google Scholar] [CrossRef]
- Chen, Y.; Guillemin, G.J. Kynurenine pathway metabolites in humans: Disease and healthy States. Int. J. Tryptophan Res. 2009, 2, IJTR-S2097. [Google Scholar] [CrossRef] [Green Version]
- Pocivavsek, A.; Baratta, A.M.; Mong, J.A.; Viechweg, S.S. Acute Kynurenine Challenge Disrupts Sleep-Wake Architecture and Impairs Contextual Memory in Adult Rats. Sleep 2017, 40, zsx141. [Google Scholar] [CrossRef]
- Bravo, R.; Matito, S.; Cubero, J.; Paredes, S.D.; Franco, L.; Rivero, M.; Rodriguez, A.B.; Barriga, C. Tryptophan-enriched cereal intake improves nocturnal sleep, melatonin, serotonin, and total antioxidant capacity levels and mood in elderly humans. Age (Dordr) 2013, 35, 1277–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Rodriguez, A.; Rubio-Arias, J.A.; Ramos-Campo, D.J.; Reche-Garcia, C.; Leyva-Vela, B.; Nadal-Nicolas, Y. Psychological and Sleep Effects of Tryptophan and Magnesium-Enriched Mediterranean Diet in Women with Fibromyalgia. Int. J. Environ. Res. Public Health 2020, 17, 2227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afaghi, A.; O’Connor, H.; Chow, C.M. High-glycemic-index carbohydrate meals shorten sleep onset. Am. J. Clin. Nutr. 2007, 85, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Lavialle, M.; Champeil-Potokar, G.; Alessandri, J.M.; Balasse, L.; Guesnet, P.; Papillon, C.; Pevet, P.; Vancassel, S.; Vivien-Roels, B.; Denis, I. An (n-3) polyunsaturated fatty acid-deficient diet disturbs daily locomotor activity, melatonin rhythm, and striatal dopamine in Syrian hamsters. J. Nutr. 2008, 138, 1719–1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato-Mito, N.; Sasaki, S.; Murakami, K.; Okubo, H.; Takahashi, Y.; Shibata, S.; Yamada, K.; Sato, K.; Freshmen in Dietetic Courses Study II Group. The midpoint of sleep is associated with dietary intake and dietary behavior among young Japanese women. Sleep Med. 2011, 12, 289–294. [Google Scholar] [CrossRef]
- Sarubbo, F.; Ramis, M.R.; Aparicio, S.; Ruiz, L.; Esteban, S.; Miralles, A.; Moranta, D. Improving effect of chronic resveratrol treatment on central monoamine synthesis and cognition in aged rats. Age (Dordr) 2015, 37, 9777. [Google Scholar] [CrossRef] [Green Version]
- Ramis, M.R.; Sarubbo, F.; Moranta, D.; Tejada, S.; Llado, J.; Miralles, A.; Esteban, S. Cognitive and Neurochemical Changes Following Polyphenol-Enriched Diet in Rats. Nutrients 2020, 13, 59. [Google Scholar] [CrossRef]
- Malhotra, R.K.; Desai, A.K. Healthy brain aging: What has sleep got to do with it? Clin. Geriatr. Med. 2010, 26, 45–56. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Sen, P.; Molinero-Perez, A.; O’Riordan, K.J.; McCafferty, C.P.; O’Halloran, K.D.; Cryan, J.F. Microbiota and sleep: Awakening the gut feeling. Trends Mol. Med. 2021, 27, 935–945. [Google Scholar] [CrossRef]
- Smith, R.P.; Easson, C.; Lyle, S.M.; Kapoor, R.; Donnelly, C.P.; Davidson, E.J.; Parikh, E.; Lopez, J.V.; Tartar, J.L. Gut microbiome diversity is associated with sleep physiology in humans. PLoS ONE 2019, 14, e0222394. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Yuan, S.; Zhang, J. The interplay between sleep and gut microbiota. Brain Res. Bull. 2022, 180, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Szentirmai, E.; Millican, N.S.; Massie, A.R.; Kapas, L. Butyrate, a metabolite of intestinal bacteria, enhances sleep. Sci. Rep. 2019, 9, 7035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Synowiec, S.; Lu, L.; Yu, Y.; Bretherick, T.; Takada, S.; Yarnykh, V.; Caplan, J.; Caplan, M.; Claud, E.C.; et al. Microbiota influence the development of the brain and behaviors in C57BL/6J mice. PLoS ONE 2018, 13, e0201829. [Google Scholar] [CrossRef]
- Tran, S.M.; Mohajeri, M.H. The Role of Gut Bacterial Metabolites in Brain Development, Aging and Disease. Nutrients 2021, 13, 732. [Google Scholar] [CrossRef]
- Agrawal, R.; Ajami, N.J.; Malhotra, S.; Chen, L.; White, D.L.; Sharafkhaneh, A.; Hoffman, K.L.; Graham, D.Y.; El-Serag, H.B.; Petrosino, J.F.; et al. Habitual Sleep Duration and the Colonic Mucosa-Associated Gut Microbiota in Humans—A Pilot Study. Clocks Sleep 2021, 3, 387–397. [Google Scholar] [CrossRef]
- Ogawa, Y.; Miyoshi, C.; Obana, N.; Yajima, K.; Hotta-Hirashima, N.; Ikkyu, A.; Kanno, S.; Soga, T.; Fukuda, S.; Yanagisawa, M. Gut microbiota depletion by chronic antibiotic treatment alters the sleep/wake architecture and sleep EEG power spectra in mice. Sci. Rep. 2020, 10, 19554. [Google Scholar] [CrossRef]
- Grosicki, G.J.; Riemann, B.L.; Flatt, A.A.; Valentino, T.; Lustgarten, M.S. Self-reported sleep quality is associated with gut microbiome composition in young, healthy individuals: A pilot study. Sleep Med. 2020, 73, 76–81. [Google Scholar] [CrossRef]
- Badran, M.; Khalyfa, A.; Ericsson, A.; Gozal, D. Fecal microbiota transplantation from mice exposed to chronic intermittent hypoxia elicits sleep disturbances in naive mice. Exp. Neurol. 2020, 334, 113439. [Google Scholar] [CrossRef]
- Kurokawa, S.; Kishimoto, T.; Mizuno, S.; Masaoka, T.; Naganuma, M.; Liang, K.C.; Kitazawa, M.; Nakashima, M.; Shindo, C.; Suda, W.; et al. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome, functional diarrhea and functional constipation: An open-label observational study. J. Affect. Disord 2018, 235, 506–512. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Requena, T.; Martinez-Cuesta, M.C.; Pelaez, C. Diet and microbiota linked in health and disease. Food Funct. 2018, 9, 688–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Govindarajulu, M.; Pinky, P.D.; Steinke, I.; Bloemer, J.; Ramesh, S.; Kariharan, T.; Rella, R.T.; Bhattacharya, S.; Dhanasekaran, M.; Suppiramaniam, V.; et al. Gut Metabolite TMAO Induces Synaptic Plasticity Deficits by Promoting Endoplasmic Reticulum Stress. Front. Mol. Neurosci. 2020, 13, 138. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ke, Y.; Zhan, R.; Liu, C.; Zhao, M.; Zeng, A.; Shi, X.; Ji, L.; Cheng, S.; Pan, B.; et al. Trimethylamine-N-oxide promotes brain aging and cognitive impairment in mice. Aging Cell 2018, 17, e12768. [Google Scholar] [CrossRef] [PubMed]
- Barber, C.; Mego, M.; Sabater, C.; Vallejo, F.; Bendezu, R.A.; Masihy, M.; Guarner, F.; Espin, J.C.; Margolles, A.; Azpiroz, F. Differential Effects of Western and Mediterranean-Type Diets on Gut Microbiota: A Metagenomics and Metabolomics Approach. Nutrients 2021, 13, 2638. [Google Scholar] [CrossRef] [PubMed]
- Haro, C.; Garcia-Carpintero, S.; Rangel-Zuniga, O.A.; Alcala-Diaz, J.F.; Landa, B.B.; Clemente, J.C.; Perez-Martinez, P.; Lopez-Miranda, J.; Perez-Jimenez, F.; Camargo, A. Consumption of Two Healthy Dietary Patterns Restored Microbiota Dysbiosis in Obese Patients with Metabolic Dysfunction. Mol. Nutr. Food Res. 2017, 61, 1700300. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.J.; Zhang, W. Role of Dietary Nutrients in the Modulation of Gut Microbiota: A Narrative Review. Nutrients 2020, 12, 381. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Aoun, A.; Darwish, F.; Hamod, N. The Influence of the Gut Microbiome on Obesity in Adults and the Role of Probiotics, Prebiotics, and Synbiotics for Weight Loss. Prev. Nutr. Food Sci. 2020, 25, 113–123. [Google Scholar] [CrossRef]
- Correa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Sarrias, A.; Romo-Vaquero, M.; Garcia-Villalba, R.; Cortes-Martin, A.; Selma, M.V.; Espin, J.C. The Endotoxemia Marker Lipopolysaccharide-Binding Protein is Reduced in Overweight-Obese Subjects Consuming Pomegranate Extract by Modulating the Gut Microbiota: A Randomized Clinical Trial. Mol. Nutr. Food Res. 2018, 62, e1800160. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.C.; Li, J.; Zhang, M.; Pan, J.C.; Yu, Y.; Zhang, J.B.; Zheng, L.; Si, J.M.; Xu, Y. Resveratrol Improves Brain-Gut Axis by Regulation of 5-HT-Dependent Signaling in the Rat Model of Irritable Bowel Syndrome. Front. Cell. Neurosci. 2019, 13, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plamada, D.; Vodnar, D.C. Polyphenols-Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Yannakoulia, M.; Kontogianni, M.; Scarmeas, N. Cognitive health and Mediterranean diet: Just diet or lifestyle pattern? Ageing Res. Rev. 2015, 20, 74–78. [Google Scholar] [CrossRef]
- Schulze, M.B.; Martinez-Gonzalez, M.A.; Fung, T.T.; Lichtenstein, A.H.; Forouhi, N.G. Food based dietary patterns and chronic disease prevention. BMJ 2018, 361, k2396. [Google Scholar] [CrossRef] [Green Version]
- Vilarnau, C.; Stracker, D.M.; Funtikov, A.; da Silva, R.; Estruch, R.; Bach-Faig, A. Worldwide adherence to Mediterranean Diet between 1960 and 2011. Eur. J. Clin. Nutr. 2019, 72, 83–91. [Google Scholar] [CrossRef]
- Wittmann, M.; Dinich, J.; Merrow, M.; Roenneberg, T. Social jetlag: Misalignment of biological and social time. Chronobiol. Int. 2006, 23, 497–509. [Google Scholar] [CrossRef]
- Zeron-Rugerio, M.F.; Cambras, T.; Izquierdo-Pulido, M. Social Jet Lag Associates Negatively with the Adherence to the Mediterranean Diet and Body Mass Index among Young Adults. Nutrients 2019, 11, 1756. [Google Scholar] [CrossRef] [Green Version]
- Heath, G.; Dorrian, J.; Coates, A. Associations between shift type, sleep, mood, and diet in a group of shift working nurses. Scand. J. Work Environ. Health 2019, 45, 402–412. [Google Scholar] [CrossRef]
| Class | Subclass | Main Representatives | Main Food Source | Intake (mg/day) |
|---|---|---|---|---|
| Flavonoids | Flavanols | Catechin Epicatechin Epigallocatechin | Apples, red wine, tea, peaches, cocoa products, beans | 26.7 ± 19.6 |
| Flavonols | Quercetin Kaempferol Myricetin | Spinach, beans, onions, lettuce | 80.4 ± 32.7 | |
| Flavanones | Hesperidin and its aglycone hesperetin Naringenin Didymin | Oranges, orange juice, red wine, tomatoes | 132 ± 125 | |
| Flavones | Apigenin Luteolin | Oranges, whole-grain wheat-flour bread, refined-grain wheat-flour bread | 41.6 ± 26.1 | |
| Isoflavones | Genistein Daidzen Glycitein | Beans, beer | 0.003 ± 0.003 | |
| Anthocyanins | Malvidin Cyanidin Delphinidin | Cherries, red wine, olives, strawberries | 38.5 ± 37.4 | |
| Phenolic alcohol and secoiridoids | Hydroxytyrosol, tyrosol Oleuropein Oleacein Oleocanthal | Olive oil | 39.46 ± 29.37 | |
| Non-flavonoids | Stilbenes | Resveratrol | Red wine, white wine, grapes, strawberries | 1.84 ± 3.39 |
| Phenolic acids | Hydroxycinnamic acids (cinnamic, p-coumaric, ferulic, caffeic, chlorogenic, and rosmarinic acids, verbascoside) | Coffee, potatoes, apples, olives | 276 ± 146 | |
| Hydroxybenzoic acids (p-hydroxybenzoic, gallic, syringic, protocatechuic, and vanilic acids) | Olives, red wine, walnuts, beer | 19.1 ± 16.8 | ||
| Lignans | Secoisolariciresinol Pinoresinol 1-Acetoxypinoresinol | Olive oil, whole-grain wheat-flour bread | 0.85 ± 0.36 | |
| Tannins | Condensed tannins or proanthocyanidins (oligomers or polymers of flavanols) Hydrolyzable tannins or gallotannins, ellagitannin | Red wine, apples, peaches, plums, orange, green beans, lentils | 117 ± 81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scoditti, E.; Tumolo, M.R.; Garbarino, S. Mediterranean Diet on Sleep: A Health Alliance. Nutrients 2022, 14, 2998. https://doi.org/10.3390/nu14142998
Scoditti E, Tumolo MR, Garbarino S. Mediterranean Diet on Sleep: A Health Alliance. Nutrients. 2022; 14(14):2998. https://doi.org/10.3390/nu14142998
Chicago/Turabian StyleScoditti, Egeria, Maria Rosaria Tumolo, and Sergio Garbarino. 2022. "Mediterranean Diet on Sleep: A Health Alliance" Nutrients 14, no. 14: 2998. https://doi.org/10.3390/nu14142998
APA StyleScoditti, E., Tumolo, M. R., & Garbarino, S. (2022). Mediterranean Diet on Sleep: A Health Alliance. Nutrients, 14(14), 2998. https://doi.org/10.3390/nu14142998