Exopolysaccharides of Lactic Acid Bacteria: Production, Purification and Health Benefits towards Functional Food
Abstract
:1. Introduction
“A food can be regarded as ‘functional’ if it is satisfactorily demonstrated to affect beneficially one or more target functions in the body, beyond adequate nutritional effects, in a way that is relevant to either an improved state of health and well-being and/or reduction of risk of disease. Functional foods must remain foods and they must demonstrate their effects in amounts that can normally be expected to be consumed in the diet: they are not pills or capsules, but part of a normal food pattern” [6].
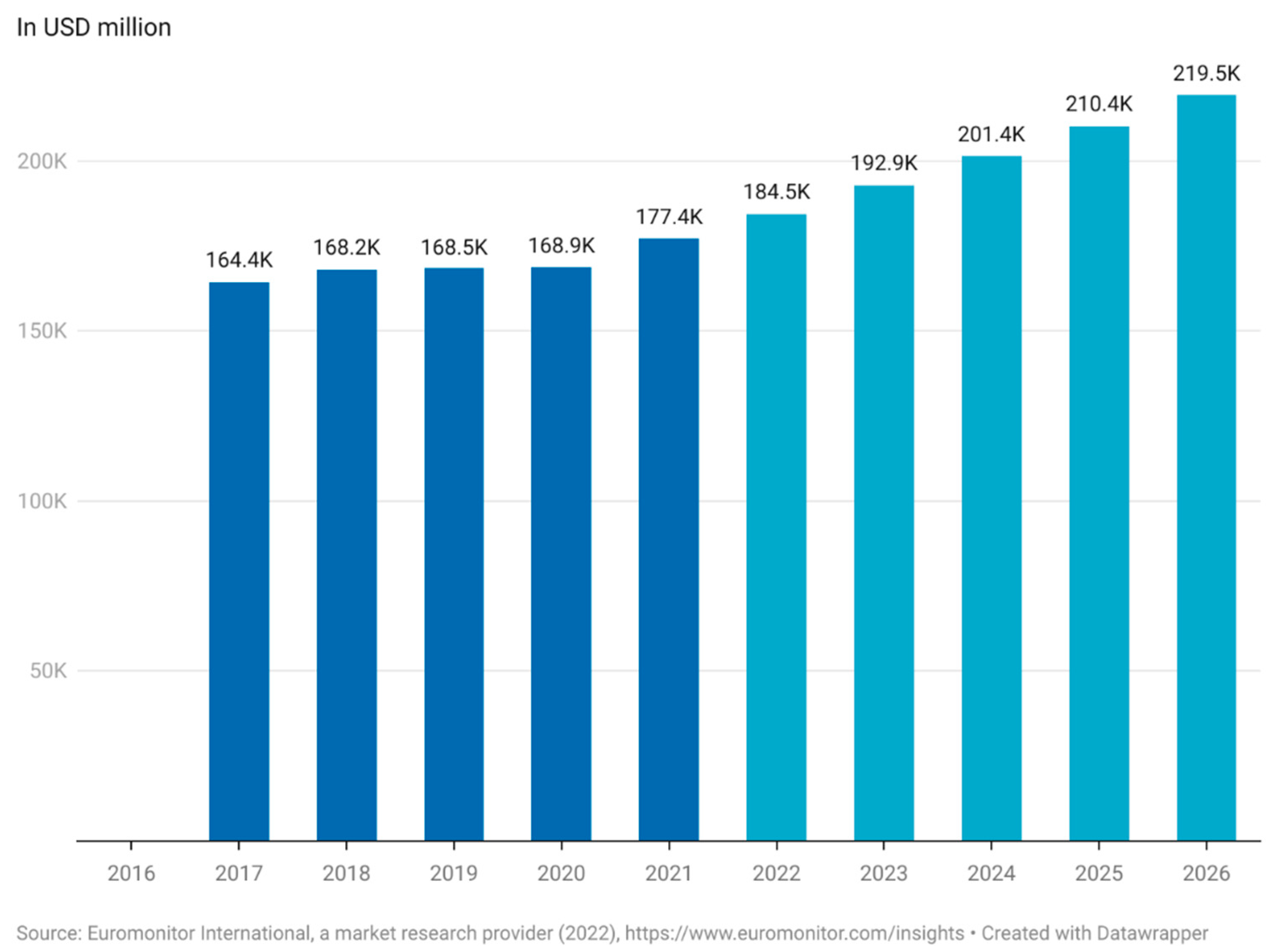
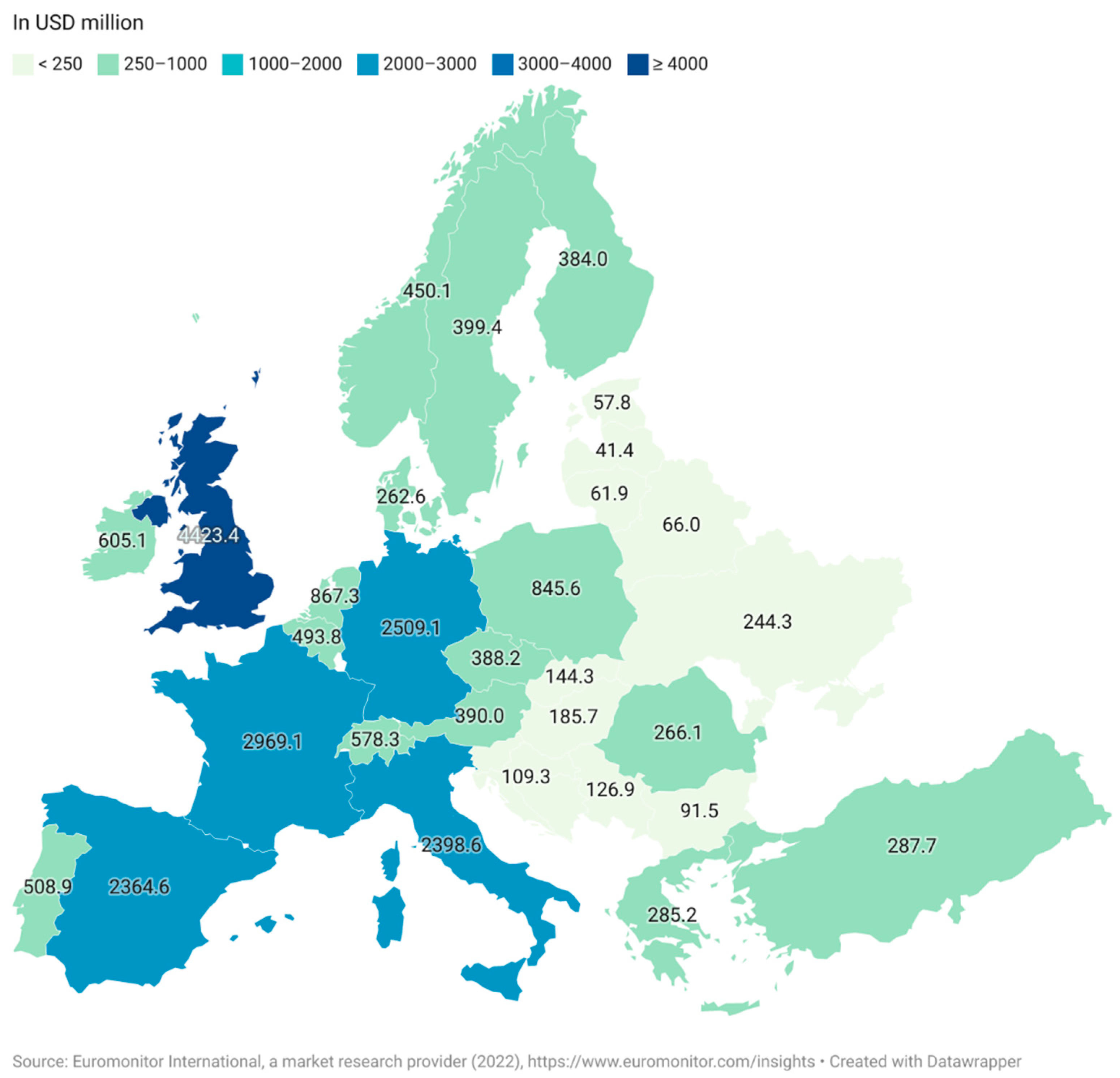
1.1. Market Value of Functional Foods
1.2. Current Commercial and Research Interest
1.3. Exopolysaccharides as a Functional Food Ingredient
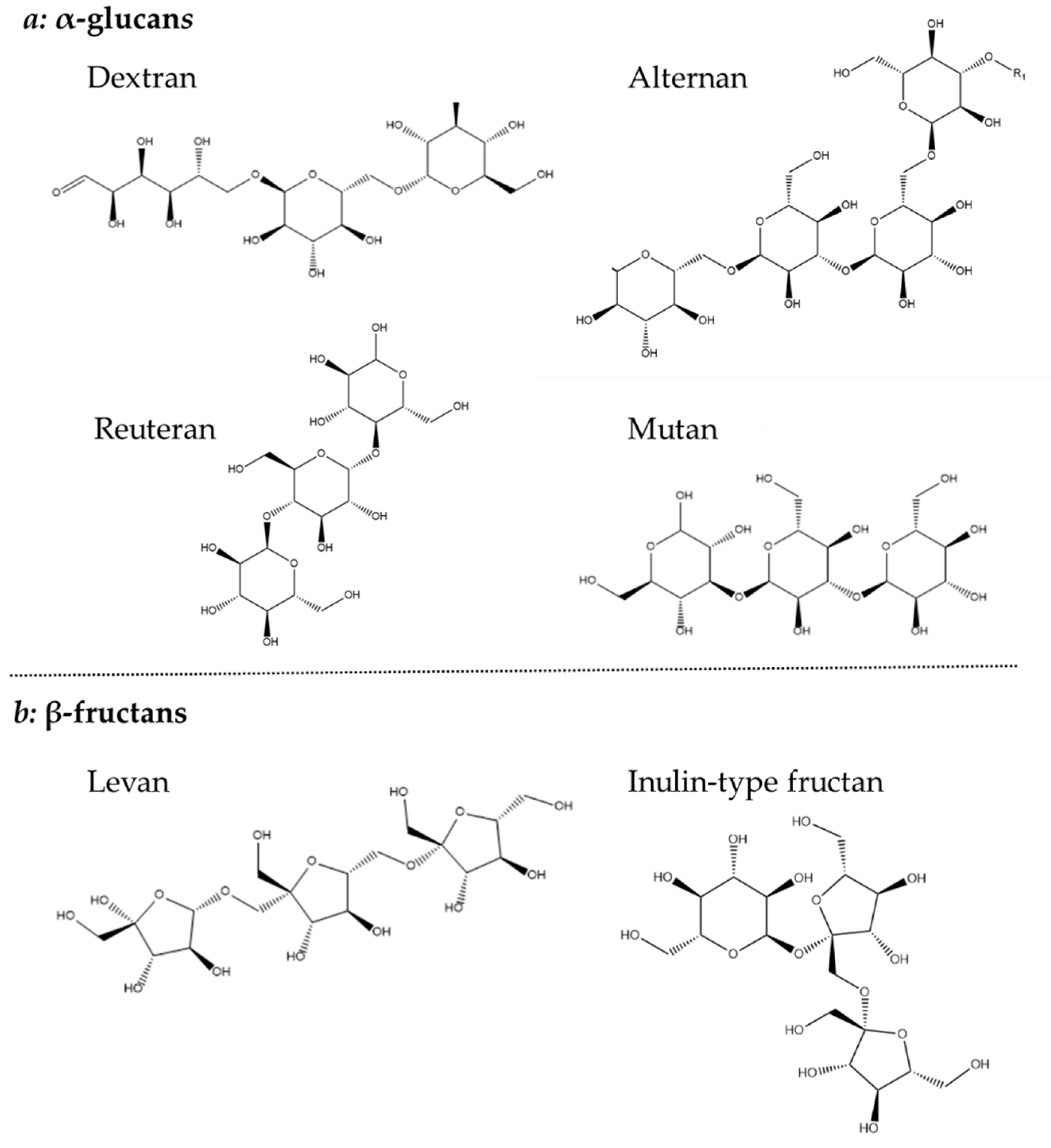
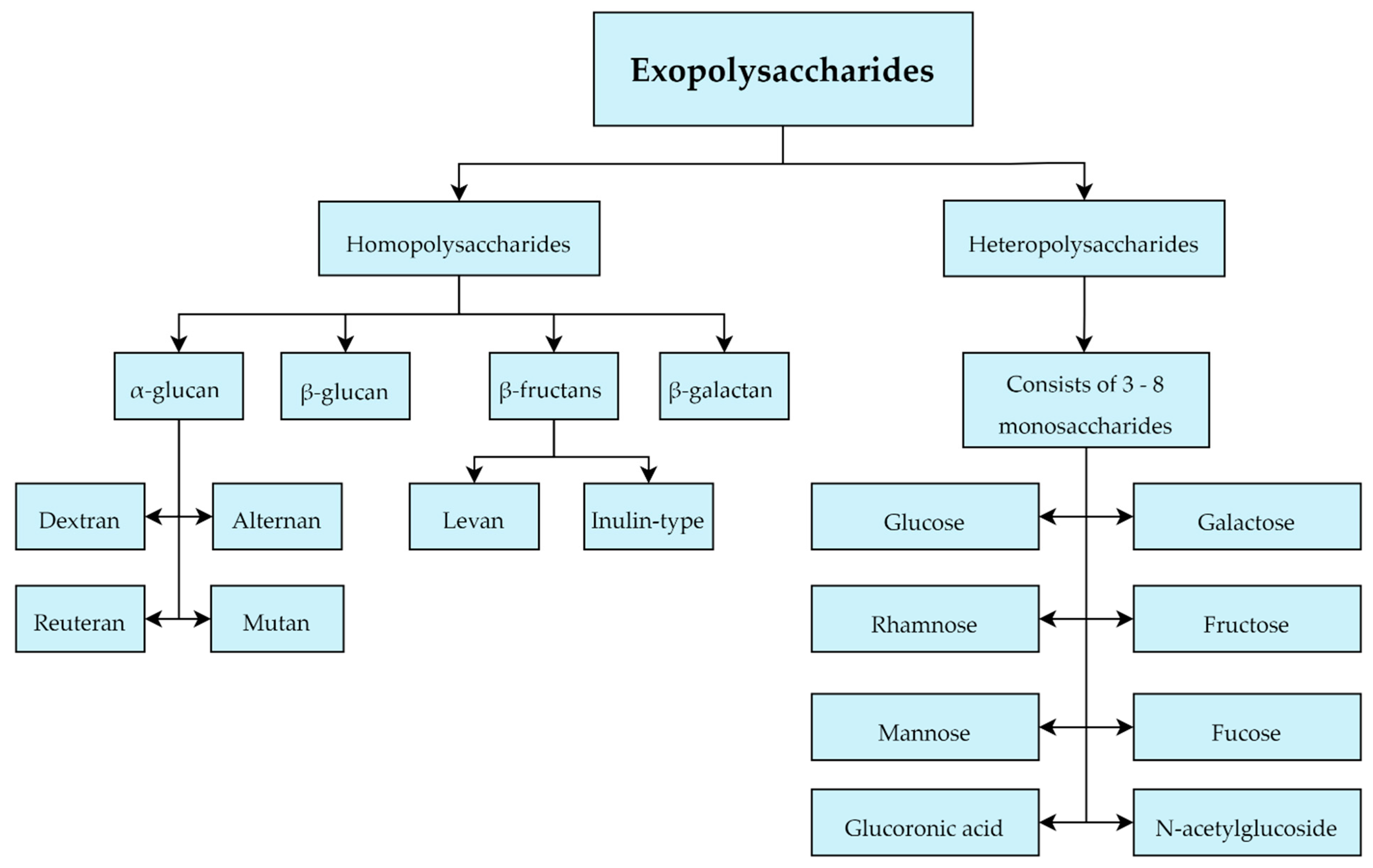
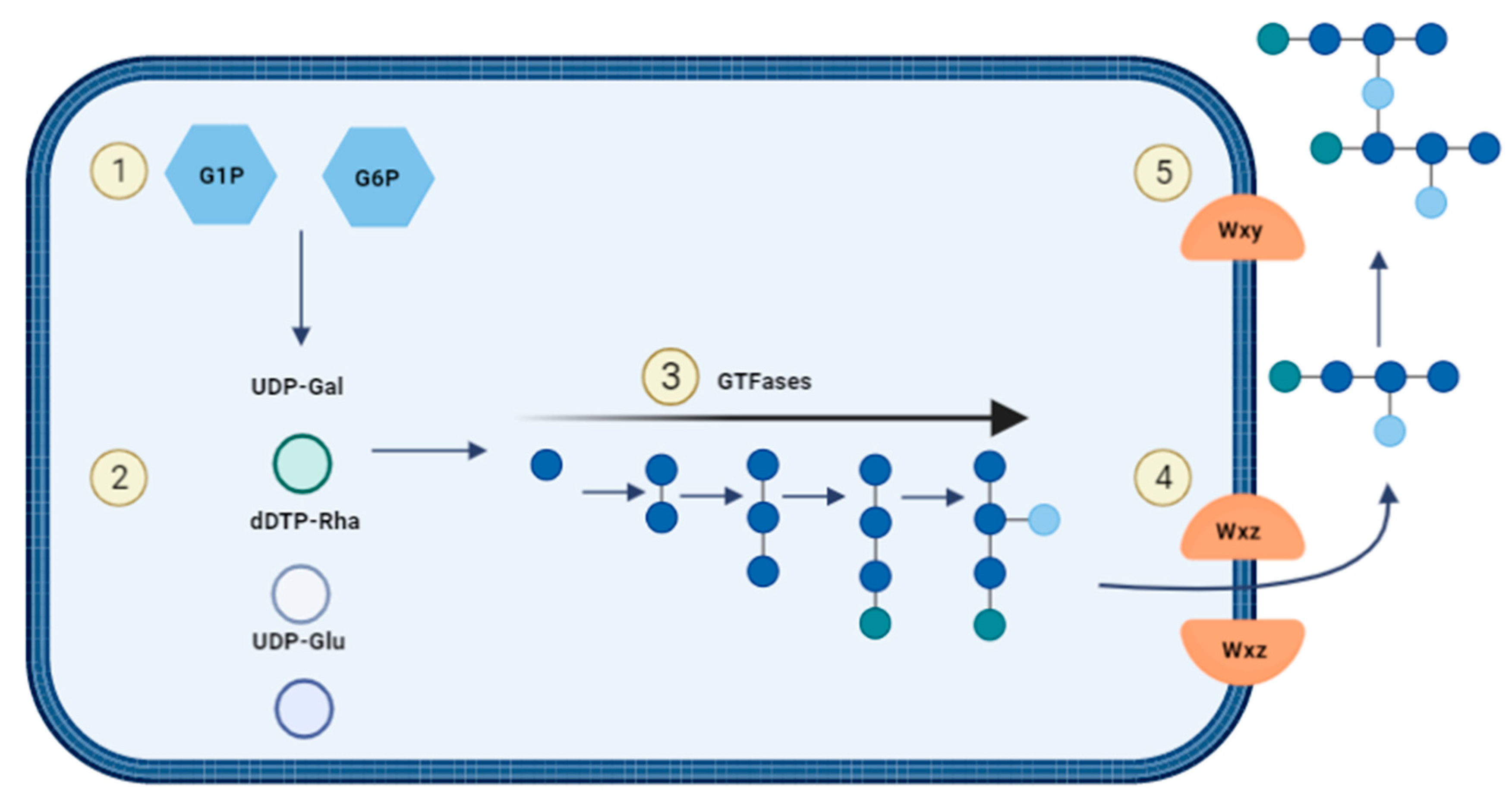
1.4. Exopolysaccharide Structures and Biosyntheses
1.5. Industrial Applications
1.6. Legal Status of EPS as a Novel Food Ingredient
2. Production and Process Conditions
2.1. Effects of Strain Selection and Carbon Sources
2.2. Nitrogen Sources
2.3. Amino Acids, Salts, and Vitamins
2.4. Temperature and pH
2.5. Fermentation Technologies
2.6. The Effect of Strain Interactions on EPS Production
2.7. Production Challenges
3. Extraction and Purification of Exopolysaccharides
3.1. Isolation and Recovery
3.1.1. Pre-Treatments
3.1.2. Precipitation and Dialysis
3.1.3. Drying and Characterisation
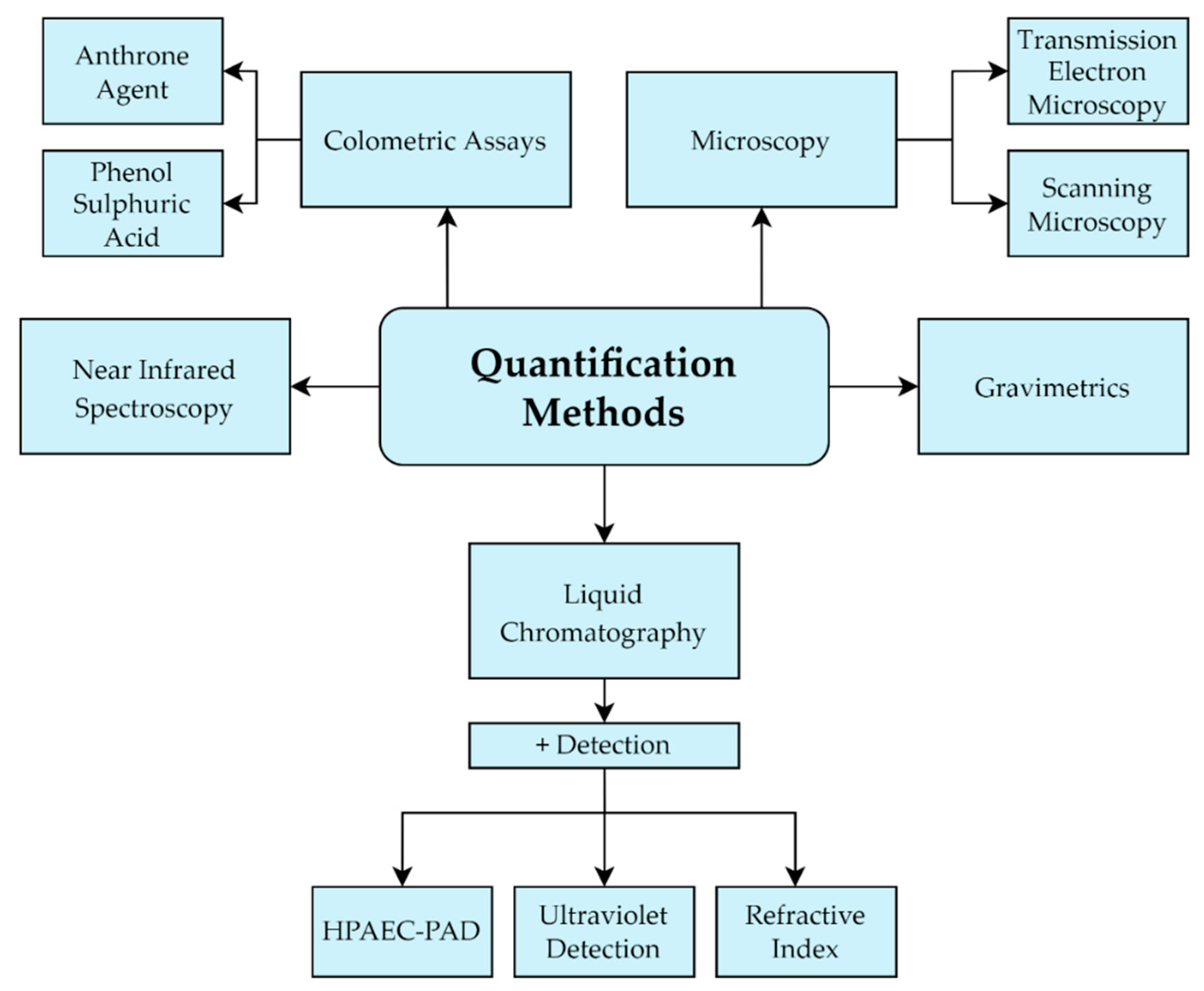
3.2. Quantification Methods
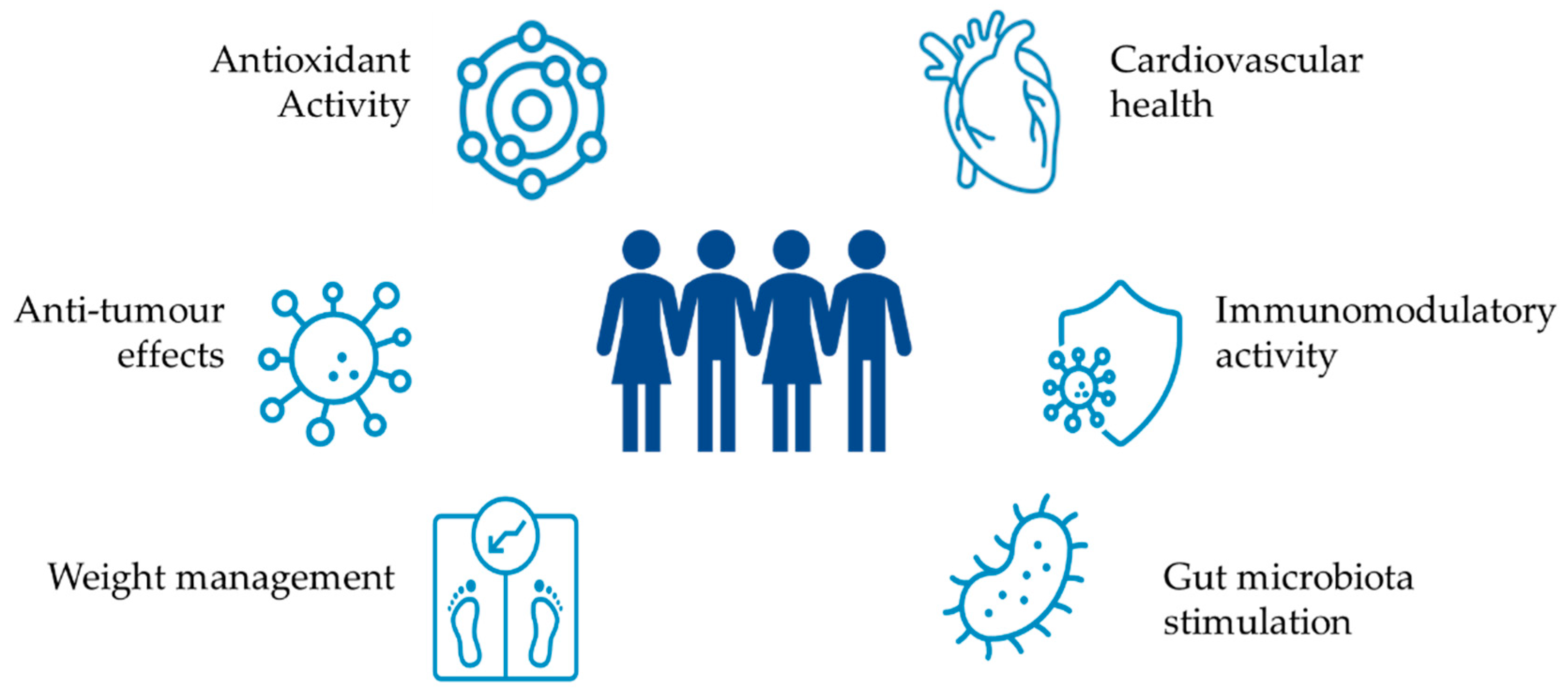
4. Health Benefits of Exopolysaccharides
4.1. Gut Microbiota Stimulation
4.2. Immunomodulatory Activity
4.3. Antioxidant Activity
4.4. Anti-Tumour Activity
4.5. Cardiovascular Health
4.6. Weight Management
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roberfroid, M.B. Global View on Functional Foods: European Perspectives. Br. J. Nutr. 2002, 88, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Tukenmez, U.; Aktas, B.; Aslim, B.; Yavuz, S. The Relationship between the Structural Characteristics of Lactobacilli-EPS and Its Ability to Induce Apoptosis in Colon Cancer Cells in Vitro. Sci. Rep. 2019, 9, 8268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- London, L.E.E.; Kumar, A.H.S.; Wall, R.; Casey, P.G.; O’Sullivan, O.; Shanahan, F.; Hill, C.; Cotter, P.D.; Fitzgerald, G.F.; Ross, P.P.; et al. Exopolysaccharide-Producing Probiotic Lactobacilli Reduce Serum Cholesterol and Modify Enteric Microbiota in ApoE-Deficient Mice. J. Nutr. 2014, 144, 1956–1962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasler, C.M. The Changing Face of Functional Foods. J. Am. Coll. Nutr. 2000, 19, 499S–506S. [Google Scholar] [CrossRef]
- Crowe, K.M.; Francis, C. Position of the Academy of Nutrition and Dietetics: Functional Foods. J. Acad. Nutr. Diet. 2013, 113, 1096–1103. [Google Scholar] [CrossRef]
- Diplock, A.T.; Aggett, P.J.; Ashwell, M.; Bornet, F.; Fern, E.B.; Roberfroid, M.B. Scientific Concepts of Functional Foods in Europe: Consensus Document. Br. J. Nutr. 1999, 81, S1–S27. [Google Scholar]
- Hasler, C.M.; Brown, A.C. Position of the American Dietetic Association: Functional Foods. J. Am. Diet. Assoc. 2009, 109, 735–746. [Google Scholar] [CrossRef]
- Kruger, M.C.; Ha, P.C.; Todd, J.M.; Schollum, L.M.; Ma, J.; Qin, G.; Lau, E. High-Calcium, Vitamin D Fortified Milk Is Effective in Improving Bone Turnover Markers and Vitamin D Status in Healthy Postmenopausal Chinese Women. Eur. J. Clin. Nutr. 2012, 66, 856–861. [Google Scholar] [CrossRef] [Green Version]
- Hertrampf, E.; Corte, F.; Erickson, J.D.; Cayazzo, M.; Freire, W.; Bailey, L.B.; Howson, C.; Kauwell, G.P.A.; Pfeiffer, C. Consumption of Folic Acid–Fortified Bread Improves Folate Status in Women of Reproductive Age in Chile. J. Nutr. 2003, 133, 3166–3169. [Google Scholar] [CrossRef] [Green Version]
- Codex Standard 72 on Infant Formula. Available online: https://www.researchgate.net/profile/Hildegard-Przyrembel/publication/237329880_Medical_Position_Paper_Global_Standard_for_the_Composition_of_Infant_Formula_Recommendations_of_an_ESPGHAN_Coordinated_International_Expert_Group/links/59d4825c0f7e9b4fd702990 (accessed on 22 April 2022).
- Giovannini, M.; Verduci, E.; Salvatici, E.; Fiori, L.; Riva, E. Phenylketonuria: Dietary and Therapeutic Challenges. J. Inherit. Metab. Dis. 2007, 30, 145–152. [Google Scholar] [CrossRef]
- Alongi, M.; Anese, M. Re-Thinking Functional Food Development through a Holistic Approach. J. Funct. Foods 2021, 81, 104466. [Google Scholar] [CrossRef]
- Kaur, S.; Das, M. Functional Foods: An Overview. Food Sci. Biotechnol. 2011, 20, 861–875. [Google Scholar] [CrossRef]
- Menrad, K. Market and Marketing of Functional Food in Europe. J. Food Eng. 2003, 56, 181–188. [Google Scholar] [CrossRef]
- Euromonitor. Available online: https://www-portal-euromonitor-com.dcu.idm.oclc.org/portal/statisticsevolution/index (accessed on 21 April 2022).
- Euromonitor. Trends Shaping the Future of the Food and Nutrition Industry; Euromonitor International: London, UK, 2021. [Google Scholar]
- Ginde, A.A.; Mansback, J.M.; Camargo, C.A. Vitamin D, Respiratory Infections, and Asthma. Curr. Allergy Asthma Rep. 2009, 9, 81–87. [Google Scholar] [CrossRef]
- Bech-Larsen, T.; Scholderer, J. Functional Foods in Europe: Consumer Experiences and Regulatory Aspects. Trends Food Sci. Technol. 2007, 18, 231–234. [Google Scholar] [CrossRef]
- Vicentini, A.; Liberatore, L.; Mastrocola, D. Functional Foods: Trends and Development of the Global Market. Ital. J. Food Sci. 2016, 28, 338–351. [Google Scholar]
- Granato, D.; Branco, G.F.; Cruz, A.G.; Faria, J.d.A.F.; Shah, N.P. Probiotic Dairy Products as Functional Foods. Compr. Rev. Food Sci. Food Saf. 2010, 9, 455–470. [Google Scholar] [CrossRef]
- Nagpal, R.; Behare, P.V.; Kumar, M.; Mohania, D.; Yadav, M.; Jain, S.; Menon, S.; Parkash, O.; Marotta, F.; Minelli, E.; et al. Milk, Milk Products, and Disease Free Health: An Updated Overview. Crit. Rev. Food Sci. Nutr. 2012, 52, 321–333. [Google Scholar] [CrossRef]
- Mathur, H.; Beresford, T.P.; Cotter, P.D. Health Benefits of Lactic Acid Bacteria (Lab) Fermentates. Nutrients 2020, 12, 1679. [Google Scholar] [CrossRef]
- Shao, L.; Wu, Z.; Zhang, H.; Chen, W.; Ai, L.; Guo, B. Partial Characterization and Immunostimulatory Activity of Exopolysaccharides from Lactobacillus rhamnosus KF5. Carbohydr. Polym. 2014, 107, 51–56. [Google Scholar] [CrossRef]
- Ruijssenaars, H.J.; Stingele, F.; Hartmans, S. Biodegradability of Food-Associated Extracellular Polysaccharides. Curr. Microbiol. 2000, 40, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Deveau, H.; Van Calsteren, M.R.; Moineau, S. Effect of Exopolysaccharides on Phage-Host Interactions in Lactococcus lactis. Appl. Environ. Microbiol. 2002, 68, 4364–4369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trabelsi, I.; Ktari, N.; Ben Slima, S.; Triki, M.; Bardaa, S.; Mnif, H.; Ben Salah, R. Evaluation of Dermal Wound Healing Activity and in Vitro Antibacterial and Antioxidant Activities of a New Exopolysaccharide Produced by Lactobacillus Sp.Ca6. Int. J. Biol. Macromol. 2017, 103, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Murofushi, M.; Shiomi, M.; Aibara, K. Effect of Orally Administered Polysaccharide from Kefir Grain on Delayed-Type Hypersensitivity and Tumor Growth in Mice. Japanese J. Med. Sci. Biol. 1983, 36, 49–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, H.; Suzuki, Y.; Kaizu, H.; Hirota, T. Cholesterol Lowering Activity of Ropy Fermented Milk. J. Food Sci. 1992, 57, 1327–1329. [Google Scholar] [CrossRef]
- Laws, A.; Gu, Y.; Marshall, V. Biosynthesis, Characterisation, and Design of Bacterial Exopolysaccharides from Lactic Acid Bacteria. Biotechnol. Adv. 2001, 19, 597–625. [Google Scholar] [CrossRef]
- Looijesteijn, P.J.; Trapet, L.; De Vries, E.; Abee, T.; Hugenholtz, J. Physiological Function of Exopolysaccharides Produced by Lactococcus lactis. Int. J. Food Microbiol. 2001, 64, 71–80. [Google Scholar] [CrossRef]
- Donot, F.; Fontana, A.; Baccou, J.C.; Schorr-Galindo, S. Microbial Exopolysaccharides: Main Examples of Synthesis, Excretion, Genetics and Extraction. Carbohydr. Polym. 2012, 87, 951–962. [Google Scholar] [CrossRef]
- Ruas-Madiedo, P.; Hugenholtz, J.; Zoon, P. An Overview of the Functionality of Exopolysaccharides Produced by Lactic Acid Bacteria. Int. Dairy J. 2002, 12, 163–171. [Google Scholar] [CrossRef]
- Forde, A.; Fitzgerald, G.F. Analysis of Exopolysaccharide (EPS) Production Mediated by the Bacteriophage Adsorption Blocking Plasmid, PCI658, Isolated from Lactococcus lactis ssp. Cremoris HO2. Int. Dairy J. 1999, 9, 465–472. [Google Scholar] [CrossRef]
- Cerning, J. Exocellular Polysaccharides Produced by Lactic Acid Bacteria. FEMS Microbiol. Lett. 1990, 87, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuevas-González, P.F.; Liceaga, A.M.; Aguilar-Toalá, J.E. Postbiotics and Paraprobiotics: From Concepts to Applications. Food Res. Int. 2020, 136, 109502. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Scott, K.P.; Rastall, R.A.; Tuohy, K.M.; Hotchkiss, A.; Dubert-ferrandon, A.; Gareau, M.; Murphy, E.F.; Saulnier, D.; Loh, G.; et al. Dietary Prebiotics: Current Status and New Definition Dietary Prebiotics: Current Status and New Definition. Food Sci. Technol. Bull. Funct. Foods 2010, 1, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Mende, S.; Rohm, H.; Jaros, D. Influence of Exopolysaccharides on the Structure, Texture, Stability and Sensory Properties of Yoghurt and Related Products. Int. Dairy J. 2016, 52, 57–71. [Google Scholar] [CrossRef]
- Korcz, E.; Varga, L. Exopolysaccharides from Lactic Acid Bacteria: Techno-Functional Application in the Food Industr. Trends Food Sci. Technol. 2021, 110, 375–384. [Google Scholar] [CrossRef]
- Abid, Y.; Joulak, I.; Ben Amara, C.; Casillo, A.; Attia, H.; Gharsallaoui, A.; Azabou, S. Study of Interactions between Anionic Exopolysaccharides Produced by Newly Isolated Probiotic Bacteria and Sodium Caseinate. Colloids Surf. B Biointerfaces 2018, 167, 516–523. [Google Scholar] [CrossRef]
- Badel, S.; Bernardi, T.; Michaud, P. New Perspectives for Lactobacilli Exopolysaccharides. Biotechnol. Adv. 2011, 29, 54–66. [Google Scholar] [CrossRef]
- Ryan, P.M.; Ross, R.P.; Fitzgerald, G.F.; Caplice, N.M.; Stanton, C. Sugar-Coated: Exopolysaccharide Producing Lactic Acid Bacteria for Food and Human Health Applications. Food Funct. 2015, 6, 679–693. [Google Scholar] [CrossRef]
- Nabot, M.; Guérin, M.; Sivakumar, D.; Remize, F.; Garcia, C. Variability of Bacterial Homopolysaccharide Production and Properties during Food Processing. Biology 2022, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; De Vin, F.; Vaningelgem, F.; Degeest, B. Recent Developments in the Biosynthesis and Applications of Heteropolysaccharides from Lactic Acid Bacteria. Int. Dairy J. 2001, 11, 687–707. [Google Scholar] [CrossRef]
- De Vuyst, L.; Degeest, B. Heteropolysaccharides from Lactic Acid Bacteria. FEMS Microbiol. Rev. 1999, 23, 153–177. [Google Scholar] [CrossRef]
- Mozzi, F.; Vaningelgem, F.; Hébert, E.M.; Van Der Meulen, R.; Moreno, M.R.F.; Font De Valdez, G.; De Vuyst, L. Diversity of Heteropolysaccharide-Producing Lactic Acid Bacterium Strains and Their Biopolymers. Appl. Environ. Microbiol. 2006, 72, 4431–4435. [Google Scholar] [CrossRef] [Green Version]
- Schmid, J.; Sieber, V.; Rehm, B. Bacterial Exopolysaccharides: Biosynthesis Pathways and Engineering Strategies. Front. Microbiol. 2015, 6, 496. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of Lactic Acid Bacteria: Structure, Bioactivity and Associations: A Review. Carbohydr. Polym. 2019, 207, 317–332. [Google Scholar] [CrossRef]
- Zannini, E.; Waters, D.M.; Coffey, A.; Arendt, E.K. Production, Properties, and Industrial Food Application of Lactic Acid Bacteria-Derived Exopolysaccharides. Appl. Microbiol. Biotechnol. 2016, 100, 1121–1135. [Google Scholar] [CrossRef]
- Moscovici, M. Present and Future Medical Applications of Microbial Exopolysaccharides. Front. Microbiol. 2015, 6, 1012. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, S.; Kavitake, D.; Devi, P.B.; Halady Shetty, P. Bacterial Exopolysaccharides for Improvement of Technological, Functional and Rheological Properties of Yoghurt. Int. J. Biol. Macromol. 2021, 183, 1585–1595. [Google Scholar] [CrossRef]
- Rühmkorf, C.; Rübsam, H.; Becker, T.; Bork, C.; Voiges, K.; Mischnick, P.; Brandt, M.J.; Vogel, R.F. Effect of Structurally Different Microbial Homoexopolysaccharides on the Quality of Gluten-Free Bread. Eur. Food Res. Technol. 2012, 235, 139–146. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F.; Lindqvist, R.; et al. Update of the List of QPS-recommended Biological Agents Intentionally Added to Food or Feed as Notified to EFSA 15: Suitability of Taxonomic Units Notified to EFSA until September 2021. EFSA J. 2022, 20, e07045. [Google Scholar] [CrossRef]
- EFSA. Reporting Food Additive Usage Data on Pullulan (E1204); EFSA: Parma, Italy, 2022.
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Domenico, A.D.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambr, C.; Leblanc, J.-C.; et al. Re-Evaluation of Xanthan Gum (E 415) as a Food Additive EFSA Panel on Food Additives and Nutrient Sources Added to Food (ANS) Panel Members. EFSA J. 2017, 15, 4909. [Google Scholar] [CrossRef] [Green Version]
- EFSA. Scientific Opinion on the Safety of ‘Yeast Beta-Glucans’ as a Novel Food Ingredient. EFSA J. 2011, 9, 2137. [Google Scholar] [CrossRef]
- EFSA. Call for Technical and Toxicological Data on Pullulan (E 1204) Authorised as a Food Additive in the EU; EFSA: Parma, Italy, 2017.
- The European Parliament and the Council of the European Union. Regulation (EC) No 258/97 of the European Parliament and of the of 27 January 1991 Concerning Novel Foods and Novel Food Ingredients. Off. J. Eur. Union 1997, L43, 1–6. [Google Scholar]
- Mårtensson, O.; Öste, R.; Olle, H. Texture Promoting Capacity and EPS Formation by Lactic Acid Bacteria in Three Different Oat-Based Non-Dairy Media. Eur. Food Res. Technol. 2002, 214, 232–236. [Google Scholar] [CrossRef]
- Macedo, M.G.; Lacroix, C.; Gardner, N.J.; Champagne, C.P. Effect of Medium Supplementation on Exopolysaccharide Production by Lactobacillus rhamnosus RW-9595M in Whey Permeate. Int. Dairy J. 2002, 12, 419–426. [Google Scholar] [CrossRef]
- Yuksekdag, Z.N.; Aslim, B. Influence of Different Carbon Sources on Exopolysaccharide Production by Lactobacillus delbrueckii subsp. Bulgaricus (B3, G12) and Streptococcus thermophilus (W22). Brazil. Arch. Biol. Technol. 2008, 51, 581–585. [Google Scholar] [CrossRef]
- Cerning, J.; Renard, C.M.G.C.; Thibault, J.F.; Bouillanne, C.; Landon, M.; Desmazeaud, M.; Topisirovic, L. Carbon Source Requirements for Exopolysaccharide Production by Lactobacillus casei CG11 and Partial Structure Analysis of the Polymer. Appl. Environ. Microbiol. 1994, 60, 3914–3919. [Google Scholar] [CrossRef] [Green Version]
- Gamar, L.; Blondeau, K.; Simonet, J.M. Physiological Approach to Extracellular Polysaccharide Production by Lactobacillus rhamnosus Strain C83. J. Appl. Microbiol. 1997, 83, 281–287. [Google Scholar] [CrossRef]
- Li, D.; Li, J.; Zhao, F.; Wang, G.; Qin, Q.; Hao, Y. The Influence of Fermentation Condition on Production and Molecular Mass of EPS Produced by Streptococcus thermophilus 05-34 in Milk-Based Medium. Food Chem. 2016, 197, 367–372. [Google Scholar] [CrossRef]
- Grobben, G.J.; Smith, M.R.; Sikkema, J.; De Bont, J.A.M. Influence of Fructose and Glucose on the Production of Exopolysaccharides and the Activities of Enzymes Involved in the Sugar Metabolism and the Synthesis of Sugar Nucleotides in Lactobacillus delbrueckii subsp. Bulgaricus NCFB 2772. Appl. Microbiol. Biotechnol. 1996, 46, 279–284. [Google Scholar] [CrossRef]
- Chervaux, C.; Ehrlich, S.D.; Maguin, E. Physiological Study of Lactobacillus delbrueckii subsp. Bulgaricus Strains in a Novel Chemically Defined Medium. Appl. Environ. Microbiol. 2000, 66, 5306–5311. [Google Scholar] [CrossRef] [Green Version]
- Mozzi, F.; Savoy De Giori, G.; Oliver, G.; Font De Valdez, G. Exopolysaccharide Production by Lactobacillus casei. I: Influence of Carbon Source. Milchwissenschaft 1995, 50, 186–188. [Google Scholar]
- Mozzi, F.; Rollán, G.; Savoy De Giori, G.; Font De Valdez, G. Effect of Galactose and Glucose on the Exopolysaccharide Production and the Activities of Biosynthetic Enzymes in Lactobacillus casei CRL 87. J. Appl. Microbiol. 2001, 91, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Cerning, J.; Bouillanne, C.; Landon, M.; Desmazeaud, M. Isolation and Characterization of Exopolysaccharides from Slime-Forming Mesophilic Lactic Acid Bacteria. J. Dairy Sci. 1992, 75, 692–699. [Google Scholar] [CrossRef]
- Imran, M.Y.M.; Reehana, N.; Jayaraj, K.A.; Ahamed, A.A.P.; Dhanasekaran, D.; Thajuddin, N.; Alharbi, N.S.; Muralitharan, G. Statistical Optimization of Exopolysaccharide Production by Lactobacillus plantarum NTMI05 and NTMI20. Int. J. Biol. Macromol. 2016, 93, 731–745. [Google Scholar] [CrossRef]
- Tallon, R.; Bressollier, P.; Urdaci, M.C. Isolation and Characterization of Two Exopolysaccharides Produced by Lactobacillus plantarum EP56. Res. Microbiol. 2003, 154, 705–712. [Google Scholar] [CrossRef]
- Polak-Berecka, M.; Waśko, A.; Kubik-Komar, A. Optimization of Culture Conditions for Exopolysaccharide Production by a Probiotic Strain of Lactobacillus rhamnosus E/N. Polish J. Microbiol. 2014, 63, 253–257. [Google Scholar] [CrossRef]
- Oleksy-Sobczak, M.; Klewicka, E. Optimization of Media Composition to Maximize the Yield of Exopolysaccharides Production by Lactobacillus rhamnosus Strains. Probiotics Antimicrob. Proteins 2020, 12, 774–783. [Google Scholar] [CrossRef] [Green Version]
- Dupont, I.; Roy, D.; Lapointe, G. Comparison of Exopolysaccharide Production by Strains of Lactobacillus rhamnosus and Lactobacillus paracasei Grown in Chemically Defined Medium and Milk. J. Ind. Microbiol. Biotechnol. 2000, 24, 251–255. [Google Scholar] [CrossRef]
- Pham, P.L.; Dupont, I.; Roy, D.; Lapointe, G.; Cerning, J. Production of Exopolysaccharide by Lactobacillus rhamnosus R and Analysis of Its Enzymatic Degradation during Prolonged Fermentation. Appl. Environ. Microbiol. 2000, 66, 2302–2310. [Google Scholar] [CrossRef] [Green Version]
- Mozzi, F. Exopolysaccharide Production by Lactobacillus casei under Controlled PH. Biotechnol. Lett. 1996, 18, 435–439. [Google Scholar] [CrossRef]
- Mozzi, F.; De Giori, G.S.; Oliver, G.; De Valdez, G. racidel. Effect of Culture PH on the Growth Characteristics and Polysaccharide Production by Lactobacillus casei. Milchwiss.-Milk Sci. Int. 1994, 49, 667–670. [Google Scholar]
- Mozzi, F.; Savoy De Giori, G.; Oliver, G.; Fónt De Valdez, G. Exopolysaccharide Production by Lactobacillus casei in Milk under Different Growth Conditions. Milchwissenschaft 1996, 51, 670–673. [Google Scholar]
- Briczinski, E.P.; Roberts, R.F. Production of an Exopolysaccharide-Containing Whey Protein Concentrate by Fermentation of Whey. J. Dairy Sci. 2002, 85, 3189–3197. [Google Scholar] [CrossRef] [Green Version]
- Gassem, M.A.; Sims, K.A.; Frank, J.F. Extracellular Polysaccharide Production by Lactobacillus delbrueckii subsp. Bulgaricus RR in a Continuous Fermentor. LWT—Food Sci. Technol. 1997, 30, 273–278. [Google Scholar] [CrossRef]
- Grobben, G.J.; Chin-Joe, I.; Kitzen, V.A.; Boels, I.C.; Boer, F.; Sikkema, J.; Smith, M.R.; De Bont, J.A.M. Enhancement of Exopolysaccharide Production by Lactobacillus delbrueckii subsp. Bulgaricus NCFB 2772 with a Simplified Defined Medium. Appl. Environ. Microbiol. 1998, 64, 1333–1337. [Google Scholar] [CrossRef] [Green Version]
- Grobben, G.J.; Sikkema, J.; Smith, M.R.; de Bont, J.A.M. Production of Extracellular Polysaccharides by Lactobacillus delbrueckii ssp. Bulgaricus NCFB 2772 Grown in a Chemically Defined Medium. J. Appl. Bacteriol. 1995, 79, 103–107. [Google Scholar] [CrossRef]
- Kimmel, S.A.; Roberts, R.F. Development of a Growth Medium Suitable for Exopolysaccharide Production by Lactobacillus delbrueckii ssp. Bulgaricus RR. Int. J. Food Microbiol. 1998, 40, 87–92. [Google Scholar] [CrossRef]
- Petry, S.; Furlan, S.; Crepeau, M.J.; Cerning, J.; Desmazeaud, M. Factors Affecting Exocellular Polysaccharide Production by Lactobacillus delbrueckii subsp. Bulgaricus Grown in a Chemically Defined Medium. Appl. Environ. Microbiol. 2000, 66, 3427–3431. [Google Scholar] [CrossRef] [Green Version]
- Shene, C.; Bravo, S. Whey Fermentation by Lactobacillus delbrueckii subsp. Bulgaricus for Exopolysaccharide Production in Continuous Culture. Enzyme Microb. Technol. 2007, 40, 1578–1584. [Google Scholar] [CrossRef]
- Bouzar, F.; Cerning, J.; Desmazeaud, M.; Lac, A. Exopolysaccharide Production and Texture-Promoting Abilities of Mixed-Strain Starter Cultures in Yogurt Production. J. Dairy Sci. 1997, 80, 2310–2317. [Google Scholar] [CrossRef]
- Torino, M.I.; Taranto, M.P.; Sesma, F.; De Valdez, G.F. Heterofermentative Pattern and Exopolysaccharide Production by Lactobacillus helveticus ATCC 15807 in Response to Environmental PH. J. Appl. Microbiol. 2001, 91, 846–852. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Ji, J.; Rui, X.; Yu, J.; Tang, W.; Chen, X.; Jiang, M.; Dong, M. Production of Exopolysaccharides by Lactobacillus helveticus MB2-1 and Its Functional Characteristics in Vitro. LWT—Food Sci. Technol. 2014, 59, 732–739. [Google Scholar] [CrossRef]
- Looijesteijn, P.J.; Hugenholtz, J. Uncoupling of Growth and Exopolysaccharide Production by Lactococcus lactis subsp, Cremoris NIZO B40 and Optimization of Its Synthesis. J. Biosci. Bioeng. 1999, 88, 178–182. [Google Scholar] [CrossRef]
- Bergmaier, D.; Champagne, C.P.; Lacroix, C. Exopolysaccharide Production during Batch Cultures with Free and Immobilized Lactobacillus rhamnosus RW-9595M. J. Appl. Microbiol. 2003, 95, 1049–1057. [Google Scholar] [CrossRef] [Green Version]
- Bergmaier, D.; Champagne, C.P.; Lacroix, C. Growth and Exopolysaccharide Production during Free and Immobilized Cell Chemostat Culture of Lactobacillus rhamnosus RW-9595M. J. Appl. Microbiol. 2005, 98, 272–284. [Google Scholar] [CrossRef]
- Gamar-Nourani, L.; Blondeau, K.; Simonet, J.M. Influence of Culture Conditions on Exopolysaccharide Production by Lactobacillus rhamnosus Strain C83. J. Appl. Microbiol. 1998, 85, 664–672. [Google Scholar] [CrossRef]
- Macedo, M.G.; Lacroix, C.; Champagne, C.P. Combined Effects of Temperature and Medium Composition on Exopolysaccharide Production by Lactobacillus rhamnosus RW-9595M in a Whey Permeate Based Medium. Biotechnol. Prog. 2002, 18, 167–173. [Google Scholar] [CrossRef]
- Polak-Berecka, M.; Waśko, A.; Szwajgier, D.; Choma, A. Bifidogenic and Antioxidant Activity of Exopolysaccharides Produced by Lactobacillus rhamnosus E/N Cultivated on Different Carbon Sources. Polish J. Microbiol. 2013, 62, 181–188. [Google Scholar] [CrossRef]
- Ng, I.S.; Xue, C. Enhanced Exopolysaccharide Production and Biological Activity of Lactobacillus rhamnosus ZY with Calcium and Hydrogen Peroxide. Process Biochem. 2017, 52, 295–304. [Google Scholar] [CrossRef]
- Haj-Mustafa, M.; Abdi, R.; Sheikh-Zeinoddin, M.; Soleimanian-Zad, S. Statistical Study on Fermentation Conditions in the Optimization of Exopolysaccharide Production by Lactobacillus rhamnosus 519 in Skimmed Milk Base Media. Biocatal. Agric. Biotechnol. 2015, 4, 521–527. [Google Scholar] [CrossRef]
- Bertsch, A.; Roy, D.; LaPointe, G. Enhanced Exopolysaccharide Production by Lactobacillus rhamnosus in Co-Culture with Saccharomyces cerevisiae. Appl. Sci. 2019, 9, 4026. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhao, X.; Tian, Z.; He, C.; Yang, Y.; Yang, Z. Isolation and Characterization of Exopolysaccharide-Producing Lactobacillus plantarum SKT109 from Tibet Kefir. Polish J. Food Nutr. Sci. 2015, 65, 269–279. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ahmed, Z.; Feng, W.; Li, C.; Song, S. Physicochemical Properties of Exopolysaccharide Produced by Lactobacillus kefiranofaciens ZW3 Isolated from Tibet Kefir. Int. J. Biol. Macromol. 2008, 43, 283–288. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Shimizu, H.; Shioya, S. Enhanced Kefiran Production by Mixed Culture of Lactobacillus kefiranofaciens and Saccharomyces cerevisiae. J. Biotechnol. 2003, 100, 43–53. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Radchabut, S. Use of Whey Lactose from Dairy Industry for Economical Kefiran Production by Lactobacillus kefiranofaciens in Mixed Cultures with Yeasts. N. Biotechnol. 2011, 28, 574–580. [Google Scholar] [CrossRef]
- Tada, S.; Katakura, Y.; Ninomiya, K.; Shioya, S. Fed-Batch Coculture of Lactobacillus kefiranofaciens with Saccharomyces cerevisiae for Effective Production of Kefiran. J. Biosci. Bioeng. 2007, 103, 557–562. [Google Scholar] [CrossRef]
- De Vuyst, L.; Vanderveken, F.; Van De Ven, S.; Degeest, B. Production by and Isolation of Exopolysaccharides from Streptococcus thermophilus Grown in a Milk Medium and Evidence for Their Growth-Associated Biosynthesis. J. Appl. Microbiol. 1998, 84, 1059–1068. [Google Scholar] [CrossRef]
- De Vuyst, L.; Zamfir, M.; Mozzi, F.; Adriany, T.; Marshall, V.; Degeest, B.; Vaningelgem, F. Exopolysaccharide-Producing Streptococcus thermophilus Strains as Functional Starter Cultures in the Production of Fermented Milks. Int. Dairy J. 2003, 13, 707–717. [Google Scholar] [CrossRef]
- Degeest, B.; Mozzi, F.; De Vuyst, L. Effect of Medium Composition and Temperature and PH Changes on Exopolysaccharide Yields and Stability during Streptococcus thermophilus LY03 Fermentations. Int. J. Food Microbiol. 2002, 79, 161–174. [Google Scholar] [CrossRef]
- Degeest, B.; De Vuyst, L. Indication That the Nitrogen Source Influences Both Amount and Size of Exopolysaccharides Produced by Streptococcus thermophilus LY03 and Modelling of the Bacterial Growth and Exopolysaccharide Production in a Complex Medium. Appl. Environ. Microbiol. 1999, 65, 2863–2870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levander, F.; Svensson, M.; Rådström, P. Small-Scale Analysis of Exopolysaccharides from Streptococcus thermophilus Grown in a Semi-Defined Medium. BMC Microbiol. 2001, 1, 23. [Google Scholar] [CrossRef]
- Ricciardi, A.; Parente, E.; Crudele, M.A.; Zanetti, F.; Scolari, G.; Mannazzu, I. Exopolysaccharide Production by Streptococcus thermophilus SY: Production and Preliminary Characterization of the Polymer. J. Appl. Microbiol. 2002, 92, 297–306. [Google Scholar] [CrossRef]
- Shene, C.; Canquil, N.; Bravo, S.; Rubilar, M. Production of the Exopolysacchzrides by Streptococcus thermophilus: Effect of Growth Conditions on Fermentation Kinetics and Intrinsic Viscosity. Int. J. Food Microbiol. 2008, 124, 279–284. [Google Scholar] [CrossRef]
- Vaningelgem, F.; Zamfir, M.; Adriany, T.; De Vuyst, L. Fermentation Conditions Affecting the Bacterial Growth and Exopolysaccharide Production by Streptococcus thermophilus ST 111 in Milk-Based Medium. J. Appl. Microbiol. 2004, 97, 1257–1273. [Google Scholar] [CrossRef]
- Simova, E.D.; Frengova, G.I.; Beshkova, D.M. Exopolysaccharides Produced by Mixed Culture of Yeast Rhodotorula Rubra GED10 and Yogurt Bacteria (Streptococcus thermophilus 13a + Lactobacillus bulgaricus 2-11). J. Appl. Microbiol. 2004, 97, 512–519. [Google Scholar] [CrossRef]
- Petry, S.; Furlan, S.; Waghorne, E.; Saulnier, L.; Cerning, J.; Maguin, E. Comparison of the Thickening Properties of Four Lactobacillus delbrueckii subsp. Bulgaricus Strains and Physicochemical Characterization of Their Exopolysaccharides. FEMS Microbiol. Lett. 2003, 221, 285–291. [Google Scholar] [CrossRef] [Green Version]
- Zisu, B.; Shah, N.P. Effects of PH, Temperature, Supplementation with Whey Protein Concentrate, and Adjunct Cultures on the Production of Exopolysaccharides by Streptococcus thermophilus 1275. J. Dairy Sci. 2003, 86, 3405–3415. [Google Scholar] [CrossRef] [Green Version]
- Ismail, B.; Nampoothiri, K.M. Production, Purification and Structural Characterization of an Exopolysaccharide Produced by a Probiotic Lactobacillus plantarum MTCC 9510. Arch. Microbiol. 2010, 192, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Farnworth, E.R.; Champagne, C.P. Exopolysaccharides from Lactic Acid Bacteria: Food Uses, Production, Chemical Structures, and Health Effects. In Probiotics in Food Safety and Human Health; CRC Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 353–371. [Google Scholar]
- Leroy, F.; Degeest, B.; De Vuyst, L. A Novel Area of Predictive Modelling: Describing the Functionality of Beneficial Microorganisms in Foods. Int. J. Food Microbiol. 2002, 73, 251–259. [Google Scholar] [CrossRef]
- Grobben, G.J.; Boels, I.C.; Sikkema, J.; Smith, M.R.; De Bont, J.A.M. Influence of Ions on Growth and Production of Exopolysaccharides by Lactobacillus delbrueckii subsp. Bulgaricus NCFB 2772. J. Dairy Res. 2000, 67, 131–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozzi, F.; Savoy De Giori, G.; Oliver, G.; Font De Valdez, G. Exopolysaccharide Production by Lactobacillus-Casei.1. Influence of Salts. Milchwiss.-Milk Sci. Int. 1995, 50, 186–188. [Google Scholar]
- Zajšek, K.; Goršek, A.; Kolar, M. Cultivating Conditions Effects on Kefiran Production by the Mixed Culture of Lactic Acid Bacteria Imbedded within Kefir Grains. Food Chem. 2013, 139, 970–977. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Shimizu, H.; Shioya, S. Modelling and Optimization of Environmental Conditions for Kefiran Production by Lactobacillus kefiranofaciens. Appl. Microbiol. Biotechnol. 2001, 57, 639–646. [Google Scholar] [CrossRef]
- Mozzi, F.; Oliver, G.; De Giori, G.; De Valdez, G. Influence of Temperature on the Production of Exopolysaccharides by Thermophilic Lactic Acid Bacteria. Milchwiss.-Milk Sci. Int. 1995, 50, 80–82. [Google Scholar]
- Looijesteijn, P.J.; Van Casteren, W.H.M.; Tuinier, R.; Doeswijk-Voragen, C.H.L.; Hugenholtz, J. Influence of Different Substrate Limitations on the Yield, Composition and Molecular Mass of Exopolysaccharides Produced by Lactococcus lactis subsp. Cremoris in Continuous Cultures. J. Appl. Microbiol. 2000, 89, 116–122. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Shoji, H.; Shimizu, H.; Shioya, S. Interactions between Lactobacillus kefiranofaciens and Saccharomyces cerevisiae in Mixed Culture for Kefiran Production. J. Biosci. Bioeng. 2003, 96, 279–284. [Google Scholar] [CrossRef]
- Freitas, F.; Alves, V.D.; Pais, J.; Costa, N.; Oliveira, C.; Mafra, L.; Hilliou, L.; Oliveira, R.; Reis, M.A.M. Characterization of an Extracellular Polysaccharide Produced by a Pseudomonas Strain Grown on Glycerol. Bioresour. Technol. 2009, 100, 859–865. [Google Scholar] [CrossRef]
- Alves, V.D.; Freitas, F.; Torres, C.A.V.; Cruz, M.; Marques, R.; Grandfils, C.; Gonçalves, M.P.; Oliveira, R.; Reis, M.A.M. Rheological and Morphological Characterization of the Culture Broth during Exopolysaccharide Production by Enterobacter sp. Carbohydr. Polym. 2010, 81, 758–764. [Google Scholar] [CrossRef]
- Seviour, R.J.; McNeil, B.; Fazenda, M.L.; Harvey, L.M. Operating Bioreactors for Microbial Exopolysaccharide Production. Crit. Rev. Biotechnol. 2011, 31, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Mcneil, B.; Harvey, L.M. Viscous Fermentation Products. Crit. Rev. Biotechnol. 1993, 13, 275–304. [Google Scholar] [CrossRef]
- Freitas, F.; Torres, C.A.V.; Reis, M.A.M. Engineering Aspects of Microbial Exopolysaccharide Production. Bioresour. Technol. 2017, 245, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- Freitas, F.; Alves, V.D.; Reis, M.A.M. Advances in Bacterial Exopolysaccharides: From Production to Biotechnological Applications. Trends Biotechnol. 2011, 29, 388–398. [Google Scholar] [CrossRef]
- Marshall, V.M.; Cowie, E.N.; Moreton, R.S. Analysis and Production of Two Exopolysaccharides from Lactococcus lactis subsp. Cremoris LC330. J. Dairy Res. 1995, 62, 621–628. [Google Scholar] [CrossRef] [Green Version]
- Leroy, F.; Vuyst, L. De Advances in Production and Simplified Methods for Recovery and Quantification of Exopolysaccharides for Applications. J. Dairy Sci. 2016, 99, 3229–3238. [Google Scholar] [CrossRef] [Green Version]
- Bajaj, I.B.; Survase, S.A.; Saudagar, P.S.; Singhal, R.S. Gellan Gum: Fermentative Production, Downstream Processing and Applications. Food Technol. Biotechnol. 2007, 45, 341–354. [Google Scholar]
- Rimada, P.S.; Abraham, A.G. Comparative Study of Different Methodologies to Determine the Exopolysaccharide Produced by Kefir Grains in Milk and Whey. Lait 2003, 83, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Ruas-Madiedo, P.; De Los Reyes-Gavilán, C.G. Invited Review: Methods for the Screening, Isolation, and Characterization of Exopolysaccharides Produced by Lactic Acid Bacteria. J. Dairy Sci. 2005, 88, 843–856. [Google Scholar] [CrossRef] [Green Version]
- Polak-Berecka, M.; Choma, A.; Waśko, A.; Górska, S.; Gamian, A.; Cybulska, J. Physicochemical Characterization of Exopolysaccharides Produced by Lactobacillus rhamnosus on Various Carbon Sources. Carbohydr. Polym. 2015, 117, 501–509. [Google Scholar] [CrossRef]
- Xiao, L.; Li, Y.; Tian, J.; Zhou, J.; Xu, Q.; Feng, L.; Rui, X.; Fan, X.; Zhang, Q.; Chen, X.; et al. Influences of Drying Methods on the Structural, Physicochemical and Antioxidant Properties of Exopolysaccharide from Lactobacillus helveticus MB2-1. Int. J. Biol. Macromol. 2020, 157, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Tuinier, R.; Zoon, P.; Olieman, C.; Stuart, M.A.C.; Fleer, G.J.; De Kruif, C.G. Isolation and Physical Characterization of an Exocellular Polysaccharide. Biopolymers 1999, 49, 1–9. [Google Scholar] [CrossRef]
- Notararigo, S.; Nácher-Vázquez, M.; Ibarburu, I.; Werning, M.; De Palencia, P.F.; Dueñas, M.T.; Aznar, R.; López, P.; Prieto, A. Comparative Analysis of Production and Purification of Homo- and Hetero-Polysaccharides Produced by Lactic Acid Bacteria. Carbohydr. Polym. 2013, 93, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciszek-Lenda, M.; Nowak, B.; Srottek, M.; Walczewska, M.; Gorska-Fraczek, S.; Gamian, A.; Marcinkiewicz, J. Further Studies on Immunomodulatory Effects of Exopolysaccharide Isolated from Lactobacillus rhamnosus KL37C. Cent. J. Immunol. 2013, 38, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhou, Z.; Li, Y.; Zhou, L.; Ding, Q.; Xu, L. Isolated Exopolysaccharides from Lactobacillus rhamnosus GG Alleviated Adipogenesis Mediated by TLR2 in Mice. Sci. Rep. 2016, 6, 36083. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wu, T.; Fang, X.; Min, W.; Yang, Z. Characterization and Immunomodulatory Activity of an Exopolysaccharide Produced by Lactobacillus plantarum JLK0142 Isolated from Fermented Dairy Tofu. Int. J. Biol. Macromol. 2018, 115, 985–993. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, N.; Handa, S.; Pathania, S. Purification and Characterization of Novel Exopolysaccharides Produced from Lactobacillus paraplantarum KM1 Isolated from Human Milk and Its Cytotoxicity. J. Genet. Eng. Biotechnol. 2020, 18.1, 1–10. [Google Scholar] [CrossRef]
- Zhou, K.; Zeng, Y.; Yang, M.; Chen, S.; He, L.; Ao, X.; Zou, L.; Liu, S. Production, Purification and Structural Study of an Exopolysaccharide from Lactobacillus plantarum BC-25. Carbohydr. Polym. 2016, 144, 205–214. [Google Scholar] [CrossRef]
- Lebeer, S.; Claes, I.; Tytgat, H.L.P.; Verhoeven, T.L.A.; Marien, E.; von Ossowski, I.; Reunanen, J.; Palva, A.; de Vos, W.M.; De Keersmaecker, S.C.J.; et al. Functional Analysis of Lactobacillus rhamnosus GG Pili in Relation to Adhesion and Immunomodulatory Interactions with Intestinal Epithelial Cells. Appl. Environ. Microbiol. 2012, 78, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Frengova, G.I.; Simova, E.D.; Beshkova, D.M.; Simov, Z.I. Production and Monomer Composition of Exopolysaccharides by Yogurt Starter Cultures. Can. J. Microbiol. 2000, 46, 1123–1127. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lynch, K.M.; Zannini, E.; Coffey, A.; Arendt, E.K. Lactic Acid Bacteria Exopolysaccharides in Foods and Beverages: Isolation, Properties, Characterization, and Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.L. Quantitative Determination of Carbohydrates with Dreywood’s Anthrone Reagent. Science 1948, 107, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, J.; Chirat, F.; Wieruszeski, J.M.; Strecker, G.; Favre, N.; Neeser, J.R. Structural Characterization of the Exocellular Polysaccharides Produced by Streptococcus thermophilus SFi39 and SFi12. Appl. Environ. Microbiol. 1997, 63, 3512–3518. [Google Scholar] [CrossRef] [Green Version]
- Macedo, M.G.; Laporte, M.F.; Lacroix, C. Quantification of Exopolysaccharide, Lactic Acid, and Lactose Concentrations in Culture Broth by near-Infrared Spectroscopy. J. Agric. Food Chem. 2002, 50, 1774–1779. [Google Scholar] [CrossRef]
- Salazar, N.; Gueimonde, M.; Hernández-Barranco, A.M.; Ruas-Madiedo, P.; De Los Reyes-Gavilán, C.G. Exopolysaccharides Produced by Intestinal Bifidobacterium Strains Act as Fermentable Substrates for Human Intestinal Bacteria. Appl. Environ. Microbiol. 2008, 74, 4737–4745. [Google Scholar] [CrossRef] [Green Version]
- Hongpattarakere, T.; Cherntong, N.; Wichienchot, S.; Kolida, S.; Rastall, R.A. In Vitro Prebiotic Evaluation of Exopolysaccharides Produced by Marine Isolated Lactic Acid Bacteria. Carbohydr. Polym. 2012, 87, 846–852. [Google Scholar] [CrossRef]
- Das, D.; Baruah, R.; Goyal, A. A Food Additive with Prebiotic Properties of an α-d-Glucan from Lactobacillus plantarum DM5. Int. J. Biol. Macromol. 2014, 69, 20–26. [Google Scholar] [CrossRef]
- Bello, F.D.; Walter, J.; Hertel, C.; Hammes, W.P. In Vitro Study of Prebiotic Properties of Levan-Type Exopolysaccharides from Lactobacilli and Non-Digestible Carbohydrates Using Denaturing Gradient Gel Electrophoresis. Syst. Appl. Microbiol. 2001, 24, 232–237. [Google Scholar] [CrossRef]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef] [Green Version]
- Salazar, N.; Ruas-Madiedo, P.; Kolida, S.; Collins, M.; Rastall, R.; Gibson, G.; de los Reyes-Gavilán, C.G. Exopolysaccharides Produced by Bifidobacterium longum IPLA E44 and Bifidobacterium animalis subsp. Lactis IPLA R1 Modify the Composition and Metabolic Activity of Human Faecal Microbiota in PH-Controlled Batch Cultures. Int. J. Food Microbiol. 2009, 135, 260–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segers, M.E.; Lebeer, S. Towards a Better Understanding of Lactobacillus rhamnosus GG—Host Interactions. Microb. Cell Fact. 2014, 13, S7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allonsius, C.N.; van den Broek, M.F.L.; De Boeck, I.; Kiekens, S.; Oerlemans, E.F.M.; Kiekens, F.; Foubert, K.; Vandenheuvel, D.; Cos, P.; Delputte, P.; et al. Interplay between Lactobacillus Rhamnosus GG and Candida and the Involvement of Exopolysaccharides. Microb. Biotechnol. 2017, 10, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Rahbar Saadat, Y.; Yari Khosroushahi, A.; Pourghassem Gargari, B. A Comprehensive Review of Anticancer, Immunomodulatory and Health Beneficial Effects of the Lactic Acid Bacteria Exopolysaccharides. Carbohydr. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; López, P.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Suárez, A.; Margolles, A.; Ruas-Madiedo, P. Immune Modulation Capability of Exopolysaccharides Synthesised by Lactic Acid Bacteria and Bifidobacteria. Probiotics Antimicrob. Proteins 2012, 4, 227–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitazawa, H.; Itoh, T.; Tomioka, Y.; Mizugaki, M.; Yamaguchi, T. Induction of IFN-γ and IL-1α Production in Macrophages Stimulated with Phosphopolysaccharide Produced by Lactococcus Lactis ssp. Cremoris. Int. J. Food Microbiol. 1996, 31, 99–106. [Google Scholar] [CrossRef]
- Kitazawa, H.; Harata, T.; Uemura, J.; Saito, T.; Kaneko, T.; Itoh, T. Phosphate Group Requirement for Mitogenic Activation of Lymphocytes by an Extracellular Phosphopolysaccharide from Lactobacillus delbrueckii ssp. Bulgaricus. Int. J. Food Microbiol. 1998, 40, 169–175. [Google Scholar] [CrossRef]
- Makino, S.; Ikegami, S.; Kano, H.; Sashihara, T.; Sugano, H.; Horiuchi, H.; Saito, T.; Oda, M. Immunomodulatory Effects of Polysaccharides Produced by Lactobacillus delbrueckii ssp. Bulgaricus OLL1073R-1. J. Dairy Sci. 2006, 89, 2873–2881. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.F.; Tseng, K.C.; Chiang, S.S.; Lee, B.H.; Hsu, W.H.; Pan, T.M. Immunomodulatory and Antioxidant Potential of Lactobacillus Exopolysaccharides. J. Sci. Food Agric. 2011, 91, 2284–2291. [Google Scholar] [CrossRef]
- Chabot, S.; Yu, H.L.; De Léséleuc, L.; Cloutier, D.; Van Calsteren, M.R.; Lessard, M.; Roy, D.; Lacroix, M.; Oth, D. Exopolysaccharides from Lactobacillus rhamnosus RW-9595M Stimulate TNF, IL-6 and IL-12 in Human and Mouse Cultured Immunocompetent Cells, and IFN-γ in Mouse Splenocytes. Lait 2001, 81, 683–697. [Google Scholar] [CrossRef] [Green Version]
- Vinderola, G.; Perdigón, G.; Duarte, J.; Farnworth, E.; Matar, C. Effects of the Oral Administration of the Exopolysaccharide Produced by Lactobacillus kefiranofaciens on the Gut Mucosal Immunity. Cytokine 2006, 36, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Ahn, K.; Lee, M.; Kim, S.; Park, B.; Kim, M.; Lee, I.; Oh, S.; Lee, H. Inhibitory Effect of Kefiran on Ovalbumin-Induced Lung Inflamma- Tion in a Murine Model of Asthma. Arch. Pharmacal Res. 2008, 31, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Bleau, C.; Monges, A.; Rashidan, K.; Laverdure, J.P.; Lacroix, M.; Van Calsteren, M.R.; Millette, M.; Savard, R.; Lamontagne, L. Intermediate Chains of Exopolysaccharides from Lactobacillus rhamnosus RW-9595M Increase IL-10 Production by Macrophages. J. Appl. Microbiol. 2010, 108, 666–675. [Google Scholar] [CrossRef] [PubMed]
- López, P.; Monteserín, D.C.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Margolles, A.; Suárez, A.; Ruas-Madiedo, P. Exopolysaccharide-Producing Bifidobacterium Strains Elicit Different in Vitro Responses upon Interaction with Human Cells. Food Res. Int. 2012, 46, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Kahl, R.; Kappus, H. Toxikologie Der Synthetischen Antioxidantien BHA Und BHT Im Vergleich Mit Dem Natürlichen Antioxidans Vitamin E. Z. Lebensm. Unters. Forsch. 1993, 196, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Sakagami, H.; Fujisawa, S. Cytotoxicity and Apoptosis Induction by Butylated Hydroxyanisole (BHA) and Butylated Hydroxytoluene (BHT). Anticancer Res. 2003, 23, 4693–4701. [Google Scholar]
- Sies, H. Oxidative Stress: Oxidants and Antioxidants. Exp. Physiol. Transl. Integr. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Kodali, V.P.; Sen, R. Antioxidant and Free Radical Scavenging Activities of an Exopolysaccharide from a Probiotic Bacterium. Biotechnol. J. 2008, 3, 245–251. [Google Scholar] [CrossRef]
- Adesulu-Dahunsi, A.T.; Sanni, A.I.; Jeyaram, K. Production, Characterization and In Vitro Antioxidant Activities of Exopolysaccharide from Weissella Cibaria GA44. LWT—Food Sci. Technol. 2018, 87, 432–442. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Liu, C.; Li, D.; Zhao, Y.; Zhang, X.; Zeng, X.; Yang, Z.; Li, S. Antioxidant Activity of an Exopolysaccharide Isolated from Lactobacillus plantarum C88. Int. J. Biol. Macromol. 2013, 54, 270–275. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, R.; Wang, L.; Zhang, H. The Antioxidative Effects of Probiotic Lactobacillus casei Zhang on the Hyperlipidemic Rats. Eur. Food Res. Technol. 2010, 231, 151–158. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Y.; Zhang, L.; Zhang, X.; Huang, L.; Li, D.; Niu, C.; Yang, Z.; Wang, Q. Antioxidant Activity of Lactobacillus plantarum Strains Isolated from Traditional Chinese Fermented Foods. Food Chem. 2012, 135, 1914–1919. [Google Scholar] [CrossRef] [PubMed]
- Patten, D.A.; Laws, A.P. Lactobacillus-Produced Exopolysaccharides and Their Potential Health Benefits: A Review. Benef. Microbes 2015, 6, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Kim, Y.; Han, K.S.; You, S.; Oh, S.; Kim, S.H. Effects of Lactobacillus Strains on Cancer Cell Proliferation and Oxidative Stress in Vitro. Lett. Appl. Microbiol. 2006, 42, 452–458. [Google Scholar] [CrossRef]
- Kim, Y.; Oh, S.; Yun, H.S.; Oh, S.; Kim, S.H. Cell-Bound Exopolysaccharide from Probiotic Bacteria Induces Autophagic Cell Death of Tumour Cells. Lett. Appl. Microbiol. 2010, 51, 123–130. [Google Scholar] [CrossRef]
- Ismail, B.; Nampoothiri, K.M. Exposition of Antitumour Activity of a Chemically Characterized Exopolysaccharide from a Probiotic Lactobacillus plantarum MTCC 9510. Biologia 2013, 68, 1041–1047. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Ji, J.; Chen, X.; Jiang, M.; Rui, X.; Dong, M. Structural Elucidation and Antioxidant Activities of Exopolysaccharides from Lactobacillus helveticus MB2-1. Carbohydr. Polym. 2014, 102, 351–359. [Google Scholar] [CrossRef]
- Wang, K.; Li, W.; Rui, X.; Chen, X.; Jiang, M.; Dong, M. Characterization of a Novel Exopolysaccharide with Antitumor Activity from Lactobacillus plantarum 70810. Int. J. Biol. Macromol. 2014, 63, 133–139. [Google Scholar] [CrossRef]
- Tok, E.; Aslim, B. Cholesterol Removal by Some Lactic Acid Bacteria That Can Be Used as Probiotic. Microbiol. Immunol. 2010, 54, 257–264. [Google Scholar] [CrossRef]
- Maeda, H.; Zhu, X.; Omura, K.; Suzuki, S.; Kitamura, S. Effects of an Exopolysaccharide (Kefiran) on Lipids, Blood Pressure, Blood Glucose, and Constipation. BioFactors 2004, 22, 197–200. [Google Scholar] [CrossRef]
- Pessione, E.; Cirrincione, S. Bioactive Molecules Released in Food by Lactic Acid Bacteria: Encrypted Peptides and Biogenic Amines. Front. Microbiol. 2016, 7, 876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ai, L.; Zhang, H.; Guo, B.; Chen, W.; Wu, Z.; Wu, Y. Preparation, Partial Characterization and Bioactivity of Exopolysaccharides from Lactobacillus casei LC2W. Carbohydr. Polym. 2008, 74, 353–357. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, J.M.; Liu, W.; Pi, X.; Zhou, Q.; Li, P.; Zhou, T.; Gu, Q. Effects of Exopolysaccharide from Lactobacillus rhamnosus on Human Gut Microbiota in in Vitro Fermentation Model. LWT 2021, 139, 110524. [Google Scholar] [CrossRef]
- Lim, J.; Kale, M.; Kim, D.H.; Kim, H.S.; Chon, J.W.; Seo, K.H.; Lee, H.G.; Yokoyama, W.; Kim, H. Antiobesity Effect of Exopolysaccharides Isolated from Kefir Grains. J. Agric. Food Chem. 2017, 65, 10011–10019. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-Talk between Akkermansia muciniphila and Intestinal epithelium Controls Diet-Induced Obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, C.J.; Healy, S.; O’Toole, P.W.; Murphy, E.F.; Cotter, P.D. The Probiotic L. Casei LC-XCALTM Improves Metabolic Health in a Diet-Induced Obesity Mouse Model without Altering the Microbiome. Gut Microbes 2020, 12, 1747330. [Google Scholar] [CrossRef]
- Byrne, C.S.; Chambers, E.S.; Morrison, D.J.; Frost, G. The Role of Short Chain Fatty Acids in Appetite Regulation and Energy Homeostasis. Int. J. Obes. 2015, 39, 1331–1338. [Google Scholar] [CrossRef] [Green Version]




| Stain | Media | Fermentation | Yield | Ref |
|---|---|---|---|---|
| L. casei | BMM w. glucose | Flask | 160 mg/L | [63] |
| L. casei | Skim milk | Flask | 600 mg/L | [70] |
| L. casei | Galactose + tryptone + MnSO4 + CaCl2 | Flask | 120 mg/L | [69] |
| L. casei | APTg broth + Ca2+ + Mn2+ | Flask | 124 mg/L | [68] |
| L. casei | Galactose + tryptone + MnSO4 + CaCl2 | Flask | 488 mg/L | [77] |
| L. casei | Skim milk + APTg broth | Flask | 120 mg/L | [78] |
| L. casei | Skim milk | Flask | 121 mg/L | [79] |
| L. delbrueckii subsp. bulgaricus | MRS + glucose | Flask | 255 mg/L | [62] |
| L. delbrueckii subsp. bulgaricus | Hydrolysed whey | Single batch—free cells | 325 mg/L | [80] |
| L. delbrueckii subsp. bulgaricus | Whey + lactose + KH2PO4 + NH4Cl + casamino acids | Continuous fermenter | 2.13 g/L | [81] |
| L. delbrueckii subsp. bulgaricus | Defined medium | Single batch—free cells | 250 mg/L | [82] |
| L. delbrueckii subsp. bulgaricus | Defined medium w. glucose | Flask | 36.8 mg/L | [83] |
| L. delbrueckii subsp. bulgaricus | Defined medium w. fructose + glucose | Flask | 80 mg/L | [66] |
| L. delbrueckii subsp. bulgaricus | Semi-defined media | Flask | 220 mg/L | [84] |
| L. delbrueckii subsp. bulgaricus | Milk Chemically defined medium | Flask Flask | 170 mg/L 174 mg/L | [85] |
| L. delbrueckii subsp. bulgaricus | Lactose (from whey) + YE + peptone + tween80 + MgSO4 + MnSO4 | Continuous culture | 830 mg/L | [86] |
| L. delbrueckii subsp. bulgaricus S. thermophilus | Reconstituted milk | Flask | 240 mg/L | [87] |
| L. helveticus | Skim milk | Single batch—free cells | 549 mg/L | [88] |
| L. helveticus | Whey + lactose + peptone + MnSO4 | Flask | 658 mg/L | [89] |
| L. lactis subsp. cremoris | Defined media | Single batch—free cells | 520 mg/L | [90] |
| L. rhamnosus | Whey + YE + tween 80 + MgSO4+ MnSO4 | Single batch—free cells Repeated-batch cultures—ICT | 2.3 g/L (110 mg/L/h) 1.7 g/L (250 mg/L/h) | [91] |
| L. rhamnosus | Whey + YE + tween 80 + MgSO4 + MnSO4 | Continuous—ICT | 1.8 g/L (542.6 mg/L/h) | [92] |
| L. rhamnosus | BMM w. mannose BMM w. fructose + glucose | Flask | 132 mg/L 111 mg/L | [64] |
| L. rhamnosus | CDM | Single batch—free cells | 251 mg/L | [93] |
| L. rhamnosus | Whey + yeast nitrogen base | Single batch—free cells | 477 mg/L | [94] |
| L. rhamnosus | Whey + YE + salts + AA | Single batch—free cells | 2767 mg/L | [61] |
| L. rhamnosus | BMM w. lactose | Single batch—free cells | 1275 mg/L | [75] |
| L. rhamnosus | Fructose + glucose + sucrose + K2HPO4, CH3COONa, C6H14N2O7, MgSO4 + MnSO4 | Flask | 987 mg/L | [74] |
| L. rhamnosus | Fructose + glucose + sucrose + YE + K2HPO4, CH3COONa, + C6H14N2O7, MgSO4 + MnSO4 | Flask | 900 mg/L | [74] |
| L. rhamnosus | Fructose + glucose + sucrose + K2HPO4, CH3COONa, C6H14N2O7, MgSO4 + MnSO4 + tween | Flask | 1138.2 mg/L | [74] |
| L. rhamnosus | MRS w. galactose + YE | Single batch—free cells | 210 mg/L | [73] |
| L. rhamnosus | MRS w. lactose | Single batch—free cells | 219 mg/L | [95] |
| L. rhamnosus | BMM | Single batch—free cells | 495 mg/L | [76] |
| L. rhamnosus | MRS + H2O2 + CaCl2 | Static flask | 2498 mg/L | [96] |
| L. rhamnosus | Skim milk + sucrose + YNB | Single batch—free cells | 256 mg/L | [97] |
| L. rhamnosus S. cerevisiae | Whey + YE + corn steep liquor + tween 80 + MgSO4 + MnSO4 | Singe batch—free cells | 1350 mg/L | [98] |
| L. paracasei | BMM w. lactose | Single batch—free cells | 85 mg/L | [75] |
| L. plantarum | CDM w. lactose | Static flask | 140.2 mg/L | [72] |
| L. plantarum | Semi-defined media | Flask | 58.7 mg/L | [99] |
| L. plantarum | Glucose + YE + NH3SO4 | Flask | 956 mg/L | [71] |
| L. kefiranofaciens | Whey + lactose + glucose + tryptone + sodium acetate + tween 80 + cysteine monohydrochloride | Flask | 1215 mg/L | [100] |
| L. kefiranofaciens S. cerevisiae | MRS w. lactose | Fed-batch | 5.4 g/L | [101] |
| L. kefiranofaciens + S. cerevisiae | MRS w. whey lactose | Single batch—free cells Fed-batch | 2580 mg/L 3260 mg/L | [102] |
| L. kefiranofaciens + S. cerevisiae | MRS w. lactose | Batch Fed-batch | 4.5 g/L 6.3 g/L | [103] |
| S. thermophilus | Milk + peptone + YE | Single batch—free cells | 166 mg/L | [104] |
| S. thermophilus | Milk + peptone + YE | Flask | 284 mg/L | [105] |
| S. thermophilus | Lactose + arginine | Single batch—free cells | 1158 mg/L | [106] |
| S. thermophilus | MRS w. lactose and 4.2% nitrogen | Single batch—free cells | 1142 mg/L | [107] |
| S. thermophilus | Semi-defined medium | Single batch—free cells | 325 mg/L | [108] |
| S. thermophilus | Sucrose + soy peptone | Flask | 250 mg/L | [65] |
| S. thermophilus | Whey + YE + tryptone | Single batch—free cells | 147 mg/L | [109] |
| S. thermophilus | Lactose from DW + YE + peptone + tween80 + MgSO4 + MnSO4 | Single batch—free cells | 106 mg/L | [110] |
| S. thermophilus | Milk + tryptone | Single batch—free cells | 507 m/L | [111] |
| S. thermophilus + L. delbrueckii subsp. bulgaricus + R. rubra | Whey + (NH4)2SO4 + KH2PO4 + MgSO4 + YE | Single batch—free cells | 19.3 g/L | [112] |
| Microorganism | Precipitation | Protein Removal | Other Treatment | Quantification | Ref |
|---|---|---|---|---|---|
| L. casei | Ethanol | TCA | - | Phenol/sulphuric acid | [63] |
| L. casei | Ethanol | Pronase digestion | Ultrafiltration | Phenol/sulphuric acid | [70] |
| L. casei | Ethanol | - | - | Phenol/sulphuric acid | [69] |
| L. casei | Ethanol | Pronase digestion | - | Phenol/sulphuric acid | [68] |
| L. casei | Ethanol | - | - | Phenol/sulphuric acid | [77] |
| L. casei | Ethanol | Pronase digestion | - | Phenol/sulphuric acid | [78] |
| L. casei | Ethanol | Pronase digestion | - | Phenol/sulphuric acid | [79] |
| L. delbrueckii subsp. bulgaricus | Ethanol | TCA | - | Phenol/sulphuric acid | [62] |
| L. delbrueckii subsp. bulgaricus | - | Pronase digestion and TCA | - | Phenol/sulphuric acid | [80] |
| L. delbrueckii subsp. bulgaricus | Ethanol | - | - | Dry weight | [81] |
| L. delbrueckii subsp. bulgaricus | Ethanol | TCA | - | Phenol/sulphuric acid | [82] |
| L. delbrueckii subsp. bulgaricus | Ethanol | TCA | - | Phenol/sulphuric acid | [83] |
| L. delbrueckii subsp. bulgaricus | Ethanol | Phenol/sulphuric acid | [66] | ||
| L. delbrueckii subsp. bulgaricus | - | Pronase digestion and TCA | - | Phenol/sulphuric acid | [84] |
| L. delbrueckii subsp. bulgaricus | Ethanol | Phenol/sulphuric acid | [85] | ||
| L. delbrueckii subsp. bulgaricus | Ethanol | TCA | - | Phenol/sulphuric acid | [86] |
| L. delbrueckii subsp. Bulgaricus + S. thermophilus | Ethanol | Pronase digestion | Ultrafiltration | Phenol/sulphuric acid | [87] |
| L. helveticus | Ethanol | Pronase | Phenol/sulphuric acid | [88] | |
| L. helveticus | Ethanol | TCA | Microfiltration | Phenol/sulphuric acid | [89] |
| L. lactis subsp. cremoris | - | - | Dialysis | Phenol/sulphuric acid | [131] |
| L. lactis subsp. cremoris | - | - | Microfiltration | Gel permeation chromatography | [90] |
| L. rhamnosus | - | - | Ultrafiltration | Phenol/sulphuric acid | [91] |
| L. rhamnosus | - | - | Ultrafiltration | Phenol/sulphuric acid | [92] |
| L. rhamnosus | Ethanol | - | - | Phenol/sulphuric acid | [64] |
| L. rhamnosus | Ethanol | - | - | Phenol/sulphuric acid | [93] |
| L. rhamnosus | - | - | Ultrafiltration | Phenol/sulphuric acid | [72] |
| L. rhamnosus | Ethanol | TCA | - | Phenol/sulphuric acid | [94] |
| L. rhamnosus | - | - | Ultrafiltration | Phenol/sulphuric acid | [61] |
| L. rhamnosus | Ethanol | - | - | Phenol/sulphuric acid | [75] |
| L. rhamnosus | Ethanol | TCA | - | Phenol/sulphuric acid | [74] |
| L. rhamnosus | Ethanol | TCA | Vacuum rotary evaporator | Phenol/sulphuric acid | [73] |
| L. rhamnosus | Ethanol | TCA | Vacuum rotary evaporator | Phenol/sulphuric acid | [95] |
| L. rhamnosus | Ethanol | Heat treatment | - | Phenol/sulphuric acid | [76] |
| L. rhamnosus | Ethanol | TCA | - | AEC | [96] |
| L. rhamnosus | Ethanol | Heat treatment + TCA | - | Phenol/sulphuric acid | [97] |
| L. rhamnosus + S. cerevisiae | Ethanol | TCA | - | Phenol/sulphuric acid | [98] |
| L. paracasei | Ethanol | - | - | Phenol/sulphuric acid | [75] |
| L. plantarum | Ethanol | - | - | Phenol-sulphuric acid | [115] |
| L. plantarum | Ethanol | TCA | - | Phenol/sulphuric acid | [72] |
| L. plantarum | Ethanol | TCA | - | Phenol/sulphuric acid | [99] |
| L. plantarum | Ethanol | Heat treatment | - | Phenol/sulphuric acid | [71] |
| L. kefiranofaciens | Ethanol | TCA | - | Phenol/sulphuric acid | [100] |
| L. kefiranofaciens + S. cerevisiae | Ethanol | - | - | Anthrone reagent | [101] |
| L. kefiranofaciens + S. cerevisiae | Ethanol | - | - | Anthrone reagent | [102] |
| L. kefiranofaciens + S. cerevisiae + | Ethanol | - | - | Anthrone reagent | [103] |
| S. thermophilus | Acetone | TCA | - | Dry weight | [104] |
| S. thermophilus | Acetone | TCA | - | Gel permeation chromatography | [105] |
| S. thermophilus | Acetone | TCA | - | Dry weight | [106] |
| S. thermophilus | Acetone | TCA | - | Dry weight | [107] |
| S. thermophilus | - | TCA | Ultracentrifugation filtration | HPAEC-PAD | [108] |
| S. thermophilus | Ethanol | TCA | - | Phenol/sulphuric acid | [65] |
| S. thermophilus | Ethanol | - | Ultrafiltration | Phenol/sulphuric acid | [109] |
| S. thermophilus | Ethanol | TCA | Ultrafiltration | Phenol/sulphuric acid | [110] |
| S. thermophilus | Acetone | TCA | - | Dry weight | [111] |
| S. thermophilus + L. delbrueckii subsp. bulgaricus + R. rubra | Acetone + ethanol | TCA | - | Phenol/sulphuric acid | [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sørensen, H.M.; Rochfort, K.D.; Maye, S.; MacLeod, G.; Brabazon, D.; Loscher, C.; Freeland, B. Exopolysaccharides of Lactic Acid Bacteria: Production, Purification and Health Benefits towards Functional Food. Nutrients 2022, 14, 2938. https://doi.org/10.3390/nu14142938
Sørensen HM, Rochfort KD, Maye S, MacLeod G, Brabazon D, Loscher C, Freeland B. Exopolysaccharides of Lactic Acid Bacteria: Production, Purification and Health Benefits towards Functional Food. Nutrients. 2022; 14(14):2938. https://doi.org/10.3390/nu14142938
Chicago/Turabian StyleSørensen, Helena Mylise, Keith D. Rochfort, Susan Maye, George MacLeod, Dermot Brabazon, Christine Loscher, and Brian Freeland. 2022. "Exopolysaccharides of Lactic Acid Bacteria: Production, Purification and Health Benefits towards Functional Food" Nutrients 14, no. 14: 2938. https://doi.org/10.3390/nu14142938
APA StyleSørensen, H. M., Rochfort, K. D., Maye, S., MacLeod, G., Brabazon, D., Loscher, C., & Freeland, B. (2022). Exopolysaccharides of Lactic Acid Bacteria: Production, Purification and Health Benefits towards Functional Food. Nutrients, 14(14), 2938. https://doi.org/10.3390/nu14142938








