Comparison of Five Oxidative Stress Biomarkers in Vegans and Omnivores from Germany and Finland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Populations
2.2. Blood and Urine Collection and Laboratory Analysis
2.3. Assessment of Diet and Lifestyle Characteristics
2.4. Statistical Analysis
3. Results
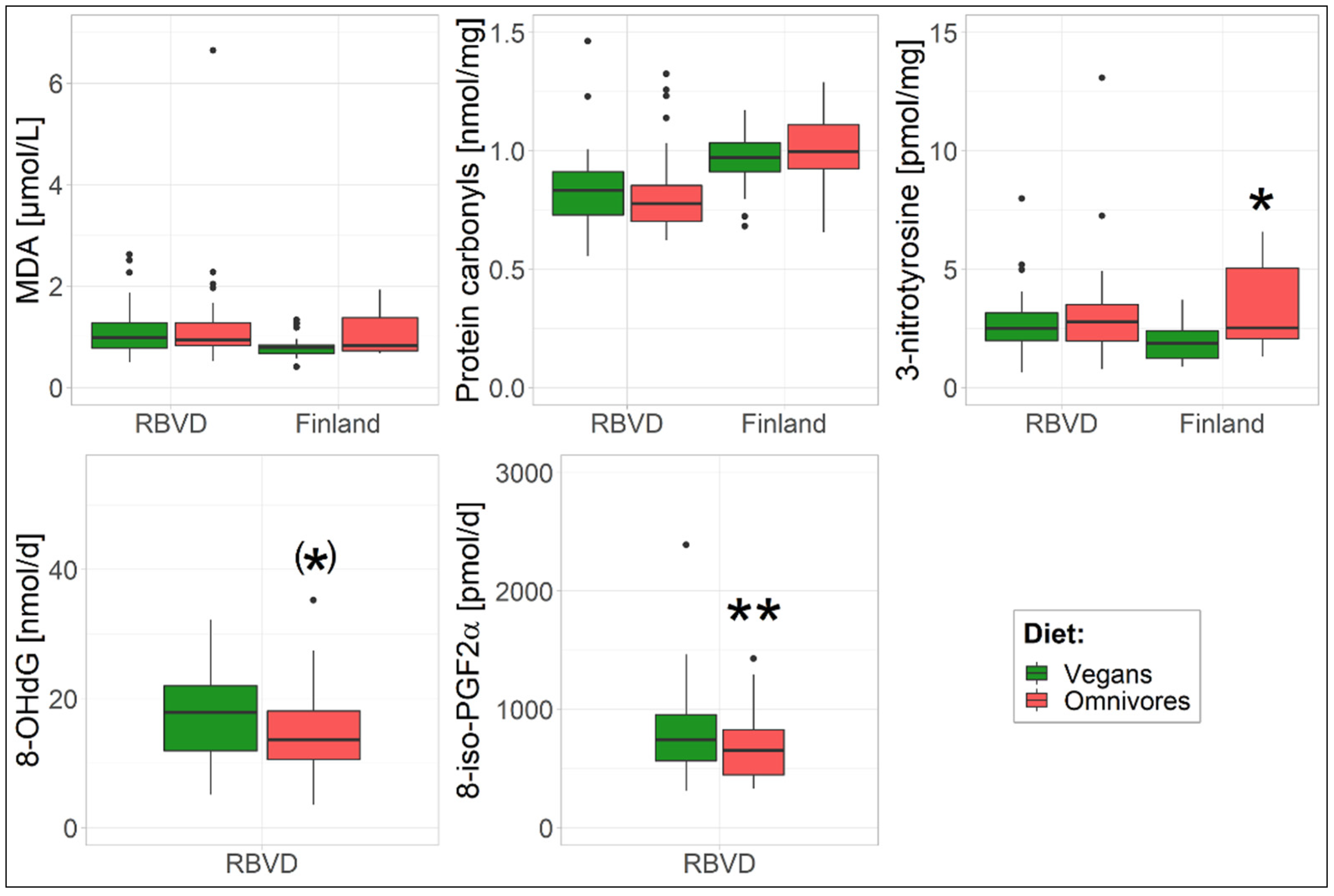
3.1. Oxidative Stress Biomarkers
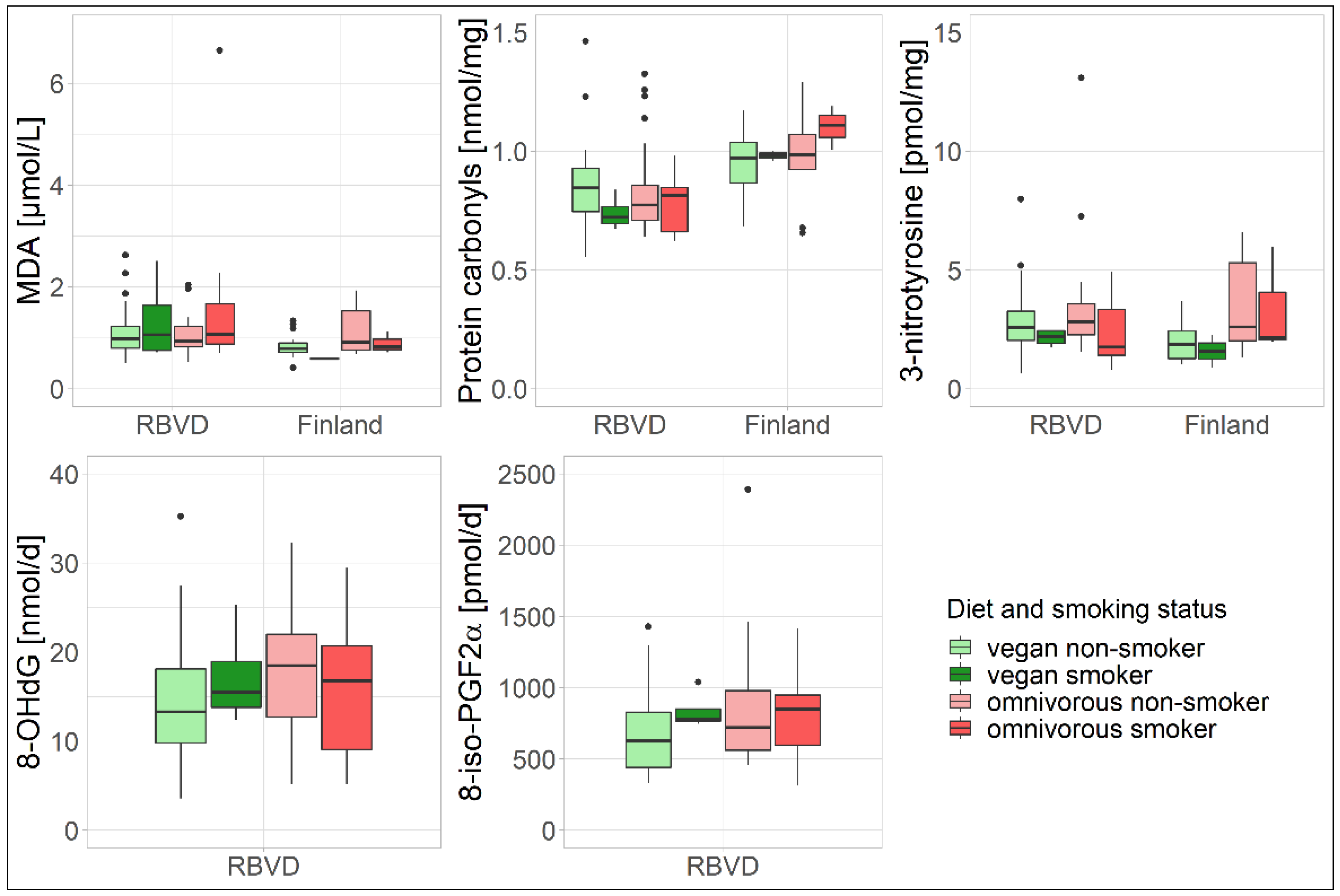
3.2. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egea, J.; Fabregat, I.; Frapart, Y.M.; Ghezzi, P.; Görlach, A.; Kietzmann, T.; Kubaichuk, K.; Knaus, U.G.; Lopez, M.G.; Olaso-Gonzalez, G.; et al. European contribution to the study of ROS: A summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS). Redox Biol. 2017, 13, 94–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical relevance of biomarkers of oxidative stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef] [Green Version]
- Madian, A.G.; Regnier, F.E. Proteomic identification of carbonylated proteins and their oxidation sites. J. Proteome Res. 2010, 9, 3766–3780. [Google Scholar] [CrossRef] [Green Version]
- Graille, M.; Wild, P.; Sauvain, J.-J.; Hemmendinger, M.; Guseva Canu, I.; Hopf, N.B. Urinary 8-OHdG as a biomarker for oxidative stress: A systematic literature review and meta-analysis. Int. J. Mol. Sci. 2020, 21, 3743. [Google Scholar] [CrossRef]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef]
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox Biol. 2021, 42, 101869. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Baiano, A.; Del Nobile, M.A. Antioxidant compounds from vegetable matrices: Biosynthesis, occurrence, and extraction systems. Crit. Rev. Food Sci. Nutr. 2016, 56, 2053–2068. [Google Scholar] [CrossRef] [PubMed]
- Kochlik, B.; Grune, T.; Weber, D. New findings of oxidative stress biomarkers in nutritional research. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Gajski, G.; Gerić, M.; Jakaša, I.; Peremin, I.; Domijan, A.M.; Vučić Lovrenčić, M.; Kežić, S.; Bituh, M.; Moraes de Andrade, V. Inflammatory, oxidative and DNA damage status in vegetarians: Is the future of human diet green? Crit. Rev. Food Sci. Nutr. 2021, 1–33. [Google Scholar] [CrossRef]
- Weikert, C.; Trefflich, I.; Menzel, J.; Obeid, R.; Longree, A.; Dierkes, J.; Meyer, K.; Herter-Aeberli, I.; Mai, K.; Stangl, G.I.; et al. Vitamin and mineral status in a vegan diet. Dtsch. Arztebl. Int. 2020, 117, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Elorinne, A.L.; Alfthan, G.; Erlund, I.; Kivimäki, H.; Paju, A.; Salminen, I.; Turpeinen, U.; Voutilainen, S.; Laakso, J. Food and nutrient intake and nutritional status of finnish vegans and non-vegetarians. PLoS ONE 2016, 11, e0148235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menzel, J.; Biemann, R.; Longree, A.; Isermann, B.; Mai, K.; Schulze, M.B.; Abraham, K.; Weikert, C. Associations of a vegan diet with inflammatory biomarkers. Sci. Rep. 2020, 10, 1933. [Google Scholar] [CrossRef]
- Wong, S.H.; Knight, J.A.; Hopfer, S.M.; Zaharia, O.; Leach, C.N., Jr.; Sunderman, F.W., Jr. Lipoperoxides in plasma as measured by liquid-chromatographic separation of malondialdehyde-thiobarbituric acid adduct. Clin. Chem. 1987, 33, 214–220. [Google Scholar] [CrossRef]
- Weber, D.; Stuetz, W.; Bernhard, W.; Franz, A.; Raith, M.; Grune, T.; Breusing, N. Oxidative stress markers and micronutrients in maternal and cord blood in relation to neonatal outcome. Eur. J. Clin. Nutr. 2014, 68, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Weber, D.; Kneschke, N.; Grimm, S.; Bergheim, I.; Breusing, N.; Grune, T. Rapid and sensitive determination of protein-nitrotyrosine by ELISA: Application to human plasma. Free Radic. Res. 2012, 46, 276–285. [Google Scholar] [CrossRef]
- Dahl, J.H.; van Breemen, R.B. Rapid quantitative analysis of 8-iso-prostaglandin-F(2alpha) using liquid chromatography-tandem mass spectrometry and comparison with an enzyme immunoassay method. Anal. Biochem. 2010, 404, 211–216. [Google Scholar] [CrossRef] [Green Version]
- The InterAct, C. Validity of a short questionnaire to assess physical activity in 10 European countries. Eur. J. Epidemiol. 2012, 27, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative stress, plant natural antioxidants, and obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef] [PubMed]
- Nebl, J.; Drabert, K.; Haufe, S.; Wasserfurth, P.; Eigendorf, J.; Tegtbur, U.; Hahn, A.; Tsikas, D. Exercise-induced oxidative stress, nitric oxide and plasma amino acid profile in recreational runners with vegetarian and non-vegetarian dietary patterns. Nutrients 2019, 11, 1875. [Google Scholar] [CrossRef] [Green Version]
- Toto, A.; Wild, P.; Graille, M.; Turcu, V.; Crézé, C.; Hemmendinger, M.; Sauvain, J.J.; Bergamaschi, E.; Guseva Canu, I.; Hopf, N.B. Urinary malondialdehyde (MDA) concentrations in the general population-a systematic literature review and meta-analysis. Toxics 2022, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Nadimi, H.; Yousefi Nejad, A.; Djazayery, A.; Hosseini, M.; Hosseini, S. Association of vegan diet with RMR, body composition and oxidative stress. Acta Sci. Pol. Technol. Aliment. 2013, 12, 311–318. [Google Scholar] [PubMed]
- Krajčovičová-Kudláčková, M.; Valachovičová, M.; Pauková, V.; Dušinská, M. Effects of diet and age on oxidative damage products in healthy subjects. Physiol. Res. 2008, 57, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Bandookwala, M.; Sengupta, P. 3-Nitrotyrosine: A versatile oxidative stress biomarker for major neurodegenerative diseases. Int J. Neurosci 2020, 130, 1047–1062. [Google Scholar] [CrossRef] [PubMed]
- Thomson, L. 3-nitrotyrosine modified proteins in atherosclerosis. Dis. Markers 2015, 2015, 708282. [Google Scholar] [CrossRef]
- Vanacore, D.; Messina, G.; Lama, S.; Bitti, G.; Ambrosio, P.; Tenore, G.; Messina, A.; Monda, V.; Zappavigna, S.; Boccellino, M.; et al. Effect of restriction vegan diet’s on muscle mass, oxidative status, and myocytes differentiation: A pilot study. J. Cell Physiol. 2018, 233, 9345–9353. [Google Scholar] [CrossRef]
- Blekkenhorst, L.C.; Prince, R.L.; Ward, N.C.; Croft, K.D.; Lewis, J.R.; Devine, A.; Shinde, S.; Woodman, R.J.; Hodgson, J.M.; Bondonno, C.P. Development of a reference database for assessing dietary nitrate in vegetables. Mol. Nutr. Food Res. 2017, 61, 1600982. [Google Scholar] [CrossRef] [Green Version]
- Pannala, A.S.; Mani, A.R.; Spencer, J.P.; Skinner, V.; Bruckdorfer, K.R.; Moore, K.P.; Rice-Evans, C.A. The effect of dietary nitrate on salivary, plasma, and urinary nitrate metabolism in humans. Free Radic Biol Med. 2003, 34, 576–584. [Google Scholar] [CrossRef]
- Korovila, I.; Höhn, A.; Jung, T.; Grune, T.; Ott, C. Reduced liver autophagy in high-fat diet induced liver steatosis in New Zealand obese mice. Antioxidants 2021, 10, 501. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, S.; Helmersson, J.; Jarosinska, D.; Sällsten, G.; Mazzolai, B.; Barregård, L. Regulatory factors of basal F(2)-isoprostane formation: Population, age, gender and smoking habits in humans. Free Radic. Res. 2009, 43, 85–91. [Google Scholar] [CrossRef]
- Burgos Alves, M.I.; Avilés Plaza, F.; Martínez-Tomás, R.; Sánchez-Campillo, M.; Larqué, E.; Pérez-Llamas, F.; Martínez Hernández, P.; Parra Pallarés, S. Oxidized LDL and its correlation with lipid profile and oxidative stress biomarkers in young healthy Spanish subjects. J. Physiol. Biochem. 2010, 66, 221–227. [Google Scholar] [CrossRef]
- Traustadóttir, T.; Davies, S.S.; Su, Y.; Choi, L.; Brown-Borg, H.M.; Roberts, L.J., 2nd; Harman, S.M. Oxidative stress in older adults: Effects of physical fitness. Age 2012, 34, 969–982. [Google Scholar] [CrossRef] [Green Version]
- Ma, E.; Ingram, K.H.; Milne, G.L.; Garvey, W.T. F2-isoprostanes reflect oxidative stress correlated with lean mass and bone density but not insulin resistance. J. Endocr. Soc. 2017, 1, 436–448. [Google Scholar] [CrossRef] [Green Version]
- Rauma, A.L.; Mykkänen, H. Antioxidant status in vegetarians versus omnivores. Nutrition 2000, 16, 111–119. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar]

| Characteristics | RBVD Study | Finnish Study | ||
|---|---|---|---|---|
| Vegans (n = 36) | Omnivores (n = 36) | Vegans (n = 21) | Omnivores (n = 18) | |
| Duration of diet (years) | 4.8 (3.1–8.7) | - | 8.0 (6.0–11.0) | - |
| Women, n (%) | 18 (50.0) | 18 (50.0) | 15 (71.4) | 10 (55.6) |
| Age (years) | 37.5 (32.5–44.0) | 38.5 (32.0–46.0) | 33.0 (28.0–38.0) | 34.0 (28.0–39.0) |
| BMI (kg/m2) a | 22.9 ± 3.2 | 24.0 ± 2.1 | 22.0 ± 2.0 | 22.7 ± 2.5 |
| Physical activity, n (%) a | ||||
| Inactive | 5 (13.9) | 4 (11.1) | 19 (90.5) | 14 (77.8) |
| Active | 31 (86.1) | 32 (88.9) | 2 (9.5) | 1 (5.6) |
| Smoking status, n (%) a | ||||
| Non-smoker | 32 (88.9) | 27 (75.0) | 19 (90.5) | 13 (72.2) |
| Smoker | 4 (11.1) | 9 (25.0) | 2 (9.5) | 3 (16.7) |
| Alcohol consumption (g/day) | 0.1 (0–3.3) | 1.3 (0–11.2) | 0 (0–4.1) | 1.9 (0–5.9) |
| Energy intake (kcal/day) a | 2297 (1800–2870) | 2386 (2081–2737) | 2135 (1888–2600) | 1992 (1730–2383) |
| Protein (g/day) a | 72.2 (54.9–91.8) | 86.3 (71.4–107.0) | 70.7 (54.5–90.4) | 93.5 (76.7–110.8) |
| Fat (g/day) a | 85.7 (63.6–111.1) | 104.1 (87.8–143.3) | 88.1 (64.2–114.5) | 90.3 (74.2–128.6) |
| Carbohydrates (g/day) a | 258.7 (211.5–371.2) | 230.3 (199.3–291.0) | 257.7 (203.4–306.6) | 180.6 (131.6–238.0) |
| Fibre (g/day) a | 45.6 (33.7–56.4) | 23.7 (18.6–29.9) | 39.9 (32.6–50.5) | 25.1 (22.7–42.0) |
| Biomarker | Model | RBVD Study | Finnish Study | ||||
|---|---|---|---|---|---|---|---|
| Vegans (n = 36) | Omnivores (n = 36) | p-Value | Vegans (n = 21) | Omnivores (n = 18) | p-Value | ||
| MDA [µmol/L] | 1 | 1.04 (0.90–1.20) | 1.08 (0.94–1.25) | 0.73 | 0.79 (0.69–0.91) | 0.97 (0.83–1.13) | 0.054 |
| 2 | 1.04 (0.90–1.20) | 1.08 (0.94–1.26) | 0.99 | 0.80 (0.69–0.92) | 1.00 (0.85–1.17) | 0.06 | |
| 3 a | 1.07 (0.93–1.24) | 1.05 (0.91–1.21) | 0.30 | 0.79 (0.69–0.90) | 0.97 (0.81–1.15) | 0.18 | |
| Protein carbonyl b [nmol/mg] | 1 | 0.82 (0.77–0.87) | 0.81 (0.75–0.86) | 0.76 | 0.95 (0.89–1.02) | 1.01 (0.94–1.08) | 0.26 |
| 2 | 0.82 (0.76–0.88) | 0.81 (0.75–0.86) | 0.73 | 0.96 (0.89–1.02) | 1.01 (0.93–1.08) | 0.61 | |
| 3 a | 0.81 (0.76–0.87) | 0.81 (0.76–0.87) | 0.15 | 0.96 (0.90–1.02) | 0.95 (0.87–1.03) | 0.34 | |
| 3-nitrotyrosine [pmol/mg] | 1 | 2.53 (2.16–2.96) | 2.72 (2.32–3.18) | 0.51 | 1.81 (1.48–2.22) | 2.98 (2.39–3.72) | 0.002 |
| 2 | 2.48 (2.12–2.91) | 2.77 (2.36–3.25) | 0.57 | 1.80 (1.45–2.25) | 3.06 (2.38–3.94) | 0.04 | |
| 3 a | 2.44 (2.08–2.85) | 2.82 (2.41–3.30) | 0.23 | 1.80 (1.47–2.21) | 3.14 (2.41–4.09) | 0.04 | |
| 8-OHdG [nmol/d] | 1 | 13.4 (11.5–15.7) | 16.0 (13.7–18.7) | 0.12 | - | - | - |
| 2 | 13.5 (11.6–15.7) | 15.8 (13.6–18.5) | 0.04 | - | - | - | |
| 3 | 13.4 (11.5–15.6) | 16.0 (13.7–18.6) | 0.05 | - | - | - | |
| 8-iso-PGF2α [pmol/d] | 1 | 645 (564–737) | 762 (666–871) | 0.08 | - | - | - |
| 2 | 657 (582–741) | 748 (663–845) | <0.001 | - | - | - | |
| 3 | 662 (585–749) | 742 (656–839) | 0.002 | - | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dietrich, S.; Elorinne, A.-L.; Bergau, N.; Abraham, K.; Grune, T.; Laakso, J.; Weber, D.; Weikert, C.; Monien, B.H. Comparison of Five Oxidative Stress Biomarkers in Vegans and Omnivores from Germany and Finland. Nutrients 2022, 14, 2918. https://doi.org/10.3390/nu14142918
Dietrich S, Elorinne A-L, Bergau N, Abraham K, Grune T, Laakso J, Weber D, Weikert C, Monien BH. Comparison of Five Oxidative Stress Biomarkers in Vegans and Omnivores from Germany and Finland. Nutrients. 2022; 14(14):2918. https://doi.org/10.3390/nu14142918
Chicago/Turabian StyleDietrich, Stefan, Anna-Liisa Elorinne, Nick Bergau, Klaus Abraham, Tilman Grune, Juha Laakso, Daniela Weber, Cornelia Weikert, and Bernhard H. Monien. 2022. "Comparison of Five Oxidative Stress Biomarkers in Vegans and Omnivores from Germany and Finland" Nutrients 14, no. 14: 2918. https://doi.org/10.3390/nu14142918
APA StyleDietrich, S., Elorinne, A.-L., Bergau, N., Abraham, K., Grune, T., Laakso, J., Weber, D., Weikert, C., & Monien, B. H. (2022). Comparison of Five Oxidative Stress Biomarkers in Vegans and Omnivores from Germany and Finland. Nutrients, 14(14), 2918. https://doi.org/10.3390/nu14142918







