Mediterranean Diet Reduces Social Isolation and Anxiety in Adult Female Nonhuman Primates
Abstract
:1. Introduction
2. Methods
2.1. Subjects
2.2. Experimental Diets
2.3. Behavioral Characterization
2.4. Statistical Analyses
3. Results
3.1. Diet Groups Did Not Significantly Differ in Any Behaviors during the Baseline Phase
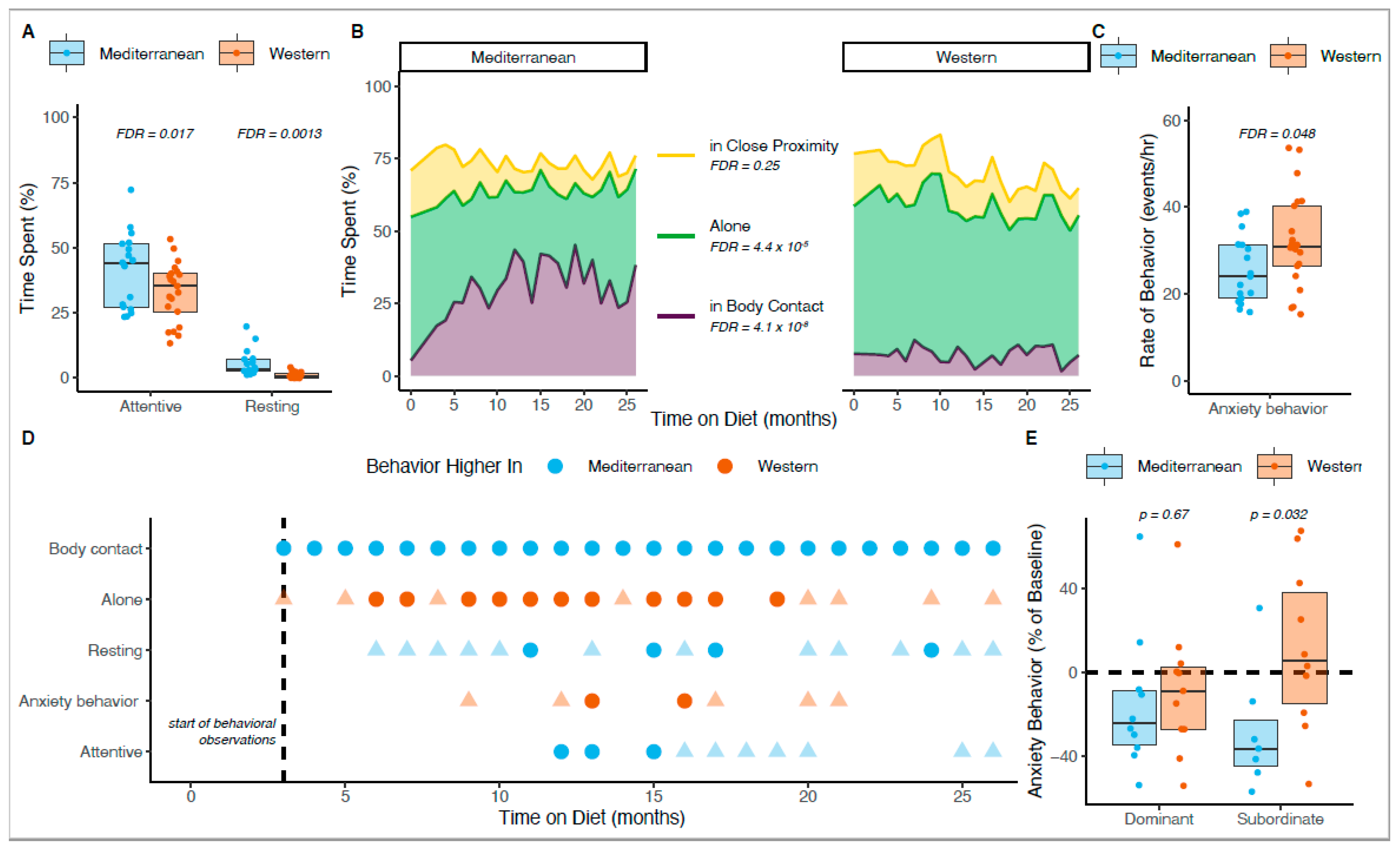
3.2. Diet-Induced Changes in Activity, Affiliation, and Anxiety
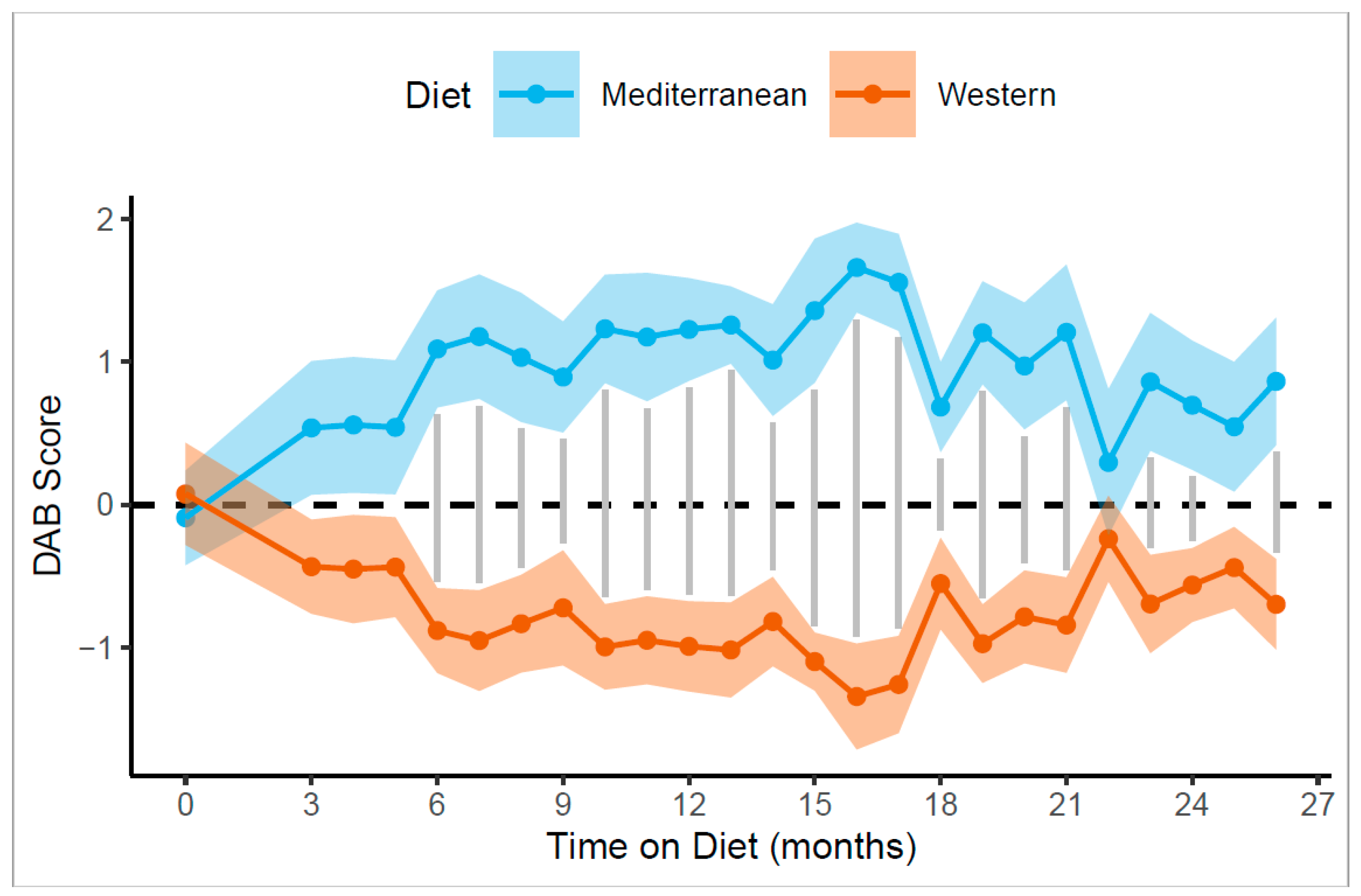
3.3. Diet-Induced Changes in DAB and Affiliation Were Rapid
3.4. Diet-Induced Behavioral Changes Were Persistent
3.5. Social Status Was Associated with Differences in Fearful Scanning, Aggression, and Submission
3.6. Social Status Altered the Effect of Diet on Anxiety Behavior
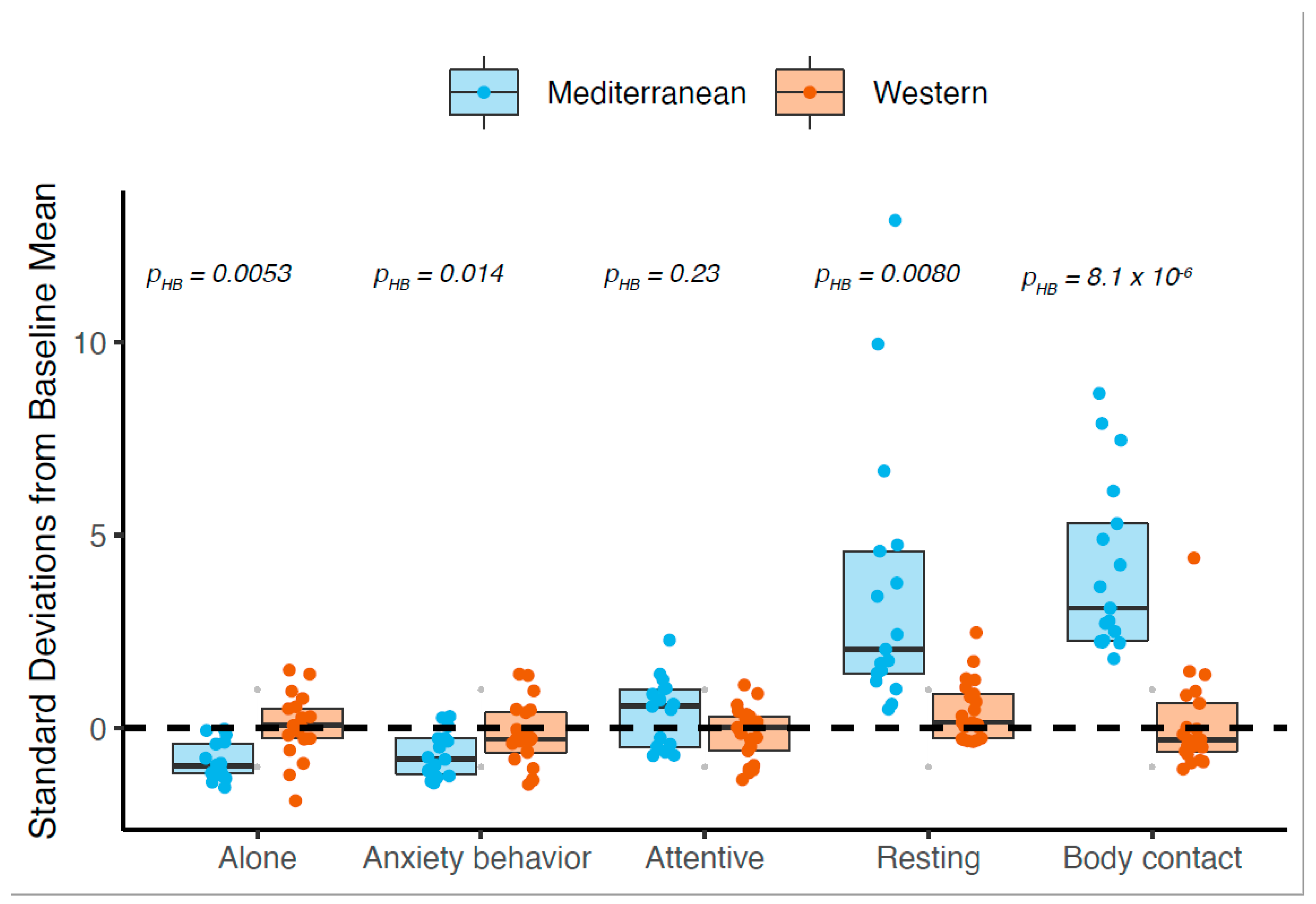
3.7. Mediterranean Diet Drove Most Behavioral Differences between Diets
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakhtiyari, M.; Ehrampoush, E.; Enayati, N.; Joodi, G.; Sadr, S.; Delpisheh, A.; Alihaydari, J.; Homayounfar, R. Anxiety as a consequence of modern dietary pattern in adults in Tehran—Iran. Eat. Behav. 2013, 14, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; O’Neil, A.; Opie, R.; Itsiopoulos, C.; Cotton, S.; Mohebbi, M.; Castle, D.; Dash, S.; Mihalopoulos, C.; Chatterton, M.L.; et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 2017, 15, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacka, F.N.; Pasco, J.A.; Mykletun, A.; Williams, L.J.; Hodge, A.M.; O’Reilly, S.L.; Nicholson, G.C.; Kotowicz, M.A.; Berk, M. Association of Western and traditional diets with depression and anxiety in women. Am. J. Psychiatry 2010, 167, 305–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fond, G.; Young, A.H.; Godin, O.; Messiaen, M.; Lançon, C.; Auquier, P.; Boyer, L. Improving diet for psychiatric patients: High potential benefits and evidence for safety. J. Affect. Disord. 2020, 265, 567–569. [Google Scholar] [CrossRef] [PubMed]
- Gibson-Smith, D.; Bot, M.; Brouwer, I.A.; Visser, M.; Giltay, E.J.; Penninx, B.W.J.H. Association of food groups with depression and anxiety disorders. Eur. J. Nutr. 2020, 59, 767–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventriglio, A.; Sancassiani, F.; Contu, M.P.; Latorre, M.; Di Slavatore, M.; Fornaro, M.; Bhuugra, D. Mediterranean Diet and its Benefits on Health and Mental Health: A Literature Review. Clin. Pract. Epidemiol. Ment. Health 2020, 16, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.E.; Edmondson, D.; Kronish, I.M. State of the Art Review: Depression, Stress, Anxiety, and Cardiovascular Disease. Am. J. Hypertens. 2015, 28, 1295–1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodwin, R.D.; Davidson, K.W.; Keyes, K. Mental disorders and cardiovascular disease among adults in the United States. J. Psychiatr. Res. 2009, 43, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Shively, C.A.; Musselman, D.L.; Willard, S.L. Stress, depression, and coronary artery disease: Modeling comorbidity in female primates. Neurosci. Biobehav. Rev. 2009, 33, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Shively, C.A.; Laber-Laird, K.; Anton, R.F. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol. Psychiatry 1997, 41, 871–882. [Google Scholar] [CrossRef]
- Vogelzangs, N.; Seldenrijk, A.; Beekman, A.T.F.; van Hout, H.P.J.; de Jonge, P.; Penninx, B.W.J.H. Cardiovascular disease in persons with depressive and anxiety disorders. J. Affect. Disord. 2010, 125, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Marrie, R.A.; Walld, R.; Bolton, J.M.; Sareen, J.; Walker, J.R.; Patten, S.B.; Singer, A.; Lix, L.M.; Hitchon, C.A.; El-Gabalawy, R.; et al. Increased incidence of psychiatric disorders in immune-mediated inflammatory disease. J. Psychosom. Res. 2017, 101, 17–23. [Google Scholar] [CrossRef]
- Marrie, R.A.; Reingold, S.; Cohen, J.; Stuve, O.; Trojano, M.; Sorensen, P.S.; Cutter, G.; Reider, N. The incidence and prevalence of psychiatric disorders in multiple sclerosis: A systematic review. Mult. Scler. J. 2015, 21, 305–317. [Google Scholar] [CrossRef] [Green Version]
- Snyder-Mackler, N.; Burger, J.R.; Gaydosh, L.; Belsky, D.W.; Noppert, G.A.; Campos, F.A.; Bartolomucci, A.; Yang, Y.C.; Aiello, A.E.; O’Rand, A.; et al. Social determinants of health and survival in humans and other animals. Science 2020, 368, eaax9553. [Google Scholar] [CrossRef]
- Berkman, L.F.; Glass, T.; Brissette, I.; Seeman, T.E. From social integration to health: Durkheim in the new millennium. Soc. Sci. Med. 2000, 51, 843–857. [Google Scholar] [CrossRef]
- Berkman, L.F.; Syme, S.L. Social networks, host resistance, and mortality: A nine-year follow-up study of Alameda County residents. Am. J. Epidemiol. 1979, 109, 186–204. [Google Scholar] [CrossRef]
- Campos, F.A.; Villavicencio, F.; Archie, E.A.; Colchero, F.; Alberts, S.C. Social bonds, social status and survival in wild baboons: A tale of two sexes. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190621. [Google Scholar] [CrossRef]
- Hawkley, L.C.; Cacioppo, J.T. Loneliness Matters: A Theoretical and Empirical Review of Consequences and Mechanisms. Ann. Behav. Med. 2010, 40, 218–227. [Google Scholar] [CrossRef] [Green Version]
- Holt-Lunstad, J.; Smith, T.B.; Baker, M.; Harris, T.; Stephenson, D. Loneliness and Social Isolation as Risk Factors for Mortality: A Meta-Analytic Review. Perspect. Psychol. Sci. 2015, 10, 227–237. [Google Scholar] [CrossRef] [Green Version]
- Holt-Lunstad, J.; Smith, T.B.; Layton, J.B. Social relationships and mortality risk: A meta-analytic review. PLoS Med. 2010, 7, e1000316. [Google Scholar] [CrossRef]
- House, J.; Landis, K.; Umberson, D. Social relationships and health. Science 1988, 241, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Shively, C.A.; Clarkson, T.B.; Kaplan, J.R. Social deprivation and coronary artery atherosclerosis in female cynomolgus monkeys. Atherosclerosis 1989, 77, 69–76. [Google Scholar] [CrossRef]
- Miller, T.M.; Abdel-Maksoud, M.F.; Crane, L.A.; Marcus, A.C.; Byers, T.E. Effects of social approval bias on self-reported fruit and vegetable consumption: A randomized controlled trial. Nutr. J. 2008, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Suchanek, P.; Poledne, R.; Hubacek, J.A. Dietary intake reports fidelity—Fact or fiction? Neuro Endocrinol. Lett. 2011, 32 (Suppl. S2), 29–31. [Google Scholar] [PubMed]
- Alcaraz, K.I.; Eddens, K.S.; Blase, J.L.; Diver, W.R.; Patel, A.V.; Teras, L.R.; Stevens, V.L.; Jacobs, E.J.; Gapstur, S.M. Social Isolation and Mortality in US Black and White Men and Women. Am. J. Epidemiol. 2019, 188, 102–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedler, B.; Crapser, J.; McCullough, L. One is the deadliest number: The detrimental effects of social isolation on cerebrovascular diseases and cognition. Acta Neuropathol. 2015, 129, 493–509. [Google Scholar] [CrossRef] [Green Version]
- Evans, I.E.M.; Martyr, A.; Collins, R.; Brayne, C.; Clare, L. Social Isolation and Cognitive Function in Later Life: A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2019, 70, S119–S144. [Google Scholar] [CrossRef] [Green Version]
- Jarczok, M.N.; Koenig, J.; Shively, C.A.; Thayer, J.F. Behavioral depression is associated with increased vagally mediated heart rate variability in adult female cynomolgus monkeys (Macaca fascicularis). Int. J. Psychophysiol. 2018, 131, 139–143. [Google Scholar] [CrossRef]
- Kromrey, S.A.; Czoty, P.W.; Nader, S.H.; Register, T.C.; Nader, M.A. Preclinical laboratory assessments of predictors of social rank in female cynomolgus monkeys. Am. J. Primatol. 2016, 78, 402–417. [Google Scholar] [CrossRef]
- Shively, C.A. Social subordination stress, behavior, and central monoaminergic function in female cynomolgus monkeys. Biol. Psychiatry 1998, 44, 882–891. [Google Scholar] [CrossRef]
- Shively, C.A.; Clarkson, T.B. The unique value of primate models in translational research. Am. J. Primatol. 2009, 71, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Shively, C.A.; Day, S.M. Social inequalities in health in nonhuman primates. Neurobiol. Stress 2015, 1, 156–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willard, S.L.; Shively, C.A. Modeling depression in adult female cynomolgus monkeys (Macaca fascicularis). Am. J. Primatol. 2012, 74, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Brent, L.J.N.; Ruiz-Lambides, A.; Platt, M.L. Family network size and survival across the lifespan of female macaques. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170515. [Google Scholar] [CrossRef] [PubMed]
- Shively, C.A.; Appt, S.E.; Vitolins, M.Z.; Uberseder, B.; Michalson, K.T.; Silverstein-Metzler, M.G.; Register, T.C. Mediterranean versus Western Diet Effects on Caloric Intake, Obesity, Metabolism, and Hepatosteatosis in Nonhuman Primates. Obesity 2019, 27, 777–784. [Google Scholar] [CrossRef]
- Nagpal, R.; Shively, C.A.; Appt, S.A.; Register, T.C.; Michalson, K.T.; Vitolins, M.Z.; Yadav, H. Gut Microbiome Composition in Non-human Primates Consuming a Western or Mediterranean Diet. Front. Nutr. 2018, 5, 28. [Google Scholar] [CrossRef]
- Shively, C.A.; Appt, S.E.; Chen, H.; Day, S.M.; Frye, B.M.; Shaltout, H.A.; Silverstein-Metzler, M.G.; Snyder-Mackler, N.; Uberseder, B.; Vitolins, M.Z.; et al. Mediterranean diet, stress resilience, and aging in nonhuman primates. Neurobiol. Stress 2020, 13, 100254. [Google Scholar] [CrossRef]
- Frye, B.M.; Craft, S.; Register, T.C.; Andrews, R.N.; Appt, S.E.; Vitolins, M.Z.; Uberseder, B.; Silverstein-Metzler, M.G.; Chen, H.; Whitlow, C.T.; et al. Diet, psychosocial stress, and Alzheimer’s disease–related neuroanatomy in female nonhuman primates. Alzheimers Dement. 2020, 17, 733–744. [Google Scholar] [CrossRef]
- Johnson, C.S.; Shively, C.; Michalson, K.T.; Lea, A.J.; DeBo, R.J.; Howard, T.D.; Hawkins, G.A.; Appt, S.E.; Liu, Y.; McCall, C.E.; et al. Contrasting effects of Western vs Mediterranean diets on monocyte inflammatory gene expression and social behavior in a primate model. eLife 2021, 10, e68293. [Google Scholar] [CrossRef]
- Thompson, J.R.; Gustafsson, H.C.; DeCapo, M.; Takahashi, D.L.; Bagley, J.L.; Dean, T.A.; Kievit, P.; Fair, D.A.; Sullivan, E.L. Maternal Diet, Metabolic State, and Inflammatory Response Exert Unique and Long-Lasting Influences on Offspring Behavior in Non-Human Primates. Front. Endocrinol. 2018, 9, 161. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, L.C.; Steptoe, A. Social Isolation, Loneliness, and Health Behaviors at Older Ages: Longitudinal Cohort Study. Ann. Behav. Med. 2018, 52, 582–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Department of Agriculture, Agricultural Research Service. Energy Intakes: Percentages of Energy from Protein, Carbohydrate, Fat, and Alcohol, by Gender and Age, What We Eat in America, NHANES 2013–2014; U.S. Department of Agriculture, Agricultural Research Service: Hyattsville, MD, USA, 2016.
- Bédard, A.; Riverin, M.; Dodin, S.; Corneau, L.; Lemieux, S. Sex differences in the impact of the Mediterranean diet on cardiovascular risk profile. Br. J. Nutr. 2012, 108, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed. Pharmacother. 2006, 60, 502–507. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Kafatos, A.; Verhagen, H.; Moschandreas, J.; Apostolaki, I.; Van Westerop, J.J. Mediterranean diet of Crete: Foods and nutrient content. J. Am. Diet. Assoc. 2000, 100, 1487–1493. [Google Scholar] [CrossRef]
- Powles, J.; Fahimi, S.; Micha, R.; Khatibzadeh, S.; Shi, P.; Ezzati, M.; Engell, R.E.; Lim, S.S.; Danaei, G.; Mozaffarian, D.; et al. Global, regional and national sodium intakes in 1990 and 2010: A systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 2013, 3, e003733. [Google Scholar] [CrossRef] [Green Version]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Altmann, J. Observational Study of Behavior: Sampling Methods. Behaviour 1974, 49, 227–266. [Google Scholar] [CrossRef] [Green Version]
- Maestripieri, D.; Schino, G.; Aureli, F.; Troisi, A. A modest proposal: Displacement activities as an indicator of emotions in primates. Anim. Behav. 1992, 44, 967–979. [Google Scholar] [CrossRef]
- Schino, G.; Perretta, G.; Taglioni, A.M.; Monaco, V.; Troisi, A. Primate displacement activities as an ethopharmacological model of anxiety. Anxiety 1996, 2, 186–191. [Google Scholar] [CrossRef]
- Shively, C.A.; Register, T.C.; Appt, S.E.; Clarkson, T.B. Effects of long-term sertraline treatment and depression on coronary artery atherosclerosis in premenopausal female primates. Psychosom. Med. 2015, 77, 267–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troisi, A. Displacement activities as a behavioral measure of stress in nonhuman primates and human subjects. Stress Amst. Neth. 2002, 5, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Troisi, A.; Belsanti, S.; Bucci, A.R.; Mosco, C.; Sinti, F.; Verucci, M. Affect regulation in alexithymia: An ethological study of displacement behavior during psychiatric interviews. J. Nerv. Ment. Dis. 2000, 188, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Storey, J.D.; Tibshirani, R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA 2003, 100, 9440–9445. [Google Scholar] [CrossRef] [Green Version]
- Cacioppo, J.T.; Cacioppo, S.; Capitanio, J.P.; Cole, S.W. The neuroendocrinology of social isolation. Annu. Rev. Psychol. 2015, 66, 733–767. [Google Scholar] [CrossRef] [Green Version]
- Cole, S.W.; Levine, M.E.; Arevalo, J.M.G.; Ma, J.; Weir, D.R.; Crimmins, E.M. Loneliness, eudaimonia, and the human conserved transcriptional response to adversity. Psychoneuroendocrinology 2015, 62, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Eisenberger, N.I.; Moieni, M.; Inagaki, T.K.; Muscatell, K.A.; Irwin, M.R. In Sickness and in Health: The Co-Regulation of Inflammation and Social Behavior. Neuropsychopharmacology 2017, 42, 242–253. [Google Scholar] [CrossRef]
- Nguyen, A.W.; Taylor, R.J.; Taylor, H.O.; Chatters, L.M. Objective and Subjective Social Isolation and Psychiatric Disorders among African Americans. Clin. Soc. Work J. 2020, 48, 87–98. [Google Scholar] [CrossRef]
- Taylor, H.O.; Taylor, R.J.; Nguyen, A.W.; Chatters, L. Social Isolation, Depression, and Psychological Distress among Older Adults. J. Aging Health 2018, 30, 229–246. [Google Scholar] [CrossRef]
- Peris-Sampedro, F.; Mounib, M.; Schéle, E.; Edvardsson, C.E.; Stoltenborg, I.; Adan, R.A.H.; Dickson, S.L. Impact of Free-Choice Diets High in Fat and Different Sugars on Metabolic Outcome and Anxiety-Like Behavior in Rats: Dietary Composition, Metabolism, and Anxiety. Obesity 2019, 27, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Veniaminova, E.; Cespuglio, R.; Cheung, C.W.; Umriukhin, A.; Markova, N.; Shevtsova, E.; Lesch, K.P.; Anthony, D.C.; Strekalova, T. Autism-Like Behaviours and Memory Deficits Result from a Western Diet in Mice. Neural Plast. 2017, 2017, 9498247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.S.; Kurti, A.; Fair, D.A.; Fryer, J.D. Dietary intervention rescues maternal obesity induced behavior deficits and neuroinflammation in offspring. J. Neuroinflamm. 2014, 11, 156. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.E.; Rocha-Gomes, A.; Pereira dos Santos, T.; Amaral, B.L.S.; da Silva, A.A.; Malagutti, A.R.; Leite, F.R.F.; Stuckert-Seixas, S.R.; Riul, T.R. Cafeteria diet administered from lactation to adulthood promotes a change in risperidone sensitivity on anxiety, locomotion, memory, and social interaction of Wistar rats. Physiol. Behav. 2020, 220, 112874. [Google Scholar] [CrossRef]
- Tsan, L.; Décarie-Spain, L.; Noble, E.E.; Kanoski, S.E. Western Diet Consumption During Development: Setting the Stage for Neurocognitive Dysfunction. Front. Neurosci. 2021, 15, 632312. [Google Scholar] [CrossRef]
- Delerue Matos, A.; Barbosa, F.; Cunha, C.; Voss, G.; Correia, F. Social isolation, physical inactivity and inadequate diet among European middle-aged and older adults. BMC Public Health 2021, 21, 924. [Google Scholar] [CrossRef]
- Ferrer-Cascales, R.; Albaladejo-Blázquez, N.; Ruiz-Robledillo, N.; Rubio-Aparicio, M.; Laguna-Pérez, A.; Zaragoza-Martí, A. Low Adherence to the Mediterranean Diet in Isolated Adolescents: The Mediation Effects of Stress. Nutrients 2018, 10, 1894. [Google Scholar] [CrossRef] [Green Version]
- Berger, M.E.; Smesny, S.; Kim, S.-W.; Davey, C.G.; Rice, S.; Sarnyai, Z.; Schlögelhofer, M.; Schäfer, M.R.; Berk, M.; McGorry, P.D.; et al. Omega-6 to omega-3 polyunsaturated fatty acid ratio and subsequent mood disorders in young people with at-risk mental states: A 7-year longitudinal study. Transl. Psychiatry 2017, 7, e1220. [Google Scholar] [CrossRef]
- Su, K.-P.; Matsuoka, Y.; Pae, C.-U. Omega-3 Polyunsaturated Fatty Acids in Prevention of Mood and Anxiety Disorders. Clin. Psychopharmacol. Neurosci. 2015, 13, 129–137. [Google Scholar] [CrossRef] [Green Version]
- dos Santos Vaz, J.; Kac, G.; Emmett, P.; Davis, J.M.; Golding, J.; Hibbeln, J.R. Dietary Patterns, n-3 Fatty Acids Intake from Seafood and High Levels of Anxiety Symptoms during Pregnancy: Findings from the Avon Longitudinal Study of Parents and Children. PLoS ONE 2013, 8, e67671. [Google Scholar]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fuulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.D.; Loughman, A.; Spencer, S.J.; Reichelt, A.C. The impact of obesity and hypercaloric diet consumption on anxiety and emotional behavior across the lifespan. Neurosci. Biobehav. Rev. 2017, 83, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; McVey Neufeld, K.-A. Gut–brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Hoehn-Saric, R.; McLeod, D.R. The peripheral sympathetic nervous system. Its role in normal and pathologic anxiety. Psychiatr. Clin. N. Am. 1988, 11, 375–386. [Google Scholar] [CrossRef]
- Juruena, M.F.; Eror, F.; Cleare, A.J.; Young, A.H. The Role of Early Life Stress in HPA Axis and Anxiety. Adv. Exp. Med. Biol. 2020, 1191, 141–153. [Google Scholar] [PubMed]
- Narita, K.; Murata, T.; Hamada, T.; Kosaka, H.; Sudo, S.; Mizukami, K.; Yoshida, H.; Wada, Y. Associations between trait anxiety, insulin resistance, and atherosclerosis in the elderly: A pilot cross-sectional study. Psychoneuroendocrinology 2008, 33, 305–312. [Google Scholar] [CrossRef]
- Rivenes, A.C.; Harvey, S.B.; Mykletun, A. The relationship between abdominal fat, obesity, and common mental disorders: Results from the HUNT Study. J. Psychosom. Res. 2009, 66, 269–275. [Google Scholar] [CrossRef]
- Giugliano, D.; Ceriello, A.; Esposito, K. The effects of diet on inflammation: Emphasis on the metabolic syndrome. J. Am. Coll. Cardiol. 2006, 48, 677–685. [Google Scholar] [CrossRef] [Green Version]
- Holt, E.M.; Steffen, L.M.; Moran, A.; Basu, S.; Steinberger, J.; Ross, J.A.; Hong, C.P.; Sinaiko, A.R. Fruit and vegetable consumption and its relation to markers of inflammation and oxidative stress in adolescents. J. Am. Diet. Assoc. 2009, 109, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Garcia, E.; Schulze, M.B.; Fung, T.T.; Meigs, J.B.; Rifai, N.; Manson, J.E.; Hu, F.B. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2004, 80, 1029–1035. [Google Scholar] [CrossRef] [Green Version]
- Nanri, A.; Moore, M.A.; Kono, S. Impact of C-reactive protein on disease risk and its relation to dietary factors. Asian Pac. J. Cancer Prev. 2007, 8, 167–177. [Google Scholar] [PubMed]
- Nettleton, J.A.; Steffen, L.M.; Mayer-Davis, E.J.; Jenny, N.S.; Jiang, R.; Herrington, D.M.; Jacobs, D.R. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Clin. Nutr. 2006, 83, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.; Delgado-Lista, J.; Garcia-Rios, A.; Cruz-Teno, C.; Yubero-Serrano, E.M.; Perez-Martinez, P.; Gutierrez-Mariscal, F.M.; Lora-Aguilar, P.; Rodriguez-Cantalejo, F.; Fuentes-Jimenez, F.; et al. Expression of proinflammatory, proatherogenic genes is reduced by the Mediterranean diet in elderly people. Br. J. Nutr. 2012, 108, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Layé, S.; Nadjar, A.; Joffre, C.; Bazinet, R.P. Anti-Inflammatory Effects of Omega-3 Fatty Acids in the Brain: Physiological Mechanisms and Relevance to Pharmacology. Pharmacol. Rev. 2018, 70, 12–38. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Gheewala, N.M.; O’Keefe, J.O. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J. Am. Coll. Cardiol. 2008, 51, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Prinz, M.; Priller, J. The role of peripheral immune cells in the CNS in steady state and disease. Nat. Neurosci. 2017, 20, 136–144. [Google Scholar] [CrossRef]
- Quan, N.; Banks, W.A. Brain-immune communication pathways. Brain Behav. Immun. 2007, 21, 727–735. [Google Scholar] [CrossRef]
- Dunphy-Doherty, F.; O’Mahony, S.M.; Peterson, V.L.; O’Sullivan, O.; Crispie, F.; Cotter, P.D.; Wingmore, P.; King, M.V.; Cryan, J.F.; Fone, K.C.F. Post-weaning social isolation of rats leads to long-term disruption of the gut microbiota-immune-brain axis. Brain Behav. Immun. 2018, 68, 261–273. [Google Scholar] [CrossRef]
- Raison, C.L.; Capuron, L.; Miller, A.H. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006, 27, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Bossak, B.H.; Turk, C.A. Spatial Variability in COVID-19 Mortality. Int. J. Environ. Res. Public Health 2021, 18, 5892. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, R.P. Cross-National Variations in COVID-19 Mortality: The Role of Diet, Obesity and Depression. Diseases 2021, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Anto, J.M.; Iaccarino, G.; Czarlewski, W.; Haahtela, T.; Anto, A.; Akdis, C.A.; Blain, H.; Canonica, W.G.; Cardona, V.; et al. Correction to: Is diet partly responsible for differences in COVID-19 death rates between and within countries? Clin. Transl. Allergy 2020, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Kamyari, N.; Soltanian, A.R.; Mahjub, H.; Moghimbeigi, A. Diet, Nutrition, Obesity, and Their Implications for COVID-19 Mortality: Development of a Marginalized Two-Part Model for Semicontinuous Data. JMIR Public Health Surveill. 2021, 7, e22717. [Google Scholar] [CrossRef] [PubMed]
- Doaei, S.; Gholami, S.; Rastgoo, S.; Gholamalizadeh, M.; Bourbour, F.; Bagheri, S.E.; Samipoor, F.; Akbari, M.E.; Shadnoush, M.; Ghorat, F.; et al. The effect of omega-3 fatty acid supplementation on clinical and biochemical parameters of critically ill patients with COVID-19: A randomized clinical trial. J. Transl. Med. 2021, 19, 128. [Google Scholar] [CrossRef]
- Kumar, A.; Salinas, J. The Long-Term Public Health Impact of Social Distancing on Brain Health: Topical Review. Int. J. Environ. Res. Public Health 2021, 18, 7307. [Google Scholar] [CrossRef]
- Schou, T.M.; Joca, S.; Wegener, G.; Bay-Richter, C. Psychiatric and neuropsychiatric sequelae of COVID-19—A systematic review. Brain Behav. Immun. 2021, 97, 328–348. [Google Scholar] [CrossRef]
| Diet Composition | Human | Nonhuman Primate | |||
|---|---|---|---|---|---|
| Western | Mediterranean | Western 1 | Mediterranean 1 | Chow 2 | |
| % of Calories | |||||
| Protein | 15 [42] | 17 [43] | 16 | 16 | 18 |
| Carbohydrate 3 | 51 [42] | 51 [43] | 54 | 54 | 69 |
| Fat | 33 [42] | 32 [43] | 31 | 31 | 13 |
| % of Total Fats | |||||
| Saturated | 33 [42] | 21 [43] | 36 | 21 | 26 |
| Monounsaturated | 36 [42] | 56 [43] | 36 | 57 | 28 |
| Polyunsaturated | 24 [42] | 15 [43] | 26 | 20 | 32 |
| Other Nutrients | |||||
| ω6:ω3 Fatty Acids | 15:1 [44] | 2.1–3:1 [45] | 14.8:1 | 2.9:1 | 12:01 |
| Cholesterol mg/Cal | 0.13 [42] | 0.16 [43] | 0.16 | 0.15 | trace |
| Fiber g/Cal | 0.01 [42] | 0.03 [46] | 0.02 | 0.04 | 0.01 |
| Sodium mg/Cal | 1.7 [42,47] | 1.3 [43,46] | 1.7 | 1.1 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, C.S.C.; Frye, B.M.; Register, T.C.; Snyder-Mackler, N.; Shively, C.A. Mediterranean Diet Reduces Social Isolation and Anxiety in Adult Female Nonhuman Primates. Nutrients 2022, 14, 2852. https://doi.org/10.3390/nu14142852
Johnson CSC, Frye BM, Register TC, Snyder-Mackler N, Shively CA. Mediterranean Diet Reduces Social Isolation and Anxiety in Adult Female Nonhuman Primates. Nutrients. 2022; 14(14):2852. https://doi.org/10.3390/nu14142852
Chicago/Turabian StyleJohnson, Corbin S. C., Brett M. Frye, Thomas C. Register, Noah Snyder-Mackler, and Carol A. Shively. 2022. "Mediterranean Diet Reduces Social Isolation and Anxiety in Adult Female Nonhuman Primates" Nutrients 14, no. 14: 2852. https://doi.org/10.3390/nu14142852
APA StyleJohnson, C. S. C., Frye, B. M., Register, T. C., Snyder-Mackler, N., & Shively, C. A. (2022). Mediterranean Diet Reduces Social Isolation and Anxiety in Adult Female Nonhuman Primates. Nutrients, 14(14), 2852. https://doi.org/10.3390/nu14142852






