The Potential Roles of Dietary Anthocyanins in Inhibiting Vascular Endothelial Cell Senescence and Preventing Cardiovascular Diseases
Abstract
:1. Introduction
2. Anthocyanin
2.1. Structure of Anthocyanin
2.2. Physiological Activities of Anthocyanins
2.2.1. Anti-Cancer
2.2.2. Anti-Inflammatory
2.2.3. Anti-Oxidation
2.2.4. Protective Effect on the Liver
2.2.5. Lowering Blood Glucose
2.2.6. Anti-Aging
2.2.7. Other Effects
3. Bioavailability of Anthocyanins and Their Interaction with the Gut Microbiome
3.1. Bioavailability of Dietary Anthocyanins
3.2. Interaction of Dietary Anthocyanins with the Gut Microbiome

4. Effects of Anthocyanins on Endothelial Cell Aging and Cardiovascular Protection
4.1. Cell Senescence and Vascular Endothelial Cell Senescence
4.2. Dietary Anthocyanins Protect Cardiovascular System through Anti-Aging
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.J.; Wu, Z.Y.; Nie, X.W.; Bian, J.S. Role of Endothelial Dysfunction in Cardiovascular Diseases: The Link Between Inflammation and Hydrogen Sulfide. Front. Pharmacol. 2019, 10, 1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, G.; Aroor, A.R.; Jia, C.; Sowers, J.R. Endothelial cell senescence in aging-related vascular dysfunction. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W. Anthocyanins and Their C6-C3-C6 Metabolites in Humans and Animals. Molecules 2019, 24, 4024. [Google Scholar] [CrossRef] [Green Version]
- Krga, I.; Milenkovic, D. Anthocyanins: From Sources and Bioavailability to Cardiovascular-Health Benefits and Molecular Mechanisms of Action. J. Agric. Food Chem. 2019, 67, 1771–1783. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.C.; Rodrigues, M.; Santos, A.O.; Alves, G.; Silva, L.R. Antioxidant Status, Antidiabetic Properties and Effects on Caco-2 Cells of Colored and Non-Colored Enriched Extracts of Sweet Cherry Fruits. Nutrients 2018, 10, 1688. [Google Scholar] [CrossRef] [Green Version]
- Masci, A.; Coccia, A.; Lendaro, E.; Mosca, L.; Paolicelli, P.; Cesa, S. Evaluation of different extraction methods from pomegranate whole fruit or peels and the antioxidant and antiproliferative activity of the polyphenolic fraction. Food Chem. 2016, 202, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Lage, N.N.; Layosa, M.A.A.; Arbizu, S.; Chew, B.P.; Pedrosa, M.L.; Mertens-Talcott, S.; Talcott, S.; Noratto, G.D. Dark sweet cherry (Prunus avium) phenolics enriched in anthocyanins exhibit enhanced activity against the most aggressive breast cancer subtypes without toxicity to normal breast cells. J. Funct. Foods 2020, 64, 103710. [Google Scholar] [CrossRef]
- Hogan, S.; Chung, H.; Zhang, L.; Li, J.; Lee, Y.; Dai, Y.; Zhou, K. Antiproliferative and antioxidant properties of anthocyanin-rich extract from açai. Food Chem. 2010, 118, 208–214. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Raudone, L.; Petrikaite, V. Phenolic Fractions from Vaccinium vitis-idaea L. and Their Antioxidant and Anticancer Activities Assessment. Antioxidants 2020, 9, 1261. [Google Scholar] [CrossRef]
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compos. Anal. 2010, 23, 554–560. [Google Scholar] [CrossRef]
- Long, H.L.; Zhang, F.F.; Wang, H.L.; Yang, W.S.; Hou, H.T.; Yu, J.K.; Liu, B. Mulberry anthocyanins improves thyroid cancer progression mainly by inducing apoptosis and autophagy cell death. Kaohsiung J. Med. Sci. 2018, 34, 255–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, J.W.; Lee, W.S.; Kim, M.J.; Lu, J.N.; Kang, M.H.; Kim, H.G.; Kim, D.C.; Choi, E.J.; Choi, J.Y.; Kim, H.G.; et al. Characterization of a profile of the anthocyanins isolated from Vitis coignetiae Pulliat and their anti-invasive activity on HT-29 human colon cancer cells. Food Chem. Toxicol. 2010, 48, 903–909. [Google Scholar] [CrossRef]
- Fragoso, M.F.; Romualdo, G.R.; Vanderveer, L.A.; Franco-Barraza, J.; Cukierma, E.; Clapper, M.L.; Carvalho, R.F.; Barbisa, L.F. Lyophilized acai pulp (Euterpe oleracea Mart) attenuates colitis-associated colon carcinogenesis while its main anthocyanin has the potential to affect the motility of colon cancer cells. Food Chem. Toxicol. 2018, 121, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Mazewski, C.; Liang, K.; de Mejia, E.G. Inhibitory potential of anthocyanin-rich purple and red corn extracts on human colorectal cancer cell proliferation in vitro. J. Funct. Foods 2017, 34, 254–265. [Google Scholar] [CrossRef]
- Su, C.-C.; Wang, C.-J.; Huang, K.-H.; Lee, Y.-J.; Chan, W.-M.; Chang, Y.-C. Anthocyanins from Hibiscus sabdariffa calyx attenuate in vitro and in vivo melanoma cancer metastasis. J. Funct. Foods 2018, 48, 614–631. [Google Scholar] [CrossRef]
- Lim, S.; Xu, J.; Kim, J.; Chen, T.Y.; Su, X.; Standard, J.; Carey, E.; Griffin, J.; Herndon, B.; Katz, B.; et al. Role of anthocyanin-enriched purple-fleshed sweet potato p40 in colorectal cancer prevention. Mol. Nutr. Food Res. 2013, 57, 1908–1917. [Google Scholar] [CrossRef] [Green Version]
- Teng, H.; Fang, T.; Lin, Q.; Song, H.; Liu, B.; Chen, L. Red raspberry and its anthocyanins: Bioactivity beyond antioxidant capacity. Trends Food Sci. Technol. 2017, 66, 153–165. [Google Scholar] [CrossRef]
- Akiyama, S.; Nesumi, A.; Maeda-Yamamoto, M.; Uehara, M.; Murakami, A. Effects of anthocyanin-rich tea “Sunrouge” on dextran sodium sulfate-induced colitis in mice. Biofactors 2012, 38, 226–233. [Google Scholar] [CrossRef]
- Folmer, F.; Basavaraju, U.; Jaspars, M.; Hold, G.; El-Omar, E.; Dicato, M.; Diederich, M. Anticancer effects of bioactive berry compounds. Phytochem. Rev. 2013, 13, 295–322. [Google Scholar] [CrossRef]
- Hou, D.X.; Kai, K.; Li, J.J.; Lin, S.; Terahara, N.; Wakamatsu, M.; Fujii, M.; Young, M.R.; Colburn, N. Anthocyanidins inhibit activator protein 1 activity and cell transformation: Structure-activity relationship and molecular mechanisms. Carcinogenesis 2004, 25, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, S.W.; Ryu, S.N.; Kim, D.H. Anti-inflammatory effects of black rice, cyanidin-3-O-beta-D-glycoside, and its metabolites, cyanidin and protocatechuic acid. Int. Immunopharmacol. 2010, 10, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.X.; Yanagita, T.; Uto, T.; Masuzaki, S.; Fujii, M. Anthocyanidins inhibit cyclooxygenase-2 expression in LPS-evoked macrophages: Structure-activity relationship and molecular mechanisms involved. Biochem. Pharmacol. 2005, 70, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Teng, H.; Fang, T.; Xiao, J. Agrimonolide from Agrimonia pilosa suppresses inflammatory responses through down-regulation of COX-2/iNOS and inactivation of NF-kappaB in lipopolysaccharide-stimulated macrophages. Phytomedicine 2016, 23, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Aboonabi, A.; Aboonabi, A. Anthocyanins reduce inflammation and improve glucose and lipid metabolism associated with inhibiting nuclear factor-kappaB activation and increasing PPAR-gamma gene expression in metabolic syndrome subjects. Free Radic. Biol. Med. 2020, 150, 30–39. [Google Scholar] [CrossRef]
- Duarte, L.J.; Chaves, V.C.; Nascimento, M.; Calvete, E.; Li, M.; Ciraolo, E.; Ghigo, A.; Hirsch, E.; Simoes, C.M.O.; Reginatto, F.H.; et al. Molecular mechanism of action of Pelargonidin-3-O-glucoside, the main anthocyanin responsible for the anti-inflammatory effect of strawberry fruits. Food Chem. 2018, 247, 56–65. [Google Scholar] [CrossRef]
- Karunarathne, W.; Lee, K.T.; Choi, Y.H.; Jin, C.Y.; Kim, G.Y. Anthocyanins isolated from Hibiscus syriacus L. attenuate lipopolysaccharide-induced inflammation and endotoxic shock by inhibiting the TLR4/MD2-mediated NF-kappaB signaling pathway. Phytomedicine 2020, 76, 153237. [Google Scholar] [CrossRef]
- Bueno, J.M.; Sáez-Plaza, P.; Ramos-Escudero, F.; Jiménez, A.M.; Fett, R.; Asuero, A.G. Analysis and Antioxidant Capacity of Anthocyanin Pigments. Part II: Chemical Structure, Color, and Intake of Anthocyanins. Crit. Rev. Anal. Chem. 2012, 42, 126–151. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Harakotr, B.; Suriharn, B.; Tangwongchai, R.; Scott, M.P.; Lertrat, K. Anthocyanins and antioxidant activity in coloured waxy corn at different maturation stages. J. Funct. Foods 2014, 9, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Matera, R.; Gabbanini, S.; Berretti, S.; Amorati, R.; De Nicola, G.R.; Iori, R.; Valgimigli, L. Acylated anthocyanins from sprouts of Raphanus sativus cv. Sango: Isolation, structure elucidation and antioxidant activity. Food Chem. 2015, 166, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Coklar, H.; Akbulut, M. Anthocyanins and phenolic compounds of Mahonia aquifolium berries and their contributions to antioxidant activity. J. Funct. Foods 2017, 35, 166–174. [Google Scholar] [CrossRef]
- Lu, X.; Zhou, Y.; Wu, T.; Hao, L. Ameliorative effect of black rice anthocyanin on senescent mice induced by D-galactose. Food Funct. 2014, 5, 2892–2897. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhu, Y.; Li, C.; Sui, Z.; Min, W. Effect of Blueberry Anthocyanins Malvidin and Glycosides on the Antioxidant Properties in Endothelial Cells. Oxid. Med. Cell. Longev. 2016, 2016, 1591803. [Google Scholar] [CrossRef] [Green Version]
- Daveri, E.; Cremonini, E.; Mastaloudis, A.; Hester, S.N.; Wood, S.M.; Waterhouse, A.L.; Anderson, M.; Fraga, C.G.; Oteiza, P.I. Cyanidin and delphinidin modulate inflammation and altered redox signaling improving insulin resistance in high fat-fed mice. Redox Biol. 2018, 18, 16–24. [Google Scholar] [CrossRef]
- Jiang, X.; Guo, H.; Shen, T.; Tang, X.; Yang, Y.; Ling, W. Cyanidin-3-O-beta-glucoside Purified from Black Rice Protects Mice against Hepatic Fibrosis Induced by Carbon Tetrachloride via Inhibiting Hepatic Stellate Cell Activation. J. Agric. Food Chem. 2015, 63, 6221–6230. [Google Scholar] [CrossRef]
- Arjinajarn, P.; Chueakula, N.; Pongchaidecha, A.; Jaikumkao, K.; Chatsudthipong, V.; Mahatheeranont, S.; Norkaew, O.; Chattipakorn, N.; Lungkaphin, A. Anthocyanin-rich Riceberry bran extract attenuates gentamicin-induced hepatotoxicity by reducing oxidative stress, inflammation and apoptosis in rats. Biomed. Pharmacother. 2017, 92, 412–420. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Lu, J.; Zheng, Y.L.; Wu, D.M.; Hu, B.; Shan, Q.; Cheng, W.; Li, M.Q.; Sun, Y.Y. Purple sweet potato color attenuates hepatic insulin resistance via blocking oxidative stress and endoplasmic reticulum stress in high-fat-diet-treated mice. J. Nutr. Biochem. 2013, 24, 1008–1018. [Google Scholar] [CrossRef]
- Cai, Z.; Song, L.; Qian, B.; Xu, W.; Ren, J.; Jing, P.; Oey, I. Understanding the effect of anthocyanins extracted from purple sweet potatoes on alcohol-induced liver injury in mice. Food Chem. 2018, 245, 463–470. [Google Scholar] [CrossRef]
- Gowd, V.; Jia, Z.Q.; Chen, W. Anthocyanins as promising molecules and dietary bioactive components against diabetes—A review of recent advances. Trends Food Sci. Technol. 2017, 68, 1–13. [Google Scholar] [CrossRef]
- Hong, S.H.; Heo, J.I.; Kim, J.H.; Kwon, S.O.; Yeo, K.M.; Bakowska-Barczak, A.M.; Kolodziejczyk, P.; Ryu, O.H.; Choi, M.K.; Kang, Y.H.; et al. Antidiabetic and Beta Cell-Protection Activities of Purple Corn Anthocyanins. Biomol. Ther. 2013, 21, 284–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarikaya, I.; Schierz, J.-H.; Sarikaya, A. Liver: Glucose metabolism and 18F-fluorodeoxyglucose PET findings in normal parenchyma and diseases. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 233–249. [Google Scholar] [PubMed]
- Choi, K.H.; Lee, H.A.; Park, M.H.; Han, J.S. Mulberry (Morus alba L.) Fruit Extract Containing Anthocyanins Improves Glycemic Control and Insulin Sensitivity via Activation of AMP-Activated Protein Kinase in Diabetic C57BL/Ksj-db/db Mice. J. Med. Food 2016, 19, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hu, C.; Ye, H.; Luo, R.; Fu, X.; Li, X.; Huang, J.; Chen, W.; Zheng, Y. Inflamm-Aging: A New Mechanism Affecting Premature Ovarian Insufficiency. J. Immunol. Res. 2019, 2019, 8069898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.G.; Yan, Q.Q.; Lu, L.Z.; Zhang, Y.Q. In vivo antioxidant, hypoglycemic, and anti-tumor activities of anthocyanin extracts from purple sweet potato. Nutr. Res. Pract. 2013, 7, 359–365. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, D.; Liu, Y.; Wang, D.; Liu, J.; Ji, B. The protective effects of berry-derived anthocyanins against visible light-induced damage in human retinal pigment epithelial cells. J. Sci. Food Agric. 2015, 95, 936–944. [Google Scholar] [CrossRef]
- Gao, J.M.; Wu, Y.T.; He, D.J.; Zhu, X.Q.; Li, H.B.; Liu, H.F.; Liu, H.L. Anti-aging effects of Ribes meyeri anthocyanins on neural stem cells and aging mice. Aging 2020, 12, 17738–17753. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, G.; Zhang, X.; Xu, D.; Gao, J.; Fan, J. Anthocyanins Delay Ageing-Related Degenerative Changes in the Liver. Plant Foods Hum. Nutr. 2017, 72, 425–431. [Google Scholar] [CrossRef]
- Qin, S.; Sun, D.; Mu, J.; Ma, D.; Tang, R.; Zheng, Y. Purple sweet potato color improves hippocampal insulin resistance via down-regulating SOCS3 and galectin-3 in high-fat diet mice. Behav. Brain Res. 2019, 359, 370–377. [Google Scholar] [CrossRef]
- Lee, M.; Sorn, S.R.; Park, Y.; Park, H.K. Anthocyanin Rich-Black Soybean Testa Improved Visceral Fat and Plasma Lipid Profiles in Overweight/Obese Korean Adults: A Randomized Controlled Trial. J. Med. Food 2016, 19, 995–1003. [Google Scholar] [CrossRef]
- Farrell, N.; Norris, G.; Lee, S.G.; Chun, O.K.; Blesso, C.N. Anthocyanin-rich black elderberry extract improves markers of HDL function and reduces aortic cholesterol in hyperlipidemic mice. Food Funct. 2015, 6, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Roder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic regulation of glucose homeostasis. Exp. Mol. Med. 2016, 48, e219. [Google Scholar] [CrossRef] [Green Version]
- Fang, J. Bioavailability of anthocyanins. Drug. Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Hair, R.; Sakaki, J.R.; Chun, O.K. Anthocyanins, Microbiome and Health Benefits in Aging. Molecules 2021, 26, 537. [Google Scholar] [CrossRef] [PubMed]
- Tena, N.; Martin, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A (13)C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, H.; Roma-Rodrigues, C.; Santos, A.; Veigas, B.; Bras, N.; Faria, A.; Calhau, C.; de Freitas, V.; Baptista, P.V.; Mateus, N.; et al. GLUT1 and GLUT3 involvement in anthocyanin gastric transport- Nanobased targeted approach. Sci. Rep. 2019, 9, 789. [Google Scholar] [CrossRef]
- Mallery, S.R.; Budendorf, D.E.; Larsen, M.P.; Pei, P.; Tong, M.; Holpuch, A.S.; Larsen, P.E.; Stoner, G.D.; Fields, H.W.; Chan, K.K.; et al. Effects of human oral mucosal tissue, saliva, and oral microflora on intraoral metabolism and bioactivation of black raspberry anthocyanins. Cancer Prev. Res. 2011, 4, 1209–1221. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, I.; Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Bioavailability of anthocyanins and derivatives. J. Funct. Foods 2014, 7, 54–66. [Google Scholar] [CrossRef]
- Lila, M.A.; Burton-Freeman, B.; Grace, M.; Kalt, W. Unraveling Anthocyanin Bioavailability for Human Health. Annu. Rev. Food Sci. Technol. 2016, 7, 375–393. [Google Scholar] [CrossRef]
- Faria, A.; Fernandes, I.; Norberto, S.; Mateus, N.; Calhau, C. Interplay between anthocyanins and gut microbiota. J. Agric. Food Chem. 2014, 62, 6898–6902. [Google Scholar] [CrossRef] [PubMed]
- de Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harmsen, H.J.; de Goffau, M.C. The Human Gut Microbiota. Adv. Exp. Med. Biol. 2016, 902, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Igwe, E.O.; Charlton, K.E.; Probst, Y.C.; Kent, K.; Netzel, M.E. A systematic literature review of the effect of anthocyanins on gut microbiota populations. J. Hum. Nutr. Diet. 2019, 32, 53–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramakrishna, B.S. Role of the gut microbiota in human nutrition and metabolism. J. Gastroenterol. Hepatol. 2013, 28, 9–17. [Google Scholar] [CrossRef]
- Aura, A.M.; Martin-Lopez, P.; O’Leary, K.A.; Williamson, G.; Oksman-Caldentey, K.M.; Poutanen, K.; Santos-Buelga, C. In vitro metabolism of anthocyanins by human gut microflora. Eur. J. Nutr. 2005, 44, 133–142. [Google Scholar] [CrossRef]
- Fernandes, I.; Faria, A.; de Freitas, V.; Calhau, C.; Mateus, N. Multiple-approach studies to assess anthocyanin bioavailability. Phytochem. Rev. 2015, 14, 899–919. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Bobryshev, Y.V.; Kozarov, E.; Sobenin, I.A.; Orekhov, A.N. Role of gut microbiota in the modulation of atherosclerosis-associated immune response. Front. Microbiol. 2015, 6, 671. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Barrio, R.; Edwards, C.A.; Crozier, A. Colonic catabolism of ellagitannins, ellagic acid, and raspberry anthocyanins: In vivo and in vitro studies. Drug. Metab. Dispos. 2011, 39, 1680–1688. [Google Scholar] [CrossRef]
- Hanske, L.; Engst, W.; Loh, G.; Sczesny, S.; Blaut, M.; Braune, A. Contribution of gut bacteria to the metabolism of cyanidin 3-glucoside in human microbiota-associated rats. Br. J. Nutr. 2013, 109, 1433–1441. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, H.; He, S.; Lou, Q.; Yu, M.; Tang, M.; Tu, L. Metabolism and prebiotics activity of anthocyanins from black rice (Oryza sativa L.) in vitro. PLoS ONE 2018, 13, e0195754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morais, C.A.; de Rosso, V.V.; Estadella, D.; Pisani, L.P. Anthocyanins as inflammatory modulators and the role of the gut microbiota. J. Nutr. Biochem. 2016, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, D.; Ji, Y.; Liu, Y.; Xu, L.; Guo, Y. Dietary Supplementation of Black Rice Anthocyanin Extract Regulates Cholesterol Metabolism and Improves Gut Microbiota Dysbiosis in C57BL/6J Mice Fed a High-Fat and Cholesterol Diet. Mol. Nutr. Food Res. 2020, 64, e1900876. [Google Scholar] [CrossRef]
- Cremonini, E.; Daveri, E.; Mastaloudis, A.; Adamo, A.M.; Mills, D.; Kalanetra, K.; Hester, S.N.; Wood, S.M.; Fraga, C.G.; Oteiza, P.I. Anthocyanins protect the gastrointestinal tract from high fat diet-induced alterations in redox signaling, barrier integrity and dysbiosis. Redox Biol. 2019, 26, 101269. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xiao, Y.; Bian, J.; Han, L.; He, C.; El-Omar, E.; Gong, L.; Wang, M. Ameliorative Effects of Gut Microbial Metabolite Urolithin A on Pancreatic Diseases. Nutrients 2022, 14, 2549. [Google Scholar] [CrossRef]
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef]
- Arnoult, N.; Karlseder, J. Complex interactions between the DNA-damage response and mammalian telomeres. Nat. Struct. Mol. Biol. 2015, 22, 859–866. [Google Scholar] [CrossRef]
- Roake, C.M.; Artandi, S.E. Control of Cellular Aging, Tissue Function, and Cancer by p53 Downstream of Telomeres. Cold Spring Harb. Perspect. Med. 2017, 7, a026088. [Google Scholar] [CrossRef] [Green Version]
- Jaskelioff, M.; Muller, F.L.; Paik, J.H.; Thomas, E.; Jiang, S.; Adams, A.C.; Sahin, E.; Kost-Alimova, M.; Protopopov, A.; Cadinanos, J.; et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 2011, 469, 102–106. [Google Scholar] [CrossRef] [Green Version]
- Shaw, A.; Doherty, M.K.; Mutch, N.J.; MacRury, S.M.; Megson, I.L. Endothelial cell oxidative stress in diabetes: A key driver of cardiovascular complications? Biochem. Soc. Trans. 2014, 42, 928–933. [Google Scholar] [CrossRef]
- Erusalimsky, J.D. Vascular endothelial senescence: From mechanisms to pathophysiology. J. Appl. Physiol. 2009, 106, 326–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, V.; Chow, M.Z.; Wang, Z.; Zhang, L.; Liu, B.; Liu, X.; Zhou, Z. Histone H4 lysine 16 hypoacetylation is associated with defective DNA repair and premature senescence in Zmpste24-deficient mice. Proc. Natl. Acad. Sci. USA 2011, 108, 12325–12330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of Vascular Aging. Circ. Res. 2018, 123, 849–867. [Google Scholar] [CrossRef] [PubMed]
- Donato, A.J.; Machin, D.R.; Lesniewski, L.A. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ. Res. 2018, 123, 825–848. [Google Scholar] [CrossRef]
- Xiao-Hong, D.; Chang-Qin, X.; Jian-Hua, H.; Wen-Jiang, Z.; Bing, S. Icariin delays homocysteine-induced endothelial cellular senescence involving activation of the PI3K/AKT-eNOS signaling pathway. Pharm. Biol. 2013, 51, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Jin, R.; Zhang, J.; You, T.; Peng, Z.; Ge, X.; Bronson, R.T.; Halperin, J.A.; Loscalzo, J.; Qin, X. The critical roles of platelet activation and reduced NO bioavailability in fatal pulmonary arterial hypertension in a murine hemolysis model. Blood 2010, 116, 1613–1622. [Google Scholar] [CrossRef] [Green Version]
- Ungvari, Z.; Kaley, G.; de Cabo, R.; Sonntag, W.E.; Csiszar, A. Mechanisms of vascular aging: New perspectives. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 1028–1041. [Google Scholar] [CrossRef] [Green Version]
- Luis, A.; Domingues, F.; Pereira, L. Association between berries intake and cardiovascular diseases risk factors: A systematic review with meta-analysis and trial sequential analysis of randomized controlled trials. Food Funct. 2018, 9, 740–757. [Google Scholar] [CrossRef]
- Garcia-Conesa, M.T.; Chambers, K.; Combet, E.; Pinto, P.; Garcia-Aloy, M.; Andres-Lacueva, C.; de Pascual-Teresa, S.; Mena, P.; Konic Ristic, A.; Hollands, W.J.; et al. Meta-Analysis of the Effects of Foods and Derived Products Containing Ellagitannins and Anthocyanins on Cardiometabolic Biomarkers: Analysis of Factors Influencing Variability of the Individual Responses. Int. J. Mol. Sci. 2018, 19, 694. [Google Scholar] [CrossRef] [Green Version]
- Fairlie-Jones, L.; Davison, K.; Fromentin, E.; Hill, A.M. The Effect of Anthocyanin-Rich Foods or Extracts on Vascular Function in Adults: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2017, 9, 908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donato, A.J.; Morgan, R.G.; Walker, A.E.; Lesniewski, L.A. Cellular and molecular biology of aging endothelial cells. J. Mol. Cell. Cardiol. 2015, 89, 122–135. [Google Scholar] [CrossRef] [Green Version]
- Parzonko, A.; Oswit, A.; Bazylko, A.; Naruszewicz, M. Anthocyans-rich Aronia melanocarpa extract possesses ability to protect endothelial progenitor cells against angiotensin II induced dysfunction. Phytomedicine 2015, 22, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, R.; Zhao, H.; Chen, G.; Jiang, Y.; Lyu, X.; Wu, T. Reduction of Aging-Induced Oxidative Stress and Activation of Autophagy by Bilberry Anthocyanin Supplementation via the AMPK-mTOR Signaling Pathway in Aged Female Rats. J. Agric. Food Chem. 2019, 67, 7832–7843. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, M.; Wang, Z.; Guo, Z.; Wang, Z.; Chen, Q. Cyanidin-3-O-glucoside attenuates endothelial cell dysfunction by modulating miR-204-5p/SIRT1-mediated inflammation and apoptosis. Biofactors 2020, 46, 803–812. [Google Scholar] [CrossRef]
- Tsai, K.L.; Hung, C.H.; Chan, S.H.; Hsieh, P.L.; Ou, H.C.; Cheng, Y.H.; Chu, P.M. Chlorogenic Acid Protects Against oxLDL-Induced Oxidative Damage and Mitochondrial Dysfunction by Modulating SIRT1 in Endothelial Cells. Mol. Nutr. Food Res. 2018, 62, e1700928. [Google Scholar] [CrossRef]
- Lee, G.H.; Hoang, T.H.; Jung, E.S.; Jung, S.J.; Han, S.K.; Chung, M.J.; Chae, S.W.; Chae, H.J. Anthocyanins attenuate endothelial dysfunction through regulation of uncoupling of nitric oxide synthase in aged rats. Aging Cell 2020, 19, e13279. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Kim, B.K.; Park, K.Y.; Yokozawa, T.; Song, Y.O.; Cho, E.J. Anti-aging Effects of Cyanidin under a Stress-Induced Premature Senescence Cellular System. Biol. Pharm. Bull. 2010, 33, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Hada, Y.; Uchida, H.A.; Otaka, N.; Onishi, Y.; Okamoto, S.; Nishiwaki, M.; Takemoto, R.; Takeuchi, H.; Wada, J. The Protective Effect of Chlorogenic Acid on Vascular Senescence via the Nrf2/HO-1 Pathway. Int. J. Mol. Sci. 2020, 21, 4527. [Google Scholar] [CrossRef]
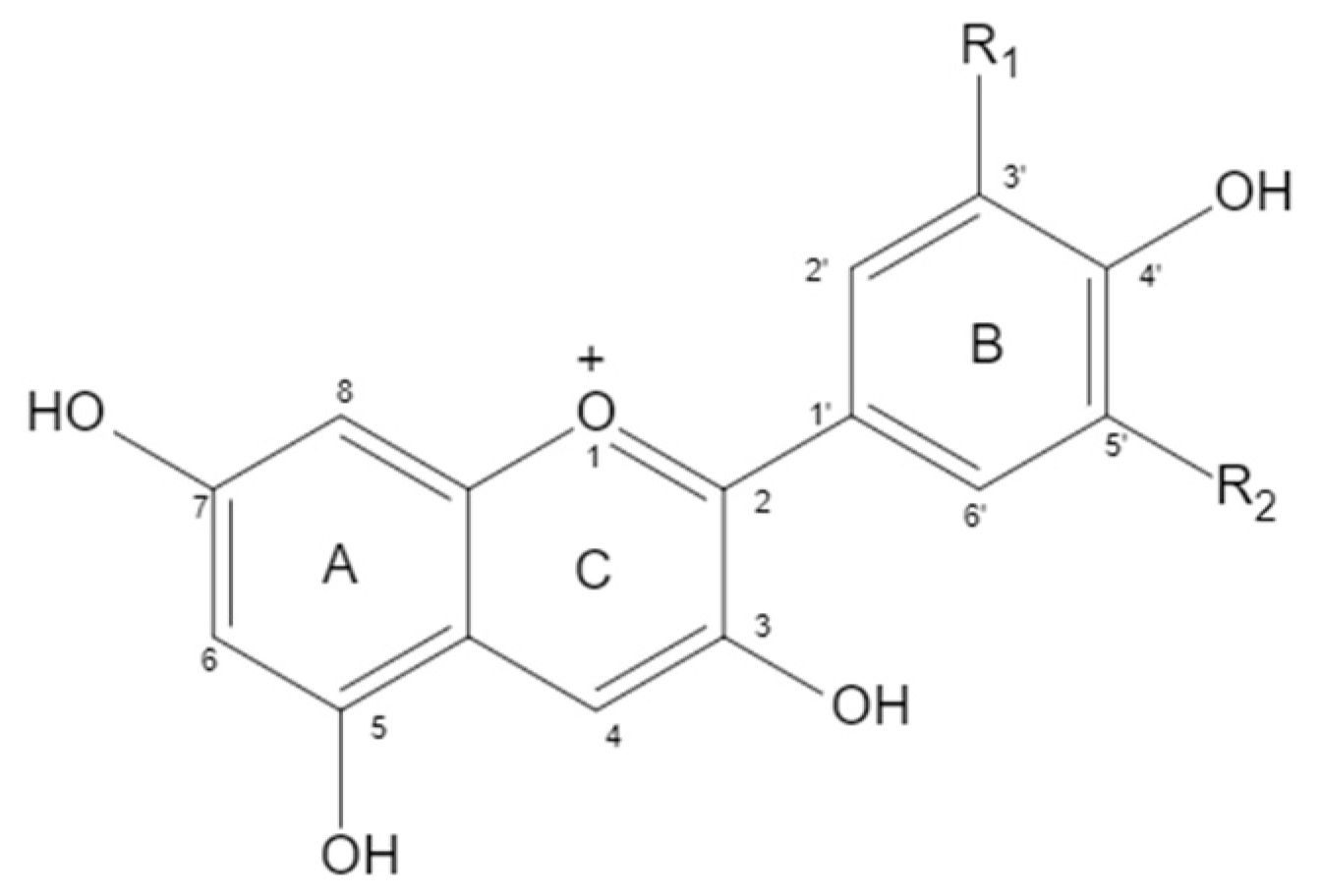

| Anthocyanin | Proportion | Substituents | |
|---|---|---|---|
| R1 | R2 | ||
| Cyanidin | 50% | OH | H |
| Delphindin | 12% | OH | OH |
| Pelargonidin | 12% | H | H |
| Peonidin | 12% | OCH3 | H |
| Petunidin | 7% | OCH3 | OH |
| Malvidin | 7% | OCH3 | OCH3 |
| Effects | Source | Mechanisms | Ref. | |
|---|---|---|---|---|
| Anti-cancer | colon cancer | Purple grape anthocyanins |
| [13] |
| colon cancer | Cyanidin-3-O-rutinoside |
| [14] | |
| colon cancer | Purple and red maize anthocyanins |
| [15] | |
| breast cancer | Black sweet cherry anthocyanins |
| [8] | |
| melanoma cancer | Hibiscus calyx anthocyanin |
| [16] | |
| Purple sweet potato anthocyanin |
| [17] | ||
| Anti-inflammatory |
| [20] | ||
| [21] [24] | |||
| Cyanidin-3-glucoside |
| [22] | ||
| Geranium pigment-3-O-glucoside in strawberry |
| [26] | ||
| Hibiscus anthocyanin |
| [27] | ||
| Anti-oxidation | Purple corn anthocyanin |
| [30] | |
| Cyanidins in radish buds |
| [31] | ||
| Mahonia aquifolium anthocyanin |
| [32] | ||
| Black rice anthocyanin extract (cyanidin-3-O-glucoside) |
| [33] | ||
| Blueberry anthocyanins |
| [34] | ||
| Protective effect on liver | Cyanidin-3-O-glucoside |
| [36] | |
| Riceberry bran anthocyanin |
| [37] | ||
| Purple sweet potato anthocyanin |
| [38] | ||
| Purple sweet potato anthocyanin |
| [39] | ||
| Lowering blood glucose | Purple corn anthocyanins |
| [52] | |
| Mulberry anthocyanin |
| [41] | ||
| Anti-aging | Purple sweet potato anthocyanin |
| [45] | |
| Cy-3-glu Pg-3-glu |
| [46] | ||
| Ribes meyeri anthocyanins |
| [47] | ||
| [48] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Wu, X.; Han, L.; Bian, J.; He, C.; El-Omar, E.; Gong, L.; Wang, M. The Potential Roles of Dietary Anthocyanins in Inhibiting Vascular Endothelial Cell Senescence and Preventing Cardiovascular Diseases. Nutrients 2022, 14, 2836. https://doi.org/10.3390/nu14142836
Dong Y, Wu X, Han L, Bian J, He C, El-Omar E, Gong L, Wang M. The Potential Roles of Dietary Anthocyanins in Inhibiting Vascular Endothelial Cell Senescence and Preventing Cardiovascular Diseases. Nutrients. 2022; 14(14):2836. https://doi.org/10.3390/nu14142836
Chicago/Turabian StyleDong, Yonghui, Xue Wu, Lin Han, Ji Bian, Caian He, Emad El-Omar, Lan Gong, and Min Wang. 2022. "The Potential Roles of Dietary Anthocyanins in Inhibiting Vascular Endothelial Cell Senescence and Preventing Cardiovascular Diseases" Nutrients 14, no. 14: 2836. https://doi.org/10.3390/nu14142836









