Thymol Alleviates LPS-Induced Liver Inflammation and Apoptosis by Inhibiting NLRP3 Inflammasome Activation and the AMPK-mTOR-Autophagy Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Chemicals
2.3. Animals and Treatment
2.4. Histopathological Analysis
2.5. Serum Biochemical Analysis
2.6. Immunohistochemistry (IHC) Staining
2.7. Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
2.8. Elisa Assay
2.9. Western Blotting Analysis
2.10. TUNEL Staining
2.11. Detection of Caspase3 and Caspase9 Activity
2.12. Isolation and Culture of Primary Hepatocytes
2.13. Detection of LDH Activity
2.14. Statistical Analysis
3. Results
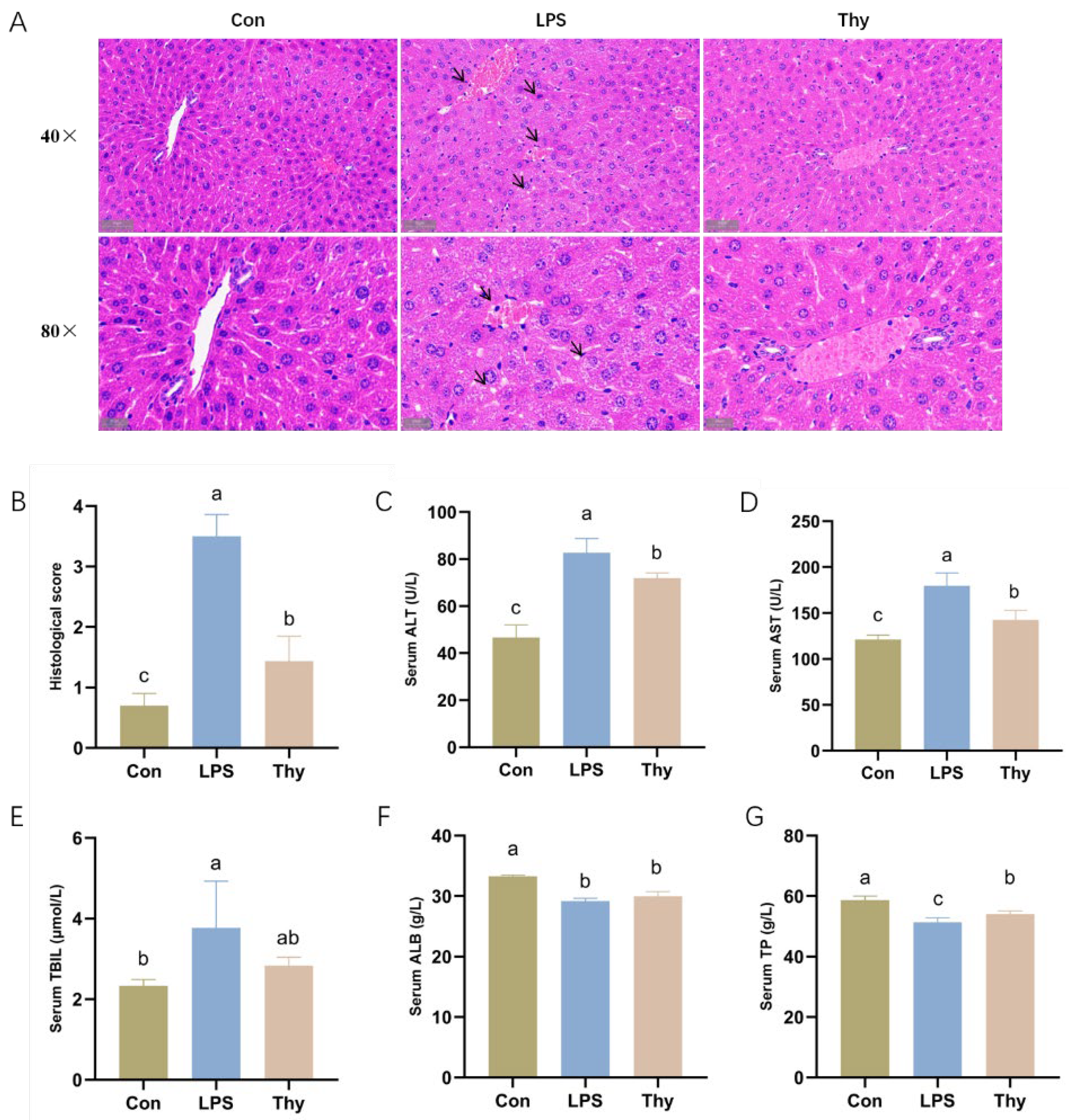
3.1. Thymol Protects Mice from LPS-Induced Liver Injury
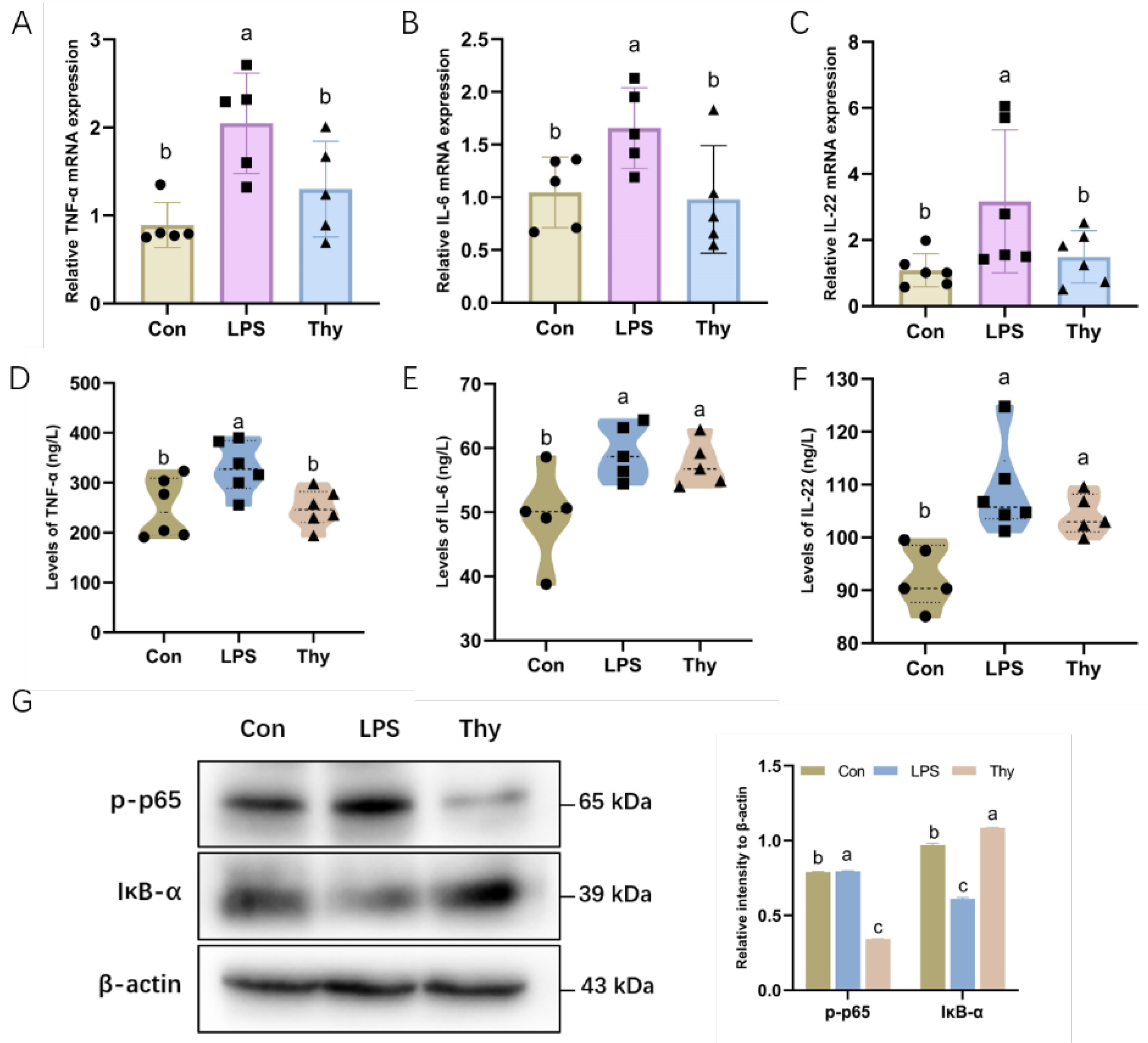
3.2. Thymol Protects Mice from Liver Injury via Suppressing Proinflammatory Cytokines
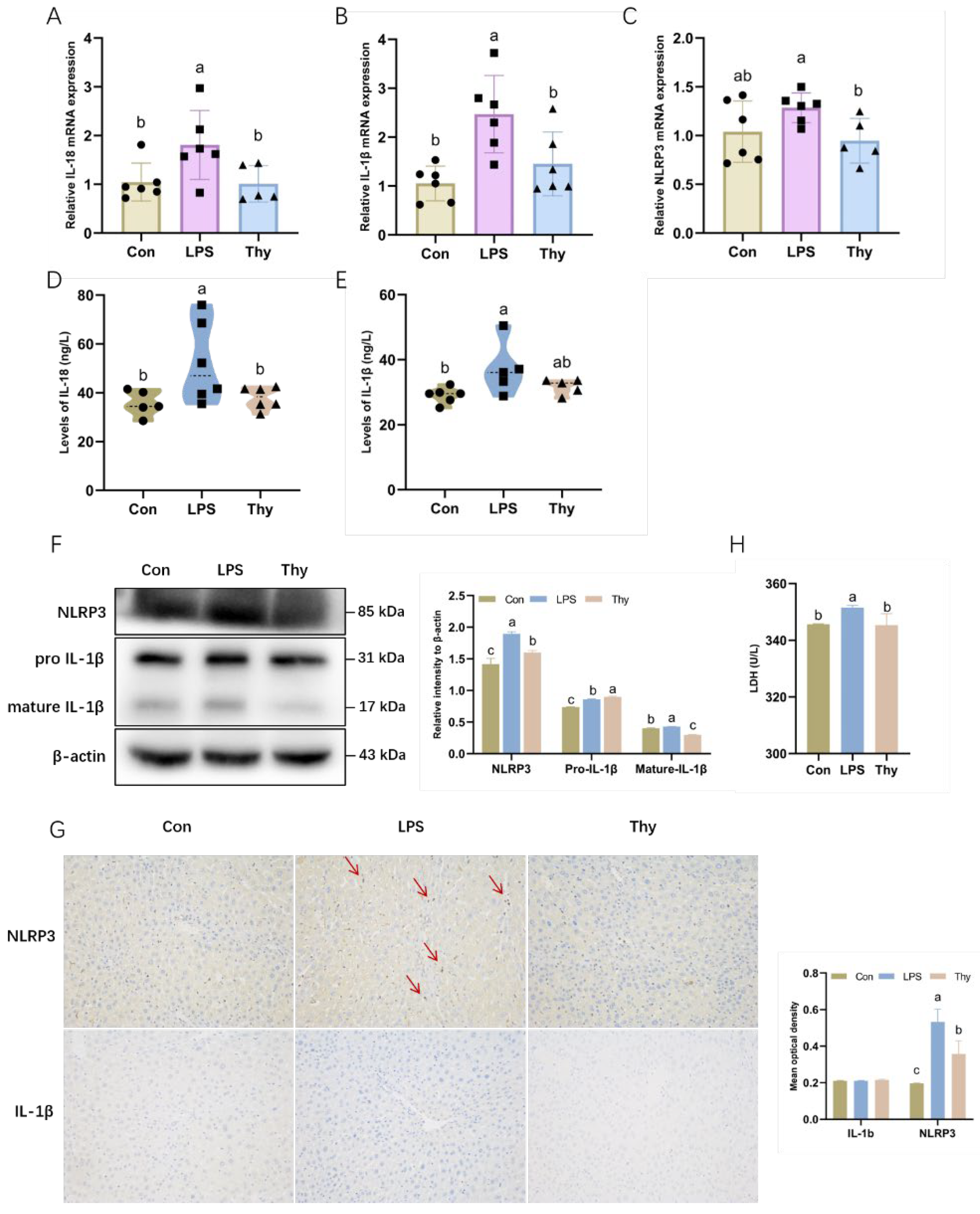
3.3. Thymol Protects Mice from Liver Injury by Inhibiting NLRP3 Inflammasome Activation
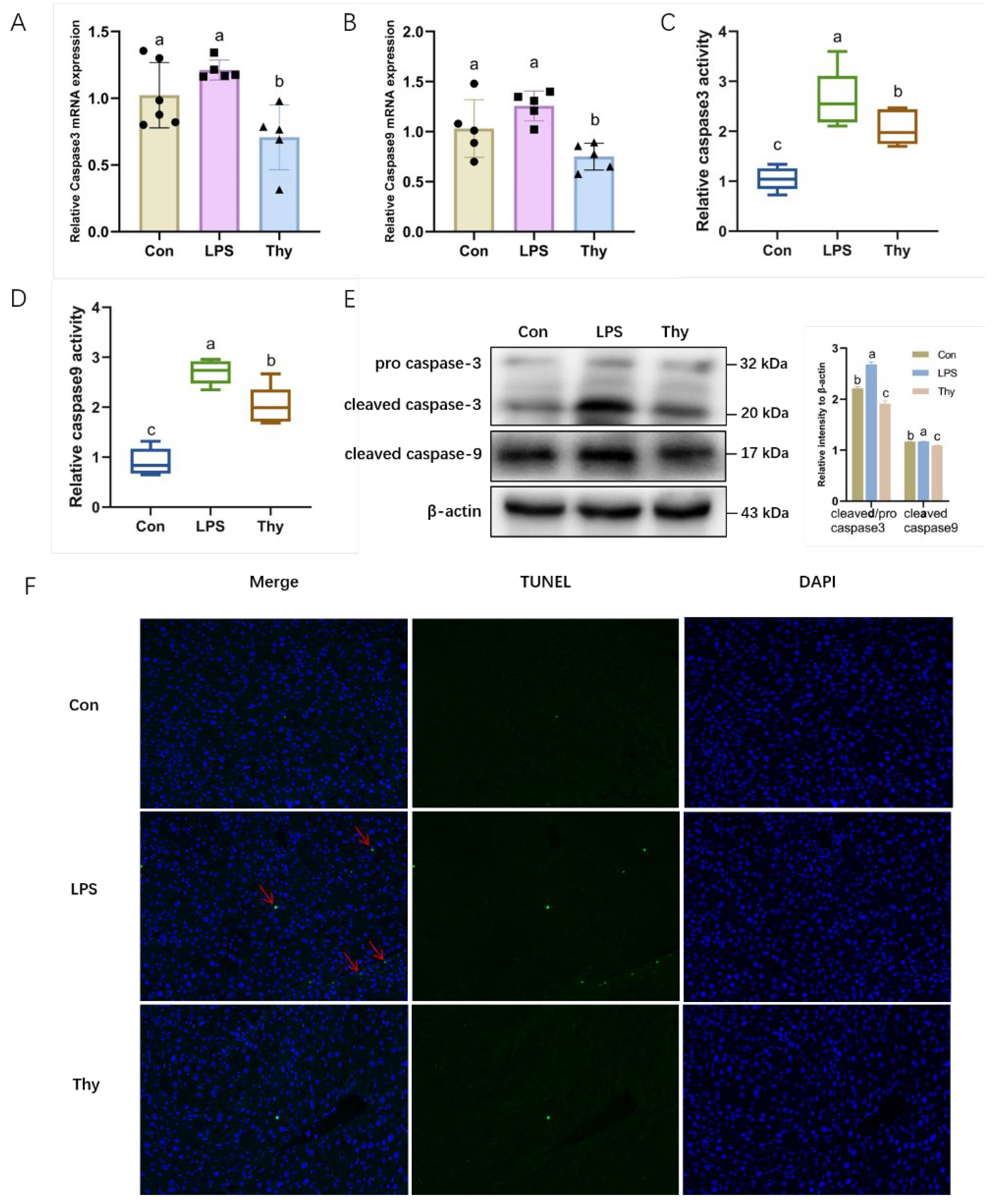
3.4. Thymol Protects Mice from Liver Injury via Suppressing Hepatocyte Apoptosis
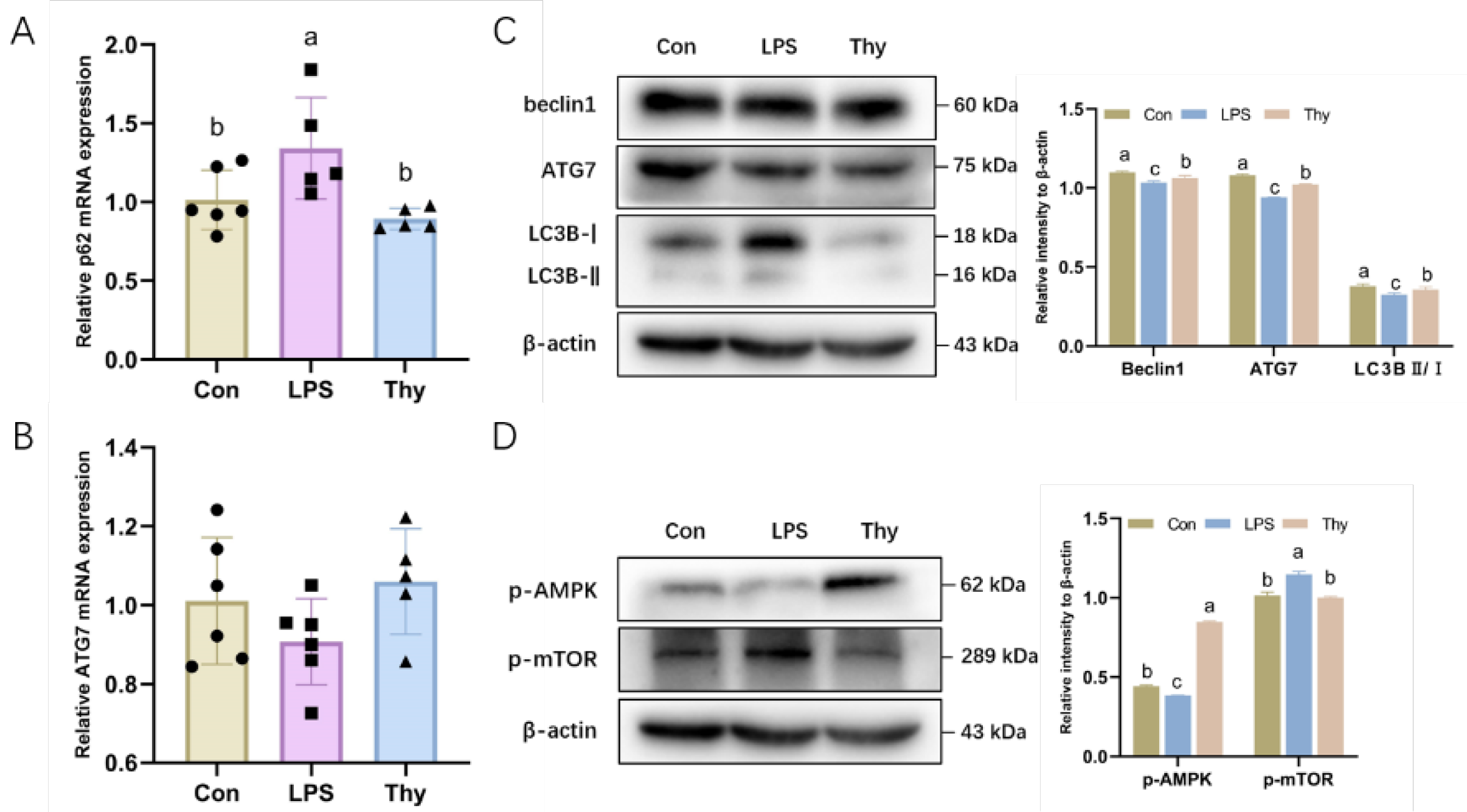
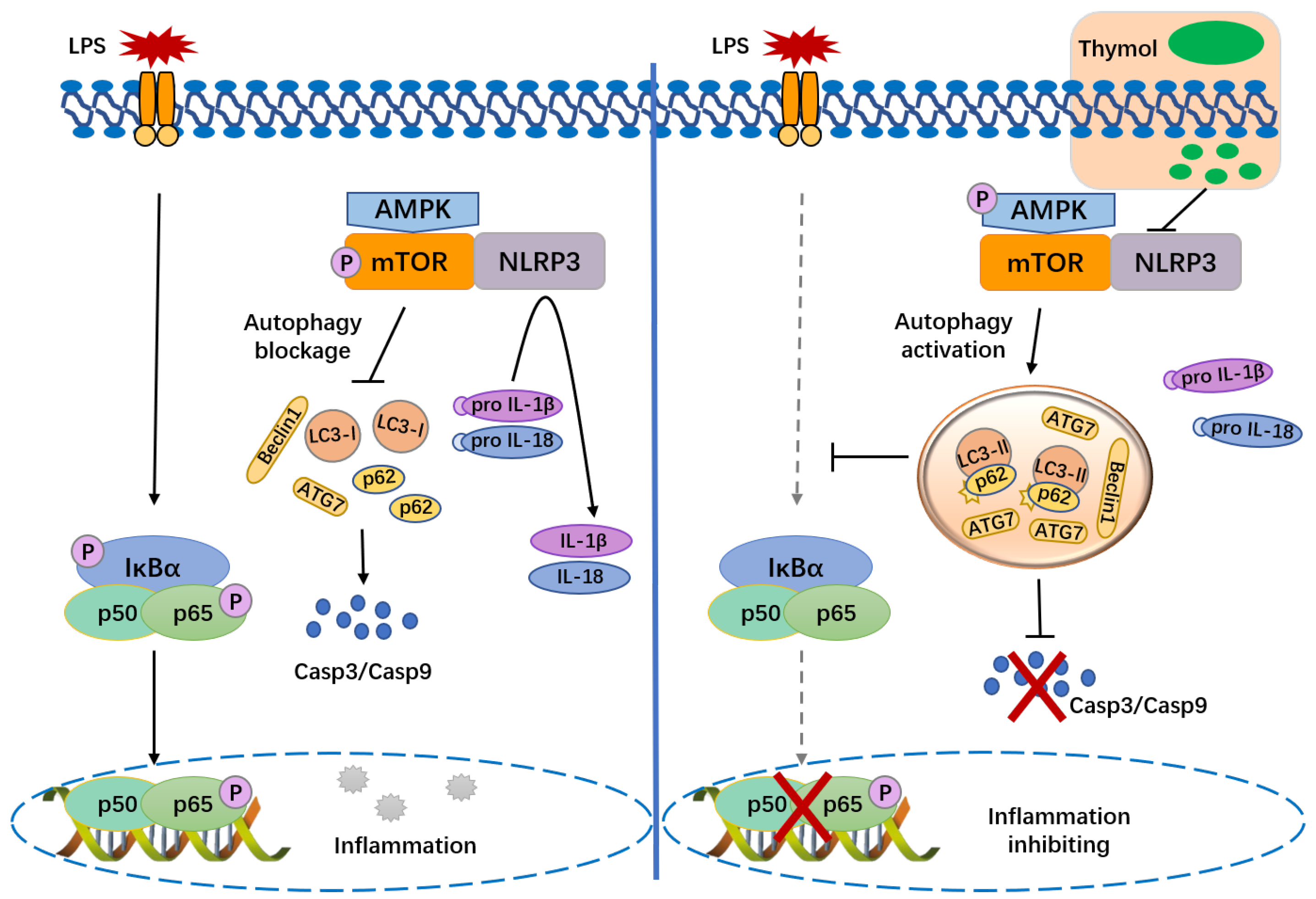
3.5. Thymol Protects Mice from Liver Injury via Modulating the AMPK-mTOR-Autophagy Pathway
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Shao, L.; Xiong, X.; Zhang, Y.; Miao, H.; Ren, Y.; Tang, X.; Song, J.; Wang, C. IL-22 ameliorates LPS-induced acute liver injury by autophagy activation through ATF4-ATG7 signaling. Cell Death Dis. 2020, 11, 970. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, L.; Zhang, X.; Xu, L.; Xie, B.; Shi, H.; Duan, Z.; Zhang, H.; Ren, F. Kaempferol protects mice from d-GalN/LPS-induced acute liver failure by regulating the ER stress-Grp78-CHOP signaling pathway. Biomed. Pharm. 2019, 111, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Li, H.; Zhao, Y.; Cai, E.; Zhu, H.; Li, P.; Liu, J. Hepatoprotective effect of α-mangostin against lipopolysaccharide/d-galactosamine-induced acute liver failure in mice. Biomed. Pharmacother. 2018, 106, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; Abdelfattah-Hassan, A.; Badawi, M.; Ismail, T.; Bendary, M.; Abdelaziz, A.; Mosbah, R.; Mohamed, D.; Arisha, A.; El-Hamid, M. Thymol nanoemulsion promoted broiler chicken’s growth, gastrointestinal barrier and bacterial community and conferred protection against Salmonella Typhimurium. Sci. Rep. 2021, 11, 7742. [Google Scholar] [CrossRef]
- Omonijo, F.; Liu, S.; Hui, Q.; Zhang, H.; Lahaye, L.; Bodin, J.; Gong, J.; Nyachoti, M.; Yang, C. Thymol improves barrier function and attenuates inflammatory responses in porcine intestinal epithelial cells during lipopolysaccharide (LPS)-induced inflammation. J. Agric. Food Chem. 2018, 67, 615–624. [Google Scholar] [CrossRef]
- Liao, H.; Du, S.; Jiang, T.; Zheng, M.; Xiang, Z.; Yang, J. UMSCs Attenuate LPS/D-GalN-Induced Acute Liver Failure in Mice by Down-regulating the MyD88/NF-κB Pathway. J. Clin. Transl. Hepatol. 2021, 9, 690–701. [Google Scholar] [CrossRef]
- Dou, X.; Yan, D.; Ma, Z.; Gao, N.; Shan, A. Sodium butyrate alleviates LPS-induced kidney injury via inhibiting TLR2/4 to regulate rBD2 expression. J. Food Biochem. 2022, 46, e14126. [Google Scholar] [CrossRef]
- Jin, L.; Wang, Q.; Zhang, H.; Tai, S.; Liu, H.; Zhang, D. A Synthetic Peptide AWRK6 Alleviates Lipopolysaccharide-Induced Liver Injury. Int. J. Mol. Sci. 2018, 19, 2661. [Google Scholar] [CrossRef] [Green Version]
- Dou, X.; Ma, Z.; Yan, D.; Gao, N.; Li, Z.; Li, Y.; Feng, X.; Meng, L.; Shan, A. Sodium butyrate alleviates intestinal injury and microbial flora disturbance induced by lipopolysaccharides in rats. Food Funct. 2022, 13, 1360–1369. [Google Scholar] [CrossRef]
- Gao, N.; Dou, X.; Yin, T.; Yang, Y.; Yan, D.; Ma, Z.; Bi, C.; Shan, A. Tryptophan Promotes Intestinal Immune Defense through Calcium-Sensing Receptor (CaSR)-Dependent Metabolic Pathways. J. Agric. Food Chem. 2021, 69, 13460–13473. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, N.; Liu, Y.; He, H.; Luo, Z.; Liu, W.; Song, N.; Ju, M. Ginsenoside Rb1 Reduces D-GalN/LPS-induced Acute Liver Injury by Regulating TLR4/NF-κB Signaling and NLRP3 Inflammasome. J. Clin. Transl. Hepatol. 2022, 10, 474–485. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, M.; Wang, T.; Cai, M.; Qian, F.; Sun, Y.; Wang, Y. Greentea polyphenols prevent lipopolysaccharide-induced inflammatory liver injury in mice by inhibiting NLRP3 inflammasome activation. Food Funct. 2019, 10, 3898–3908. [Google Scholar] [CrossRef]
- Qin, Y.; Li, Q.; Liang, W.; Yan, R.; Tong, L.; Jia, M.; Zhao, C.; Zhao, W. TRIM28 SUMOylates and stabilizes NLRP3 to facilitate inflammasome activation. Nat. Commun. 2021, 12, 4794. [Google Scholar] [CrossRef]
- Zhao, J.; He, B.; Zhang, S.; Huang, W.; Li, X. Ginsenoside Rg1 alleviates acute liver injury through the induction of autophagy and suppressing NF-κB/NLRP3 inflammasome signaling pathway. Biomed. Pharmacother. 2021, 18, 1382–1389. [Google Scholar] [CrossRef]
- Hou, Y.; Lin, S.; Qiu, J.; Sun, W.; Dong, M.; Xiang, Y.; Wang, L.; Du, P. NLRP3 inflammasome negatively regulates podocyte autophagy in diabetic nephropathy. Biochem. Biophys. Res. Commun. 2019, 521, 791–798. [Google Scholar] [CrossRef]
- Wan, S.; Shi, B.; Lou, X.; Liu, J.; Ma, G.; Liang, D.; Ma, S. Ghrelin protects small intestinal epithelium against sepsis-induced injury by enhancing the autophagy of intestinal epithelial cells. Biomed. Pharmacother. 2016, 83, 1315–1320. [Google Scholar] [CrossRef]
- Shi, T.; Song, W.; Xu, R. Autophagy and ER stress in LPS/GalN-induced acute liver injury. Mol. Med. Rep. 2017, 16, 7001–7005. [Google Scholar] [CrossRef] [Green Version]
- Lalazar, G.; Ilyas, G.; Malik, S.; Liu, K.; Zhao, E.; Amri, M.; Lin, Y.; Tanaka, K.; Czaja, M. Autophagy confers resistance to lipopolysaccharide-induced mouse hepatocyte injury. Am. J. Physiol. Gastrointest Liver Physiol. 2016, 311, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Feng, X.; Li, B.; Sha, J.; Wang, C.; Yang, T.; Cui, H.; Fan, H. Dexmedetomidine Protects Against Lipopolysaccharide-Induced Acute Kidney Injury by Enhancing Autophagy through Inhibition of the PI3K/AKT/mTOR Pathway. Front. Pharmacol. 2020, 11, 128–141. [Google Scholar] [CrossRef]
- Lei, Q.; Yi, T.; Chen, C. NF-κB-Gasdermin D (GSDMD) Axis Couples Oxidative Stress and NACHT, LRR and PYD Domains-Containing Protein 3 (NLRP3) Inflammasome-Mediated Cardiomyocyte Pyroptosis Following Myocardial Infarction. Med. Sci. Monit. 2018, 24, 6044–6052. [Google Scholar] [CrossRef]
- Anders, L.; Lang, A.; Anwar-Mohamed, A.; Douglas, A.; Bushau, A.; Falkner, K.; Hill, B.; Warner, N.; Arteel, G.; Cave, M.; et al. Vinyl Chloride Metabolites Potentiate Inflammatory Liver Injury Caused by LPS in Mice. Toxicol. Sci. 2016, 151, 312–323. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Jia, H.; Jin, Y.; Liu, N.; Chen, J.; Yang, Y.; Dai, Z.; Wang, C. Glycine Attenuates LPS-Induced Apoptosis and Inflammatory Cell Infiltration in Mouse Liver. J. Nutr. 2020, 150, 1116–1125. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Liu, M.; Zhou, X.; Wang, M.; Cao, K.; Jin, S.; Shan, A.; Feng, X. Curcumin mitigates aflatoxin B1-induced liver injury via regulating the NLRP3 inflammasome and Nrf2 signaling pathway. Food Chem. Toxicol. 2022, 161, 112823. [Google Scholar] [CrossRef]
- Li, X.; Lv, Z.; Chen, J.; Nepovimova, E.; Long, M.; Wu, W.; Kuca, K. Bacillus amyloliquefaciens B10 can alleviate liver apoptosis and oxidative stress induced by aflatoxin B1. Food Chem. Toxicol. 2021, 151, 112124. [Google Scholar] [CrossRef]
- Niu, Y.; Sun, Q.; Zhang, G.; Liu, X.; Shang, Y.; Xiao, Y.; Liu, S. Fowl adenovirus serotype 4-induced apoptosis, autophagy, and a severe inflammatory response in liver. Vet. Microbiol. 2018, 223, 34–41. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Q.; Wang, Y.; Li, P. A potent protective effect of baicalein on liver injury by regulating mitochondria-related apoptosis. Apoptosis 2020, 25, 412–425. [Google Scholar] [CrossRef]
- Li, Q.; Tan, Y.; Chen, S.; Xiao, X.; Zhang, M.; Wu, Q.; Dong, M. Irisin alleviates LPS-induced liver injury and inflammation through inhibition of NLRP3 inflammasome and NF-κB signaling. J. Recept. Signal Transduct. 2021, 41, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Li, X.; Ren, X.; Ding, N.; Tao, L.; Dong, X.; Chen, Z. GBP5 promotes liver injury and inflammation by inducing hepatocyte apoptosis. FASEB J. 2022, 36, e22119. [Google Scholar] [CrossRef]
- Wu, M.; Wang, C.; Mai, C.; Chen, J.; Lai, X.; He, L.; Huang, S.; Zhang, X. Flavonoids from Livistona chinensis fruit ameliorates LPS/D-GalN-induced acute liver injury by inhibiting oxidative stress and inflammation. J. Funct. Foods 2019, 61, 103460. [Google Scholar] [CrossRef]
- Utaipan, T.; Suksamrarn, A.; Kaemchantuek, P.; Chokchaisiri, R.; Stremmel, W.; Chamulitrat, W.; Chunglok, W. Diterpenoid trigonoreidon B isolated from Trigonostemon reidioides alleviates inflammation in models of LPS-stimulated murine macrophages and inflammatory liver injury in mice. Biomed. Pharm. 2018, 101, 961–971. [Google Scholar] [CrossRef]
- Du, X.; Li, H.; Yang, X.; Li, J.; Xu, J.; Chen, Y.; Xu, Q.; Yang, L.; He, C.; Huang, C. Wogonin attenuates liver fibrosis via regulating hepatic stellate cell activation and apoptosis. Int. Immunopharmacol. 2019, 75, 105671. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Jiang, Z.; Tong, Z.; Zhou, Y.; Chai, X.; Xiao, X. Shikonin Ameliorates LPS-Induced Cardiac Dysfunction by SIRT1-Dependent Inhibition of NLRP3 Inflammasome. Front. Physiol. 2020, 11, 570441. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Pan, M.; Zheng, B.; Chen, Y.; Li, W.; Yang, Q.; Zheng, Z.; Sun, N.; Zhang, Y.; Li, X. Autophagy attenuates angiotensin II-induced pulmonary fibrosis by inhibiting redox imbalance-mediated NLRP3 inflammasome activation. Antioxid. Redox Signal. 2018, 30, 520–541. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Yin, P.; Yao, H.; Chen, L.; Chang, X.; Li, H.; Hou, X. Punicalin Ameliorates Cell Pyroptosis Induced by LPS/ATP Through Suppression of ROS/NLRP3 Pathway. J. Inflamm. Res. 2021, 14, 711–718. [Google Scholar] [CrossRef]
- Tastan, B.; Arioz, B.; Tufekci, K.; Tarakcioglu, E.; Gonul, C.; Genc, K.; Genc, S. Dimethyl Fumarate Alleviates NLRP3 Inflammasome Activation in Microglia and Sickness Behavior in LPS-Challenged Mice. Front. Immunol. 2021, 12, 737065. [Google Scholar] [CrossRef]
- Han, X.; Song, J.; Lian, L.; Yao, Y.; Shao, D.; Fan, Y.; Hou, L.; Wang, G.; Zheng, S.; Wu, Y. Ginsenoside 25-OCH3-PPD Promotes Activity of LXRs To Ameliorate P2X7R-Mediated NLRP3 Inflammasome in the Development of Hepatic Fibrosis. J. Agric. Food Chem. 2018, 66, 7023–7035. [Google Scholar] [CrossRef]
- Zhang, W.; Tao, S.; Wang, T.; Li, Y.; Chen, H.; Zhan, Y.; Yu, M.; Ge, C.; Li, C.; Ren, G. NLRP3 is dispensable for D-galactosamine/lipopolysaccharide-induced acute liver failure. Biochem. Biophys. Res. Commun. 2020, 533, 1184–1190. [Google Scholar] [CrossRef]
- Gallego, P.; Castejón-Vega, B.; Del, C.; Cordero, M. The Absence of NLRP3-inflammasome Modulates Hepatic Fibrosis Progression, Lipid Metabolism, and Inflammation in KO NLRP3 Mice during Aging. Cells 2020, 9, 2148. [Google Scholar] [CrossRef]
- Hou, L.; Yang, L.; Chang, N.; Zhao, X.; Zhou, X.; Dong, C.; Liu, F.; Yang, L.; Li, L. Macrophage Sphingosine 1-Phosphate Receptor 2 Blockade Attenuates Liver Inflammation and Fibrogenesis Triggered by NLRP3 Inflammasome. Front. Immunol. 2020, 11, 1149. [Google Scholar] [CrossRef]
- Dda, B.; Lv, W.; Jing, X.; Ma, X.; Wuen, J.; Hasi, S. Dietary supplementation of camel whey protein attenuates heat stress-induced liver injury by inhibiting NLRP3 inflammasome activation through the HMGB1/RAGE signaling pathway. J. Funct. Foods 2021, 84, 104584. [Google Scholar]
- Cosin-Roger, J.; Simmen, S.; Melhem, H.; Atrott, K.; Frey-Wagner, I.; Hausmann, M.; de Vallière, C.; Spalinger, M.; Spielmann, P.; Wenger, R.; et al. Hypoxia ameliorates intestinal inflammation through NLRP3/mTOR downregulation and autophagy activation. Nat. Commun. 2017, 8, 98. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhu, Y.; Wu, Y.; Fu, M.; Wu, Y.; Wu, Y.; Qiu, Y.; Zhang, H.; Ding, M. Oridonin Alleviates LPS-Induced Depression by Inhibiting NLRP3 Inflammasome via Activation of Autophagy. Front. Med. 2021, 8, 813047. [Google Scholar] [CrossRef]
- Peng, W.; Peng, F.; Lou, Y.; Li, Y.; Zhao, N.; Shao, Q.; Chen, J.; Qian, K.; Zeng, Z.; Zhan, Y.; et al. Autophagy alleviates mitochondrial DAMP-induced acute lung injury by inhibiting NLRP3 inflammasome. Life Sci. 2021, 265, 118833. [Google Scholar] [CrossRef]
- Hu, J.; Wu, H.; Wang, D.; Yang, Z.; Zhuang, L.; Yang, N.; Dong, J. Weicao capsule ameliorates renal injury through increasing autophagy and NLRP3 degradation in UAN rats. Int. J. Biochem. Cell Biol. 2018, 96, 1–8. [Google Scholar] [CrossRef]
- Li, W.; Li, N.; Song, D.; Rong, J.; Qian, A.; Li, X. Metformin inhibits endothelial progenitor cell migration by decreasing matrix metalloproteinases, MMP-2 and MMP-9, via the AMPK/mTOR/autophagy pathway. Int. J. Mol. Med. 2017, 39, 1262–1268. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Li, N.; Chen, T.; Zhang, C.; Liu, L.; Qi, Y.; Bu, P. Trimetazidine ameliorates sunitinib-induced cardiotoxicity in mice via the AMPK/mTOR/autophagy pathway. Pharm. Biol. 2019, 57, 625–631. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Yao, X.; Zhang, Q.; Zhu, M.; Liu, Z.; Ci, B.; Xie, Y.; Carlson, D.; Rothermel, B.; Sun, Y.; et al. Beclin-1-Dependent Autophagy Protects the Heart during Sepsis. Circulation 2018, 138, 2247–2262. [Google Scholar] [CrossRef]
- Chung, K.; Kim, K.; Choi, Y.; An, H.; Lee, B.; Kim, D.; Lee, E.; Im, E.; Lee, J.; Im, D.; et al. The critical role played by endotoxin-induced liver autophagy in the maintenance of lipid metabolism during sepsis. Autophagy 2017, 13, 1113–1129. [Google Scholar] [CrossRef] [Green Version]
- Qiu, T.; Pei, P.; Yao, X.; Jiang, L.; Wei, S.; Wang, Z.; Bai, J.; Yang, G.; Gao, N.; Yang, L.; et al. Taurine attenuates arsenic-induced pyroptosis and nonalcoholic steatohepatitis by inhibiting the autophagic-inflammasomal pathway. Cell Death Dis. 2018, 9, 946. [Google Scholar] [CrossRef] [Green Version]
| Gene | Primer (5′-3′) | Gene Bank Accession |
|---|---|---|
| β-actin | F: GTGCTATGTTGCTCTAGACTTCG | NM_007393.5 |
| R: ATGCCACAGGATTCCATACC | ||
| TNF-α | F: GCCTCTTCTCATTCCTGCTTGTGG | NM_001278601.1 |
| R: GTGGTTTGTGAGTGTGAGGGTCTG | ||
| IL-6 | F: CTTCTTGGGACTGATGCTGGTGAC | NM_001314054.1 |
| R: AGGTCTGTTGGGAGTGGTATCCTC | ||
| IL-22 | F: TTCCAGCAGCCATACATCGTCAAC | XM_006513865.4 |
| R: GGTAGCACTGATCCTTAGCACTGAC | ||
| IL-1β | F: TCGCAGCAGCACATCAACAAGAG | XM_006498795.5 |
| R: AGGTCCACGGGAAAGACACAGG | ||
| IL-18 | F: GTTAGGTGGGGAGGGTTTGTGTTC | XM_036154619.1 |
| R: GCAGCCTCGGGTATTCTGTTATGG | ||
| NLRP3 | F: CCTGGTCTGCTGGATTGTGTGC | XM_039085397.1 |
| R: AGTCGTGGTCTTGGAGGTCTGG | ||
| Caspase3 | F: TCTGACTGGAAAGCCGAAACTCTTC | XM_017312543.3 |
| R: GTCCCACTGTCTGTCTCAATGCC | ||
| Caspase9 | F: ATGCTGTGTCAAGTTTGCCTACCC | NM_001355176.1 |
| R: GCTCCAGAATGCCATCCAAGGTC | ||
| p62(SQSTM1) | F: TGGAGTCGGATAACTGCTCAGGAG | NM_175843.4 |
| R: AGACTGGAGTTCACCTGTGGATGG | ||
| ATG7 | F: GGCACGAACTGACCCAGAAGAAG | XM_036152370.1 |
| R: GCAGACCAGCAGAGTCACCATTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, X.; Yan, D.; Liu, S.; Gao, L.; Shan, A. Thymol Alleviates LPS-Induced Liver Inflammation and Apoptosis by Inhibiting NLRP3 Inflammasome Activation and the AMPK-mTOR-Autophagy Pathway. Nutrients 2022, 14, 2809. https://doi.org/10.3390/nu14142809
Dou X, Yan D, Liu S, Gao L, Shan A. Thymol Alleviates LPS-Induced Liver Inflammation and Apoptosis by Inhibiting NLRP3 Inflammasome Activation and the AMPK-mTOR-Autophagy Pathway. Nutrients. 2022; 14(14):2809. https://doi.org/10.3390/nu14142809
Chicago/Turabian StyleDou, Xiujing, Di Yan, Siqi Liu, Lujia Gao, and Anshan Shan. 2022. "Thymol Alleviates LPS-Induced Liver Inflammation and Apoptosis by Inhibiting NLRP3 Inflammasome Activation and the AMPK-mTOR-Autophagy Pathway" Nutrients 14, no. 14: 2809. https://doi.org/10.3390/nu14142809





