Development of Food Group Tree-Based Analysis and Its Association with Non-Alcoholic Fatty Liver Disease (NAFLD) and Co-Morbidities in a South Indian Population: A Large Case-Control Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethical Approval
2.3. Clinical Outcomes
2.4. Dietary Data
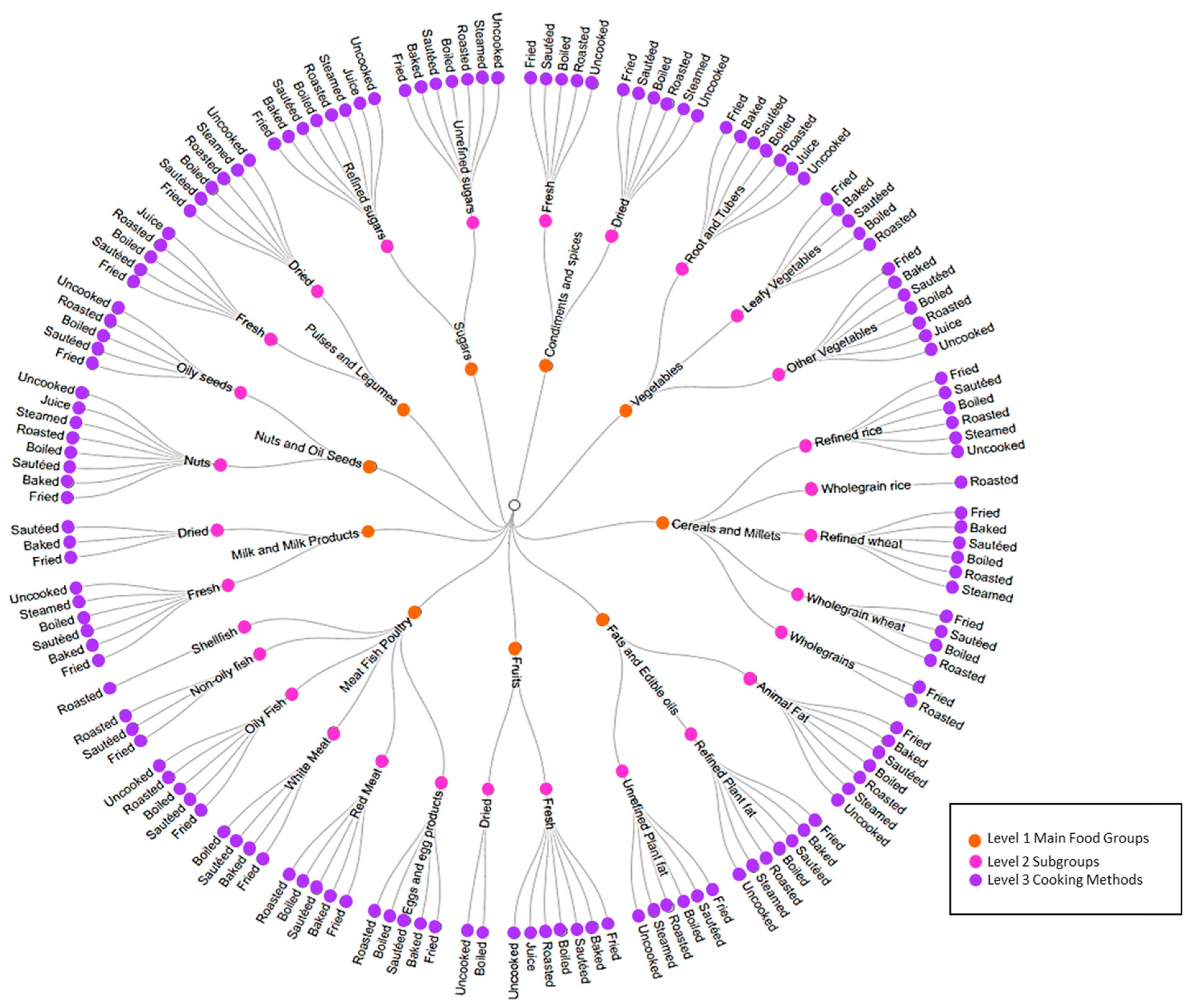
2.5. Deconstruction of the Recipes into Ingredients, and Food-Groups Tree Development
2.6. Ingredient’s Intake Calculations
2.7. Identification of Outliers
2.8. Statistical Analysis
3. Results
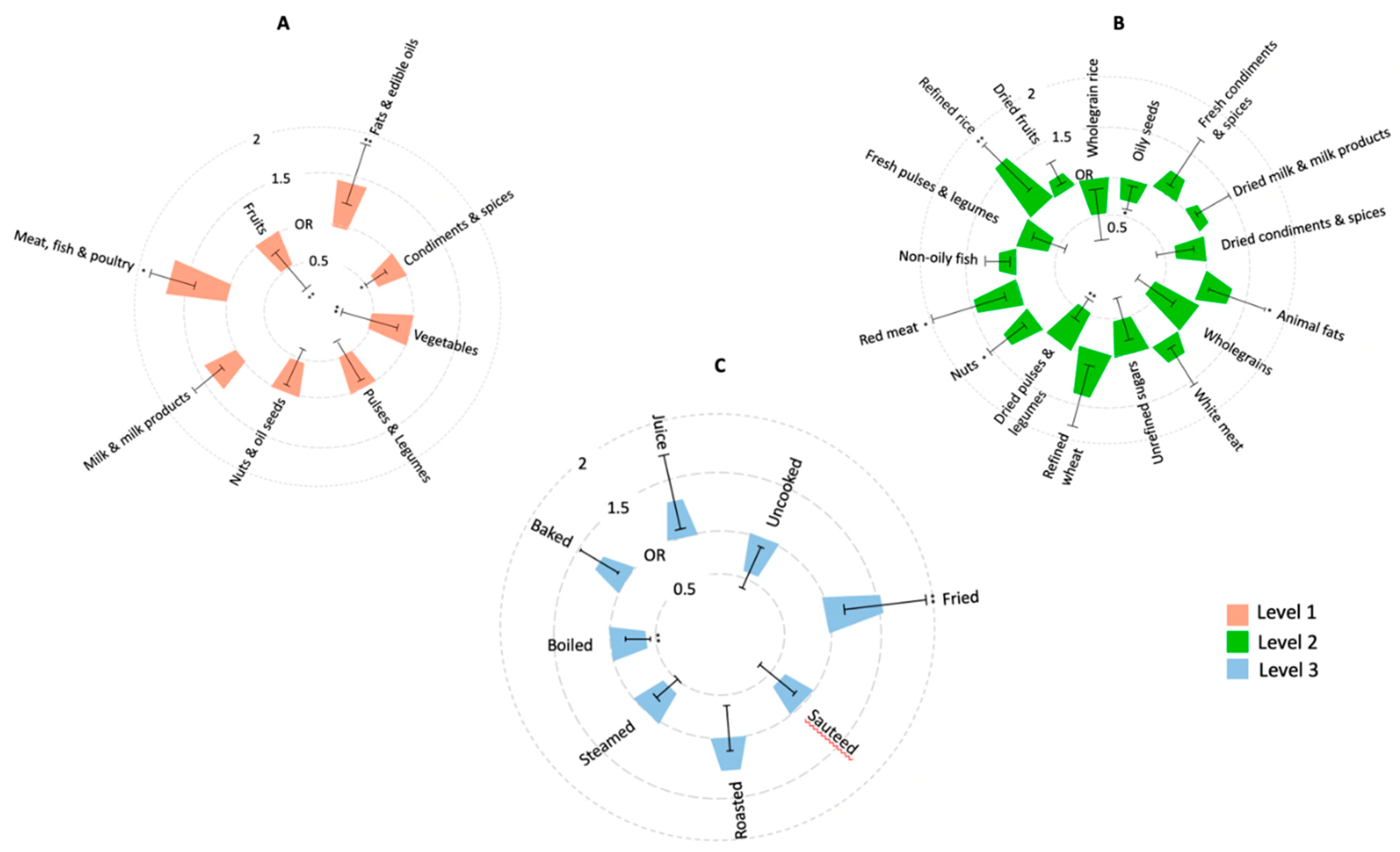
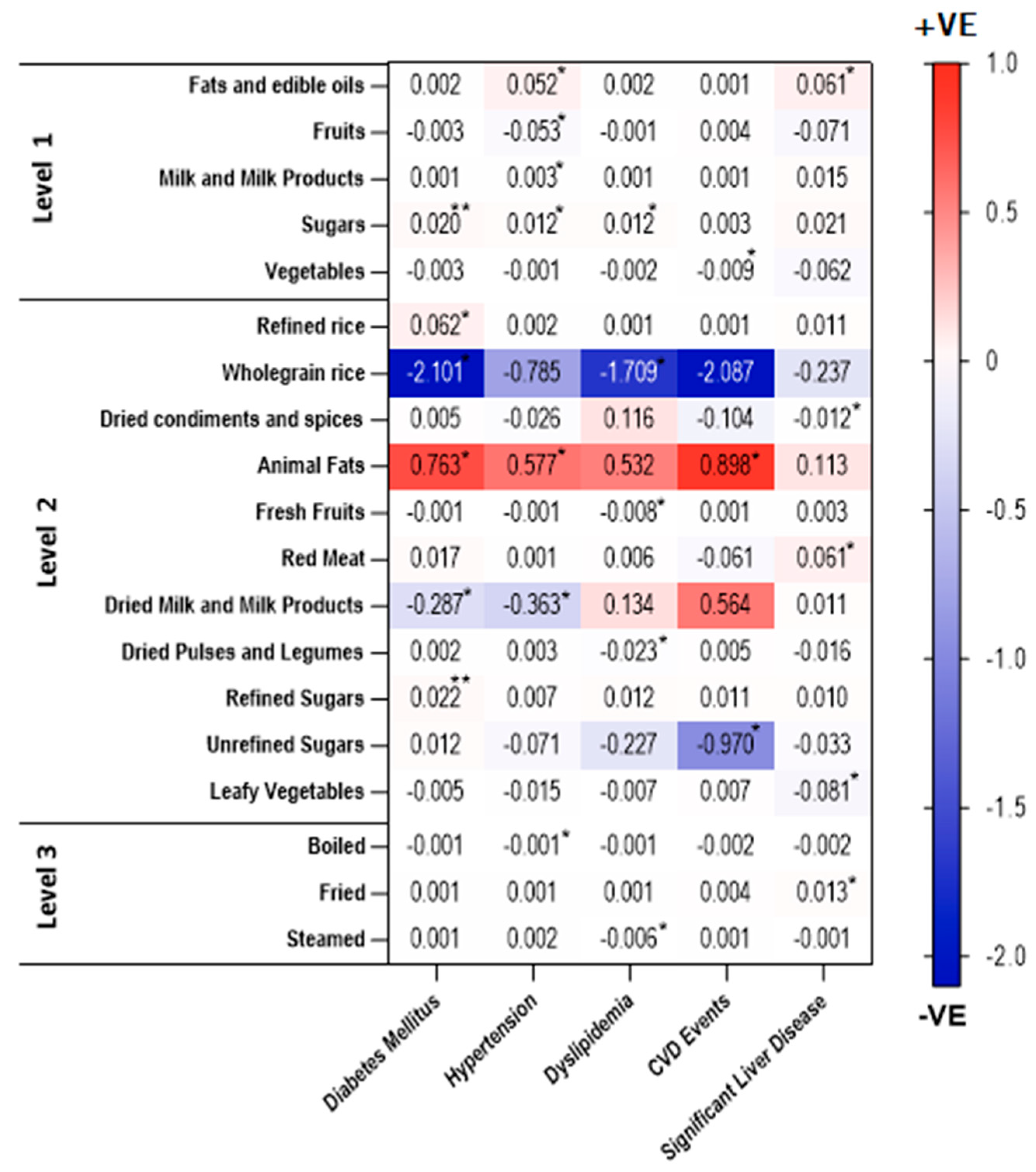
3.1. Association of Food Intakes and Cooking Methods with NAFLD
3.2. Association of Food Groups Intakes with Presence of Significant Liver Fibrosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, F.B.; van Dam, R.M.; Liu, S. Diet and risk of Type II diabetes: The role of types of fat and carbohydrate. Diabetologia 2001, 44, 805–817. [Google Scholar] [CrossRef]
- Shulman, G.I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 2014, 371, 1131–1141. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; De Michieli, F.; Cassader, M.; Rizzetto, M.; Durazzo, M.; Fagà, E.; Silli, B.; Pagano, G. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology 2003, 37, 909–916. [Google Scholar] [CrossRef]
- Heath, R.B.; Karpe, F.; Milne, R.W.; Burdge, G.C.; Wootton, S.A.; Frayn, K.N. Selective partitioning of dietary fatty acids into the VLDL TG pool in the early postprandial period. J. Lipid Res. 2003, 44, 2065–2072. [Google Scholar] [CrossRef] [Green Version]
- Rush, E.; World Health Organization (WHO) Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar]
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef]
- Popkin, B.M. The nutrition transition and obesity in the developing world. J. Nutr. 2001, 131, 871s–873s. [Google Scholar] [CrossRef]
- Mohan, V.; Farooq, S.; Deepa, M.; Ravikumar, R.; Pitchumoni, C.S. Prevalence of non-alcoholic fatty liver disease in urban south Indians in relation to different grades of glucose intolerance and metabolic syndrome. Diabetes Res. Clin. Pract. 2009, 84, 84–91. [Google Scholar] [CrossRef]
- Majumdar, A.; Misra, P.; Sharma, S.; Kant, S.; Krishnan, A.; Pandav, C.S. Prevalence of nonalcoholic fatty liver disease in an adult population in a rural community of Haryana, India. Indian J. Public Health 2016, 60, 26–33. [Google Scholar] [CrossRef]
- Toshimitsu, K.; Matsuura, B.; Ohkubo, I.; Niiya, T.; Furukawa, S.; Hiasa, Y.; Kawamura, M.; Ebihara, K.; Onji, M. Dietary habits and nutrient intake in non-alcoholic steatohepatitis. Nutrition 2007, 23, 46–52. [Google Scholar] [CrossRef]
- Solga, S.; Alkhuraishe, A.R.; Clark, J.M.; Torbenson, M.; Greenwald, A.; Diehl, A.M.; Magnuson, T. Dietary composition and nonalcoholic fatty liver disease. Dig. Dis. Sci. 2004, 49, 1578–1583. [Google Scholar] [CrossRef]
- Machado, M.V.; Ravasco, P.; Jesus, L.; Marques-Vidal, P.; Oliveira, C.R.; Proença, T.; Baldeiras, I.; Camilo, M.E.; Cortez-Pinto, H. Blood oxidative stress markers in non-alcoholic steatohepatitis and how it correlates with diet. Scand. J. Gastroenterol. 2008, 43, 95–102. [Google Scholar] [CrossRef]
- Lim, J.S.; Mietus-Snyder, M.; Valente, A.; Schwarz, J.M.; Lustig, R.H. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 251–264. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Subramonian, A.M.; O’Keefe, J.H. Added fructose as a principal driver of non-alcoholic fatty liver disease: A public health crisis. Open Heart 2017, 4, e000631. [Google Scholar] [CrossRef] [Green Version]
- Herman, R.H.; Zakim, D.; Stifel, F.B. Effect of diet on lipid metabolism in experimental animals and man. Fed. Proc. 1970, 29, 1302–1307. [Google Scholar]
- Elia, M.; Stratton, R.J. Geographical inequalities in nutrient status and risk of malnutrition among English people aged 65 y and older. Nutrition 2005, 21, 1100–1106. [Google Scholar] [CrossRef]
- Yang, C.-Q.; Shu, L.; Wang, S.; Wang, J.-J.; Zhou, Y.; Xuan, Y.-J.; Wang, S.-F. Dietary Patterns Modulate the Risk of Non-Alcoholic Fatty Liver Disease in Chinese Adults. Nutrients 2015, 7, 4778–4791. [Google Scholar] [CrossRef] [Green Version]
- Chung, G.E.; Youn, J.; Kim, Y.S.; Lee, J.E.; Yang, S.Y.; Lim, J.H.; Song, J.H.; Doo, E.Y.; Kim, J.S. Dietary patterns are associated with the prevalence of nonalcoholic fatty liver disease in Korean adults. Nutrition 2019, 62, 32–38. [Google Scholar] [CrossRef]
- Trovato, F.M.; Martines, G.F.; Brischetto, D.; Trovato, G.; Catalano, D. Neglected features of lifestyle: Their relevance in non-alcoholic fatty liver disease. World J. Hepatol. 2016, 8, 1459–1465. [Google Scholar] [CrossRef]
- Kontogianni, M.D.; Tileli, N.; Margariti, A.; Georgoulis, M.; Deutsch, M.; Tiniakos, D.; Fragopoulou, E.; Zafiropoulou, R.; Manios, Y.; Papatheodoridis, G. Adherence to the Mediterranean diet is associated with the severity of non-alcoholic fatty liver disease. Clin. Nutr. 2014, 33, 678–683. [Google Scholar] [CrossRef]
- Yki-Järvinen, H.; Luukkonen, P.K.; Hodson, L.; Moore, J.B. Dietary Carbohydrates and Fats in Nonalcoholic Fatty Liver Disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 770–786. [Google Scholar] [CrossRef]
- Vecchio, M.G.; Paramesh, E.C.; Paramesh, H.; Loganes, C.; Ballali, S.; Gafare, C.E.; Verduci, E.; Gulati, A. Types of food and nutrient intake in India: A literature review. Indian J. Pediatrics 2014, 81, 17–22. [Google Scholar] [CrossRef]
- Daniel, C.R.; Kapur, K.; McAdams, M.J.; Dixit-Joshi, S.; Devasenapathy, N.; Shetty, H.; Hariharan, S.; George, P.S.; Mathew, A.; Sinha, R. Development of a field-friendly automated dietary assessment tool and nutrient database for India. Br. J. Nutr. 2014, 111, 160–171. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.J.; Vangay, P.; Al-Ghalith, G.A.; Hillmann, B.M.; Ward, T.L.; Shields-Cutler, R.R.; Kim, A.D.; Shmagel, A.K.; Syed, A.N.; Personalized Microbiome Class Students; et al. Daily Sampling Reveals Personalized Diet-Microbiome Associations in Humans. Cell Host Microbe 2019, 25, 789–802.e785. [Google Scholar] [CrossRef]
- Vijay, A.; Mohan, L.; Taylor, M.A.; Grove, J.I.; Valdes, A.M.; Aithal, G.P.; Shenoy, K.T. The Evaluation and Use of a Food Frequency Questionnaire among the Population in Trivandrum, South Kerala, India. Nutrients 2020, 12, 383. [Google Scholar] [CrossRef] [Green Version]
- Chalmers, J.; Ban, L.; Leena, K.B.; Edwards, K.L.; Grove, J.L.; Aithal, G.P.; Shenoy, K.T. Cohort profile: The Trivandrum non-alcoholic fatty liver disease (NAFLD) cohort. BMJ Open 2019, 9, e027244. [Google Scholar] [CrossRef]
- Das, K.; Sarkar, R.; Ahmed, S.M.; Mridha, A.R.; Mukherjee, P.S.; Das, K.; Dhali, G.K.; Santra, A.; Chowdhury, A. “Normal” liver stiffness measure (LSM) values are higher in both lean and obese individuals: A population-based study from a developing country. Hepatology 2012, 55, 584–593. [Google Scholar] [CrossRef]
- Longvah, T.; Anantan, I.; Bhaskarachary, K.; Venkaiah, K.; Longvah, T. Indian Food Composition Tables; ICMR-National Institute of Nutrition: Hyderabad, India, 2017. [Google Scholar]
- Allowances, R.D. Nutrient Requirements and Recommended Dietary Allowances for Indians; ICMR-National Institute of Nutrition: Hyderabad, India, 2009. [Google Scholar]
- Welch, A.A.; Luben, R.; Khaw, K.T.; Bingham, S.A. The CAFE computer program for nutritional analysis of the EPIC-Norfolk food frequency questionnaire and identification of extreme nutrient values. J. Hum. Nutr. Diet. 2005, 18, 99–116. [Google Scholar] [CrossRef]
- Mirmiran, P.; Amirhamidi, Z.; Ejtahed, H.S.; Bahadoran, Z.; Azizi, F. Relationship between Diet and Non-alcoholic Fatty Liver Disease: A Review Article. Iran. J. Public Health 2017, 46, 1007–1017. [Google Scholar]
- Riani, R.; Djagat Purnomo, H.; Muis, S. Food Intake and Visceral Fat Deposition are Risk Factors of Incidence and Severity of Non-Alcoholic Fatty Liver Disease. J. Biomed. Transl. Res. 2017, 3, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.H.; Kallman, J.B.; Bai, C.; Pawloski, L.; Gewa, C.; Arsalla, A.; Sabatella, M.E.; Younossi, Z.M. Nutritional assessments of patients with non-alcoholic fatty liver disease. Obes. Surg. 2010, 20, 154–160. [Google Scholar] [CrossRef]
- He, K.; Li, Y.; Guo, X.; Zhong, L.; Tang, S. Food groups and the likelihood of non-alcoholic fatty liver disease: A systematic review and meta-analysis. Br. J. Nutr. 2020, 124, 1–13. [Google Scholar] [CrossRef]
- Fan, J.G.; Cao, H.X. Role of diet and nutritional management in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2013, 28, 81–87. [Google Scholar] [CrossRef]
- de Meijer, V.E.; Le, H.D.; Meisel, J.A.; Sharif, M.R.A.; Pan, A.; Nosé, V.; Puder, M. Dietary fat intake promotes the development of hepatic steatosis independently from excess caloric consumption in a murine model. Metabolism 2010, 59, 1092–1105. [Google Scholar] [CrossRef] [Green Version]
- European Association for the Study of the Liver; European Association for the Study of Diabetes; European Association for the Study of Obesity. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Hernández, E.; Kahl, S.; Seelig, A.; Begovatz, P.; Irmler, M.; Kupriyanova, Y.; Nowotny, B.; Nowotny, P.; Herder, C.; Barosa, C.; et al. Acute dietary fat intake initiates alterations in energy metabolism and insulin resistance. J. Clin. Investig. 2017, 127, 695–708. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Li, Y.; Satija, A.; Pan, A.; Sotos-Prieto, M.; Rimm, E.; Willett, W.C.; Hu, F.B. Association of changes in red meat consumption with total and cause specific mortality among US women and men: Two prospective cohort studies. BMJ 2019, 365, l2110. [Google Scholar] [CrossRef] [Green Version]
- Freidoony, L.; Kong, I.D. Practical approaches to the nutritional management of nonalcoholic fatty liver disease. Integr. Med. Res. 2014, 3, 192–197. [Google Scholar] [CrossRef] [Green Version]
- Plaz Torres, M.C.; Bodini, G.; Furnari, M.; Marabotto, E.; Zentilin, P.; Giannini, E.G. Nuts and Non-Alcoholic Fatty Liver Disease: Are Nuts Safe for Patients with Fatty Liver Disease? Nutrients 2020, 12, 3363. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, J.; Zhang, Q.; Liu, L.; Meng, G.; Yao, Z.; Wu, H.; Bao, X.; Gu, Y.; Lu, M. Association between nut consumption and non-alcoholic fatty liver disease in adults. Liver Int. 2019, 39, 1732–1741. [Google Scholar] [CrossRef]
- Chen, B.B.; Han, Y.; Pan, X.; Yan, J.; Liu, W.; Li, Y.; Lin, X.; Xu, S.; Peng, X. Association between nut intake and non-alcoholic fatty liver disease risk: A retrospective case-control study in a sample of Chinese Han adults. BMJ Open 2019, 9, e028961. [Google Scholar] [CrossRef]
- Asbaghi, O.; Emamat, H.; Kelishadi, M.R.; Hekmatdoost, A. The Association between Nuts Intake and Non-Alcoholic Fatty Liver Disease (NAFLD) Risk: A Case-Control Study. Clin. Nutr. Res. 2020, 9, 195–204. [Google Scholar] [CrossRef]
- Schlörmann, W.; Birringer, M.; Böhm, V.; Löber, K.; Jahreis, G.; Lorkowski, S.; Müller, A.K.; Schöne, F.; Glei, M. Influence of roasting conditions on health-related compounds in different nuts. Food Chem. 2015, 180, 77–85. [Google Scholar] [CrossRef]
- Hosseini Bai, S.; Darby, I.; Nevenimo, T.; Hannet, G.; Hannet, D.; Poienou, M.; Grant, E.; Brooks, P.; Walton, D.; Randall, B.; et al. Effects of roasting on kernel peroxide value, free fatty acid, fatty acid composition and crude protein content. PLoS ONE 2017, 12, e0184279. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.P.; Singh, A.; Misra, D.; Misra, B.; Pati, G.K.; Panigrahi, M.K.; Kar, S.K.; Bhuyan, P.; Pattnaik, K.; Meher, C.; et al. Risk Factors Associated With Non-Alcoholic Fatty Liver Disease in Indians: A Case-Control Study. J. Clin. Exp. Hepatol. 2015, 5, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Falade, A.O.; Oboh, G.; Ademiluyi, A.O.; Odubanjo, O.V. Consumption of thermally oxidized palm oil diets alters biochemical indices in rats. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Judd, R.C. Protein I: Structure, function, and genetics. Clin. Microbiol. Rev. 1989, 2, S41–S48. [Google Scholar] [CrossRef]
- Maduelosi, N.; Obediah, G. Effects of Vegetable Oil Reused for Frying on the Liver of Albino Rats. Sci. J. Chem. 2019, 7, 11–14. [Google Scholar]
- Soleimani, D.; Ranjbar, G.; Rezvani, R.; Goshayeshi, L.; Razmpour, F.; Nematy, M. Dietary patterns in relation to hepatic fibrosis among patients with nonalcoholic fatty liver disease. Diabetes Metab. Syndr. Obes. 2019, 12, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wang, X.; Ye, M.; Zhang, S.; Zhang, Q.; Meng, G.; Liu, L.; Wu, H.; Gu, Y.; Wang, Y.; et al. Does a high intake of green leafy vegetables protect from NAFLD? Evidence from a large population study. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1691–1701. [Google Scholar] [CrossRef]
- Cordero-Herrera, I.; Kozyra, M.; Zhuge, Z.; Haworth, S.M.; Moretti, C.; Peleli, M.; Caldeira-Dias, M.; Jahandideh, A.; Huirong, H.; Cruz, J.D.C.; et al. AMP-activated protein kinase activation and NADPH oxidase inhibition by inorganic nitrate and nitrite prevent liver steatosis. Proc. Natl. Acad. Sci. USA 2019, 116, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Whole grain and refined grain consumption and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Eur. J. Epidemiol. 2013, 28, 845–858. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef] [Green Version]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Knüppel, S.; Iqbal, K.; Andriolo, V.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food Groups and Risk of Hypertension: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Adv. Nutr. 2017, 8, 793–803. [Google Scholar] [CrossRef]
- Hu, E.A.; Pan, A.; Malik, V.; Sun, Q. White rice consumption and risk of type 2 diabetes: Meta-analysis and systematic review. BMJ 2012, 344, e1454. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Naik, S. Ghee: Its Properties, Importance and Health Benefits. 2018. Available online: https://www.researchgate.net/publication/339499398_Ghee_Its_Properties_Importance_and_Health_Benefits (accessed on 17 March 2022).
- Luukkonen, P.K.; Sädevirta, S.; Zhou, Y.; Kayser, B.; Ali, A.; Ahonen, L.; Lallukka, S.; Pelloux, V.; Gaggini, M.; Jian, C.; et al. Saturated Fat Is More Metabolically Harmful for the Human Liver Than Unsaturated Fat or Simple Sugars. Diabetes Care 2018, 41, 1732–1739. [Google Scholar] [CrossRef] [Green Version]
- Ross, A.B.; Godin, J.P.; Minehira, K.; Kirwan, J.P. Increasing whole grain intake as part of prevention and treatment of nonalcoholic Fatty liver disease. Int. J. Endocrinol. 2013, 2013, 585876. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, S.; Bani, T.; Aeri, B. Prevalence of cardiovascular disease in India and its economic impact—A review. Int. J. Sci. Res. Publ. 2013, 3, 2–4. [Google Scholar]
| NAFLD (n = 993) | Control (n = 973) | p-Value | |
|---|---|---|---|
| Age, years, mean (SD) | 48.16 (10.73) * | 45.90 (12.32) | <0.001 |
| Male gender (n, %) | 453 (45.62) * | 316 (32.48) | <0.001 |
| Weight (kg, mean SD) | 68.36 (11.57) * | 60.78 (11.12) | <0.001 |
| BMI (kg/m2, mean SD) - Underweight (n, %) - Normal weight (n, %) - Overweight (n, %) - Obese (n, %) | 26.87 (4.13) * 3 (0.30) 158 (15.91) 187 (18.83) 645 (64.95) | 24.33 (4.07) 61 (6.27) 310 (31.86) 206 (21.17) 396 (40.70) | <0.001 <0.001 <0.001 0.195 <0.001 |
| Significant liver fibrosis (n, %) n = 688 | 157 (22.82) | N/A | |
| Diabetes (n, %) | 334 (33.64) * | 180 (18.50) | <0.001 |
| Hypertension (n, %) | 396 (39.88) * | 288 (29.60) | <0.001 |
| Dyslipidaemia (n, %) | 695 (69.99) * | 520 (53.44) | <0.001 |
| Cardiovascular disease (n, %) | 25 (2.52) | 21 (2.16) | 0.598 |
| CONTROL (n = 973) Adjusted Mean Intake g/kg/day (±SD) | NAFLD (n = 993) Adjusted Mean Intake g/kg/day (±SD) | |
|---|---|---|
| FOOD GROUP LEVEL-1 | ||
| Cereals and Millets | 5.34 (±1.68) | 6.16 * (±1.98) |
| Condiments and Spices | 0.37 (±0.18) | 0.33 * (±0.17) |
| Fats and Edible Oils | 0.32 (±0.14) | 1.48 * (±1.13) |
| Fruits | 1.15 (±1.07) | 1.13 (±1.12) |
| Meat, Fish, and Poultry | 1.96 (±1.37) | 2.20 * (±1.48) |
| Milk and Milk Products | 2.55 (±1.56) | 2.33 * (±1.46) |
| Nuts and Oil Seeds | 1.24 (±0.50) | 1.21 * (±0.32) |
| Pulses and Legumes | 0.74 (±0.33) | 0.70 * (±0.36) |
| Sugars | 0.5 (±0.35) | 0.62 * (±0.40) |
| Vegetables | 3.48 (±1.66) | 3.24 * (±1.51) |
| FOOD GROUP LEVEL-2 | ||
| Refined Rice | 4.61 (±1.68) | 5.48 * (±1.97) |
| Refined Wheat | 0.14 (±0.10) | 0.13 * (±0.11) |
| Wholegrain Rice | 0.002 (±0.00) | 0.001 (±0.00) |
| Wholegrain Wheat | 0.56 (±0.64) | 0.5 (±0.59) |
| Wholegrains | 0.02 (±0.03) | 0.021 (±0.04) |
| Dried Condiments and Spices | 0.32 (±0.17) | 0.29 * (±0.15) |
| Fresh Condiments and Spices | 0.051 (±0.02) | 0.04 * (±0.02) |
| Animal Fats | 0.008 (±0.01) | 0.01 (±0.01) |
| Refined Plant Fat | 0.22 (±0.12) | 0.21 (±0.11) |
| Unrefined Plant Fat | 0.1 (±0.056) | 0.09 * (±0.05) |
| Dried Fruits | 0.02 (±0.026) | 0.01 * (±0.02) |
| Fresh Fruits | 1.13 (±1.06) | 1.11 (±1.12) |
| Eggs and Egg Products | 0.15 (±0.13) | 0.13 * (±0.13) |
| Non-Oily Fish | 0.03 (±0.039) | 0.02 * (±0.04) |
| Oily Fish | 1.72 (±1.38) | 1.52 * (±0.23) |
| Shellfish | 0.006 (±0.054) | 0.008 (±1.24) |
| Red Meat | 0.08 (±0.12) | 0.11 * (±0.011) |
| White Meat | 0.19 (±0.23) | 0.21 (±0.20) |
| Dried Milk and Milk Products | 0.009 (±0.015) | 0.006 * (±0.01) |
| Fresh Milk and Milk Products | 2.54 (±1.56) | 2.33 * (±1.45) |
| Nuts | 1.22 (±0.49) | 1.10 * (±0.42) |
| Oily Seeds | 0.01 (±0.008) | 0.02 (±0.011) |
| Dried Pulses and Legumes | 0.58 (±0.33) | 0.56 (±0.30) |
| Fresh Pulses and Legumes | 0.16 (±0.15) | 0.14 (±0.12) |
| Refined Sugars | 0.48 (±0.34) | 0.59 * (±0.39) |
| Unrefined Sugars | 0.02 (±0.027) | 0.02 (±0.027) |
| Leafy Vegetables | 0.27 (±0.25) | 0.24 (±0.21) |
| Other Vegetables | 1.83 (±0.93) | 1.70 * (±0.87) |
| Roots and Tubers | 1.38 (±0.75) | 1.29 * (±0.68) |
| FOOD GROUP LEVEL-3 | ||
| Baked | 0.08 (±0.12) | 0.07 (±0.10) |
| Boiled | 8.38 (±2.64) | 7.26 * (±2.37) |
| Fried | 0.77 (±0.50) | 0.79 * (±0.53) |
| Roasted | 1.25 (±0.67) | 1.35 * (±0.73) |
| Sauteed | 5.04 (±2.18) | 4.59 (±1.97) |
| Steamed | 1.36 (±0.75) | 1.27 * (±0.68) |
| Juice | 0.09 (±0.11) | 0.1 (±0.11) |
| Uncooked | 1.78 (±1.53) | 1.67 * (±1.47) |
| Absence of Fibrosis (≤8.4 kPa) (N = 543) (g/kg/day) ^ | Presence of Fibrosis (>8.5 Pa) N = 161 (g/kg/day) ^ | Beta Coefficient | S.E. | p-Value | |
|---|---|---|---|---|---|
| t-Test | Regression Analysis | ||||
| FOOD GROUP LEVEL-1 | |||||
| Cereals and Millets | 1.27 | 1.29 | 0.012 | 0.008 | 0.652 |
| Condiments and Spices | 0.16 | 0.11 | −0.086 | 0.011 | 0.092 |
| Fats and Edible Oils | 0.21 | 0.45 * | 0.061 | 0.031 | 0.021 * |
| Fruits | 1.21 | 0.20 | −0.071 | 0.022 | 0.782 |
| Meat, Fish, and Poultry | 1.10 | 1.17 | 0.051 | 0.041 | 0.075 |
| Milk and Milk Products | 1.21 | 1.15 | 0.015 | 0.011 | 0.148 |
| Nuts and Oil Seeds | 0.07 | 0.06 | −0.007 | 0.003 | 0.614 |
| Pulses and Legumes | 0.10 | 0.08 | −0.015 | 0.006 | 0.425 |
| Sugars | 0.26 | 0.29 | 0.021 | 0.016 | 0.512 |
| Vegetables | 1.21 | 1.18 | −0.062 | 0.046 | 0.091 |
| FOOD GROUP LEVEL-2 | |||||
| Refined Rice | 2.18 | 2.20 | 0.011 | 0.007 | 0.081 |
| Refined Wheat | 0.03 | 0.02 | 0.032 | 0.028 | 0.318 |
| Wholegrain Rice | 0.001 | 0.001 | −0.237 | 0.204 | 0.317 |
| Wholegrain Wheat | 0.22 | 0.21 | −0.021 | 0.011 | 0.421 |
| Wholegrains | 0.001 | 0.001 | −0.041 | 0.021 | 0.211 |
| Dried Condiments and Spices | 0.11 | 0.19 | −0.012 | 0.021 | 0.011 * |
| Fresh Condiments and Spices | 0.01 | 0.02 | −0.207 | 0.116 | 0.076 |
| Animal Fats | 0.002 | 0.006 | 0.113 | 0.108 | 0.137 |
| Refined Plant Fat | 0.12 | 0.11 | 0.001 | 0.021 | 0.719 |
| Unrefined Plant Fat | 0.04 | 0.02 | 0.023 | 0.014 | 0.241 |
| Dried Fruits | 0.005 | 0.003 | 0.002 | 0.013 | 0.712 |
| Fresh Fruits | 0.08 | 0.05 | 0.003 | 0.001 | 0.112 |
| Eggs and Egg Products | 0.08 | 0.10 | 0.003 | 0.006 | 0.182 |
| Non-Oily Fish | 0.001 | 0.002 | 0.004 | 0.011 | 0.641 |
| Oily Fish | 1.06 | 1.05 | 0.006 | 0.004 | 0.251 |
| Shellfish | 0.002 | 0.001 | −0.021 | 0.011 | 0.237 |
| Red Meat | 0.05 | 0.09 * | 0.061 | 0.033 | 0.031 * |
| White Meat | 0.09 | 0.10 | 0.005 | 0.003 | 0.214 |
| Dried Milk and Milk Products | 0.001 | 0.002 | 0.011 | 0.018 | 0.341 |
| Fresh Milk and Milk Products | 0.02 | 0.03 | 0.012 | 0.011 | 0.719 |
| Nuts | 0.07 | 0.06 | 0.003 | 0.001 | 0.733 |
| Oily Seeds | 0.004 | 0.003 | −0.512 | 0.319 | 0.202 |
| Dried Pulses and Legumes | 0.20 | 0.18 | −0.016 | 0.008 | 0.111 |
| Fresh Pulses and Legumes | 0.08 | 0.04 | −0.010 | 0.004 | 0.261 |
| Refined Sugars | 0.19 | 0.21 | 0.010 | 0.008 | 0.211 |
| Unrefined Sugars | 0.002 | 0.001 | −0.033 | 0.026 | 0.191 |
| Leafy Vegetables | 0.11 | 0.10 * | −0.081 | 0.032 | 0.029 * |
| Other Vegetables | 1.11 | 1.13 | −0.016 | 0.013 | 0.082 |
| Roots and Tubers | 1.05 | 1.08 | −0.008 | 0.002 | 0.282 |
| FOOD GROUP LEVEL-3 | |||||
| Baked | 0.01 | 0.02 | 0.002 | 0.001 | 0.818 |
| Boiled | 1.18 | 1.21 | −0.002 | 0.001 | 0.111 |
| Fried | 0.14 | 0.22 * | 0.013 | 0.002 | 0.031 * |
| Roasted | 1.15 | 1.18 | 0.001 | 0.001 | 0.457 |
| Sautéed | 1.19 | 1.18 | 0.001 | 0.003 | 0.614 |
| Steamed | 1.11 | 1.08 | −0.001 | 0.001 | 0.365 |
| Juice | 0.02 | 0.04 | 0.006 | 0.007 | 0.44 |
| Uncooked | 1.18 | 1.16 | −0.002 | 0.001 | 0.912 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vijay, A.; Al-Awadi, A.; Chalmers, J.; Balakumaran, L.; Grove, J.I.; Valdes, A.M.; Taylor, M.A.; Shenoy, K.T.; Aithal, G.P. Development of Food Group Tree-Based Analysis and Its Association with Non-Alcoholic Fatty Liver Disease (NAFLD) and Co-Morbidities in a South Indian Population: A Large Case-Control Study. Nutrients 2022, 14, 2808. https://doi.org/10.3390/nu14142808
Vijay A, Al-Awadi A, Chalmers J, Balakumaran L, Grove JI, Valdes AM, Taylor MA, Shenoy KT, Aithal GP. Development of Food Group Tree-Based Analysis and Its Association with Non-Alcoholic Fatty Liver Disease (NAFLD) and Co-Morbidities in a South Indian Population: A Large Case-Control Study. Nutrients. 2022; 14(14):2808. https://doi.org/10.3390/nu14142808
Chicago/Turabian StyleVijay, Amrita, Amina Al-Awadi, Jane Chalmers, Leena Balakumaran, Jane I. Grove, Ana M. Valdes, Moira A. Taylor, Kotacherry T. Shenoy, and Guruprasad P. Aithal. 2022. "Development of Food Group Tree-Based Analysis and Its Association with Non-Alcoholic Fatty Liver Disease (NAFLD) and Co-Morbidities in a South Indian Population: A Large Case-Control Study" Nutrients 14, no. 14: 2808. https://doi.org/10.3390/nu14142808
APA StyleVijay, A., Al-Awadi, A., Chalmers, J., Balakumaran, L., Grove, J. I., Valdes, A. M., Taylor, M. A., Shenoy, K. T., & Aithal, G. P. (2022). Development of Food Group Tree-Based Analysis and Its Association with Non-Alcoholic Fatty Liver Disease (NAFLD) and Co-Morbidities in a South Indian Population: A Large Case-Control Study. Nutrients, 14(14), 2808. https://doi.org/10.3390/nu14142808







