Food Selectivity and Its Implications Associated with Gastrointestinal Disorders in Children with Autism Spectrum Disorders
Abstract
1. Introduction
2. Materials and Methods
3. Eating Problems and Eating Behavior in Children with ASD
Food Neophobia in ASD
4. Sensory Sensitivity in Children with ASD
5. Food Selectivity “Peaking Eating/Fussy Eating” in Children with ASD
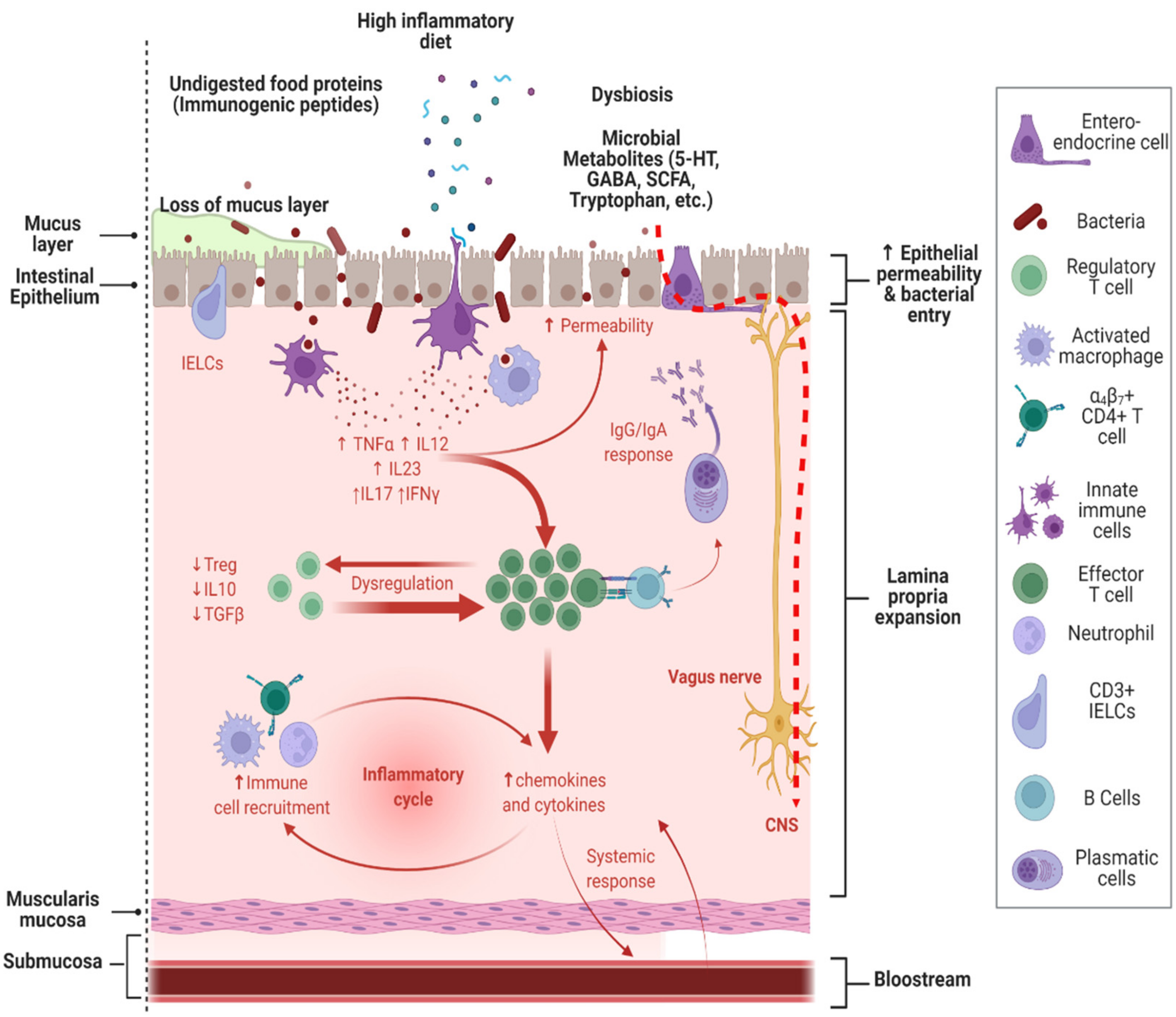
6. Food Selectivity and Its Relationship with Gastrointestinal Disorders in ASD
| Author | Sample Size (ASD/NT) | Prevalence of GIS | Most Prevalent GIS | Prevalence of FS | Possible Trigger of GIS | Study Type | Limitations |
|---|---|---|---|---|---|---|---|
| Ferguson et al., 2016 [78] | 120 children with ASD (average age 11.8) | Constipation (42.5%) and low abdominal pain (9.2%). | FS not evaluated | Not discussed | The study is based on an indirect questionnaire, without directly assessing GIS or food intake by phone (QPGS Rome III questionnaire). | No food intake nor FS is evaluated; no eating behavior is evaluated, and the subjectivity of self-administered questionnaires. | |
| Prosperi et al., 2017 [15] | 163 preschoolers with ASD | 28.5% | Constipation (22.1%) and low abdominal pain (7.4%) | 27.0% | A relationship between GIS and FS (12.27%) is found | Study based on indirect questionnaire, without direct assessment of GIS or food intake. | No food intake is evaluated; FS is considered only in one item from CBCL 1 ½-5 score. |
| Ferguson et al., 2019 [5] | 340 children with ASD (ages 2–18) | General prevalence not shown | Constipation (65%), stomachaches (47.9%), nausea (23.2%), and diarrhea (29.7%) | FS evaluated | Not discussed | Study based on indirect questionnaire, without direct assessment of GIS or food intake. | No FS is evaluated, no eating behavior is evaluated, and the subjectivity of self-administered questionnaires. |
| Babinska et al., 2020 [14] | 247 subjects with ASD (2–17 years) vs. 267 controls (p 0.000) | 88.7% of ASD subjects experienced GIS in the last 3 months, and 47.6% of ASD individuals present severe GIS | Constipation/hard stool consistency (61.9%), voluminous stools (51.0%), and bloating (49.4%) | High prevalence of FS (69.1%) compared to NT controls (37.1%), p = 0.000 | FS and mealtime problems have a significant correlation with the severity of GIS. Children who exhibit FS have more GIS | Study based on indirect questionnaire, without direct assessment of GIS or food intake. | The sample is not randomly selected. No medical evaluation of GIS is performed, and the subjectivity of self-administered questionnaires |
| Tomova et al., 2020 [73] | 46 children with ASD vs. 16 non-autistic children control | 89.4% of ASD children vs. 87.5% of non-autistic children (p = 0.838) experience GI symptoms | Constipation (28.9%), bloating (35.6%), and abdominal pain (35.6%). Differences are observed only in constipation in ASD (p = 0.014) | 57.7% of ASD children are “picky eaters” compared to controls (25%), p = 0.02. | FS modifies fecal microbiota composition. Children who exhibit FS have more GIS. | Study based on indirect questionnaire, without direct assessment of GIS. A food frequency questionnaire (FFQ) is used for dietary analysis. | A low number of participants, and subjectivity of self-administered questionnaires |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; Pan American Medical Editorial: Arlington, TX, USA, 2013. [Google Scholar]
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Robinson, C.R.; White, T.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill. Summ. 2018, 67, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Loomes, R.; Hull, L.; Mandy, W.P.L. What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Hodges, H.; Fealko, C.; Soares, N. Autism spectrum disorder: Definition, epidemiology, causes, and clinical evaluation. Transl. Pediatr. 2020, 9 (Suppl. 1), S55–S65. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.J.; Dovgan, K.; Takahashi, N.; Beversdorf, D.Q. The Relationship Among Gastrointestinal Symptoms, Problem Behaviors, and Internalizing Symptoms in Children and Adolescents with Autism Spectrum Disorder. Front. Psychiatry 2019, 10, 194. [Google Scholar] [CrossRef] [PubMed]
- Madra, M.; Ringel, R.; Margolis, K.G. Gastrointestinal Issues and Autism Spectrum Disorder. Child Adolesc. Psychiatr. Clin. N. Am. 2019, 29, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Lefter, R.; Ciobica, A.; Timofte, D.; Stanciu, C.; Trifan, A. A Descriptive Review on the Prevalence of Gastrointestinal Disturbances and Their Multiple Associations in Autism Spectrum Disorder. Medicina 2019, 56, 11. [Google Scholar] [CrossRef] [PubMed]
- Leader, G.; Tuohy, E.; Chen, J.L.; Mannion, A.; Gilroy, S.P. Feeding Problems, Gastrointestinal Symptoms, Challenging Behavior and Sensory Issues in Children and Adolescents with Autism Spectrum Disorder. J. Autism Dev. Disord. 2020, 50, 1401–1410. [Google Scholar] [CrossRef]
- Sharp, W.G.; Postorino, V.; McCracken, C.E.; Berry, R.C.; Criado, K.K.; Burrell, T.L.; Scahill, L. Dietary Intake, Nutrient Status, and Growth Parameters in Children with Autism Spectrum Disorder and Severe Food Selectivity: An Electronic Medical Record Review. J. Acad. Nutr. Diet. 2018, 118, 1943–1950. [Google Scholar] [CrossRef]
- Keen, D.V. Childhood autism, feeding problems and failure to thrive in early infancy. Eur. Child Adolesc. Psychiatry 2008, 17, 209–216. [Google Scholar] [CrossRef]
- Cherif, L.; Boudabous, J.; Khemekhem, K.; Mkawer, S.; Ayadi, H.; Moalla, Y. Feeding Problems in Children with Autism Spectrum Disorders. J. Fam. Med. 2018, 1, 30–39. [Google Scholar] [CrossRef]
- Fields, V.L.; Soke, G.N.; Reynolds, A.; Tian, L.H.; Wiggins, L.; Maenner, M.; DiGuiseppi, C.; Kral, T.V.; Hightshoe, K.; Schieve, L.A. Pica, Autism, and Other Disabilities. Pediatrics 2021, 147, e20200462. [Google Scholar] [CrossRef]
- Mayes, S.D.; Zickgraf, H. Atypical eating behaviors in children and adolescents with autism, ADHD, other disorders, and typical development. Res. Autism Spectr. Disord. 2019, 64, 76–83. [Google Scholar] [CrossRef]
- Inoue, T.; Otani, R.; Iguchi, T.; Ishii, R.; Uchida, S.; Okada, A.; Kitayama, S.; Koyanagi, K.; Suzuki, Y.; Suzuki, Y.; et al. Prevalence of autism spectrum disorder and autistic traits in children with anorexia nervosa and avoidant/restrictive food intake disorder. Biopsychosoc. Med. 2021, 15, 9. [Google Scholar] [CrossRef]
- Babinska, K.; Celusakova, H.; Belica, I.; Szapuova, Z.; Waczulikova, I.; Nemcsicsova, D.; Tomova, A.; Ostatnikova, D. Gastrointestinal Symptoms and Feeding Problems and Their Associations with Dietary Interventions, Food Supplement Use, and Behavioral Characteristics in a Sample of Children and Adolescents with Autism Spectrum Disorders. Int. J. Environ. Res. Public Health 2020, 17, 6372. [Google Scholar] [CrossRef]
- Prosperi, M.; Santocchi, E.; Balboni, G.; Narzisi, A.; Bozza, M.; Fulceri, F.; Apicella, F.; Igliozzi, R.; Cosenza, A.; Tancredi, R.; et al. Behavioral Phenotype of ASD Preschoolers with Gastrointestinal Symptoms or Food Selectivity. J. Autism Dev. Disord. 2017, 47, 3574–3588. [Google Scholar] [CrossRef]
- Huke, V.; Turk, J.; Saeidi, S.; Kent, A.; Morgan, J.F. Autism Spectrum Disorders in Eating Disorder Populations: A Systematic Review. Eur. Eat. Disord. Rev. 2013, 21, 345–351. [Google Scholar] [CrossRef]
- Sedgewick, F.; Kerr-Gaffney, J.; Leppanen, J.; Tchanturia, K. Anorexia Nervosa, Autism, and the ADOS: How Appropriate Is the New Algorithm in Identifying Cases? Front. Psychiatry 2019, 10, 507. [Google Scholar] [CrossRef]
- Lord, C.; Brugha, T.S.; Charman, T.; Cusack, J.; Dumas, G.; Frazier, T.; Jones, E.J.H.; Jones, R.M.; Pickles, A.; State, M.W.; et al. Autism spectrum disorder. Nat. Rev. Dis. Prim. 2020, 6, 5. [Google Scholar] [CrossRef]
- Cermak, S.A.; Curtin, C.; Bandini, L.G. Food Selectivity and Sensory Sensitivity in Children with Autism Spectrum Disorders. J. Am. Diet. Assoc. 2010, 110, 238–246. [Google Scholar] [CrossRef]
- Bandini, L.G.; Anderson, S.E.; Curtin, C.; Cermak, S.; Evans, E.W.; Scampini, R.; Maslin, M.; Must, A. Food Selectivity in Children with Autism Spectrum Disorders and Typically Developing Children. J. Pediatr. 2010, 157, 259–264. [Google Scholar] [CrossRef]
- Wallace, G.; Llewellyn, C.; Fildes, A.; Ronald, A. Autism spectrum disorder and food neophobia: Clinical and subclinical links. Am. J. Clin. Nutr. 2018, 108, 701–707. [Google Scholar] [CrossRef]
- Suarez, M.A.; Nelson, N.W.; Curtis, A.B. Longitudinal follow-up of factors associated with food selectivity in children with autism spectrum disorders. Autism 2013, 18, 924–932. [Google Scholar] [CrossRef]
- Zimmer, M.H.; Hart, L.C.; Manning-Courtney, P.; Murray, D.S.; Bing, N.M.; Summer, S. Food Variety as a Predictor of Nutritional Status Among Children with Autism. J. Autism Dev. Disord. 2011, 42, 549–556. [Google Scholar] [CrossRef]
- Chawner, L.R.; Blundell-Birtill, P.; Hetherington, M.M. Interventions for Increasing Acceptance of New Foods Among Children and Adults with Developmental Disorders: A Systematic Review. J. Autism Dev. Disord. 2019, 49, 3504–3525. [Google Scholar] [CrossRef]
- Łoboś, P.; Januszewicz, A. Food neophobia in children. Neofobia żywieniowa u dzieci. Pediatr. Endocrinol. Diabetes Metab. 2019, 25, 150–154. [Google Scholar] [CrossRef]
- Nicklaus, S.; Boggio, V.; Chabanet, C.; Issanchou, S. A prospective study of food variety seeking in childhood, adolescence and early adult life. Appetite 2005, 44, 289–297. [Google Scholar] [CrossRef]
- Chung, L.M.Y.; Law, Q.P.S.; Fong, S.S.M. Using Physical Food Transformation to Enhance the Sensory Approval of Children with Autism Spectrum Disorders for Consuming Fruits and Vegetables. J. Altern. Complement. Med. 2020, 26, 1074–1079. [Google Scholar] [CrossRef]
- Marlow, C.S.; Forestell, C.A. The effect of parental food neophobia on children’s fruit and vegetable consumption: A serial mediation model. Appetite 2022, 172, 105942. [Google Scholar] [CrossRef]
- Baraskewich, J.; von Ranson, K.M.; McCrimmon, A.; McMorris, C.A. Feeding and eating problems in children and adolescents with autism: A scoping review. Autism 2021, 25, 1505–1519. [Google Scholar] [CrossRef]
- Coulthard, H.; Thakker, D. Enjoyment of Tactile Play Is Associated with Lower Food Neophobia in Preschool Children. J. Acad. Nutr. Diet. 2015, 115, 1134–1140. [Google Scholar] [CrossRef]
- Leekam, S.R.; Nieto, C.; Libby, S.J.; Wing, L.; Gould, J. Describing the Sensory Abnormalities of Children and Adults with Autism. J. Autism Dev. Disord. 2006, 37, 894–910. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sasson, A.; Hen, L.; Fluss, R.; Cermak, S.A.; Engel-Yeger, B.; Gal, E. A Meta-Analysis of Sensory Modulation Symptoms in Individuals with Autism Spectrum Disorders. J. Autism Dev. Disord. 2008, 39, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Imamura, A.; Yamamoto, N.; Kanegae, S.; Ozawa, H.; Iwanaga, R. Atypical Sensory Characteristics in Autism Spectrum Disorders. In Autism Spectrum Disorders; Grabrucker, A.M., Ed.; Exon Publications: Brisbane, Australia, 2021; Chapter 5. Available online: https://www.ncbi.nlm.nih.gov/books/NBK573615/doi:10.36255/exonpublications.autismspectrumdisorders.2021.atypicalsensorycharacteristics (accessed on 25 May 2022). [CrossRef]
- Smith, B.; Rogers, S.L.; Blissett, J.; Ludlow, A.K. The relationship between sensory sensitivity, food fussiness and food preferences in children with neurodevelopmental disorders. Appetite 2020, 150, 104643. [Google Scholar] [CrossRef] [PubMed]
- Dellapiazza, F.; Michelon, C.; Oreve, M.-J.; Robel, L.; Schoenberger, M.; Chatel, C.; Vesperini, S.; Maffre, T.; Schmidt, R.; Blanc, N.; et al. The Impact of Atypical Sensory Processing on Adaptive Functioning and Maladaptive Behaviors in Autism Spectrum Disorder During Childhood: Results from the ELENA Cohort. J. Autism Dev. Disord. 2020, 50, 2142–2152. [Google Scholar] [CrossRef]
- Dellapiazza, F.; Michelon, C.; Vernhet, C.; Muratori, F.; Blanc, N.; Picot, M.-C.; Baghdadli, A.; for ELENA study group. Sensory processing related to attention in children with ASD, ADHD, or typical development: Results from the ELENA cohort. Eur. Child Adolesc. Psychiatry 2021, 30, 283–291. [Google Scholar] [CrossRef]
- Thye, M.D.; Bednarz, H.M.; Herringshaw, A.J.; Sartin, E.B.; Kana, R.K. The impact of atypical sensory processing on social impairments in autism spectrum disorder. Dev. Cogn. Neurosci. 2018, 29, 151–167. [Google Scholar] [CrossRef]
- Smith, B.; Rogers, S.L.; Blissett, J.; Ludlow, A.K. The role of sensory sensitivity in predicting food selectivity and food preferences in children with Tourette syndrome. Appetite 2019, 135, 131–136. [Google Scholar] [CrossRef]
- Little, L.M.; Dean, E.; Tomchek, S.; Dunn, W. Sensory Processing Patterns in Autism, Attention Deficit Hyperactivity Disorder, and Typical Development. Phys. Occup. Ther. Pediatr. 2018, 38, 243–254. [Google Scholar] [CrossRef]
- Malhi, P.; Saini, S.; Bharti, B.; Attri, S.; Sankhyan, N. Sensory Processing Dysfunction and Mealtime Behavior Problems in Children with Autism. Indian Pediatr. 2021, 58, 842–845. [Google Scholar] [CrossRef]
- Zobel-Lachiusa, J.; Andrianopoulos, M.V.; Mailloux, Z.; Cermak, S.A. Sensory Differences and Mealtime Behavior in Children with Autism. Am. J. Occup. Ther. 2015, 69, 6905185050. [Google Scholar] [CrossRef]
- Miller, L.J.; Anzalone, M.E.; Lane, S.J.; Cermak, S.A.; Osten, E.T. Concept Evolution in Sensory Integration: A Proposed Nosology for Diagnosis. Am. J. Occup. Ther. 2007, 61, 135–140. [Google Scholar] [CrossRef]
- Margari, L.; Marzulli, L.; Gabellone, A.; de Giambattista, C. Eating and Mealtime Behaviors in Patients with Autism Spectrum Disorder: Current Perspectives. Neuropsychiatr. Dis. Treat. 2020, 16, 2083–2102. [Google Scholar] [CrossRef]
- Page, S.D.; Souders, M.C.; Kral, T.V.E.; Chao, A.M.; Pinto-Martin, J. Correlates of Feeding Difficulties Among Children with Autism Spectrum Disorder: A Systematic Review. J. Autism Dev. Disord. 2021, 52, 255–274. [Google Scholar] [CrossRef]
- Chistol, L.T.; Bandini, L.G.; Must, A.; Phillips, S.; Cermak, S.A.; Curtin, C. Sensory sensitivity and food selectivity in children with autism spectrum disorder. J. Autism Dev. Disord. 2018, 48, 583–591. [Google Scholar] [CrossRef]
- Johnson, C.R.; Turner, K.; Stewart, P.A.; Schmidt, B.; Shui, A.; Macklin, E.; Reynolds, A.; James, J.; Johnson, S.L.; Courtney, P.M.; et al. Relationships Between Feeding Problems, Behavioral Characteristics and Nutritional Quality in Children with ASD. J. Autism Dev. Disord. 2014, 44, 2175–2184. [Google Scholar] [CrossRef]
- Dudova, I.; Vodička, J.; Havlovicova, M.; Sedlacek, Z.; Urbanek, T.; Hrdlicka, M. Odor detection threshold, but not odor identification, is impaired in children with autism. Eur. Child Adolesc. Psychiatry 2011, 20, 333–340. [Google Scholar] [CrossRef][Green Version]
- Sena, A.D.; Santos, G.; Santos, C.S.; Santos, T.S.; Pereira, G.B.; Alves, T.P.; Milagres, M.P. Sensory threshold evaluation for sweet taste in childhood autism. J. Prof. Nurs. Online 2019, 13. [Google Scholar] [CrossRef][Green Version]
- Bennetto, L.; Kuschner, E.S.; Hyman, S.L. Olfaction and Taste Processing in Autism. Biol. Psychiatry 2007, 62, 1015–1021. [Google Scholar] [CrossRef]
- Kral, T.V.E.; Souders, M.C.; Tompkins, V.H.; Remiker, A.M.; Eriksen, W.T.; Pinto-Martin, J.A. Child Eating Behaviors and Caregiver Feeding Practices in Children with Autism Spectrum Disorders. Public Health Nurs. 2015, 32, 488–497. [Google Scholar] [CrossRef]
- Muratori, F.; Tonacci, A.; Billeci, L.; Catalucci, T.; Igliozzi, R.; Calderoni, S.; Narzisi, A. Olfactory Processing in Male Children with Autism: Atypical Odor Threshold and Identification. J. Autism Dev. Disord. 2017, 47, 3243–3251. [Google Scholar] [CrossRef]
- Thomas, J.J.; Lawson, E.; Micali, N.; Misra, M.; Deckersbach, T.; Eddy, K.T. Avoidant/Restrictive Food Intake Disorder: A Three-Dimensional Model of Neurobiology with Implications for Etiology and Treatment. Curr. Psychiatry Rep. 2017, 19, 54. [Google Scholar] [CrossRef]
- Dovey, T.M.; Kumari, V.; Blissett, J. Eating behaviour, behavioural problems and sensory profiles of children with avoidant/restrictive food intake disorder (ARFID), autistic spectrum disorders or picky eating: Same or different? Eur. Psychiatry 2019, 61, 56–62. [Google Scholar] [CrossRef]
- Kanner, L. Autistic disturbances of affective contact. Acta Paedopsychiatr. 1968, 35, 100–136. [Google Scholar]
- Zulkifli, M.N.; Kadar, M.; Fenech, M.; Hamzaid, N.H. Interrelation of food selectivity, oral sensory sensitivity, and nutri-ent intake in children with autism spectrum disorder: A scoping review. Res. Autism Spectr. Disord. 2022, 93, 101928. [Google Scholar] [CrossRef]
- Martins, Y.; Young, R.L.; Robson, D.C. Feeding and Eating Behaviors in Children with Autism and Typically Developing Children. J. Autism Dev. Disord. 2008, 38, 1878–1887. [Google Scholar] [CrossRef]
- Hubbard, K.L.; Anderson, S.E.; Curtin, C.; Must, A.; Bandini, L.G. A Comparison of Food Refusal Related to Characteristics of Food in Children with Autism Spectrum Disorder and Typically Developing Children. J. Acad. Nutr. Diet. 2014, 114, 1981–1987. [Google Scholar] [CrossRef]
- Molina-López, J.; Leiva-García, B.; Planells, E.; Planells, P. Food selectivity, nutritional inadequacies, and mealtime behavioral problems in children with autism spectrum disorder compared to neurotypical children. Int. J. Eat. Disord. 2021, 54, 2155–2166. [Google Scholar] [CrossRef]
- Hartman, J.S.; Silver, A.H. Nutritional Rickets Due to Severe Food Selectivity in Autism Spectrum Disorder. J. Dev. Behav. Pediatr. 2021, 42, 66–72. [Google Scholar] [CrossRef]
- Chaidez, V.; Hansen, R.L.; Hertz-Picciotto, I. Gastrointestinal Problems in Children with Autism, Developmental Delays or Typical Development. J. Autism Dev. Disord. 2014, 44, 1117–1127. [Google Scholar] [CrossRef]
- Hyman, S.L.; Stewart, P.A.; Schmidt, B.; Cain, U.; Lemcke, N.; Foley, J.T.; Peck, R.; Clemons, T.; Reynolds, A.; Johnson, C.; et al. Nutrient Intake from Food in Children with Autism. Pediatrics 2012, 130 (Suppl. 2), S145–S153. [Google Scholar] [CrossRef]
- Marí-Bauset, S.; Llopis-González, A.; Zazpe, I.; Marí-Sanchis, A.; Suárez-Varela, M.M. Comparison of nutritional status between children with autism spectrum disorder and typically developing children in the Mediterranean Region (Valencia, Spain). Autism 2017, 21, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Leader, G.; Forde, J.; Naughton, K.; Maher, L.; Arndt, S.; Mannion, A. Relationships among gastrointestinal symptoms, sleep problems, challenging behaviour, comorbid psychopathology and autism spectrum disorder symptoms in children and adolescents with 15q duplication syndrome. J. Intellect. Disabil. Res. 2021, 65, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Holingue, C.; Newill, C.; Lee, L.-C.; Pasricha, P.J.; Fallin, M.D. Gastrointestinal symptoms in autism spectrum disorder: A review of the literature on ascertainment and prevalence. Autism Res. 2018, 11, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.A.; Micali, N.; Moll, H.A.; van Berckelaer-Onnes, I.; Hillegers, M.; Jansen, P.W. The role of food selectivity in the association between child autistic traits and constipation. Int. J. Eat. Disord. 2021, 54, 981–985. [Google Scholar] [CrossRef]
- Postorino, V.; Sanges, V.; Giovagnoli, G.; Fatta, L.M.; De Peppo, L.; Armando, M.; Vicari, S.; Mazzone, L. Clinical differences in children with autism spectrum disorder with and without food selectivity. Appetite 2015, 92, 126–132. [Google Scholar] [CrossRef]
- Rose, D.R.; Yang, H.; Serena, G.; Sturgeon, C.; Ma, B.; Careaga, M.; Hughes, H.; Angkustsiri, K.; Rose, M.; Hertz-Picciotto, I.; et al. Differential immune responses and microbiota profiles in children with autism spectrum disorders and co-morbid gastrointestinal symptoms. Brain, Behav. Immun. 2018, 70, 354–368. [Google Scholar] [CrossRef]
- Ristori, M.V.; Quagliariello, A.; Reddel, S.; Ianiro, G.; Vicari, S.; Gasbarrini, A.; Putignani, L. Autism, Gastrointestinal Symptoms and Modulation of Gut Microbiota by Nutritional Interventions. Nutrients 2019, 11, 2812. [Google Scholar] [CrossRef]
- De Magistris, L.; Picardi, A.; Siniscalco, D.; Riccio, M.P.; Sapone, A.; Cariello, R.; Abbadessa, S.; Medici, N.; Lammers, K.M.; Schiraldi, C.; et al. Antibodies against Food Antigens in Patients with Autistic Spectrum Disorders. BioMed Res. Int. 2013, 2013, 729349. [Google Scholar] [CrossRef]
- Barbaro, M.R.; Cremon, C.; Wrona, D.; Fuschi, D.; Marasco, G.; Stanghellini, V.; Barbara, G. Non-Celiac Gluten Sensitivity in the Context of Functional Gastrointestinal Disorders. Nutrients 2020, 12, 3735. [Google Scholar] [CrossRef]
- Quan, J.; Panaccione, N.; Jeong, J.; Underwood, F.E.; Coward, S.; Windsor, J.W.; Ronksley, P.E.; Gidrewicz, D.; Debruyn, J.; Turner, J.M.; et al. Association Between Celiac Disease and Autism Spectrum Disorder: A Systematic Review. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 704–711. [Google Scholar] [CrossRef]
- Tomova, A.; Soltys, K.; Kemenyova, P.; Karhanek, M.; Babinska, K. The Influence of Food Intake Specificity in Children with Autism on Gut Microbiota. Int. J. Mol. Sci. 2020, 21, 2797. [Google Scholar] [CrossRef]
- Martínez-González, A.E.; Andreo-Martínez, P. The Role of Gut Microbiota in Gastrointestinal Symptoms of Children with ASD. Medicina 2019, 55, 408. [Google Scholar] [CrossRef]
- Tomova, A.; Husarova, V.; Lakatosova, S.; Bakos, J.; Vlkova, B.; Babinska, K.; Ostatnikova, D. Gastrointestinal microbiota in children with autism in Slovakia. Physiol. Behav. 2015, 138, 179–187. [Google Scholar] [CrossRef]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef]
- Luna, R.A.; Oezguen, N.; Balderas, M.; Venkatachalam, A.; Runge, J.K.; Versalovic, J.; Veenstra-VanderWeele, J.; Anderson, G.M.; Savidge, T.; Williams, K.C. Distinct Microbiome-Neuroimmune Signatures Correlate with Functional Abdominal Pain in Children with Autism Spectrum Disorder. Cell. Mol. Gastroenterol. Hepatol. 2016, 3, 218–230. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Marler, S.; Altstein, L.L.; Lee, E.B.; Mazurek, M.O.; McLaughlin, A.; Macklin, E.A.; McDonnell, E.; Davis, D.J.; Belenchia, A.M.; et al. Associations between cytokines, endocrine stress response, and gastrointestinal symptoms in autism spectrum disorder. Brain, Behav. Immun. 2016, 58, 57–62. [Google Scholar] [CrossRef]
- Buie, T.; Campbell, D.B.; Fuchs, G.J., 3rd; Furuta, G.T.; Levy, J.; Vandewater, J.; Whitaker, A.H.; Atkins, D.; Bauman, M.L.; Beaudet, A.L.; et al. Evaluation, Diagnosis, and Treatment of Gastrointestinal Disorders in Individuals with ASDs: A Consensus Report. Pediatrics 2010, 125 (Suppl. 1), S1–S18. [Google Scholar] [CrossRef]
- Heifert, T.A.; Susi, A.; Hisle-Gorman, E.; Erdie-Lalena, C.R.; Gorman, G.; Min, S.B.; Nylund, C.M. Feeding Disorders in Children with Autism Spectrum Disorders Are Associated with Eosinophilic Esophagitis. J. Pediatr. Gastroenterol. Nutr. 2016, 63, e69–e73. [Google Scholar] [CrossRef]
- Sharp, W.G.; Burrell, T.L.; Berry, R.C.; Stubbs, K.H.; McCracken, C.E.; Gillespie, S.E.; Scahill, L. The Autism Managing Eating Aversions and Limited Variety Plan vs Parent Education: A Randomized Clinical Trial. J. Pediatr. 2019, 211, 185–192.e1. [Google Scholar] [CrossRef]
- Peterson, K.M.; Piazza, C.C.; Ibañez, V.F.; Fisher, W.W. Randomized controlled trial of an applied behavior analytic intervention for food selectivity in children with autism spectrum disorder. J. Appl. Behav. Anal. 2019, 52, 895–917. [Google Scholar] [CrossRef]
- Galpin, J.; Osman, L.; Paramore, C. Sensory Snack Time: A School-Based Intervention Addressing Food Selectivity in Autistic Children. Front. Educ. 2018, 3. [Google Scholar] [CrossRef]
- Ghalichi, F.; Ghaemmaghami, J.; Malek, A.; Ostadrahimi, A. Effect of gluten free diet on gastrointestinal and behavioral indices for children with autism spectrum disorders: A randomized clinical trial. World J. Pediatr. 2016, 12, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Thorsteinsdottir, S.; Njardvik, U.; Bjarnason, R.; Haraldsson, H.; Olafsdottir, A.S. Taste education—A food-based intervention in a school setting, focusing on children with and without neurodevelopmental disorders and their families. A randomized controlled trial. Appetite 2021, 167, 105623. [Google Scholar] [CrossRef] [PubMed]
- Santocchi, E.; Guiducci, L.; Prosperi, M.; Calderoni, S.; Gaggini, M.; Apicella, F.; Tancredi, R.; Billeci, L.; Mastromarino, P.; Grossi, E.; et al. Effects of Probiotic Supplementation on Gastrointestinal, Sensory and Core Symptoms in Autism Spectrum Disorders: A Randomized Controlled Trial. Front. Psychiatry 2020, 11. [Google Scholar] [CrossRef]
- Johnson, C.R.; Foldes, E.; DeMand, A.; Brooks, M. Behavioral Parent Training to Address Feeding Problems in Children with Autism Spectrum Disorder: A Pilot Trial. J. Dev. Phys. Disabil. 2015, 27, 591–607. [Google Scholar] [CrossRef]
- Johnson, C.R.; Brown, K.; Hyman, S.L.; Brooks, M.M.; Aponte, C.; Levato, L.; Schmidt, B.; Evans, V.; Huo, Z.; Bendixen, R.; et al. Parent Training for Feeding Problems in Children with Autism Spectrum Disorder: Initial Randomized Trial. J. Pediatr. Psychol. 2019, 44, 164–175. [Google Scholar] [CrossRef]
- González-Domenech, P.J.; Atienza, F.D.; Pablos, C.G.; Soto, M.L.F.; Martínez-Ortega, J.M.; Gutiérrez-Rojas, L. Influence of a Combined Gluten-Free and Casein-Free Diet on Behavior Disorders in Children and Adolescents Diagnosed with Autism Spectrum Disorder: A 12-Month Follow-Up Clinical Trial. J. Autism Dev. Disord. 2020, 50, 935–948. [Google Scholar] [CrossRef]
- Kim, S.Y.; Chung, K.-M.; Jung, S. Effects of repeated food exposure on increasing vegetable consumption in preschool children with autism spectrum disorder. Res. Autism Spectr. Disord. 2018, 47, 26–35. [Google Scholar] [CrossRef]
| Eating Problem | Prevalence ASD/NT (%) | Author | 95% CI/p-Value |
|---|---|---|---|
| Food neophobia | 58–67%/57.89% | Cherif et al., 2018 [11] | 0.008 |
| Pica | 23.2%/8.4% | Fields et al., 2021 [12] | 6.7 (5.1–8.8) |
| 11.8%/0% | Mayes and Zickgraf, 2019 [13] | NC | |
| Food selectivity (fussy eating) | 12.5%/NR | Inoue et al., 2021 [14] | 0.778 |
| 69.1%/37.1% | Babinska et al., 2020 [15] | 0.0001 | |
| 27.0%/NC | Prosperi et al., 2017 [16] | N/R | |
| 22.8%/3.5% | Cherif et al., 2018 [11] | 0.008 | |
| Anorexia nervosa | 22.9%/1% | Huke et al., 2013 [17] | NC |
| 23.65%/NC | Sedgewick et al., 2019 [18] | NC | |
| 16.3%/NR | Inoue et al., 2021 [14] | 0.778 |
| Classification | Definition | Questionary |
|---|---|---|
| Food refusal | There are a few foods that children with or without ASD will not consume, for preference or sensory reasons | Modified FFQ |
| Limited food repertoire | Foods consumed in 3 days, accepted for sensory reasons | 3-day food diary, based on the NDSR |
| Severe food selectivity restricted to a single type of food | Foods consumed more than 5 times per day selectively | Modified FFQ |
| Study | Total (N) | ASD (Group) | Age (Years) | Eating Problem | Intervention | Time (Weeks) | Control Group (TD) | Food Selectivity (95% CI)/Value p | Disruptive Mealtime Behaviors (95% CI)/Value p |
|---|---|---|---|---|---|---|---|---|---|
| Sharp et al., 2019 [80] | 38 | 38 | 3–8 | Moderate food selectivity | MEAL & PEP | 16 | NOT | −2.76 to −0.25 | −6.16 to −0.69 |
| Peterson et al., 2019 [81] | 6 | 3 | 3–5 | Mealtime behaviors | BAI | 24 | YES | N/A | 0.001 |
| Galpin et al., 2018 [82] | 19 | 19 | 4–10 | Feeding problems | SSN | 12 | NOT | 0.001 | 0.13 |
| Ghalichi et al., 2016 [83] | 76 | 76 | 4–16 | Stereotyped behaviors and social interaction | Gluten-free diet and regular diet | 6 | NOT | 0.001 | 0.001 |
| Thorsteinsdottir et al., 2021 [84] | 81 | 33 | 8–12 | Fussy eating | Taste education | 7 | YES | 1.37 to 2.26 | N/A |
| El-Meany et al., 2022 [85] | 50 | 25 | ≥18 | Feeding problems | Virgin coconut oil | 12 | YES | 0.001 | N/A |
| Santocchi et al., 2020 [86] | 85 | 85 | 2–6 | GI symptoms by food selectivity | DSF | 20 | YES | −0.68 to + 0.08 | N/A |
| Johnson et al., 2015 [87] | 14 | 14 | 2–7 | Feeding problems | PT-F | 16 | NOT | 0.05 | N/A |
| Johnson et al., 2018 [88] | 42 | 21 | 2–11 | Feeding and mealtime problems | PT-F | 20 | YES | 0.01 | 0.03 |
| Gonzalez-Domenech et al., 2020 [89] | 37 | 17 | 2–18 | Behavior disorders | GFCF | 24 | YES | N/A | 0.07 |
| Kim et al., 2018 [90] | 27 | 13 | 2–5 | Food selectivity | Preventive program (exposure to vegetables) | 24 | YES | 0.47 | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenzuela-Zamora, A.F.; Ramírez-Valenzuela, D.G.; Ramos-Jiménez, A. Food Selectivity and Its Implications Associated with Gastrointestinal Disorders in Children with Autism Spectrum Disorders. Nutrients 2022, 14, 2660. https://doi.org/10.3390/nu14132660
Valenzuela-Zamora AF, Ramírez-Valenzuela DG, Ramos-Jiménez A. Food Selectivity and Its Implications Associated with Gastrointestinal Disorders in Children with Autism Spectrum Disorders. Nutrients. 2022; 14(13):2660. https://doi.org/10.3390/nu14132660
Chicago/Turabian StyleValenzuela-Zamora, Angel F., David G. Ramírez-Valenzuela, and Arnulfo Ramos-Jiménez. 2022. "Food Selectivity and Its Implications Associated with Gastrointestinal Disorders in Children with Autism Spectrum Disorders" Nutrients 14, no. 13: 2660. https://doi.org/10.3390/nu14132660
APA StyleValenzuela-Zamora, A. F., Ramírez-Valenzuela, D. G., & Ramos-Jiménez, A. (2022). Food Selectivity and Its Implications Associated with Gastrointestinal Disorders in Children with Autism Spectrum Disorders. Nutrients, 14(13), 2660. https://doi.org/10.3390/nu14132660






