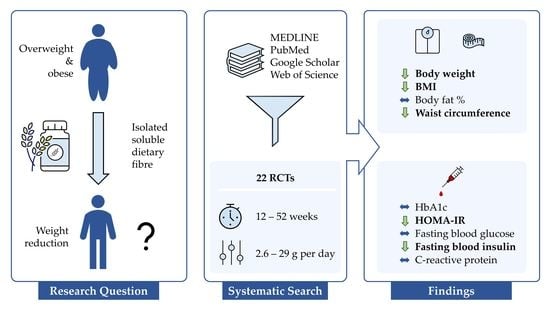
Prolonged Isolated Soluble Dietary Fibre Supplementation in Overweight and Obese Patients: A Systematic Review with Meta-Analysis of Randomised Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Eligibility
2.3. Data Extraction and Risk of Bias Assessment
- Report: title, author, publication journal, Digital Object Identifier (DOI);
- Study: design, intention-to-treat or per-protocol analysis;
- Participants: group size, BMI range, age, sex distribution;
- Intervention: DF, placebo, dose, intervention duration, mode of delivery, background treatment;
- Outcome: mean and standard deviation of the outcomes at baseline and end of the intervention.
2.4. Data Synthesis and Analysis
3. Results
3.1. Study Selection

3.2. Study and Participant Characteristics
| Author, Year | Intervention, Dose | Duration [Weeks] | Study Population BMI [kg/m2] Age [Years] | Comorbidities | Sample Size (Intervention/Control); % Female | Outcome Measures Evaluated | Additional Treatment | Country | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BW | BM | BF | WC | Hb | HO | BG | BI | CR | ||||||||
| Bomhof, 2019 [52] | FOS orally, 8–16 g/day | 36 | BMI ≥ 25 (Caucasian) or 23 (Asians) Age ≥ 18 | NASH | n = 14 (8/6); 42.8% female | x | x | x | x | x | x | U | Canada | |||
| Bongartz, 2022 [53] | Flaxseed mucilage powder in water, 5.1 g/day | 12 | BMI 25–35 Age 18–65 | NS | n = 67 (34/33); 70.1% female | x | x | x | x | x | x | ERD | Germany | |||
| Flaxseed mucilage powder in water, 2.6 g/day | 12 | BMI 25–35 Age 18–65 | NS | n = 66 (33/33); 65.2% female | x | x | x | x | x | x | ERD | Germany | ||||
| Calikoglu, 2021 [54] | Inulin + FOS powder in yogurt, 10 g/day | 26 | BMI ≥ 35 Age NR | NS | n = 30 (14/16); NR females | x | x | x | x | x | x | BS | Turkey | |||
| Cicero, 2010 [51] | Guar gum orally, 7 g/day | 26 | BMI 25–30 Age 50–70 | Metabolic syndrome | n = 91 (46/45); 52.8% female | x | x | x | x | x | x | ERD | Italy | |||
| Dewulf, 2013 [55] | Inulin + FOS powder in fluid, 16 g/day | 12 | BMI ≥ 30 Age 18–65 | NS | n = 30 (15/15); 100% female | x | x | x | x | x | x | x | x | U | Belgium | |
| Genta, 2009 [56] | FOS syrup, 0.14 g/kg BW/day | 17 | BMI ≥ 30 Age 31–49 | Dyslipidemia, constipation | n = 35 (20/15); 100% female | x | x | x | x | x | x | ERD, no FOS rich food | Argentina | |||
| Grube, 2013 [57] | Litramine IQP G-002AS tablets, 3 g/day | 12 | BMI 25–35 Age 18–60 | NS | n = 118 (59/59); 75.6% females | x | x | x | x | ERD, PA | Germany | |||||
| Grunberger, 2007 [58] | α-Cyclodextrin tablets, 6 g/day | 12 | BMI ≥ 30 Age ≥ 30 | T2D | n = 47 (20/27); 53.2% female | x | x | x | x | U | USA | |||||
| Guérin-Deremaux, 2011 [59,60] | Nutriose 2 powder in fruit juice, 29 g/day | 12 | BMI 24–28 Age 20–35 | NS | n = 113 (57/56); 0% female | x | x | U | China | |||||||
| Hassan, 2020 [61] | Inulin + RS pasta, 15 g/100 g pasta/day | 12 | BMI ≥30 Age 43.4 ± 10.3 1 | NS | n = 30 (15/15); 80.0% female | x | x | x | x | x | ERD | Italy | ||||
| Hess, 2020 [62,63] | Inulin powder in milk, 20 g/day | 12 | BMI 28–45 Age 18–60 | NS | n = 86 (42/44); 64.0% female | x | x | x | x | x | x | x | x | x | ERD, FI | Denmark |
| Jensen, 2012 [64] | Alginate powder in water, 15 g/day | 12 | BMI 30–45 Age 20–55 | NS | n = 80 (38/42); 67.5% female | x | x | x | x | x | x | x | x | x | ERD, Calcium | Denmark |
| Kristensen, 2017 [65] | Flaxseed DF powder in fluid or food, 5 g/day | 12 | BMI 30–40 Age 20–60 | NS | n = 16 (10/6); 80.7% female | x | x | x | x | x | x | x | ERD, Orlistat, Vitamins | Denmark | ||
| Flaxseed DF powder in fluid or food, 5 g/day | 12 | BMI 30–40 Age 20–60 | NS | n = 22 (10/12); 80.6% female | x | x | x | x | x | x | x | ERD, Orlistat, Vitamins, Calcium | Denmark | |||
| Pal, 2016 [66] | PGX 3 powder in water, 15 g/day | 52 | BMI 19–68 Age 25–47 | NS | n = 57 (25/32); 53.6% female | x 4 | U | Australia | ||||||||
| Parnell, 2009 [67,68] | FOS powder in fluid, 21 g/day | 12 | BMI > 25 Age 20–70 | NS | n = 39 (21/18); 82.0% female | x | x | x | U | Canada | ||||||
| Pol, 2018 [69] | FOS bar, 16 g/day | 12 | BMI 25–35 Age 20–60 | NS | n = 55 (29/26) 65.5% female | x | x | x | U | Netherlands | ||||||
| Reimer, 2021 [70] | PGX 3 sprinkled on food, 15–20 g/day | 52 | BMI 27–60 Age 18–75 | T2D | n = 100 (53/47); 68.3% female | x | x | x | x | ERD | Canada | |||||
| Reimer, 2017 [71] | FOS + Inulin bar, 8 g/day | 12 | BMI > 25 Age 18–75 | NS | n = 53 (26/27); 54.2% female | x | x | x | x | x | U | Canada | ||||
| FOS + Inulin protein bar, 8 g/day | 12 | BMI > 25 Age 18–75 | NS | n = 43 (21/22); 51.5% female | x | x | x | x | x | U | Canada | |||||
| Reimer, 2013 [72] | PGX 3 powder with yogurt, 15 g/day | 14 | BMI 24–30 Age 20–65 | NS | n = 64 (32/32); 56.3% female | x | x | x | x | x | x | U | Japan | |||
| Solah, 2017 [73] | PGX 3 softgel capsules with water, 8–11 g/day | 12 | BMI 25–35 Age 25–70 | NS | n = 51 (32/19); 77.5% female | x 4 | U | Australia | ||||||||
| PGX 3 granules in food or fluid, 12 g/day | 12 | BMI 25–35 Age 25–70 | NS | n = 51 (32/19); 76.3% female | x 4 | U | Australia | |||||||||
| Tovar, 2012 [74] | Inulin powder in milk with PMR powder, 10 g/day | 12 | BMI > 25 Age 18–50 | NS | n = 51 (23/28); 100% female | x | ERD, PMR | Mexico | ||||||||
| Inulin powder in fluid, 10 g/day | 12 | BMI > 25 Age 18–50 | NS | n = 59 (30/29); 100% female | x | ERD | Mexico | |||||||||
| Wood, 2006 [75,76] | Glucomannan capsules with water, 3 g/day | 12 | BMI 25–35 Age 20–69 | NS | n = 29 (14/15); 0% female | x | x | x | x | x | x | x | x | ERD, vitamins | USA | |
3.3. Quality and Risk of Bias of Primary Outcome Body Weight
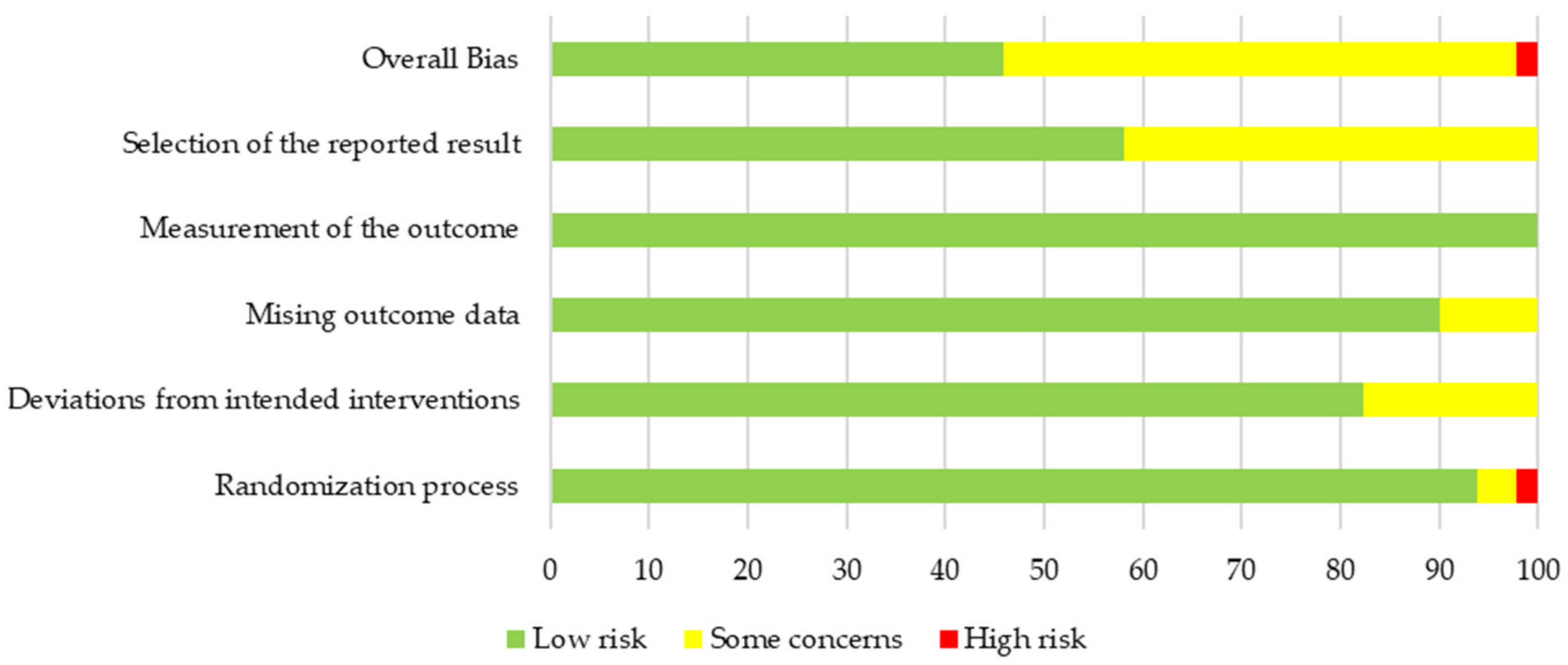
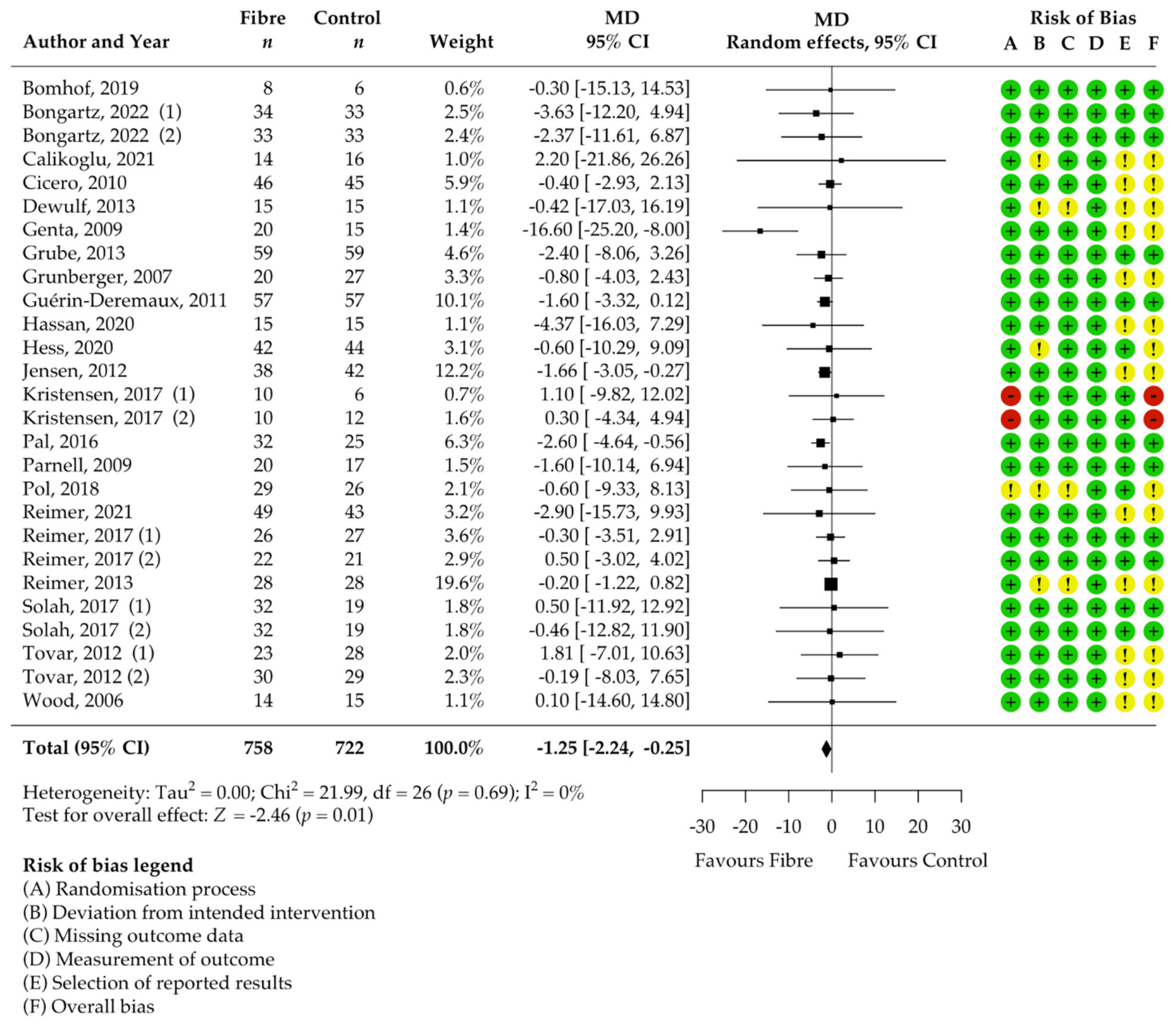
3.4. Pooled Results on Primary Outcome Body Weight
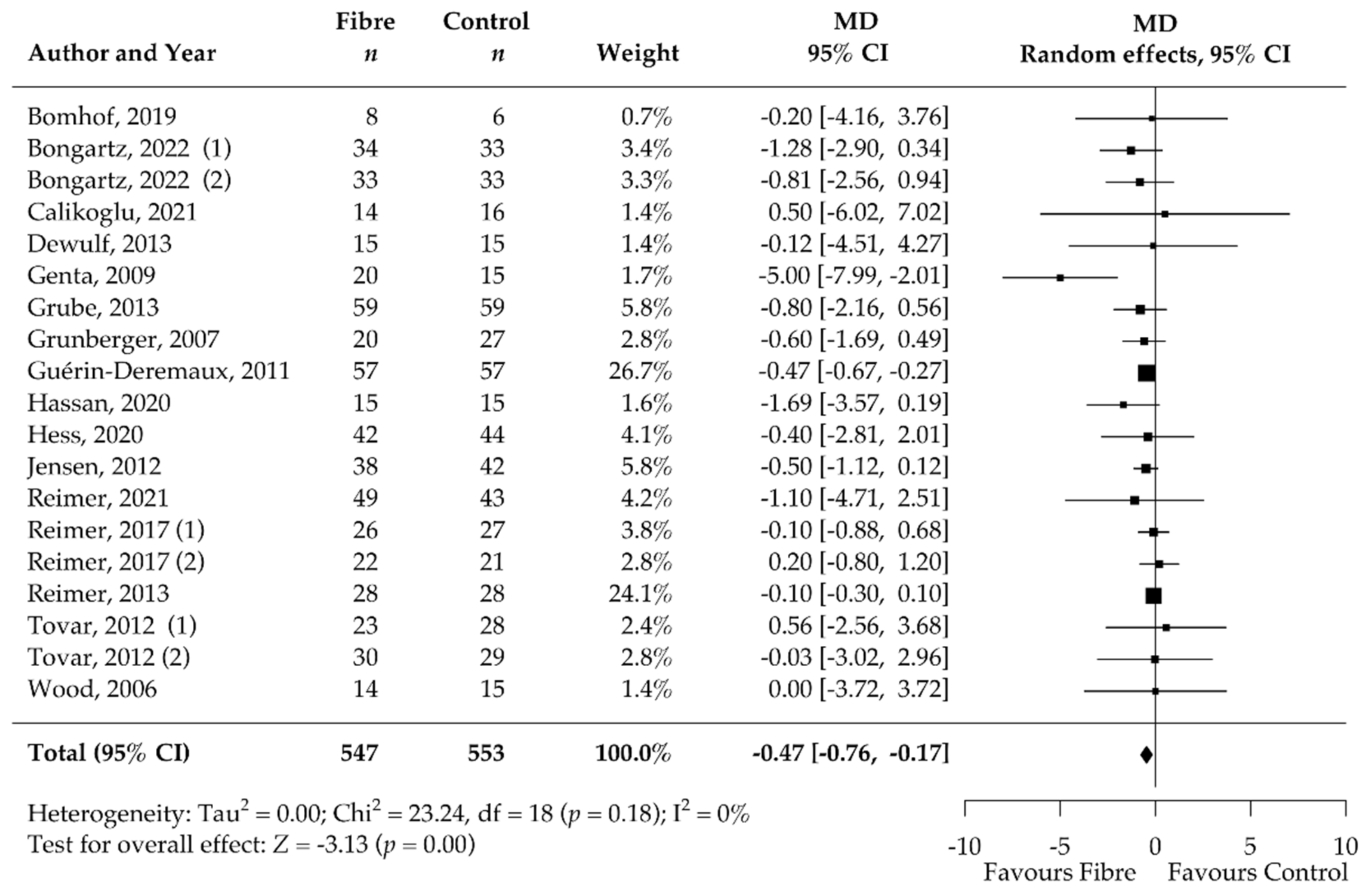
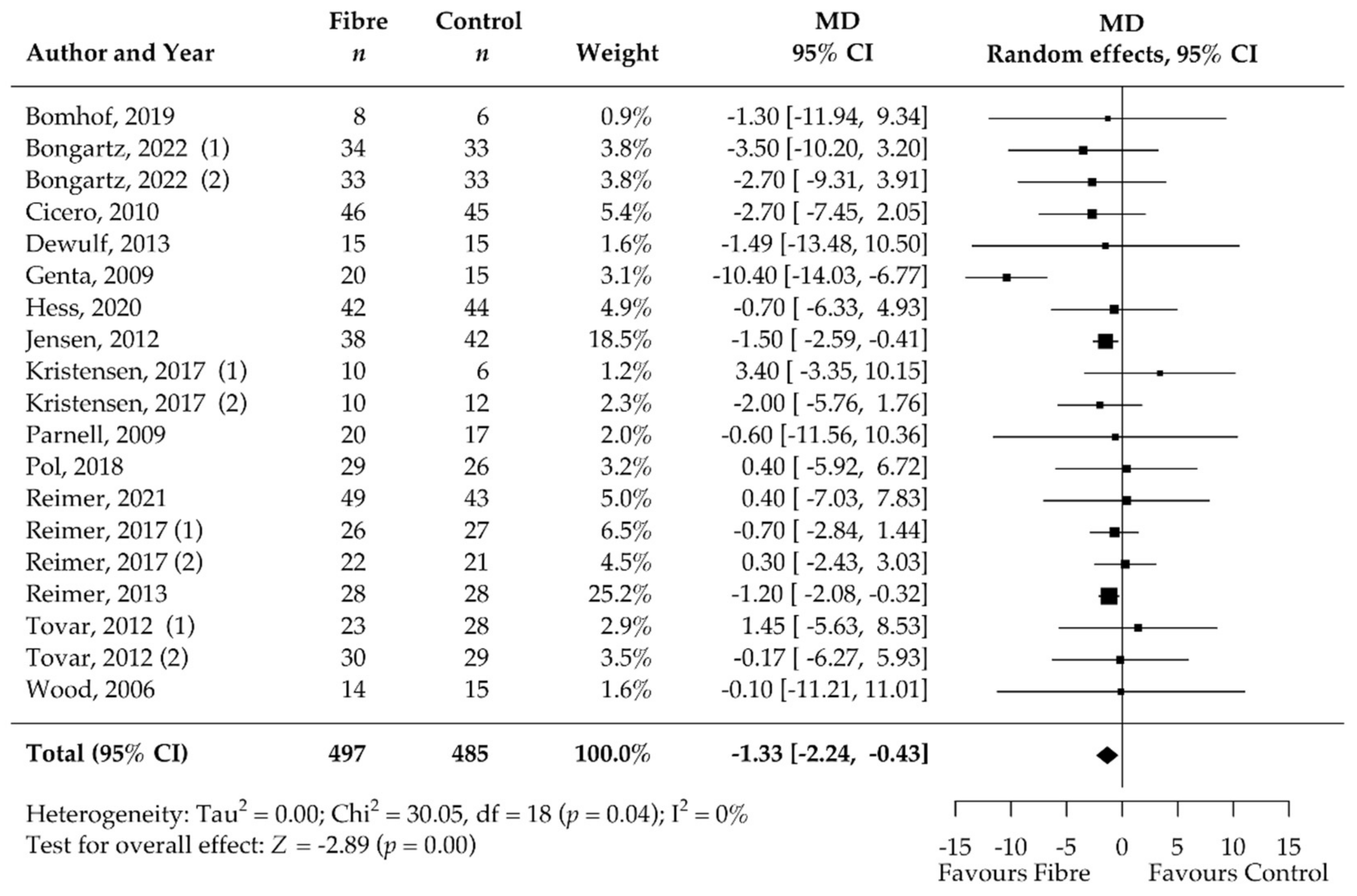
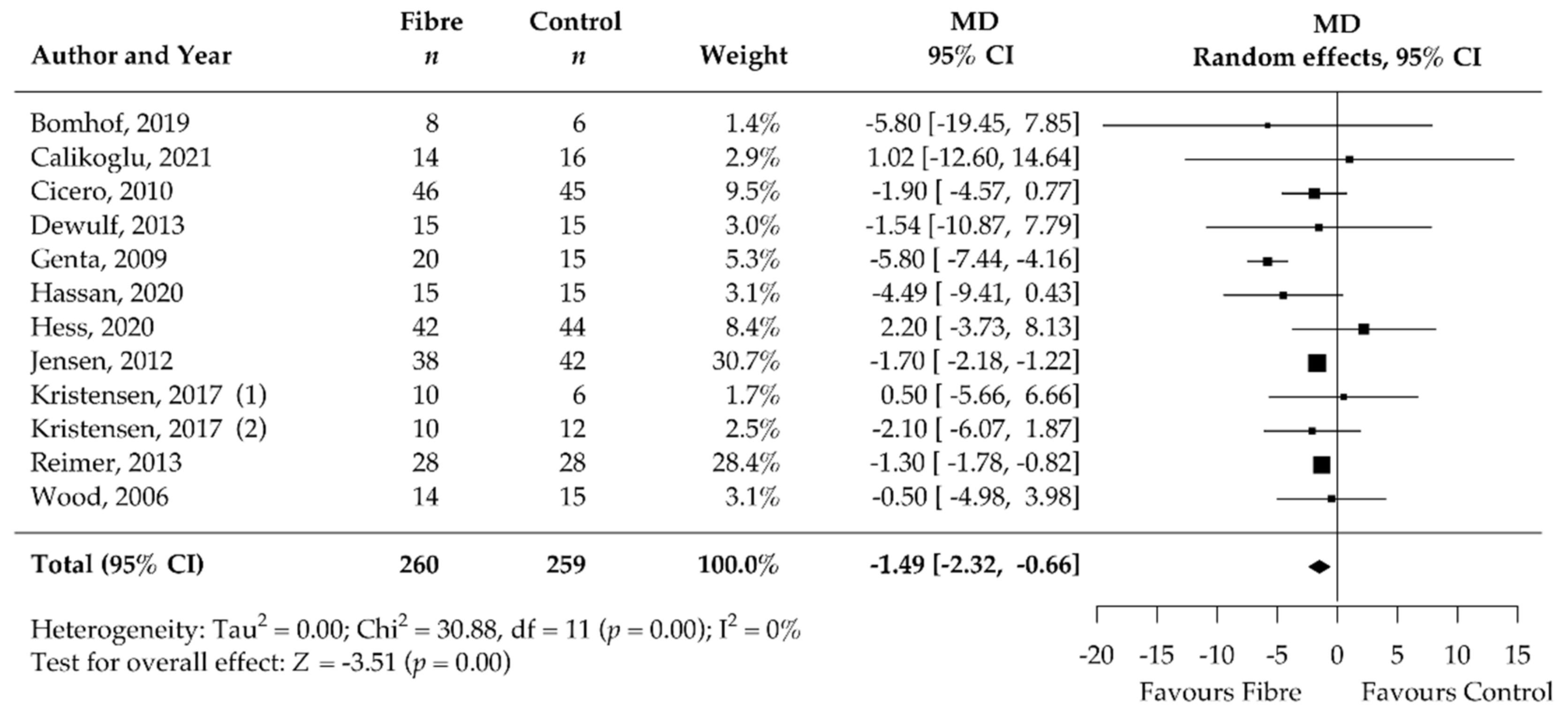
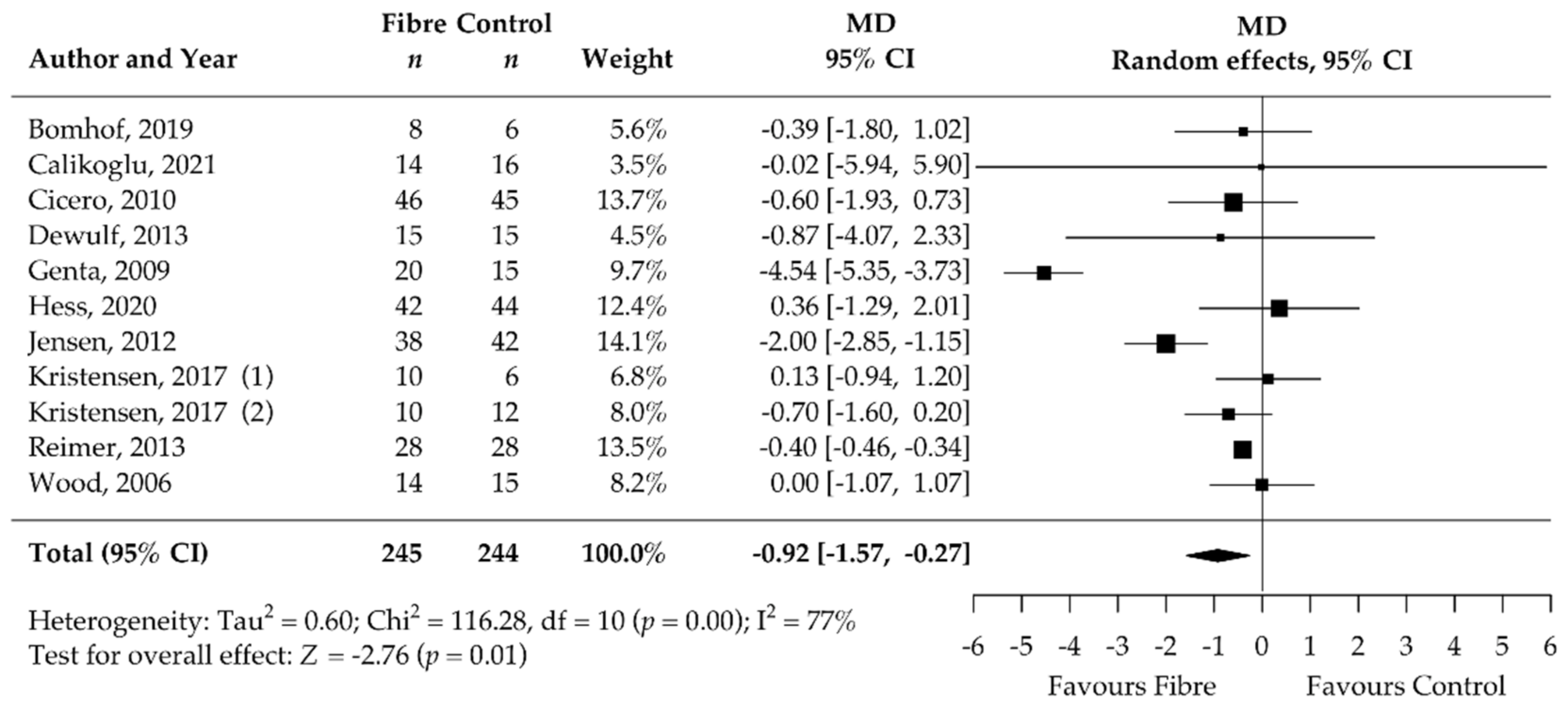
3.5. Pooled Results of Secondary Outcomes (BMI, Body Fat, Waist Circumference, HbA1c, HOMA-IR, Fasting Blood Glucose, Fasting Blood Insulin, CRP, and Stool Calprotectin)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- exp adiposity/or exp Obesity/(249464)
- (adipos* or obes* or (excess adj3 weight) or overweight).ti,ab,kw. (449982)
- exp dietary fiber/or exp Inulin/or exp xylans/or exp beta-Glucans/or exp beta-Glucans/or exp Pectins/or exp guar gum/or exp 4’-galactooligosaccharide/or exp fructooligosaccharide/or exp Psyllium/or exp Psyllium/or exp Flax/(50110)
- ((diet* adj4 fib*) or (Wheat adj bran*) or Roughage* or “alimentary fib*” or “opti?fib*” or “stimulance multi fib*” or alant* or “dahlin” or inulin* or “synanthrin” or xylan* or arabinoxylan or xyloarabinan or hemixylan or (beta and glucan*) or “beta dextroglucan” or macrogard or Pectin* or Methoxy?pectin or guar or Glucotard or “GU-052” or slocose or supercol or “Cal-Ban 3000” or “burtonite v 7 e” or “cyamopsis gum” or decorpa or fibraguar or galactasol or “gum cyamopsis” or “hepart hp 7000” or lejguar or prefill or galacto?oligosaccharide* or “galactose oligomer” or oligogalactose or GOS or fructo?oligosaccharide* or Idolax or “Raftilose P95” or neosugar or oligofructose or Metamucil or Plantaglucide or Ispaghul* or (Plantago adj Seed*) or Iso?gel or Reguval or agiocur or arcolax or betajel or fybogel or konsyl or metamucil or mucilax or mucilose or mucofalk or “plantaginis semen” or plantaglucid* or “plantago ovata extract” or “plantago ovata seed” or psyllium or regulan or transilane or “vi siblin” or volcolon or Flax* or Linum* or Lin?seed*).ti,ab,kw. (79488)
- exp Weight Loss/(46792)
- ((weight or BMI) adj3 (los* or decrea* or reduc* or watching)).ti,ab,kw. (161745)
- 1 or 2 (494979)
- 3 or 4 (100446)
- 5 or 6 (175368)
- 7 and 8 and 9 (817)
- exp animals/not exp humans/(4991746)
- 10 not 11 (665)
- #14
- #12 NOT #13 1202
- #13
- ‘conference abstract’/it 4374972
- #12
- #10 NOT #11 1450
- #11
- ‘animals’/exp NOT ‘humans’/exp 5764029
- #10
- #7 AND #8 AND #9 1709
- #9
- #5 OR #6 322875
- #8
- #3 OR #4 196845
- #7
- #1 OR #2 809870
- #6
- ((weight OR bmi) NEAR/3 (los* OR decrea* OR reduc* OR watching)):ti,ab,kw 249922
- #5
- ‘weight loss’/exp 210306
- #4
- ((diet* NEAR/3 fib*):ti,ab,kw) OR (wheat:ti,ab,kw AND near:ti,ab,kw AND bran*:ti,ab,kw) OR roughage*:ti,ab,kw OR ‘alimentary fib*’:ti,ab,kw OR opti?fib*:ti,ab,kw OR ‘stimulance multi fib*’:ti,ab,kw OR alant*:ti,ab,kw OR dahlin:ti,ab,kw OR inulin*:ti,ab,kw OR synanthrin:ti,ab,kw OR xylan*:ti,ab,kw OR arabinoxylan*:ti,ab,kw OR xyloarabinan*:ti,ab,kw OR hemixylan*:ti,ab,kw OR (beta:ti,ab,kw AND glucan*:ti,ab,kw) OR ‘beta dextroglucan’:ti,ab,kw OR macrogard:ti,ab,kw OR pectin*:ti,ab,kw OR methoxy?pectin:ti,ab,kw OR guar*:ti,ab,kw OR glucotard:ti,ab,kw OR ‘gu-052’:ti,ab,kw OR slocose:ti,ab,kw OR supercol:ti,ab,kw OR ‘cal-ban 3000’:ti,ab,kw OR ‘burtonite v 7 e’:ti,ab,kw OR ‘cyamopsis gum’:ti,ab,kw OR decorpa:ti,ab,kw OR fibraguar:ti,ab,kw OR galactasol:ti,ab,kw OR ‘gum cyamopsis’:ti,ab,kw OR ‘hepart hp 7000’:ti,ab,kw OR lejguar:ti,ab,kw OR prefill:ti,ab,kw OR galacto?oligosaccharide*:ti,ab,kw OR ‘galactose oligomer’:ti,ab,kw OR oligogalactose:ti,ab,kw OR gos:ti,ab,kw OR fructo?oligosaccharide*:ti,ab,kw OR idolax:ti,ab,kw OR ‘raftilose p95’:ti,ab,kw OR neosugar:ti,ab,kw OR oligofructose:ti,ab,kw OR plantaglucid*:ti,ab,kw OR ispaghul*:ti,ab,kw OR (plantago:ti,ab,kw AND near:ti,ab,kw AND seed:ti,ab,kw) OR ‘seed plantago’:ti,ab,kw OR iso?gel:ti,ab,kw OR reguval:ti,ab,kw OR agiocur:ti,ab,kw OR arcolax:ti,ab,kw OR betajel:ti,ab,kw OR fybogel:ti,ab,kw OR konsyl:ti,ab,kw OR metamucil:ti,ab,kw OR mucilax:ti,ab,kw OR mucilose:ti,ab,kw OR mucofalk:ti,ab,kw OR ‘plantaginis semen’:ti,ab,kw OR plantaglucide:ti,ab,kw OR ‘plantago ovata extract’:ti,ab,kw OR ‘plantago ovata seed’:ti,ab,kw OR psyllium:ti,ab,kw OR regulan:ti,ab,kw OR transilane:ti,ab,kw OR ‘vi siblin’:ti,ab,kw OR volcolon:ti,ab,kw OR flax*:ti,ab,kw OR linum*:ti,ab,kw OR lin?seed*:ti,ab,kw 164504
- #3
- ‘dietary fibre’/exp OR ‘inulin’/exp OR ‘xylans’/exp OR ‘beta-glucans’/exp OR ‘pectins’/exp OR ‘4-galactooligosaccharide’ OR ‘fructooligosaccharide’/exp OR ‘psyllium’/exp OR ‘flax’/exp 67816
- #2
- adipos*:ti,ab,kw OR obes*:ti,ab,kw OR ((excess NEAR/3 weight):ti,ab,kw) OR overweight:ti,ab,kw 657886
- #1
- ‘adiposity’/de OR ‘obesity’/exp 601338
Appendix B
| Outcome | Certainty Assessment | Number of Patients | Absolute Effect (95% CI) | Certainty | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Cohorts | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Dietary Fibre | Placebo or No Intervention | |||
| Body weight [kg] | 27 | randomised trials | not serious a | not serious b | not serious | not serious c | none | 758 | 670 | MD 1.25 SD lower (2.24 lower to 0.25 lower) | High |
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 6 May 2022).
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Waters, H.; Graf, M. America’s obesity crisis. In The Health and Economic Costs of Excess Weight; Milken Institute: Santa Monica, CA, USA, 2018. [Google Scholar]
- National Clinical Guideline Centre (UK). Obesity: Identification, Assessment and Management of Overweight and Obesity in Children, Young People and Adults: Partial Update of CG43; National Institute for Health and Care Excellence (NICE): London, UK, 2014. [Google Scholar]
- Varady, K.A.; Cienfuegos, S.; Ezpeleta, M.; Gabel, K. Clinical application of intermittent fasting for weight loss: Progress and future directions. Nat. Rev. Endocrinol. 2022, 18, 309–321. [Google Scholar] [CrossRef]
- Dent, R.; McPherson, R.; Harper, M.E. Factors affecting weight loss variability in obesity. Metabolism 2020, 113, 154388. [Google Scholar] [CrossRef]
- Schlesinger, S.; Neuenschwander, M.; Schwedhelm, C.; Hoffmann, G.; Bechthold, A.; Boeing, H.; Schwingshackl, L. Food Groups and Risk of Overweight, Obesity, and Weight Gain: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Adv. Nutr. 2019, 10, 205–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Wang, D.; Long, J.; Yang, F.; Si, L. Meta-analysis of the association between whole grain intake and coronary heart disease risk. Am. J. Cardiol. 2015, 115, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Trowell, H. Crude fibre, dietary fibre and atherosclerosis. Atherosclerosis 1972, 16, 138–140. [Google Scholar] [CrossRef]
- World Health Organization. Diet, Nutrition, and the Pprevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; World Health Organization: Geneva, Switzerland, 2003; Volume 916. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific opinion on dietary reference values for carbohydrates and dietary fibre. EFSA J. 2010, 8, 1462. [Google Scholar]
- DACH. Referenzwerte für die Nährstoffzufuhr, Gesellschaften für Ernährung in Deutschland (DGE), Österreich (ÖGE) und der Schweiz (SGE); Deutsche Gesellschaft für Ernährung (DGE): Bonn, Germany, 2021; Volume 2. [Google Scholar]
- Nordic Council of Ministers. Nordic Nutrition Recommendations 2012: Integrating Nutrition and Physical Activity; Nordisk Ministerråd: Copenhagen, Denmark, 2014; Volume 5, p. 627. [Google Scholar]
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; USDA: Washington, DC, USA, 2020.
- National Health and Medical Research Council, Australian Government Department of Health and Ageing, New Zealand Ministry of Health. Nutrient Reference Values for Australia and New Zealand: Including Recommended Dietary Intakes; National Health and Medical Research Council: Canberra, Australia, 2006.
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Stephen, A.M.; Champ, M.M.; Cloran, S.J.; Fleith, M.; van Lieshout, L.; Mejborn, H.; Burley, V.J. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017, 30, 149–190. [Google Scholar] [CrossRef]
- Balmer, M.L.; Ma, E.H.; Bantug, G.R.; Grählert, J.; Pfister, S.; Glatter, T.; Jauch, A.; Dimeloe, S.; Slack, E.; Dehio, P.; et al. Memory CD8(+) T Cells Require Increased Concentrations of Acetate Induced by Stress for Optimal Function. Immunity 2016, 44, 1312–1324. [Google Scholar] [CrossRef] [Green Version]
- Balmer, M.L.; Ma, E.H.; Thompson, A.J.; Epple, R.; Unterstab, G.; Lötscher, J.; Dehio, P.; Schürch, C.M.; Warncke, J.D.; Perrin, G.; et al. Memory CD8(+) T Cells Balance Pro- and Anti-inflammatory Activity by Reprogramming Cellular Acetate Handling at Sites of Infection. Cell Metab. 2020, 32, 457–467.e455. [Google Scholar] [CrossRef]
- Koh, A.; de Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [Green Version]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Overby, H.B.; Ferguson, J.F. Gut Microbiota-Derived Short-Chain Fatty Acids Facilitate Microbiota: Host Cross Talk and Modulate Obesity and Hypertension. Curr. Hypertens. Rep. 2021, 23, 8. [Google Scholar] [CrossRef]
- Ho, H.V.; Sievenpiper, J.L.; Zurbau, A.; Blanco Mejia, S.; Jovanovski, E.; Au-Yeung, F.; Jenkins, A.L.; Vuksan, V. The effect of oat β-glucan on LDL-cholesterol, non-HDL-cholesterol and apoB for CVD risk reduction: A systematic review and meta-analysis of randomised-controlled trials. Br. J. Nutr. 2016, 116, 1369–1382. [Google Scholar] [CrossRef] [Green Version]
- Ho, H.V.T.; Jovanovski, E.; Zurbau, A.; Blanco Mejia, S.; Sievenpiper, J.L.; Au-Yeung, F.; Jenkins, A.L.; Duvnjak, L.; Leiter, L.; Vuksan, V. A systematic review and meta-analysis of randomized controlled trials of the effect of konjac glucomannan, a viscous soluble fiber, on LDL cholesterol and the new lipid targets non-HDL cholesterol and apolipoprotein B. Am. J. Clin. Nutr. 2017, 105, 1239–1247. [Google Scholar] [CrossRef] [Green Version]
- Khan, K.; Jovanovski, E.; Ho, H.V.T.; Marques, A.C.R.; Zurbau, A.; Mejia, S.B.; Sievenpiper, J.L.; Vuksan, V. The effect of viscous soluble fiber on blood pressure: A systematic review and meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 3–13. [Google Scholar] [CrossRef]
- Thompson, S.V.; Hannon, B.A.; An, R.; Holscher, H.D. Effects of isolated soluble fiber supplementation on body weight, glycemia, and insulinemia in adults with overweight and obesity: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2017, 106, 1514–1528. [Google Scholar] [CrossRef] [Green Version]
- Jovanovski, E.; Mazhar, N.; Komishon, A.; Khayyat, R.; Li, D.; Blanco Mejia, S.; Khan, T.; Jenkins, A.L.; Smircic-Duvnjak, L.; Sievenpiper, J.L.; et al. Can dietary viscous fiber affect body weight independently of an energy-restrictive diet? A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2020, 111, 471–485. [Google Scholar] [CrossRef]
- McGill, C.R.; Fulgoni, V.L., 3rd; Devareddy, L. Ten-year trends in fiber and whole grain intakes and food sources for the United States population: National Health and Nutrition Examination Survey 2001–2010. Nutrients 2015, 7, 1119–1130. [Google Scholar] [CrossRef]
- Dreher, M.L.; Davenport, A.J. Hass avocado composition and potential health effects. Crit. Rev. Food Sci. Nutr. 2013, 53, 738–750. [Google Scholar] [CrossRef] [Green Version]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [Green Version]
- National Clinical Guideline Centre (UK). Obesity in Adults: Prevention and Lifestyle Wweight Management Programmes; National Institute for Health and Care Excellence (NICE): London, UK, 2016. [Google Scholar]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Cooper, H. Research Synthesis and Meta-Analysis: A Step-by-Step Approach; Sage Publications: Newbury Park, CA, USA, 2015; Volume 2. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Hedges, L.V. Meta-analysis. J. Educ. Stat. 1992, 17, 279–296. [Google Scholar] [CrossRef]
- Hedges, L.V. A random effects model for effect sizes. Psychol. Bull. 1983, 93, 388. [Google Scholar] [CrossRef]
- Cochran, W.G. The combination of estimates from different experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Baujat, B.; Mahé, C.; Pignon, J.P.; Hill, C. A graphical method for exploring heterogeneity in meta-analyses: Application to a meta-analysis of 65 trials. Stat. Med. 2002, 21, 2641–2652. [Google Scholar] [CrossRef]
- Schünemann, H.B.J.; Guyatt, G.; Oxman, A. (Eds.) GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations; Updated October 2013; The GRADE Working Group: Adelaide, Australia, 2013. [Google Scholar]
- GRADEpro GDT. GRADEpro Guideline Development Tool [Software]: McMaster University and Evidence Prime, Canada. Available online: https://www.Gradepro.org/ (accessed on 18 May 2022).
- Cicero, A.F.G.; Derosa, G.; Bove, M.; Imola, F.; Borghi, C.; Gaddi, A.V. Psyllium improves dyslipidaemia, hyperglycaemia and hypertension, while guar gum reduces body weight more rapidly in patients affected by metabolic syndrome following an AHA Step 2 diet. Mediterr. J. Nutr. Metab. 2010, 3, 47–54. [Google Scholar] [CrossRef]
- Bomhof, M.R.; Parnell, J.A.; Ramay, H.R.; Crotty, P.; Rioux, K.P.; Probert, C.S.; Jayakumar, S.; Raman, M.; Reimer, R.A. Histological improvement of non-alcoholic steatohepatitis with a prebiotic: A pilot clinical trial. Eur. J. Nutr. 2019, 58, 1735–1745. [Google Scholar] [CrossRef]
- Bongartz, U.; Hochmann, U.; Grube, B.; Uebelhack, R.; Alt, F.; Erlenbeck, C.; Peng, L.V.; Chong, P.W.; de Costa, P. Flaxseed Mucilage (IQP-LU-104) Reduces Body Weight in Overweight and Moderately Obese Individuals in a 12-Week, Three-Arm, Double-Blind, Randomized and Placebo-Controlled Clinical Study. Obes. Facts 2022, 15, 395–404. [Google Scholar] [CrossRef]
- Calikoglu, F.; Barbaros, U.; Uzum, A.K.; Tutuncu, Y.; Satman, I. The Metabolic Effects of Pre-probiotic Supplementation After Roux-en-Y Gastric Bypass (RYGB) Surgery: A Prospective, Randomized Controlled Study. Obes. Surg. 2021, 31, 215–223. [Google Scholar] [CrossRef]
- Dewulf, E.M.; Cani, P.D.; Claus, S.P.; Fuentes, S.; Puylaert, P.G.; Neyrinck, A.M.; Bindels, L.B.; de Vos, W.M.; Gibson, G.R.; Thissen, J.-P. Insight into the prebiotic concept: Lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 2013, 62, 1112–1121. [Google Scholar] [CrossRef]
- Genta, S.; Cabrera, W.; Habib, N.; Pons, J.; Carillo, I.M.; Grau, A.; Sanchez, S. Yacon syrup: Beneficial effects on obesity and insulin resistance in humans. Clin. Nutr. 2009, 28, 182–187. [Google Scholar] [CrossRef]
- Grube, B.; Chong, P.W.; Lau, K.Z.; Orzechowski, H.D. A natural fiber complex reduces body weight in the overweight and obese: A double-blind, randomized, placebo-controlled study. Obesity 2013, 21, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Grunberger, G.; Jen, K.L.C.; Artiss, J.D. The benefits of early intervention in obese diabetic patients with FBCx™—A new dietary fibre. Diabetes/Metab. Res. Rev. 2007, 23, 56–62. [Google Scholar] [CrossRef]
- Guérin-Deremaux, L.; Li, S.; Pochat, M.; Wils, D.; Mubasher, M.; Reifer, C.; Miller, L.E. Effects of NUTRIOSE® dietary fiber supplementation on body weight, body composition, energy intake, and hunger in overweight men. Int. J. Food Sci. Nutr. 2011, 62, 628–635. [Google Scholar] [CrossRef]
- Li, S.; Guerin-Deremaux, L.; Pochat, M.; Wils, D.; Reifer, C.; Miller, L.E. NUTRIOSE dietary fiber supplementation improves insulin resistance and determinants of metabolic syndrome in overweight men: A double-blind, randomized, placebo-controlled study. Appl. Physiol. Nutr. Metab. 2010, 35, 773–782. [Google Scholar] [CrossRef]
- Hassan, O.M.S.; di Folco, U.; Nardone, M.R.; Tubili, F.; Tubili, C. Fiber enrichment of pasta: Metabolic effects and diet adherence in obese subjects. Mediterr. J. Nutr. Metab. 2020, 13, 53–62. [Google Scholar] [CrossRef]
- Hess, A.L.; Benítez-Páez, A.; Blædel, T.; Larsen, L.H.; Iglesias, J.R.; Madera, C.; Sanz, Y.; Larsen, T.M. The effect of inulin and resistant maltodextrin on weight loss during energy restriction: A randomised, placebo-controlled, double-blinded intervention. Eur. J. Nutr. 2020, 59, 2507–2524. [Google Scholar] [CrossRef]
- Benítez-Páez, A.; Hess, A.L.; Krautbauer, S.; Liebisch, G.; Christensen, L.; Hjorth, M.F.; Larsen, T.M.; Sanz, Y. Sex, Food, and the Gut Microbiota: Disparate Response to Caloric Restriction Diet with Fiber Supplementation in Women and Men. Mol. Nutr. Food Res. 2021, 65, e2000996. [Google Scholar] [CrossRef]
- Georg Jensen, M.; Kristensen, M.; Astrup, A. Effect of alginate supplementation on weight loss in obese subjects completing a 12-wk energy-restricted diet: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 96, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, M.; Juul, S.R.; Sorensen, K.V.; Lorenzen, J.K.; Astrup, A. Supplementation with dairy calcium and/or flaxseed fibers in conjunction with orlistat augments fecal fat excretion without altering ratings of gastrointestinal comfort. Nutr. Metab. 2017, 14, 13. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.; Ho, S.; Gahler, R.J.; Wood, S. Effect on body weight and composition in overweight/obese Australian adults over 12 months consumption of two different types of fibre supplementation in a randomized trial. Nutr. Metab. 2016, 13, 82. [Google Scholar] [CrossRef] [Green Version]
- Parnell, J.A.; Reimer, R.A. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am. J. Clin. Nutr. 2009, 89, 1751–1759. [Google Scholar] [CrossRef] [Green Version]
- Parnell, J.A.; Klancic, T.; Reimer, R.A. Oligofructose decreases serum lipopolysaccharide and plasminogen activator inhibitor-1 in adults with overweight/obesity. Obesity 2017, 25, 510–513. [Google Scholar] [CrossRef] [Green Version]
- Pol, K.; Christensen, R.; Bartels, E.M.; Raben, A.; Tetens, I.; Kristensen, M. Whole grain and body weight changes in apparently healthy adults: A systematic review and meta-analysis of randomized controlled studies. Am. J. Clin. Nutr. 2013, 98, 872–884. [Google Scholar] [CrossRef] [Green Version]
- Reimer, R.A.; Wharton, S.; Green, T.J.; Manjoo, P.; Ramay, H.R.; Lyon, M.R.; Gahler, R.J.; Wood, S. Effect of a functional fibre supplement on glycemic control when added to a year-long medically supervised weight management program in adults with type 2 diabetes. Eur. J. Nutr. 2021, 60, 1237–1251. [Google Scholar] [CrossRef]
- Reimer, R.A.; Willis, H.J.; Tunnicliffe, J.M.; Park, H.; Madsen, K.L.; Soto-Vaca, A. Inulin-type fructans and whey protein both modulate appetite but only fructans alter gut microbiota in adults with overweight/obesity: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61, 11. [Google Scholar] [CrossRef]
- Reimer, R.A.; Yamaguchi, H.; Eller, L.K.; Lyon, M.R.; Gahler, R.J.; Kacinik, V.; Juneja, P.; Wood, S. Changes in visceral adiposity and serum cholesterol with a novel viscous polysaccharide in Japanese adults with abdominal obesity. Obesity 2013, 21, E379–E387. [Google Scholar] [CrossRef]
- Solah, V.A.; Kerr, D.A.; Hunt, W.J.; Johnson, S.K.; Boushey, C.J.; Delp, E.J.; Meng, X.; Gahler, R.J.; James, A.P.; Mukhtar, A.S.; et al. Effect of Fibre Supplementation on Body Weight and Composition, Frequency of Eating and Dietary Choice in Overweight Individuals. Nutrients 2017, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Tovar, A.R.; Caamano Mdel, C.; Garcia-Padilla, S.; Garcia, O.P.; Duarte, M.A.; Rosado, J.L. The inclusion of a partial meal replacement with or without inulin to a calorie restricted diet contributes to reach recommended intakes of micronutrients and decrease plasma triglycerides: A randomized clinical trial in obese Mexican women. Nutr. J. 2012, 11, 44. [Google Scholar] [CrossRef] [Green Version]
- Wood, R.J.; Volek, J.S.; Davis, S.R.; Dell’Ova, C.; Fernandez, M.L. Effects of a carbohydrate-restricted diet on emerging plasma markers for cardiovascular disease. Nutr. Metab. 2006, 3, 19. [Google Scholar] [CrossRef] [Green Version]
- Wood, R.J.; Fernandez, M.L.; Sharman, M.J.; Silvestre, R.; Greene, C.M.; Zern, T.L.; Shrestha, S.; Judelson, D.A.; Gomez, A.L.; Kraemer, W.J.; et al. Effects of a carbohydrate-restricted diet with and without supplemental soluble fiber on plasma low-density lipoprotein cholesterol and other clinical markers of cardiovascular risk. Metabolism 2007, 56, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: Abingdon, UK, 1988. [Google Scholar]
- Magkos, F.; Fraterrigo, G.; Yoshino, J.; Luecking, C.; Kirbach, K.; Kelly, S.C.; de Las Fuentes, L.; He, S.; Okunade, A.L.; Patterson, B.W.; et al. Effects of Moderate and Subsequent Progressive Weight Loss on Metabolic Function and Adipose Tissue Biology in Humans with Obesity. Cell Metab. 2016, 23, 591–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warkentin, L.M.; Majumdar, S.R.; Johnson, J.A.; Agborsangaya, C.B.; Rueda-Clausen, C.F.; Sharma, A.M.; Klarenbach, S.W.; Karmali, S.; Birch, D.W.; Padwal, R.S. Weight loss required by the severely obese to achieve clinically important differences in health-related quality of life: Two-year prospective cohort study. BMC Med. 2014, 12, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014, 129, S102–S138. [Google Scholar] [CrossRef] [Green Version]
- Hamman, R.F.; Wing, R.R.; Edelstein, S.L.; Lachin, J.M.; Bray, G.A.; Delahanty, L.; Hoskin, M.; Kriska, A.M.; Mayer-Davis, E.J.; Pi-Sunyer, X.; et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006, 29, 2102–2107. [Google Scholar] [CrossRef] [Green Version]
- Koutoukidis, D.A.; Jebb, S.A.; Zimmerman, M.; Otunla, A.; Henry, J.A.; Ferrey, A.; Schofield, E.; Kinton, J.; Aveyard, P.; Marchesi, J.R. The association of weight loss with changes in the gut microbiota diversity, composition, and intestinal permeability: A systematic review and meta-analysis. Gut Microbes 2022, 14, 2020068. [Google Scholar] [CrossRef]
- Tilg, H.; Zmora, N.; Adolph, T.E.; Elinav, E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 2020, 20, 40–54. [Google Scholar] [CrossRef]
- Shaw, K.; Gennat, H.; O’Rourke, P.; del Mar, C. Exercise for overweight or obesity. Cochrane Database Syst. Rev. 2006, 4, CD003817. [Google Scholar] [CrossRef] [Green Version]
- Franz, M.J.; VanWormer, J.J.; Crain, A.L.; Boucher, J.L.; Histon, T.; Caplan, W.; Bowman, J.D.; Pronk, N.P. Weight-loss outcomes: A systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J. Am. Diet. Assoc. 2007, 107, 1755–1767. [Google Scholar] [CrossRef]
- Khera, R.; Murad, M.H.; Chandar, A.K.; Dulai, P.S.; Wang, Z.; Prokop, L.J.; Loomba, R.; Camilleri, M.; Singh, S. Association of Pharmacological Treatments for Obesity with Weight Loss and Adverse Events: A Systematic Review and Meta-analysis. JAMA 2016, 315, 2424–2434. [Google Scholar] [CrossRef]
- Gloy, V.L.; Briel, M.; Bhatt, D.L.; Kashyap, S.R.; Schauer, P.R.; Mingrone, G.; Bucher, H.C.; Nordmann, A.J. Bariatric surgery versus non-surgical treatment for obesity: A systematic review and meta-analysis of randomised controlled trials. BMJ 2013, 347, f5934. [Google Scholar] [CrossRef] [Green Version]
- Deehan, E.C.; Duar, R.M.; Armet, A.M.; Perez-Muñoz, M.E.; Jin, M.; Walter, J. Modulation of the gastrointestinal microbiome with nondigestible fermentable carbohydrates to improve human health. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Venkataraman, A.; Sieber, J.R.; Schmidt, A.W.; Waldron, C.; Theis, K.R.; Schmidt, T.M. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome 2016, 4, 33. [Google Scholar] [CrossRef] [Green Version]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A.; et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011, 5, 220–230. [Google Scholar] [CrossRef]
- Martínez, I.; Kim, J.; Duffy, P.R.; Schlegel, V.L.; Walter, J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS ONE 2010, 5, e15046. [Google Scholar] [CrossRef] [Green Version]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Miyamoto, N.; Shibata, K.; Valasek, M.A.; Motoike, T.; Kedzierski, R.M.; Yanagisawa, M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc. Natl. Acad. Sci. USA 2004, 101, 1045–1050. [Google Scholar] [CrossRef] [Green Version]
- Zaibi, M.S.; Stocker, C.J.; O’Dowd, J.; Davies, A.; Bellahcene, M.; Cawthorne, M.A.; Brown, A.J.; Smith, D.M.; Arch, J.R. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett. 2010, 584, 2381–2386. [Google Scholar] [CrossRef] [Green Version]
- Roager, H.M.; Vogt, J.K.; Kristensen, M.; Hansen, L.B.S.; Ibrügger, S.; Mærkedahl, R.B.; Bahl, M.I.; Lind, M.V.; Nielsen, R.L.; Frøkiær, H.; et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: A randomised cross-over trial. Gut 2019, 68, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Suhr, J.; Vuholm, S.; Iversen, K.N.; Landberg, R.; Kristensen, M. Wholegrain rye, but not wholegrain wheat, lowers body weight and fat mass compared with refined wheat: A 6-week randomized study. Eur. J. Clin. Nutr. 2017, 71, 959–967. [Google Scholar] [CrossRef]
- Burley, V.J.; Paul, A.W.; Blundell, J.E. Influence of a high-fibre food (myco-protein) on appetite: Effects on satiation (within meals) and satiety (following meals). Eur. J. Clin. Nutr. 1993, 47, 409–418. [Google Scholar]
- Wanders, A.J.; Jonathan, M.C.; van den Borne, J.J.; Mars, M.; Schols, H.A.; Feskens, E.J.; de Graaf, C. The effects of bulking, viscous and gel-forming dietary fibres on satiation. Br. J. Nutr. 2013, 109, 1330–1337. [Google Scholar] [CrossRef] [Green Version]
- Odunsi, S.T.; Vázquez-Roque, M.I.; Camilleri, M.; Papathanasopoulos, A.; Clark, M.M.; Wodrich, L.; Lempke, M.; McKinzie, S.; Ryks, M.; Burton, D.; et al. Effect of alginate on satiation, appetite, gastric function, and selected gut satiety hormones in overweight and obesity. Obesity 2010, 18, 1579–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Outcome | Included Cohorts [n] | Pooled MD [95% CI] | Heterogeneity I2 [%] | Publication Bias | |
|---|---|---|---|---|---|
| Egger’s [p] | Rank’s [p] | ||||
| Body weight [kg] | 27 | −1.25 [−2.24, −0.25] | 0 | 0.87 | 1.00 |
| BMI [kg/m2] | 19 | −0.47 [−0.76, −0.17] | 0 | NA | NA |
| Body fat [%] | 13 | −0.37 [−2.46, 1.71] | 0 | NA | NA |
| Waist circumference [cm] | 19 | −1.33 [−2.24, −0.43] | 0 | NA | NA |
| HbA1c [%] | 12 | −0.11 [−0.23, 0.01] | 0 | NA | NA |
| HOMA-IR | 11 | −0.92 [−1.57, −0.27] | 77 | NA | NA |
| Fasting blood glucose [mmol/L] | 14 | −0.08 [−0.17, 0.01] | 0 | NA | NA |
| Fasting blood insulin [mIU/L] | 12 | −1.49 [−2.32, −0.66] | 0 | NA | NA |
| CRP [mg/L] | 7 | 0.02 [−0.64, 0.67] | 0 | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huwiler, V.V.; Schönenberger, K.A.; Segesser von Brunegg, A.; Reber, E.; Mühlebach, S.; Stanga, Z.; Balmer, M.L. Prolonged Isolated Soluble Dietary Fibre Supplementation in Overweight and Obese Patients: A Systematic Review with Meta-Analysis of Randomised Controlled Trials. Nutrients 2022, 14, 2627. https://doi.org/10.3390/nu14132627
Huwiler VV, Schönenberger KA, Segesser von Brunegg A, Reber E, Mühlebach S, Stanga Z, Balmer ML. Prolonged Isolated Soluble Dietary Fibre Supplementation in Overweight and Obese Patients: A Systematic Review with Meta-Analysis of Randomised Controlled Trials. Nutrients. 2022; 14(13):2627. https://doi.org/10.3390/nu14132627
Chicago/Turabian StyleHuwiler, Valentina V., Katja A. Schönenberger, Alexander Segesser von Brunegg, Emilie Reber, Stefan Mühlebach, Zeno Stanga, and Maria L. Balmer. 2022. "Prolonged Isolated Soluble Dietary Fibre Supplementation in Overweight and Obese Patients: A Systematic Review with Meta-Analysis of Randomised Controlled Trials" Nutrients 14, no. 13: 2627. https://doi.org/10.3390/nu14132627
APA StyleHuwiler, V. V., Schönenberger, K. A., Segesser von Brunegg, A., Reber, E., Mühlebach, S., Stanga, Z., & Balmer, M. L. (2022). Prolonged Isolated Soluble Dietary Fibre Supplementation in Overweight and Obese Patients: A Systematic Review with Meta-Analysis of Randomised Controlled Trials. Nutrients, 14(13), 2627. https://doi.org/10.3390/nu14132627