Effect of Cholecalciferol Supplementation on the Clinical Features and Inflammatory Markers in Hospitalized COVID-19 Patients: A Randomized, Open-Label, Single-Center Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Data
2.3. Laboratory Tests
2.4. Instrumental Data
2.5. Concomitant Medication
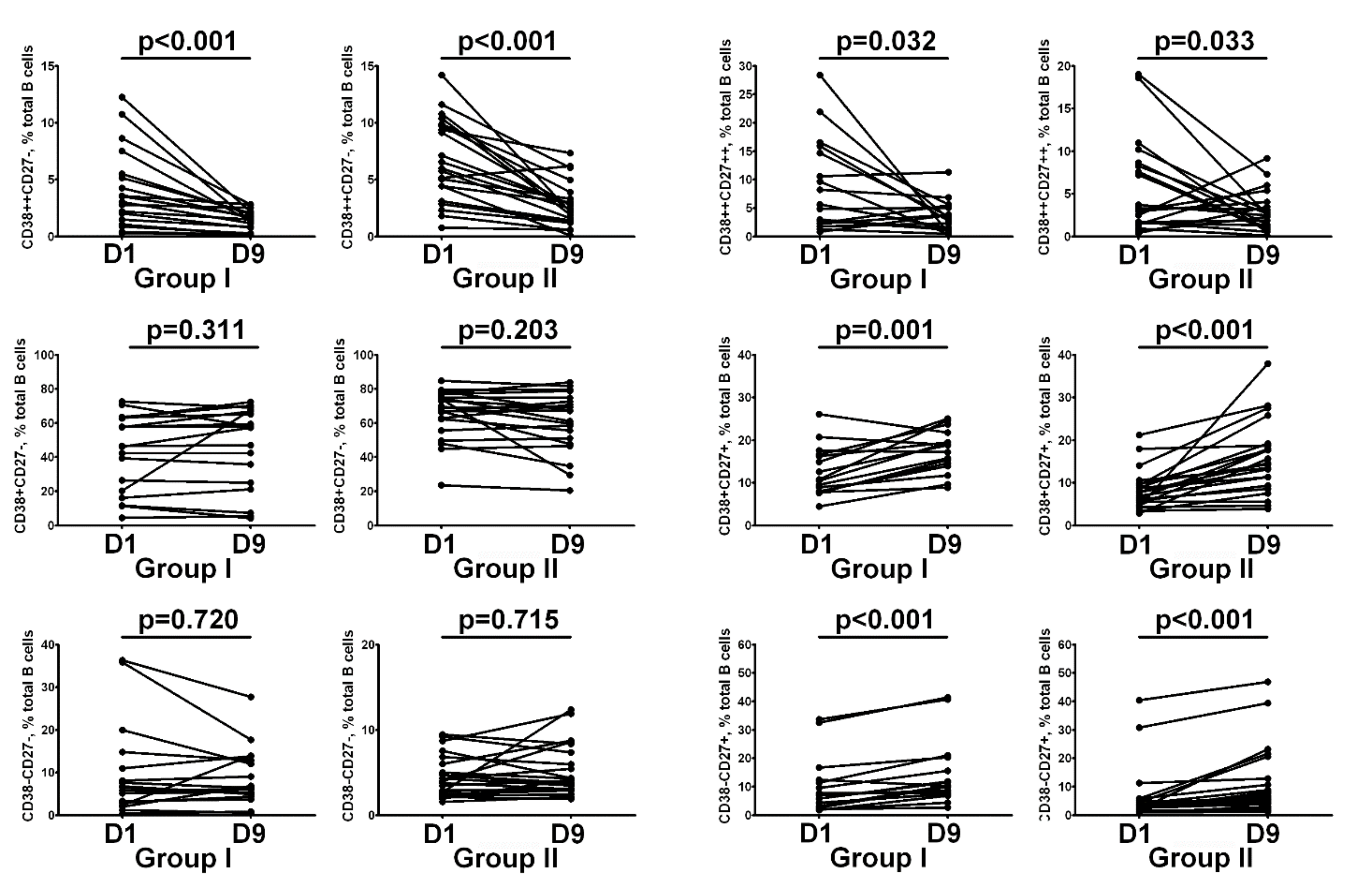
2.6. Immunological Data
2.7. Study Objective
2.8. Statistical Analysis
3. Results
4. Discussion
5. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kazemi, A.; Mohammadi, V.; Aghababaee, S.K.; Golzarand, M.; Clark, C.C.T.; Babajafari, S. Association of Vitamin D status with SARS-CoV-2 infection or COVID-19 severity: A systematic review and meta-analysis. Adv. Nutr. 2021, 12, 1636–1658. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.J.; Hesketh, K.; Power, C.; Hypponen, E. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br. J. Nutr. 2011, 106, 1433–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufman, H.W.; Niles, J.K.; Kroll, M.H.; Bi, C.; Holick, M.F. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS ONE 2020, 15, e0239252. [Google Scholar] [CrossRef] [PubMed]
- Mercola, J.; Grant, W.B.; Wagner, C.L. Evidence Regarding Vitamin D and Risk of COVID-19 and Its Severity. Nutrients 2020, 12, 3361. [Google Scholar] [CrossRef]
- Khammissa, R.; Fourie, J.; Motswaledi, M.H.; Ballyram, R.; Lemmer, J.; Feller, L. The Biological Activities of Vitamin D and Its Receptor in Relation to Calcium and Bone Homeostasis, Cancer, Immune and Cardiovascular Systems, Skin Biology, and Oral Health. BioMed Res. Int. 2018, 2018, 9276380. [Google Scholar] [CrossRef]
- Agraz-Cibrian, J.M.; Giraldo, D.M.; Urcuqui-Inchima, S. 25-Dihydroxyvitamin D3 induces formation of neutrophil extracellular trap-like structures and modulates the transcription of genes whose products are neutrophil extracellular trap-associated proteins: A pilot study. Steroids 2019, 141, 14–22. [Google Scholar] [CrossRef]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and extraskeletal actions of Vitamin D: Current evidence and outstanding questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Yang, J.; Chen, J.; Luo, Q.; Zhang, Q.; Zhang, H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol. Med. Rep. 2017, 16, 7432–7438. [Google Scholar] [CrossRef] [Green Version]
- Karonova, T.L.; Andreeva, A.T.; Golovatuk, K.A.; Bykova, E.S.; Simanenkova, A.V.; Vashukova, M.A.; Grant, W.B.; Shlyakhto, E.V. Low 25(OH)D Level Is Associated with Severe Course and Poor Prognosis in COVID-19. Nutrients 2021, 13, 3021. [Google Scholar] [CrossRef]
- Karonova, T.L.; Kudryavtsev, I.V.; Golovatyuk, K.A.; Aquino, A.D.; Kalinina, O.V.; Chernikova, A.T.; Zaikova, E.K.; Lebedev, D.A.; Bykova, E.S.; Golovkin, A.S.; et al. Vitamin D Status and Immune Response in Hospitalized Patients with Moderate and Severe COVID-19. Pharmaceuticals 2022, 15, 305. [Google Scholar] [CrossRef]
- Carpagnano, G.E.; Di Lecce, V.; Quaranta, V.N.; Zito, A.; Buonamico, E.; Capozza, E.; Palumbo, A.; Di Gioia, G.; Valerio, V.N.; Resta, O. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J. Endocrinol. Investig. 2021, 44, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, H.A.; de Silva, N.L.; Sumanatilleke, M.; de Silva, S.D.N.; Gamage, K.K.K.; Dematapitiya, C.; Kuruppu, D.C.; Ranasinghe, P.; Pathmanathan, S.; Katulanda, P. Prognostic and therapeutic role of vitamin D in COVID-19: Systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2021, 107, 1484–1502. [Google Scholar] [CrossRef] [PubMed]
- Prevention, diagnosis and treatment of new coronavirus infection (COVID-19). In Temporary Guidelines; Version 9; Moscow, Russia, 2020. (In Russian)
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golovkin, A.; Kalinina, O.; Bezrukikh, V.; Aquino, A.; Zaikova, E.; Karonova, T.; Melnik, O.; Vasilieva, E.; Kudryavtsev, I. Imbalanced Immune Response of T-Cell and B-Cell Subsets in Patients with Moderate and Severe COVID-19. Viruses 2021, 13, 1966. [Google Scholar] [CrossRef]
- Hanley, P.; Sutter, J.A.; Goodman, N.G.; Du, Y.; Sekiguchi, D.R.; Meng, W.; Rickels, M.R.; Naji, A.; Luning Prak, E.T. Circulating B cells in type 1 diabetics exhibit fewer maturation-associated phenotypes. Clin. Immunol. 2017, 183, 336–343. [Google Scholar] [CrossRef]
- Kaya, M.O.; Pamukçu, E.; Yakar, B. The role of vitamin D deficiency on COVID-19: A systematic review and meta-analysis of observational studies. Epidemiol. Health 2021, 43, e2021074. [Google Scholar] [CrossRef]
- Grant, W.B.; Anouti, F.A.; Boucher, B.J.; Dursun, E.; Gezen-Ak, D.; Jude, E.B.; Karonova, T.; Pludowski, P. A Narrative Review of the Evidence for Variations in Serum 25-Hydroxyvitamin D Concentration Thresholds for Optimal Health. Nutrients 2022, 14, 639. [Google Scholar] [CrossRef]
- Seal, K.H.; Bertenthal, D.; Carey, E.; Grunfeld, C.; Bikle, D.D.; Lu, C.M. Association of Vitamin D Status and COVID-19-Related Hospitalization and Mortality. J. Gen. Intern. Med. 2022, 37, 853–861. [Google Scholar] [CrossRef]
- Annweiler, G.; Corvaisier, M.; Gautier, J.; Dubée, V.; Legrand, E.; Sacco, G.; Annweiler, C. Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study. Nutrients 2020, 12, 3377. [Google Scholar] [CrossRef]
- Efird, J.T.; Anderson, E.J.; Jindal, C.; Redding, T.S.; Thompson, A.D.; Press, A.M.; Upchurch, J.; Williams, C.D.; Choi, Y.M.; Suzuki, A. The Interaction of Vitamin D and Corticosteroids: A Mortality Analysis of 26,508 Veterans Who Tested Positive for SARS-CoV-2. Int. J. Environ. Res. Public Health 2022, 19, 447. [Google Scholar] [CrossRef]
- Rastogi, A.; Bhansali, A.; Khare, N.; Suri, V.; Yaddanapudi, N.; Sachdeva, N.; Puri, G.D.; Malhotra, P. Short term, high-dose vitamin D supplementation for COVID-19 disease: A randomised, placebo-controlled, study (SHADE study). Postgrad. Med. J. 2022, 98, 87–90. [Google Scholar] [CrossRef]
- Shah, K.; Varna, V.P.; Sharma, U.; Mavalankar, D. Does vitamin D supplementation reduce COVID-19 severity?: A systematic review. QJM Int. J. Med. 2022, 15, hcac040. [Google Scholar] [CrossRef] [PubMed]
- Murai, I.H.; Fernandes, A.L.; Sales, L.P.; Pinto, A.J.; Goessler, K.F.; Duran, C.S.C.; Silva, C.B.R.; Franco, A.S.; Macedo, M.B.; Dalmolin, H.H.H.; et al. Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.F.; Broad, E.; Murphy, R.; Pappachan, J.M.; Pardesi-Newton, S.; Kong, M.F.; Jude, E.B. High-Dose Cholecalciferol Booster Therapy is Associated with a Reduced Risk of Mortality in Patients with COVID-19: A Cross-Sectional Multi-Centre Observational Study. Nutrients 2020, 12, 3799. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Casado, G.; Vigón, L.; Rodríguez-Mora, S.; Mateos, E.; Ramos-Martín, F.; López-Wolf, D.; Sanz-Moreno, J.; Ryan-Murua, P.; Taboada-Martínez, M.L.; et al. Changes in the immune response against SARS-CoV-2 in individuals with severe COVID-19 treated with high dose of vitamin D. Biomed. Pharmacother. 2022, 150, 112965. [Google Scholar] [CrossRef] [PubMed]
- Gonen, M.S.; Alaylioglu, M.; Durcan, E.; Ozdemir, Y.; Sahin, S.; Konukoglu, D.; Nohut, O.K.; Urkmez, S.; Kucukece, B.; Balkan, I.I.; et al. Rapid and Effective Vitamin D Supplementation May Present Better Clinical Outcomes in COVID-19 (SARS-CoV-2) Patients by Altering Serum INOS1, IL1B, IFNg, Cathelicidin-LL37, and ICAM1. Nutrients 2021, 13, 4047. [Google Scholar] [CrossRef]
- Alcala-Diaz, J.F.; Limia-Perez, L.; Gomez-Huelgas, R.; Martin-Escalante, M.D.; Cortes-Rodriguez, B.; Zambrana-Garcia, J.L.; Entrenas-Castillo, M.; Perez-Caballero, A.I.; López-Carmona, M.D.; Garcia-Alegria, J.; et al. Calcifediol Treatment and Hospital Mortality Due to COVID-19: A Cohort Study. Nutrients 2021, 13, 1760. [Google Scholar] [CrossRef]
- Oristrell, J.; Oliva, J.C.; Casado, E.; Subirana, I.; Domínguez, D.; Toloba, A.; Balado, A.; Grau, M. Vitamin D supplementation and COVID-19 risk: A population-based, cohort study. J. Endocrinol. Investig. 2022, 45, 167–179. [Google Scholar] [CrossRef]
- Soliman, A.R.; Abdelaziz, T.S.; Fathy, A. Impact of Vitamin D Therapy on the Progress COVID-19: Six Weeks Follow-Up Study of Vitamin D Deficient Elderly Diabetes Patients. Proc. Singap. Healthc. 2021, 31, 1–5. [Google Scholar] [CrossRef]
- Mazziotti, G.; Formenti, A.M.; Frara, S.; Doga, M.; Giustina, A. Vitamin D and Glucocorticoid-Induced Osteoporosis. Front. Horm. Res. 2018, 50, 149–160. [Google Scholar] [CrossRef]
- Kudryavtsev, I.; Kalinina, O.; Bezrukikh, V.; Melnik, O.; Golovkin, A. The significance of phenotyping and quantification of plasma extracellular vesicles levels using high-sensitivity flow cytometry during covid-19 treatment. Viruses 2021, 13, 767. [Google Scholar] [CrossRef]
- Gao, Y.D.; Ding, M.; Dong, X.; Zhang, J.J.; Kursat Azkur, A.; Azkur, D.; Gan, H.; Sun, Y.L.; Fu, W.; Li, W.; et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef] [PubMed]
- Maghbooli, Z.; Sahraian, M.A.; Jamalimoghadamsiahkali, S.; Asadi, A.; Zarei, A.; Zendehdel, A.; Varzandi, T.; Mohammadnabi, S.; Alijani, N.; Karimi, M.; et al. Treatment With 25-Hydroxyvitamin D3 (Calcifediol) Is Associated With a Reduction in the Blood Neutrophil-to-Lymphocyte Ratio Marker of Disease Severity in Hospitalized Patients With COVID-19: A Pilot Multicenter, Randomized, Placebo-Controlled, Double-Blinded Clinical Trial. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2021, 27, 1242–1251. [Google Scholar] [CrossRef]
- Deluca, H.F.; Cantorna, M.T. Vitamin D: Its role and uses in immunology. FASEB J. 2001, 15, 2579–2585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef]
- Hayes, C.E.; Hubler, S.L.; Moore, J.R.; Barta, L.E.; Praska, C.E.; Nashold, F.E. Vitamin D Actions on CD4(+) T Cells in Autoimmune Disease. Front. Immunol. 2015, 6, 100. [Google Scholar] [CrossRef]
- Todosenko, N.; Vulf, M.; Yurova, K.; Khaziakhmatova, O.; Mikhailova, L.; Litvinova, L. Causal Links between Hypovitaminosis D and Dysregulation of the T Cell Connection of Immunity Associated with Obesity and Concomitant Pathologies. Biomedicines 2021, 9, 1750. [Google Scholar] [CrossRef]
- Geldmeyer-Hilt, K.; Heine, G.; Hartmann, B.; Baumgrass, R.; Radbruch, A.; Worm, M. 1,25-dihydroxyvitamin D3 impairs NF-κB activation in human naïve B cells. Biochem. Biophys. Res. Commun. 2011, 407, 699–702. [Google Scholar] [CrossRef]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef] [Green Version]
- Heine, G.; Niesner, U.; Chang, H.D.; Steinmeyer, A.; Zügel, U.; Zuberbier, T.; Radbruch, A.; Worm, M. 1,25-dihydroxyvitamin D(3) promotes IL-10 production in human B cells. Eur. J. Immunol. 2008, 38, 2210–2218. [Google Scholar] [CrossRef]
- Rolf, L.; Muris, A.H.; Hupperts, R.; Damoiseaux, J. Vitamin D effects on B cell function in autoimmunity. Ann. N. Y. Acad. Sci. 2014, 1317, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Tonacci, A.; Negrini, S.; Greco, M.; Borro, M.; Puppo, F.; Gangemi, S. Emerging role of vitamin D in autoimmune diseases: An update on evidence and therapeutic implications. Autoimmun. Rev. 2019, 18, 102350. [Google Scholar] [CrossRef]
- Lakkireddy, M.; Gadiga, S.G.; Malathi, R.D.; Karra, M.L.; Raju, I.; Ragini; Chinapaka, S.; Baba, K.; Kandakatla, M. Author Correction: Impact of daily high dose oral vitamin D therapy on the inflammatory markers in patients with COVID-19 disease. Sci. Rep. 2021, 11, 17652. [Google Scholar] [CrossRef] [PubMed]
- Sabico, S.; Enani, M.A.; Sheshah, E.; Aljohani, N.J.; Aldisi, D.A.; Alotaibi, N.H.; Alshingetti, N.; Alomar, S.Y.; Alnaami, A.M.; Amer, O.E.; et al. Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate Covid-19: A Randomized Clinical Trial. Nutrients 2021, 13, 2170. [Google Scholar] [CrossRef]
- Elamir, Y.M.; Amir, H.; Lim, S.; Rana, Y.P.; Lopez, C.G.; Feliciano, N.V.; Omar, A.; Grist, W.P.; Via, M.A. A randomized pilot study using calcitriol in hospitalized COVID-19 patients. Bone 2022, 154, 116175. [Google Scholar] [CrossRef]
- Taefehshokr, N.; Taefehshokr, S.; Heit, B. Mechanisms of Dysregulated Humoral and Cellular Immunity by SARS-CoV-2. Pathogens 2020, 9, 1027. [Google Scholar] [CrossRef]
- Mangge, H.; Kneihsl, M.; Schnedl, W.; Sendlhofer, G.; Curcio, F.; Domenis, R. Immune Responses against SARS-CoV-2—Questions and Experiences. Biomedicines 2021, 9, 1342. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A.; Solomatina, L.; Chereshnev, V. SARS-CoV-2-Specific Immune Response and the Pathogenesis of COVID-19. Int. J. Mol. Sci. 2022, 23, 1716. [Google Scholar] [CrossRef]
- Shafqat, A.; Shafqat, S.; Salameh, S.A.; Kashir, J.; Alkattan, K.; Yaqinuddin, A. Mechanistic Insights Into the Immune Pathophysiology of COVID-19; An In-Depth Review. Front. Immunol. 2022, 13, 835104. [Google Scholar] [CrossRef]
- Kudryavtsev, I.; Rubinstein, A.; Golovkin, A.; Kalinina, O.; Vasilyev, K.; Rudenko, L.; Isakova-Sivak, I. Dysregulated Immune Responses in SARS-CoV-2-Infected Patients: A Comprehensive Overview. Viruses 2022, 14, 1082. [Google Scholar] [CrossRef] [PubMed]
- Terrier, B.; Derian, N.; Schoindre, Y.; Chaara, W.; Geri, G.; Zahr, N.; Mariampillai, K.; Rosenzwajg, M.; Carpentier, W.; Musset, L.; et al. Restoration of regulatory and effector T cell balance and B cell homeostasis in systemic lupus erythematosus patients through vitamin D supplementation. Arthritis Res. Ther. 2012, 17, R221. [Google Scholar] [CrossRef] [Green Version]
- Dankers, W.; Colin, E.M.; van Hamburg, J.P.; Lubberts, E. Vitamin D in Autoimmunity: Molecular Mechanisms and Therapeutic Potential. Front. Immunol. 2017, 7, 697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuwa, H.A.; Shaw, T.N.; Knight, S.B.; Wemyss, K.; McClure, F.A.; Pearmain, L.; Prise, I.; Jagger, C.; Morgan, D.J.; Khan, S.; et al. Alterations in T and B cell function persist in convalescent COVID-19 patients. Med 2021, 2, 720–735.e4. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtsev, I.V.; Arsentieva, N.A.; Batsunov, O.K.; Korobova, Z.R.; Khamitova, I.V.; Isakov, D.V.; Kuznetsova, R.N.; Rubinstein, A.A.; Stanevich, O.V.; Lebedeva, A.A.; et al. Alterations in B Cell and Follicular T-Helper Cell Subsets in Patients with Acute COVID-19 and COVID-19 Convalescents. Curr. Issues Mol. Biol. 2022, 44, 194–205. [Google Scholar] [CrossRef] [PubMed]
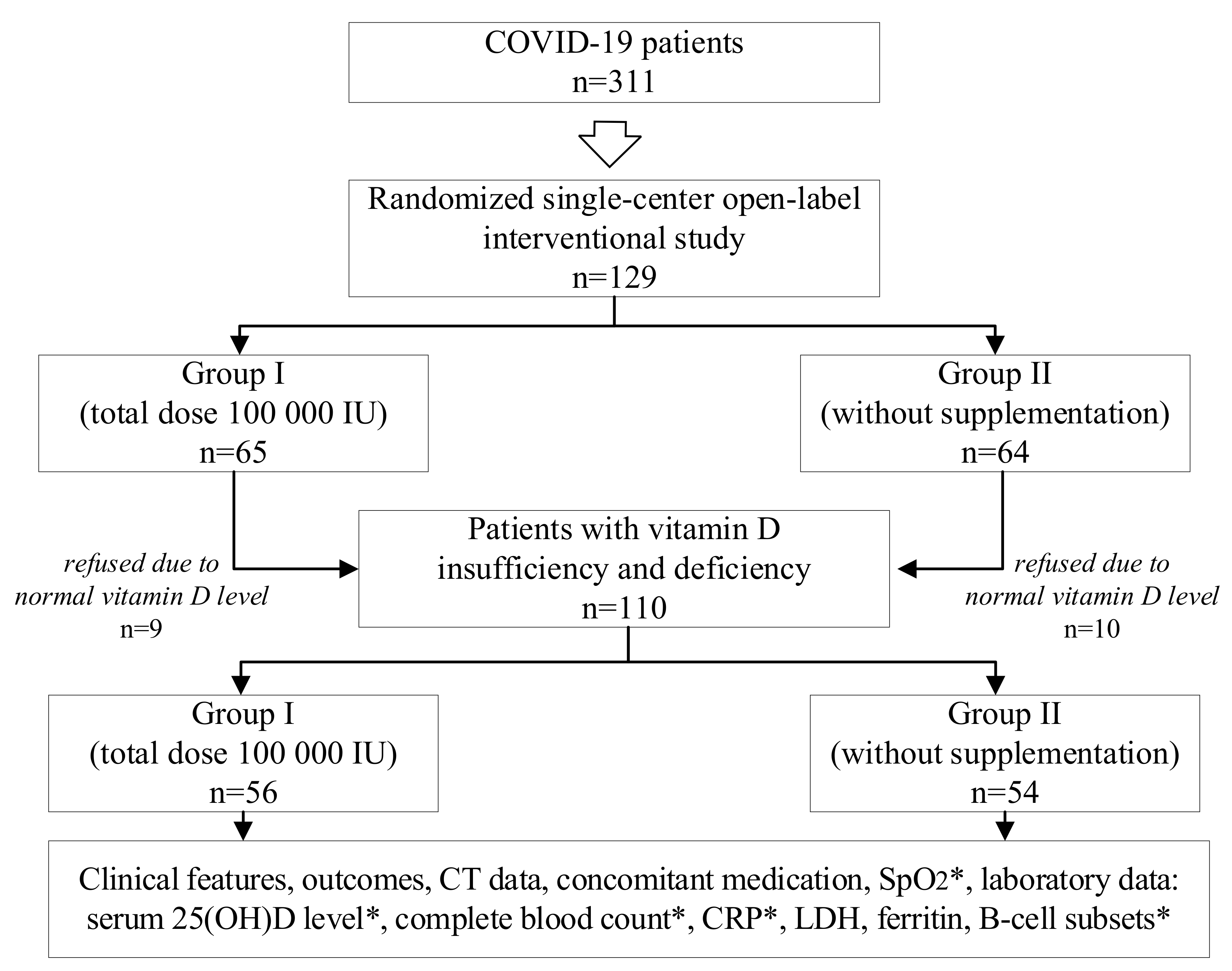
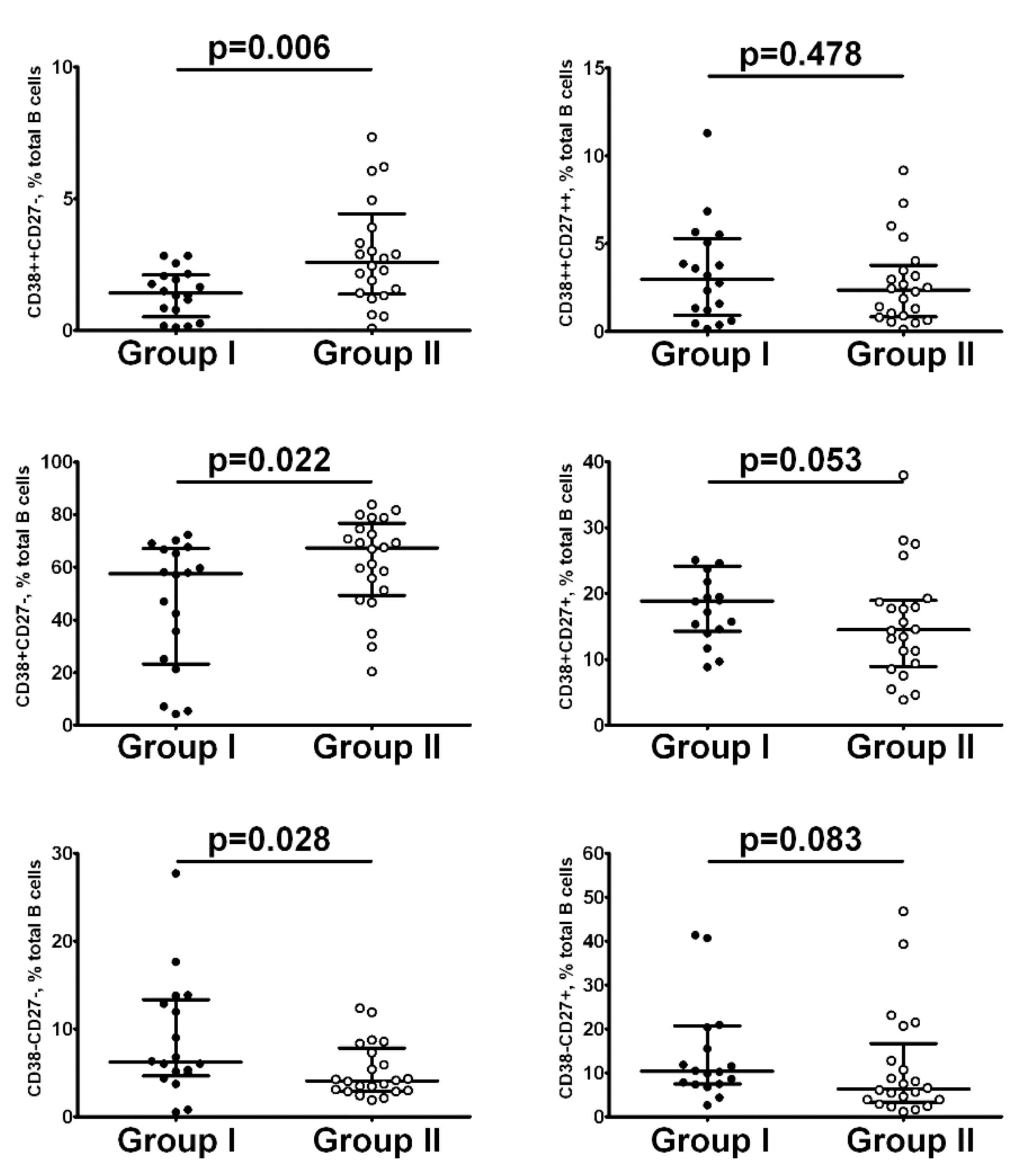
| Parameters | Group I n = 65 | Group II n = 64 | p |
|---|---|---|---|
| Age, years, Me and IQR [25; 75] | 57 [51; 66] | 64 [55; 70] | 0.03 |
| Gender, female, n (%) | 31 (47.7) | 32 (50.0) | 0.86 |
| Days from symptoms onset to hospitalization, days, Me and IQR [25; 75] | 8 [6;10] | 8 [6;10] | 0.37 |
| Severe clinical course, n (%) | 13 (20) | 13 (20) | 0.36 |
| CT lung involvement, %, Me and IQR [25; 75] | 39 [30; 50] | 30 [20; 45] | 0.06 |
| CT grading, n (%) | 0.29 | ||
| 0 | 4 (6) | 2 (3) | |
| 1 | 10 (15) | 20 (30) | |
| 2 | 37 (57) | 33 (52) | |
| 3 | 12 (17) | 6 (10) | |
| 4 | 2 (3) | 3 (5) | |
| SpO2, %, Me and IQR [25; 75] | 95 [92; 97] | 95 [92; 97] | 0.51 |
| Supplemental Oxygenation, n (%) | 38 (58.4) | 32 (50) | 0.35 |
| BMI, kg/m2, Me and IQR [25; 75] | 29.5 [25.5; 32.9] | 28.9 [25.5; 31.4] | 0.41 |
| Obesity, n (%) | 28 (43.1) | 22 (34.9) | 0.42 |
| DM type 2, n (%) | 17 (26.2) | 24 (38.1) | 0.84 |
| AH, n (%) | 46 (70.8) | 49 (76.6) | 0.31 |
| IHD, n (%) | 16 (24.6) | 14 (21.9) | 0.12 |
| Neutrophils, ×109/L, Me and IQR [25; 75] | 4.5 [2.4; 7.1] | 4.2 [2.9; 5.9] | 0.80 |
| Lymphocytes, ×109/L, Me and IQR [25; 75] | 1.3 [0.8; 1.5] | 1.04 [0.7; 1.4] | 0.25 |
| NLR, Me and IQR [25; 75] | 3.7 [2.5; 7.6] | 4.3 [2.7; 8] | 0.15 |
| CRP, mg/L, Me and IQR [25; 75] | 48 [21; 134] | 49 [18; 107] | 0.73 |
| Ferritin, ng/mL, Me and IQR [25; 75] | 610 [243; 610] | 446.1 [237; 825] | 0.12 |
| LDH, µ/L, Me and IQR [25; 75] | 351 [261; 483] | 327.5 [265; 495] | 0.80 |
| 25(OH)D, ng/mL, Me and IQR [25; 75] | 17.8 [11.7; 25.4] | 15.4 [11.0; 22.9] | 0.47 |
| Vitamin D status, n (%) | 0.07 | ||
| Normal | 9 (13.8) | 10 (15.6) | |
| Insufficiency | 20 (30.8) | 11 (17.2) | |
| Deficiency | 36 (55.4) | 43 (67.2) |
| Parameters | Group I n = 56 | Group II n = 54 | p |
|---|---|---|---|
| Age, years, Me and IQR [25; 75] | 58 [50; 65] | 64 [55; 70] | 0.03 |
| 25(OH)D, ng/mL, Me and IQR [25; 75] | 16.4 [11.0; 21.8] | 13.9 [9.7; 17.4] | 0.08 |
| Vitamin D status, n (%) | 0.07 | ||
| Insufficiency | 20 (36) | 11 (20) | |
| Deficiency | 36 (64) | 43 (80) | |
| CT lung involvement, %, Me and IQR [25; 75] | 42 [30; 48.5] | 32.5 [20.5; 45] | 0.21 |
| CT grading, n (%) | 0.77 | ||
| 1 | 11 (19.6) | 19 (35.2) | |
| 2 | 33 (58.9) | 26 (48.1) | |
| 3 | 11 (19.6) | 6 (11.1) | |
| 4 | 1 (1.9) | 3 (5.6) | |
| SpO2, %, Me and IQR [25; 75] | 95 [92; 97] | 95 [92; 97] | 0.50 |
| Supplemental Oxygenation, n (%) | 38 (68) | 32 (59) | 0.35 |
| Neutrophils, ×109/L, Me and IQR [25; 75] | 4.3 [2.9; 6.0] | 4.3 [2.9; 5.8] | 0.53 |
| Lymphocytes, ×109/L, Me and IQR [25; 75] | 1.3 [0.9; 1.5] | 1.0 [0.7; 1.3] | 0.16 |
| NLR, Me + IQR [25; 75] | 3.5 [2.2; 5.3] | 4.7 [2.6; 7.3] | 0.09 |
| CRP, mg/L, Me and IQR [25; 75] | 48.2 [22.7; 135.3] | 47.5 [17.5; 99.0] | 0.97 |
| Ferritin, ng/mL, Me and IQR [25; 75] | 559 [217; 925] | 365 [229; 765] | 0.21 |
| LDH, µ/L, Me and IQR [25; 75] | 351 [261; 516] | 327 [261; 496] | 0.84 |
| Concomitant medication | |||
| GC | |||
| Dexamethasone, n (%) | 47 (84) | 43 (80) | 0.67 |
| Dexamethasone, mg | 136 [72; 214] | 149 [112; 234] | 0.99 |
| Prednisolone, n (%) | 15 (26.7) | 11 (20.3) | 0.53 |
| Prednisolone, mg | 1295 [846; 1658] | 1140 [375; 1703] | 0.60 |
| Anti-IL-6 receptor monoclonal antibodies, n (%) | 16 (28.5) | 18 (33.3) | 0.59 |
| Olokizumab, n (%) | 13 (23.2) | 15 (27.7) | 0.39 |
| Levilimab, n (%) | 2 (3.5) | 2 (3.7) | 0.38 |
| Tocilizumab, n (%) | 4 (7.14) | 3 (5.55) | 0.74 |
| Anticoagulant therapy, n (%) | 56 (100) | 54 (100) | - |
| Antibiotics therapy, n (%) | 8 (12.5) | 11 (20.4) | 0.26 |
| Parameters | Group I n = 56 | Group II n = 54 | p |
|---|---|---|---|
| Vitamin D status, n (%) | |||
| Normal | 13 (23) | 1 (2) | |
| Insufficiency | 20 (36) | 3 (6) | |
| Deficiency | 23 (41) | 50 (92) | <0.001 |
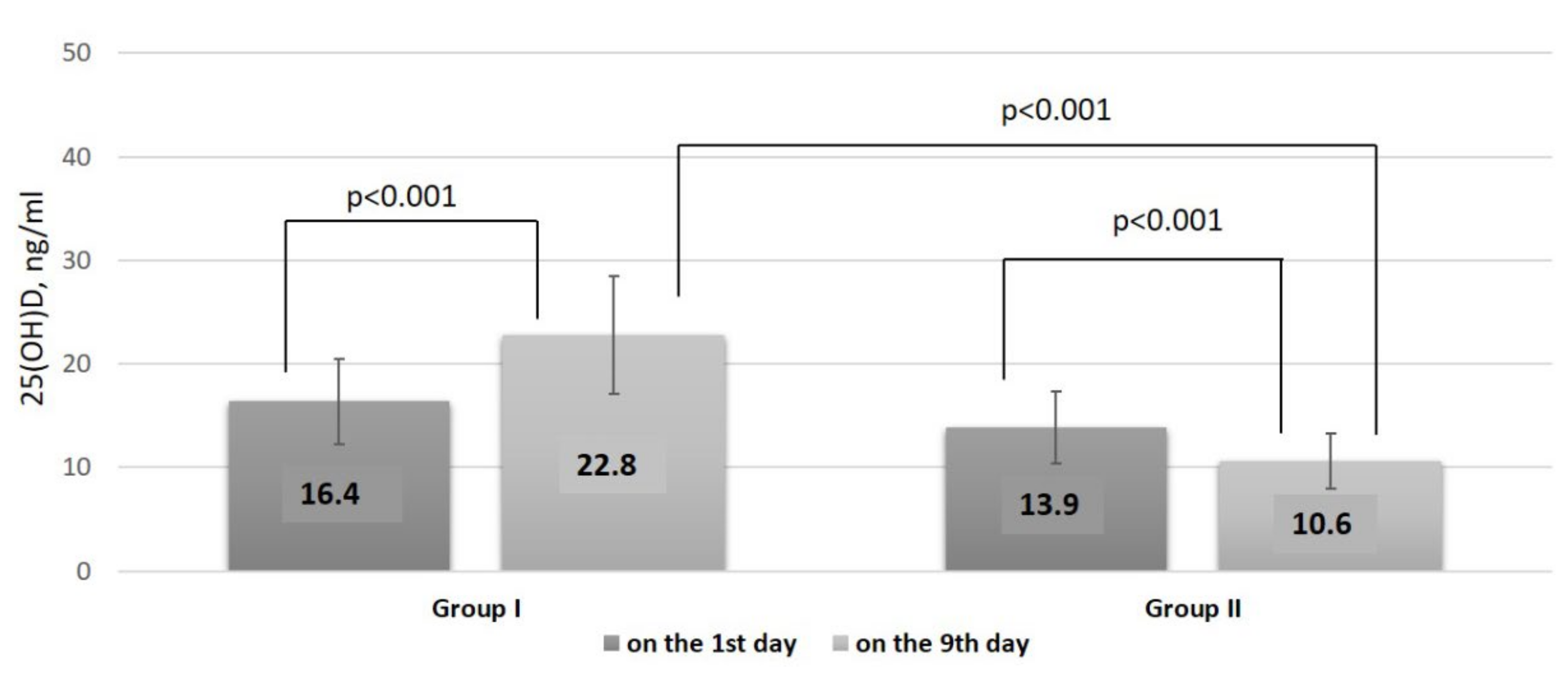
| 25(OH)D, ng/mL, Me and IQR [25; 75] | 22.8 [17.7; 27.7] | 10.6 [8.4; 14.9] | <0.001 |
| Bed days, Me and IQR [25; 75] | 18 [14; 22] | 17 [14; 23] | 0.87 |
| Discharged, n (%) | 56 (100) | 54 (100) | 0.93 |
| ICU admission rates, n (%) | 0 | 3 (6) | - |
| SpO2, %, Me and IQR [25; 75] | 97 [96; 98] | 97 [96; 98] | 0.56 |
| Supplemental Oxygenation, n (%) | 27 (48) | 28 (52) | 0.70 |
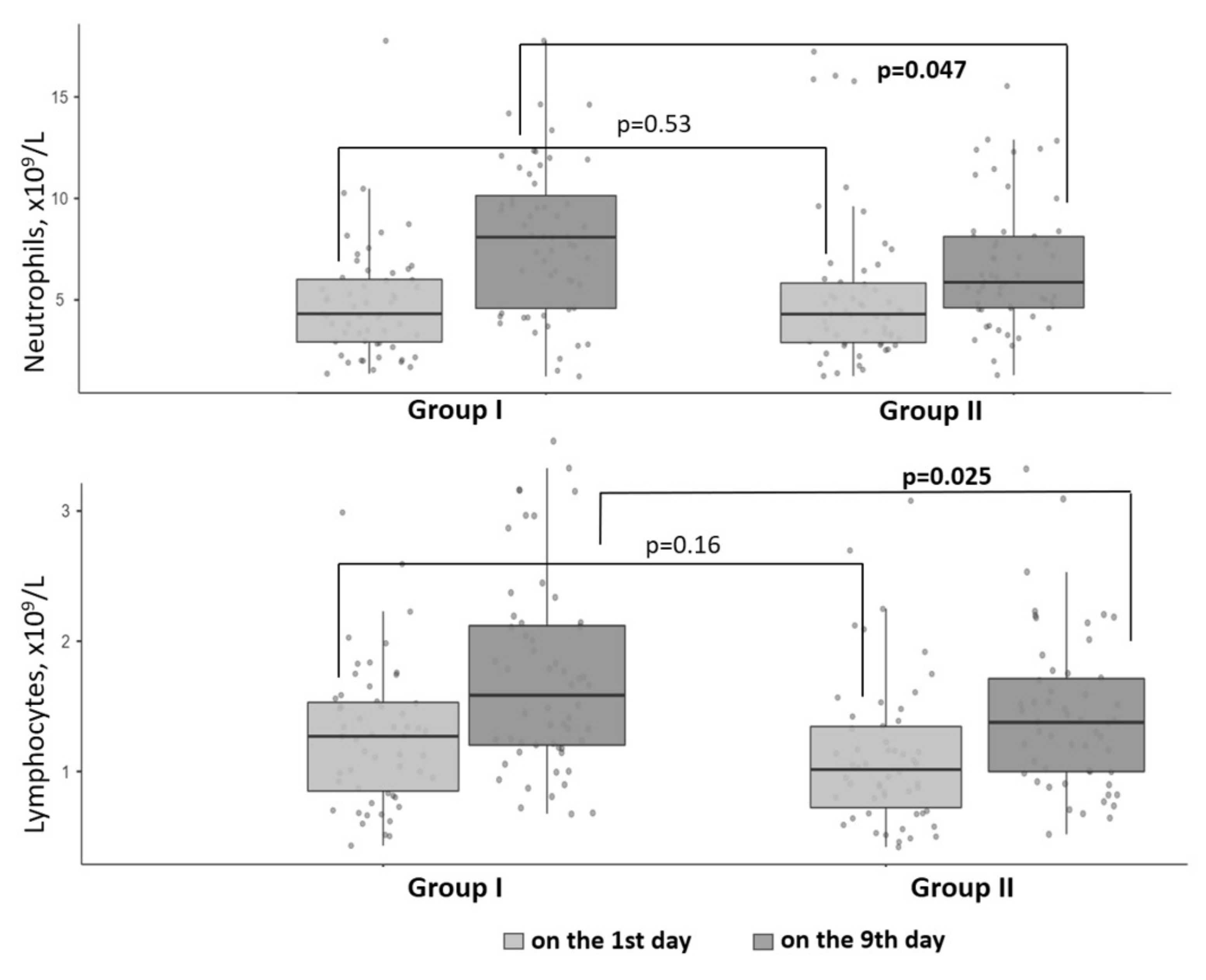
| Neutrophils, ×109/L, Me and IQR [25; 75] | 8.6 [5.1; 10.6] | 6.4 [5.2; 8.6] | 0.04 |
| Lymphocytes, ×109/L, Me and IQR [25; 75] | 1.8 [1.3; 2.6] | 1.58 [1.0; 2.0] | 0.02 |
| NLR, Me and IQR [25; 75] | 4.5 [2.6; 6.9] | 4.4 [2.7; 7.0] | 0.71 |
| CRP, mg/L, Me and IQR [25; 75] | 2 [0.8; 4.7] | 3 [1; 9] | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karonova, T.L.; Golovatyuk, K.A.; Kudryavtsev, I.V.; Chernikova, A.T.; Mikhaylova, A.A.; Aquino, A.D.; Lagutina, D.I.; Zaikova, E.K.; Kalinina, O.V.; Golovkin, A.S.; et al. Effect of Cholecalciferol Supplementation on the Clinical Features and Inflammatory Markers in Hospitalized COVID-19 Patients: A Randomized, Open-Label, Single-Center Study. Nutrients 2022, 14, 2602. https://doi.org/10.3390/nu14132602
Karonova TL, Golovatyuk KA, Kudryavtsev IV, Chernikova AT, Mikhaylova AA, Aquino AD, Lagutina DI, Zaikova EK, Kalinina OV, Golovkin AS, et al. Effect of Cholecalciferol Supplementation on the Clinical Features and Inflammatory Markers in Hospitalized COVID-19 Patients: A Randomized, Open-Label, Single-Center Study. Nutrients. 2022; 14(13):2602. https://doi.org/10.3390/nu14132602
Chicago/Turabian StyleKaronova, Tatiana L., Ksenia A. Golovatyuk, Igor V. Kudryavtsev, Alena T. Chernikova, Arina A. Mikhaylova, Arthur D. Aquino, Daria I. Lagutina, Ekaterina K. Zaikova, Olga V. Kalinina, Alexey S. Golovkin, and et al. 2022. "Effect of Cholecalciferol Supplementation on the Clinical Features and Inflammatory Markers in Hospitalized COVID-19 Patients: A Randomized, Open-Label, Single-Center Study" Nutrients 14, no. 13: 2602. https://doi.org/10.3390/nu14132602
APA StyleKaronova, T. L., Golovatyuk, K. A., Kudryavtsev, I. V., Chernikova, A. T., Mikhaylova, A. A., Aquino, A. D., Lagutina, D. I., Zaikova, E. K., Kalinina, O. V., Golovkin, A. S., Grant, W. B., & Shlyakhto, E. V. (2022). Effect of Cholecalciferol Supplementation on the Clinical Features and Inflammatory Markers in Hospitalized COVID-19 Patients: A Randomized, Open-Label, Single-Center Study. Nutrients, 14(13), 2602. https://doi.org/10.3390/nu14132602








