Drug D, a Diosgenin Derive, Inhibits L-Arginine-Induced Acute Pancreatitis through Meditating GSDMD in the Endoplasmic Reticulum via the TXNIP/HIF-1α Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Experimental Animal
2.3. Experimental Model of AP
2.4. Pancreatic Primary Acinar Cells Isolation
2.5. Endoplasmic Reticulum Protein Extraction
2.6. Histopathological Examination
2.7. Immunofluorescence Staining
2.8. Necrotic Cell Death Measurement
2.9. Oxidative Stress Measurement
2.10. IL-1β Level Measurement
2.11. Western Blot Analysis
2.12. Serum Amylase, Amylase and LDH Secretion Measurement
2.13. Molecular Docking Studies
2.14. Statistical Analysis
3. Results
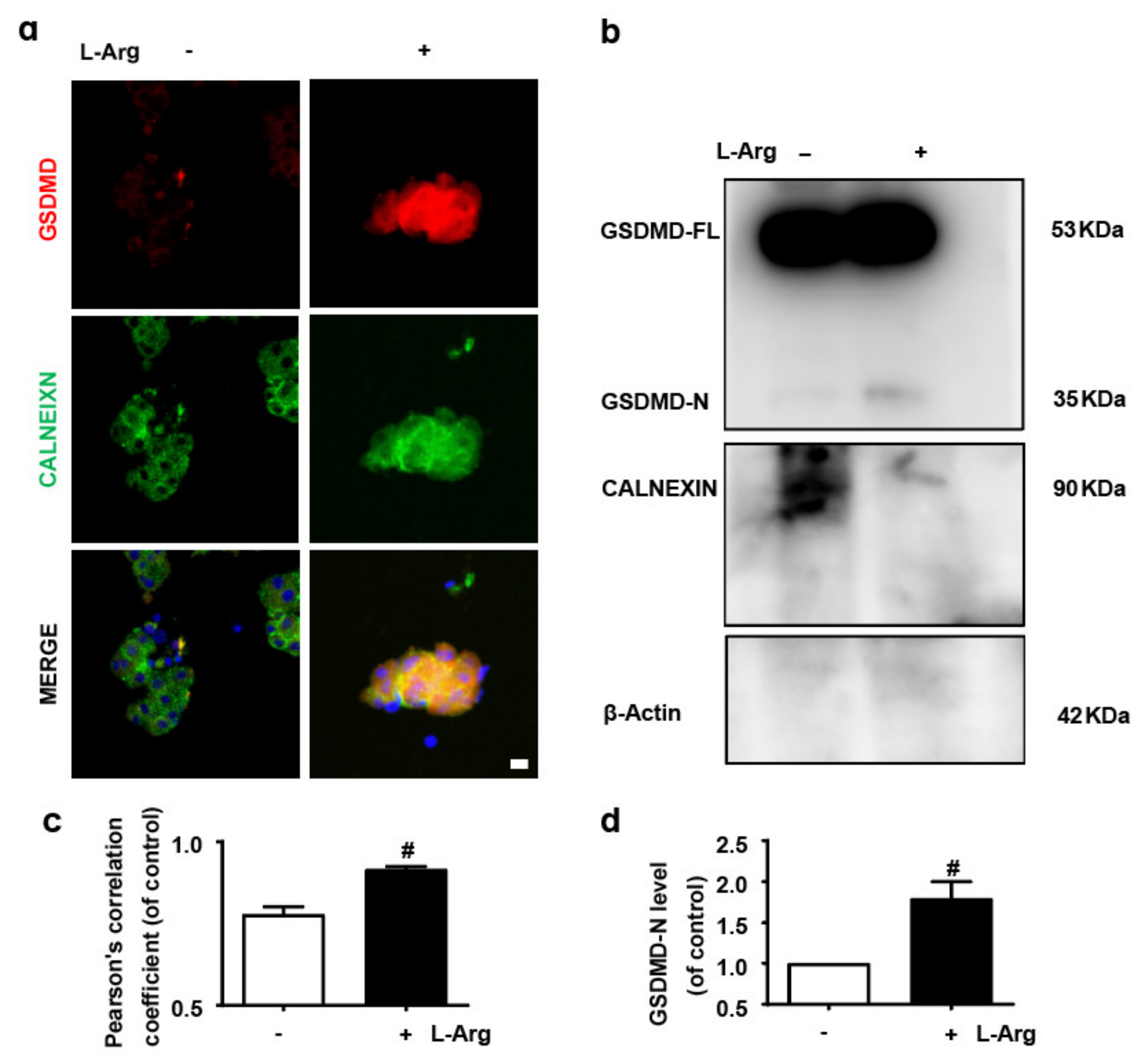
3.1. GSDMD in Endoplasmic Reticulum of Acinar Cells Is Up-Regulated in L-Arginine-Acute Pancreatitis
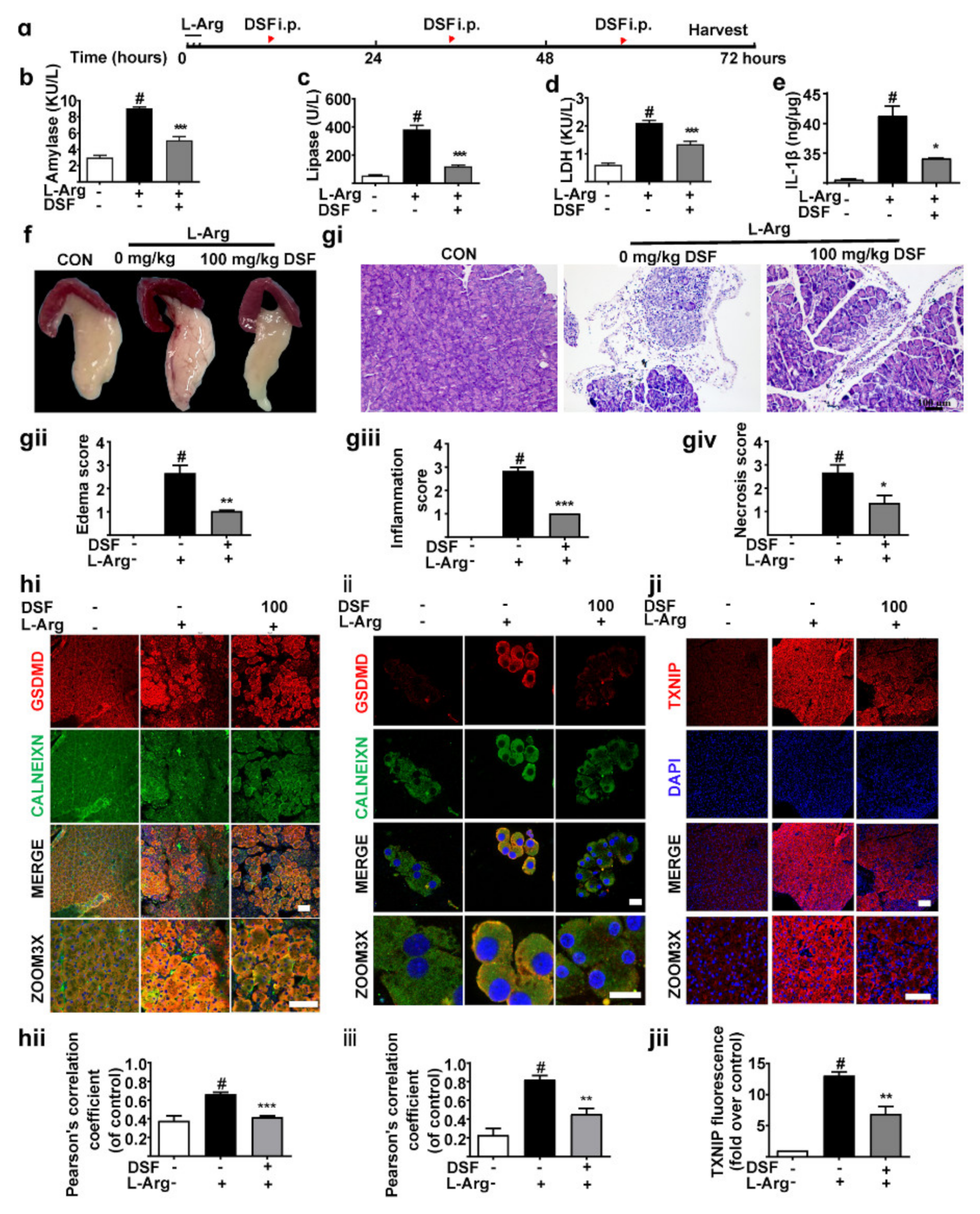
3.2. Inhibition of GSDMD Accumulation in Endoplasmic Reticulum Ameliorates Acute Pancreatitis with the Down-Regulation of TXNIP
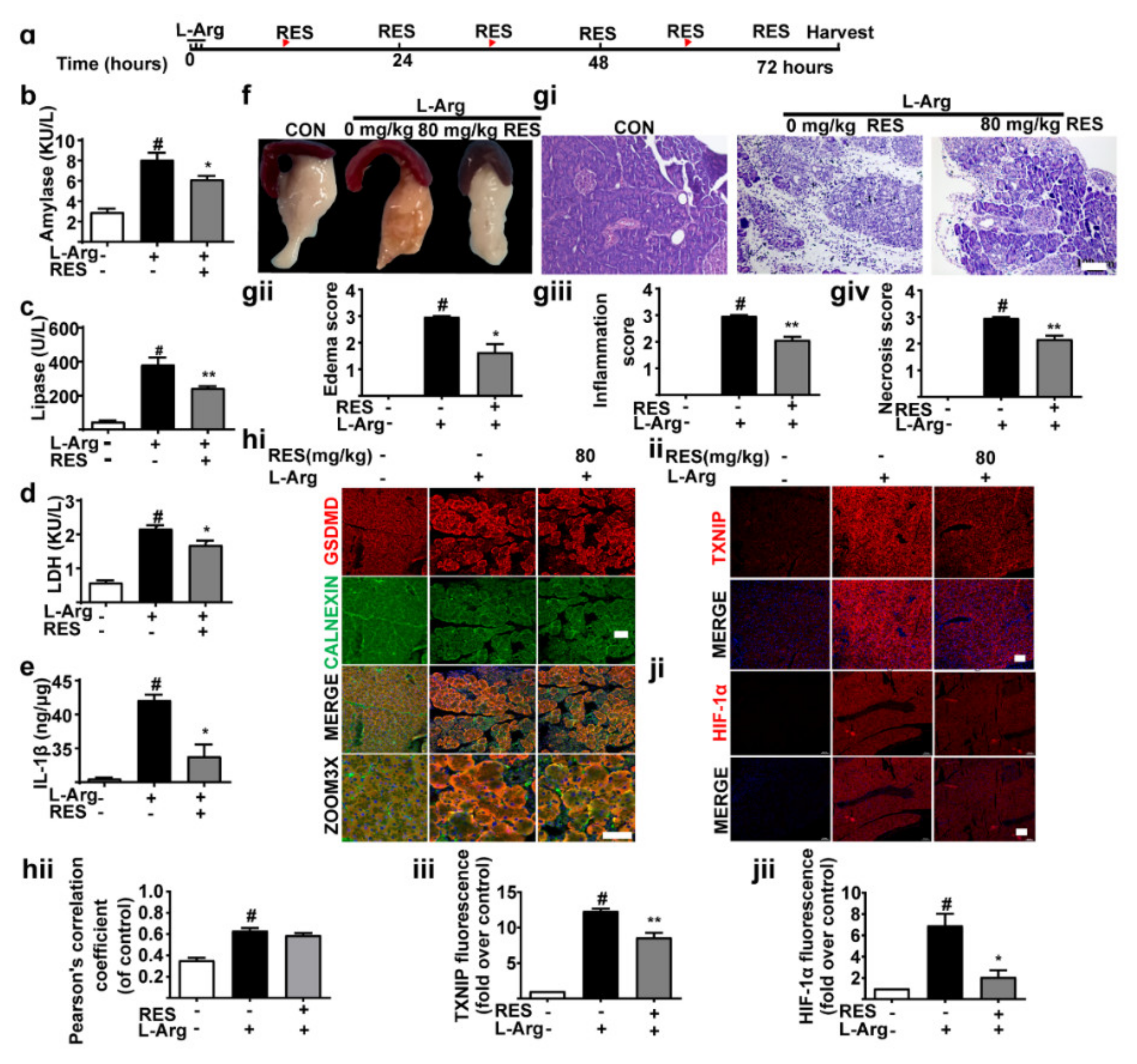
3.3. TXNIP Inhibitor (Resveratrol) Alleviates Pancreatic Necrosis, Systemic Inflammation and Oxidative Stress in L-Arginine Induced Acute Pancreatitis, without Changes of GSDMD
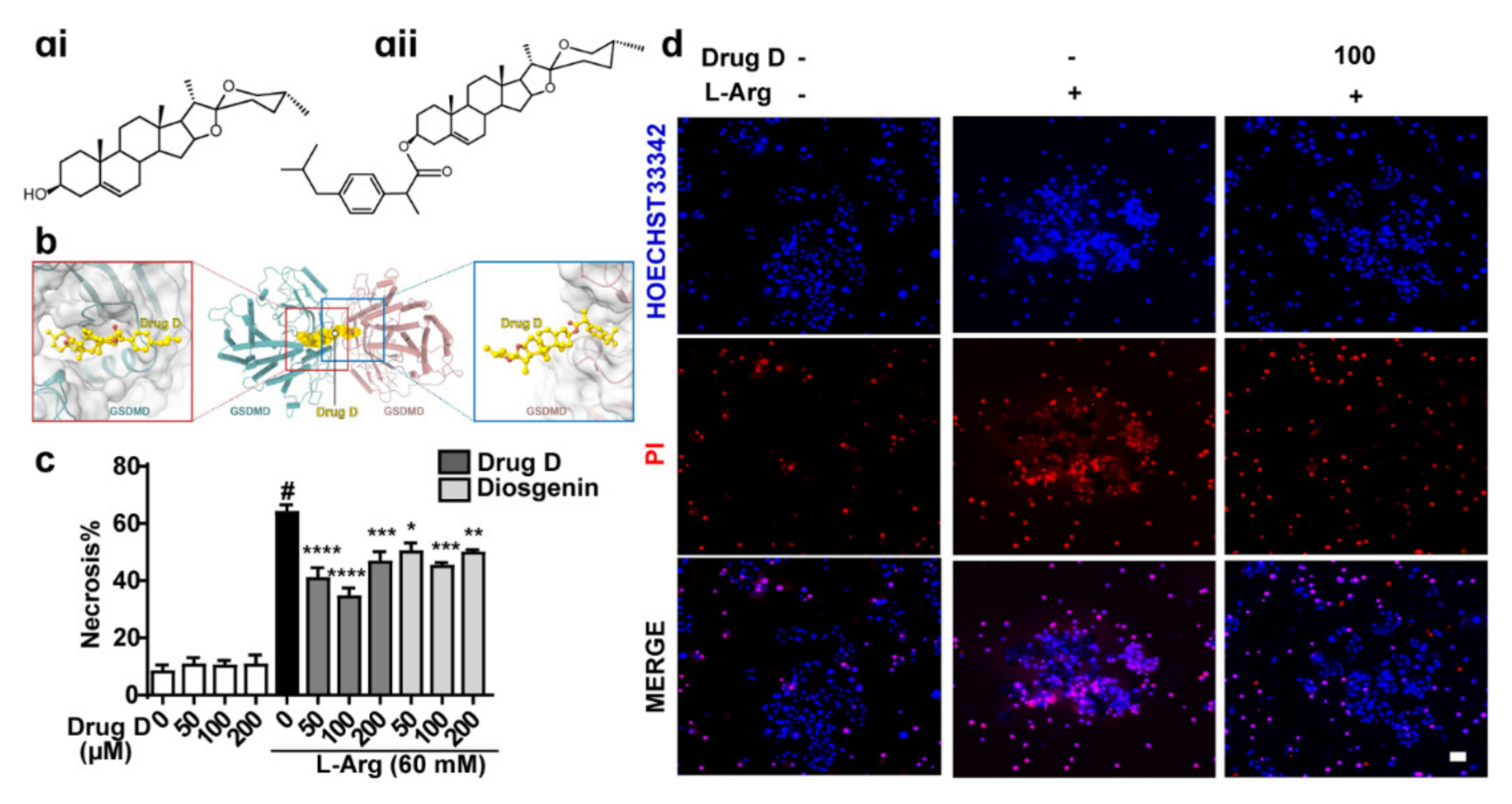
3.4. Drug D, Targeting GSDMD, Protects against L-Arginine-Induced Necrosis of Primary Acinar Cells
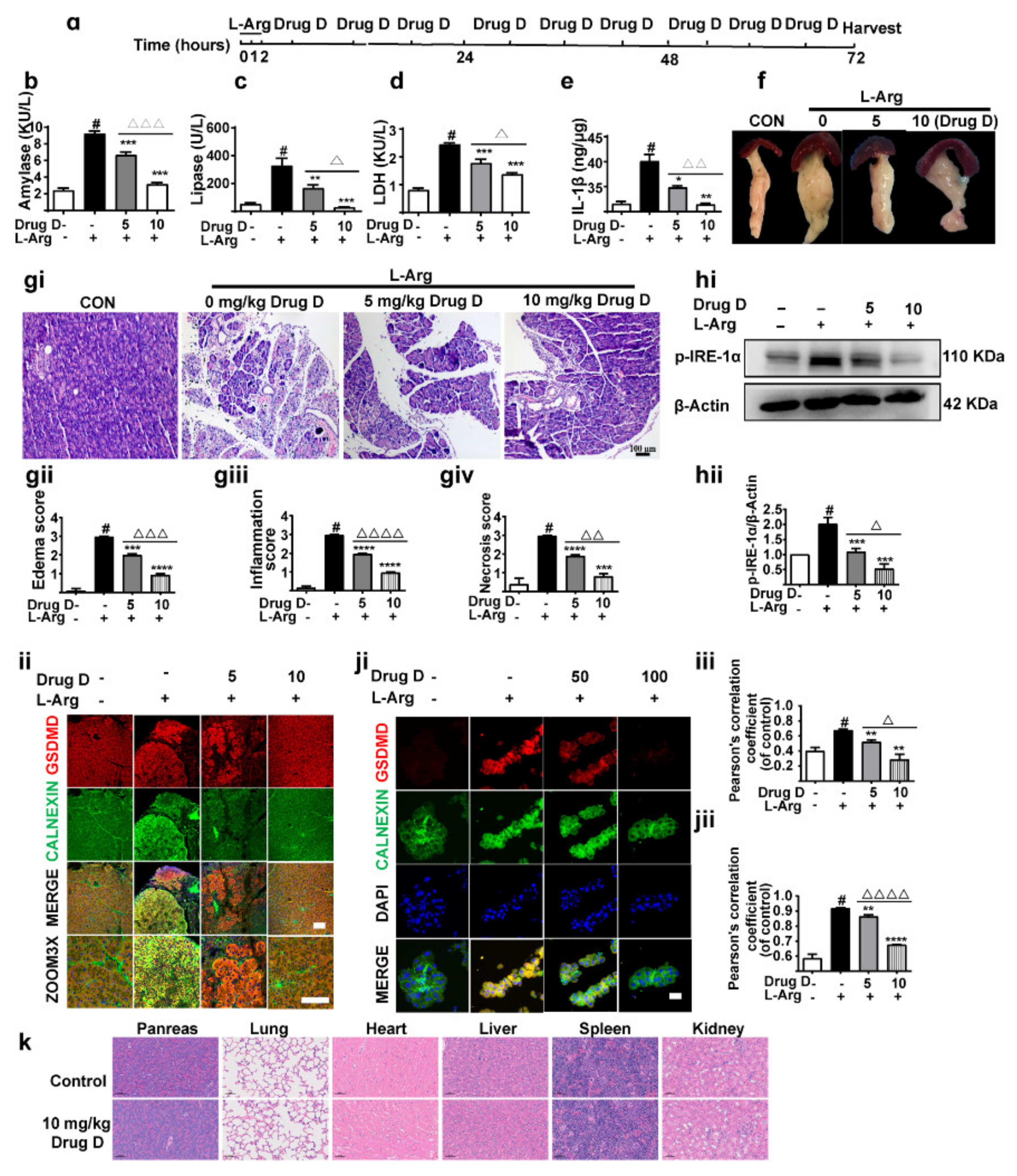
3.5. Drug D Mitigates Pancreatic Necrosis and Systemic Inflammation with the GSDMD Down-Accumulation of Endoplasmic Reticulum in Acute Pancreatitis
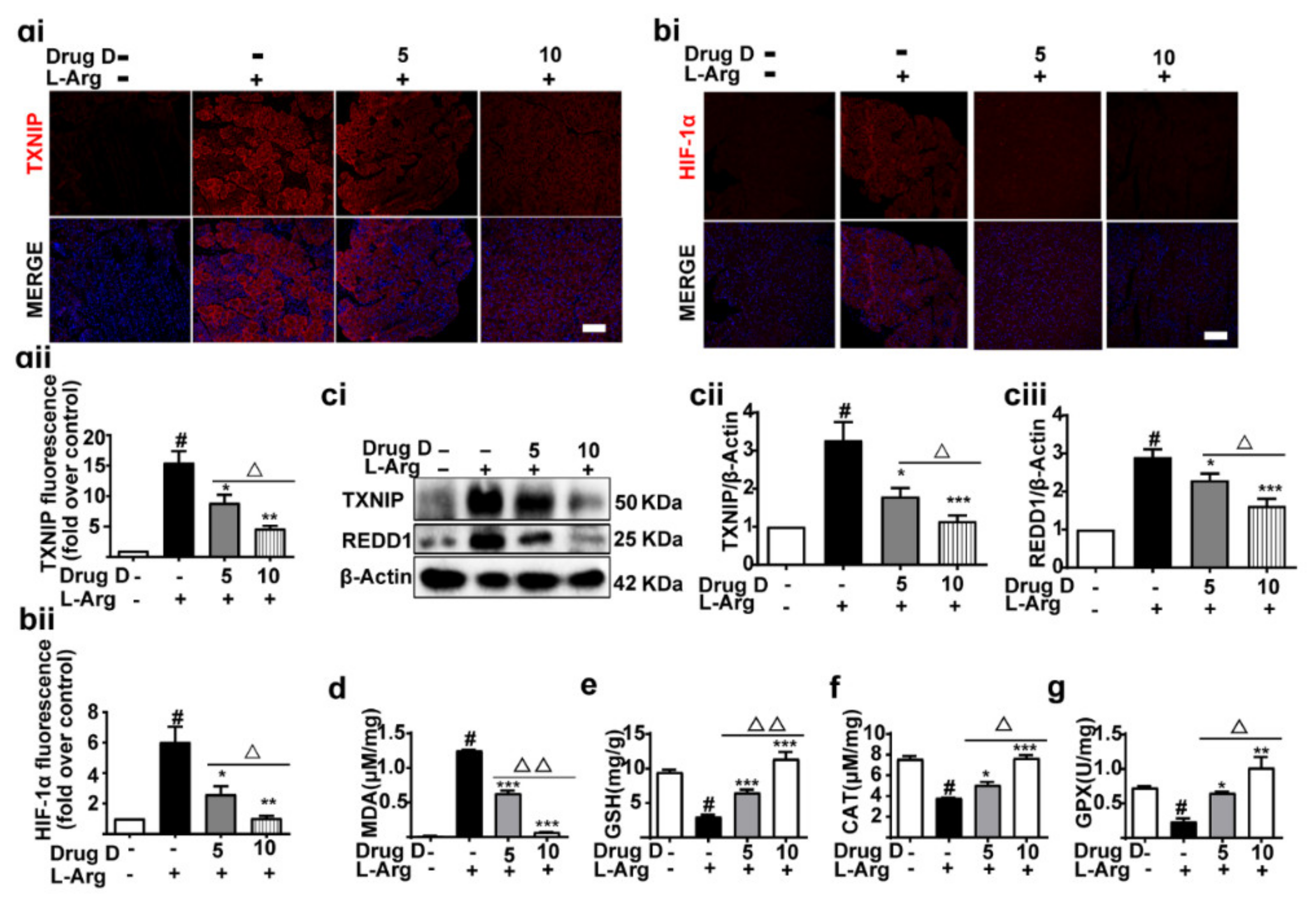
3.6. Drug D Alleviates TXNIP Up-Regulation and Oxidative Stress in Acute Pancreatitis
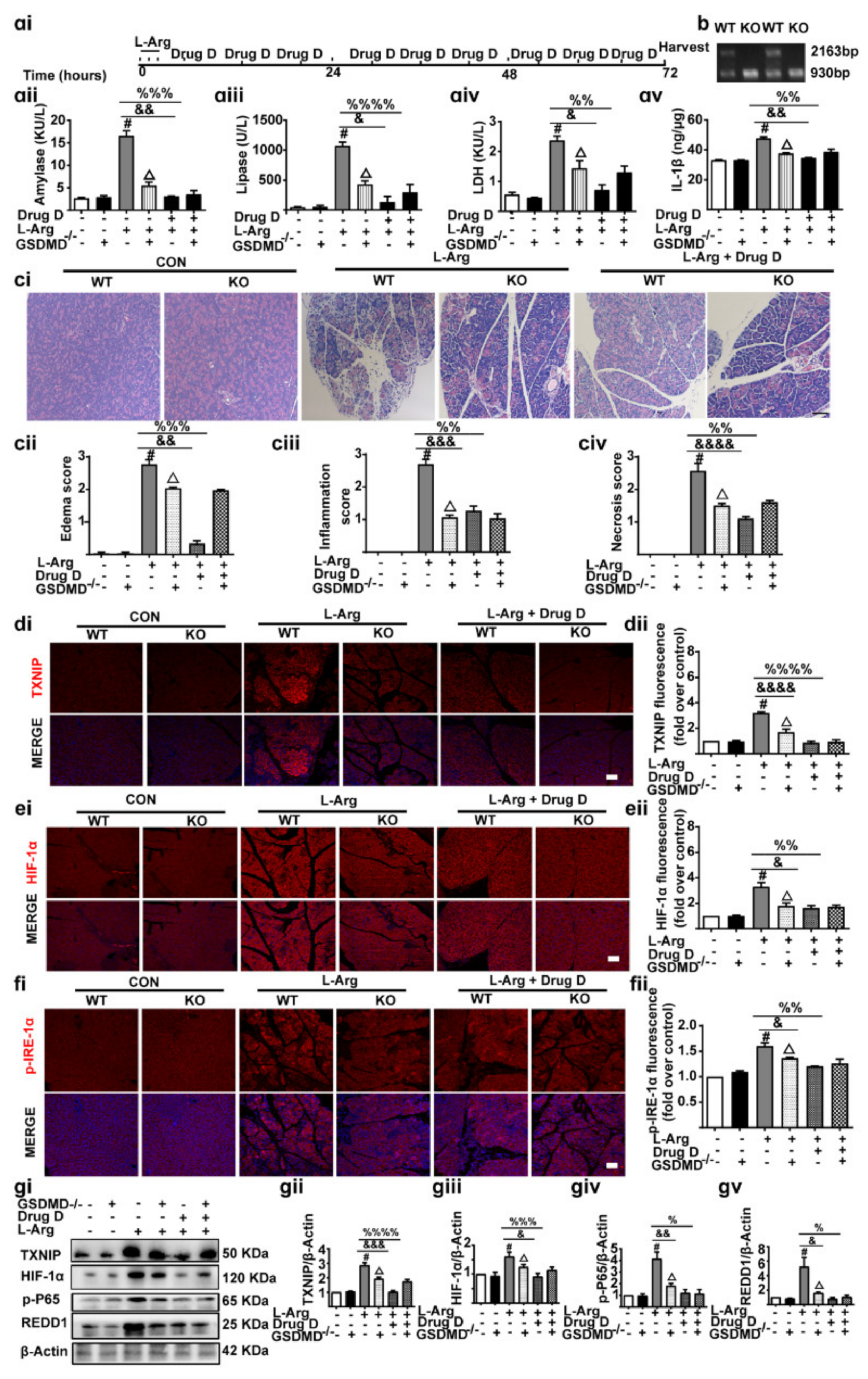
3.7. Knockout of GSDMD Reduces Drug D-Inhibited Pancreatic Necrosis, Systemic Inflammation and Oxidative Stress in Pancreas Tissues
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Barlass, U.; Dutta, R.; Cheema, H.; George, J.; Sareen, A.; Dixit, A.; Yuan, Z.; Giri, B.; Meng, J.; Banerjee, S. Morphine worsens the severity and prevents pancreatic regeneration in mouse models of acute pancreatitis. Gut 2017, 67, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Manohar, M.; Jones, E.K.; Rubin, S.J.S.; Subrahmanyam, P.B.; Swaminathan, G.; Mikhail, D.; Bai, L.; Singh, G.; Wei, Y.; Sharma, V. Novel circulating and tissue monocytes as well as macrophages in pancreatitis and recovery. Gastroenterology 2021, 161, 2014–2029. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Dong, X.; Gong, W.; Huang, W.; Xue, J.; Zhu, Q.; Ma, N.; Chen, W.; Fu, X.; Gao, X. Acinar cell NLRP3 inflammasome and gasdermin D (GSDMD) activation mediates pyroptosis and systemic inflammation in acute pancreatitis. Br. J. Pharmacol. 2021, 178, 3533–3552. [Google Scholar] [CrossRef]
- Fan, R.; Sui, J.; Dong, X.; Jing, B.; Gao, Z. Wedelolactone alleviates acute pancreatitis and associated lung injury via GPX4 mediated suppression of pyroptosis and ferroptosis. Free Radic. Biol. Med. 2021, 173, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.B. Acute pancreatitis. Ann. Intern Med. 2021, 174, Itc17–Itc32. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Cui, R.; Wang, C.; Feng, Y.; Li, Z.; Tong, Y.; Qu, K.; Liu, C.; Zhang, J. Metformin protects against intestinal ischemia-reperfusion injury and cell pyroptosis via TXNIP-NLRP3-GSDMD pathway. Redox Biol. 2020, 32, 101534. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xu, X.; Tang, C.; Gao, P.; Chen, X.; Xiong, X.; Yang, M.; Yang, S.; Zhu, X.; Yuan, S. Reactive oxygen species promote tubular injury in diabetic nephropathy: The role of the mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol. 2018, 16, 32–46. [Google Scholar] [CrossRef]
- Sun, L.; Xiao, L.; Nie, J.; Liu, F.Y.; Ling, G.H.; Zhu, X.J.; Tang, W.B.; Chen, W.C.; Xia, Y.C.; Zhan, M. p66Shc mediates high-glucose and angiotensin II-induced oxidative stress renal tubular injury via mitochondrial-dependent apoptotic pathway. Am. J. Physiol. Ren. Physiol. 2010, 299, F1014–F1025. [Google Scholar] [CrossRef]
- Swentek, L.; Chung, D.; Ichii, H. Antioxidant therapy in pancreatitis. Antioxidants 2021, 10, 657. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Bechard, M.E.; Smalling, R.; Hayashi, A.; Zhong, Y.; Word, A.E.; Campbell, S.L.; Tran, A.V.; Weiss, V.L.; Iacobuzio-Donahue, C.; Wellen, K.E. Pancreatic cancers suppress negative feedback of glucose transport to reprogram chromatin for metastasis. Nat. Commun. 2020, 11, 4055. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Liao, R.; Liu, C.; Liu, S.; Huang, H.; Liu, J.; Jin, T.; Guo, H.; Zheng, Z.; Xia, M. Epigenetic regulation of TXNIP-mediated oxidative stress and NLRP3 inflammasome activation contributes to SAHH inhibition-aggravated diabetic nephropathy. Redox Biol. 2021, 45, 102033. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.; Erkes, D.A.; Nardone, A.; Aplin, A.E.; Fernandes-Alnemri, T.; Alnemri, E.S. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat. Commun. 2019, 10, 1689. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Zhao, Y.; Li, X.; Bai, T.; Wang, S.; Wang, W.; Sun, Y. Effects of penehyclidine hydrochloride on severe acute pancreatitis-associated acute lung injury in rats. Biomed. Pharm. 2018, 97, 1689–1693. [Google Scholar] [CrossRef]
- Mahmoudi, N.; Kiasalari, Z.; Rahmani, T.; Sanaierad, A.; Afshin-Majd, S.; Naderi, G.; Baluchnejadmojarad, T.; Roghani, M. Diosgenin attenuates cognitive impairment in streptozotocin-induced diabetic rats: Underlying mechanisms. Neuropsychobiology 2021, 80, 25–35. [Google Scholar] [CrossRef]
- Gupta, D.D.; Mishra, S.; Verma, S.S.; Shekher, A.; Rai, V.; Awasthee, N.; Das, T.J.; Paul, D.; Das, S.K.; Tag, H.; et al. Evaluation of antioxidant, anti-inflammatory and anticancer activities of diosgenin enriched Paris polyphylla rhizome extract of Indian Himalayan landraces. J. Ethnopharmacol. 2021, 270, 113842. [Google Scholar] [CrossRef]
- Michalak, O.; Krzeczyński, P.; Cieślak, M.; Cmoch, P.; Cybulski, M.; Królewska-Golińska, K.; Kaźmierczak-Barańska, J.; Trzaskowski, B.; Ostrowska, K. Synthesis and anti-tumour, immunomodulating activity of diosgenin and tigogenin conjugates. J. Steroid Biochem. Mol. Biol. 2020, 198, 105573. [Google Scholar] [CrossRef]
- Shen, Y.; Wen, L.; Zhang, R.; Wei, Z.; Shi, N.; Xiong, Q.; Xia, Q.; Xing, Z.; Zeng, Z.; Niu, H. Dihydrodiosgenin protects against experimental acute pancreatitis and associated lung injury through mitochondrial protection and PI3Kγ/Akt inhibition. Br. J. Pharmacol. 2018, 175, 1621–1636. [Google Scholar] [CrossRef] [Green Version]
- Dawra, R.; Sharif, R.; Phillips, P.; Dudeja, V.; Dhaulakhandi, D.; Saluja, A.K. Development of a new mouse model of acute pancreatitis induced by administration of L-arginine. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G1009–G1018. [Google Scholar] [CrossRef] [Green Version]
- Kui, B.; Balla, Z.; Vasas, B.; Végh, E.T.; Pallagi, P.; Kormányos, E.S.; Venglovecz, V.; Iványi, B.; Takács, T.; Hegyi, P. New insights into the methodology of L-arginine-induced acute pancreatitis. PLoS ONE 2015, 10, e0117588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerasimenko, J.V.; Gryshchenko, O.; Ferdek, P.E.; Stapleton, E.; Hébert, T.O.; Bychkova, S.; Peng, S.; Begg, M.; Gerasimenko, O.V.; Petersen, O.H. Ca2+ release-activated Ca2+ channel blockade as a potential tool in antipancreatitis therapy. Proc. Natl. Acad. Sci. USA 2013, 110, 13186–13191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Jin, T.; Shi, N.; Yao, L.; Yang, X.; Han, C.; Wen, L.; Du, D.; Szatmary, P.; Mukherjee, R. Mechanisms of pancreatic injury induced by basic amino acids differ between L-Arginine, L-Ornithine, and L-Histidine. Front. Physiol. 2018, 9, 1922. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Wei, Z.; Xin, G.; Ji, C.; Wen, L.; Xia, Q.; Niu, H.; Huang, W. Preventive effect of a novel diosgenin derivative on arterial and venous thrombosis in vivo. Bioorganic Med. Chem. Lett. 2016, 26, 3364–3369. [Google Scholar] [CrossRef] [PubMed]
- Ceranowicz, P.; Warzecha, Z.; Dembinski, A.; Cieszkowski, J.; Dembinski, M.; Sendur, R.; Kusnierz-Cabala, B.; Tomaszewska, R.; Kuwahara, A.; Kato, I. Pretreatment with obestatin inhibits the development of cerulein-induced pancreatitis. J. Physiol. Pharmacol. 2009, 60, 95–101. [Google Scholar] [PubMed]
- Dunn, K.W.; Kamocka, M.M.; McDonald, J.H. A practical guide to evaluating colocalization in biological microscopy. Am. J. Physiol. Cell Physiol. 2011, 300, C723–C742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, D.M.; Murphy, J.A.; Mukherjee, R.; Awais, M.; Neoptolemos, J.P.; Gerasimenko, O.V.; Tepikin, A.V.; Petersen, O.H.; Sutton, R.; Criddle, D.N. Reactive oxygen species induced by bile acid induce apoptosis and protect against necrosis in pancreatic acinar cells. Gastroenterology 2011, 140, 2116–2125. [Google Scholar] [CrossRef]
- Curtis, M.J.; Bond, R.A.; Spina, D.; Ahluwalia, A.; Alexander, S.P.; Giembycz, M.A.; Gilchrist, A.; Hoyer, D.; Insel, P.A.; Izzo, A.A. Experimental design and analysis and their reporting: New guidance for publication in BJP. Br. J. Pharmacol. 2015, 172, 3461–3471. [Google Scholar] [CrossRef] [Green Version]
- Xin, G.; Wei, Z.L.; Ji, C.J.; Zheng, H.J.; Gu, J.; Ma, L.M.; Huang, W.F.; Morris-Natschke, S.L.; Yeh, J.L.; Zhang, R. Metformin uniquely prevents thrombosis by inhibiting platelet activation and mtDNA release. Sci. Rep. 2016, 6, 36222. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, T.; Qi, H.; Yap, M.C.; Tahbaz, N.; Milburn, L.A.; Lucchinetti, E.; Lou, P.H.; Zaugg, M.; LaPointe, P.G.; Mercier, P. The ER chaperone calnexin controls mitochondrial positioning and respiration. Sci. Signal. 2020, 13, eaax6660. [Google Scholar] [CrossRef]
- Hu, J.J.; Liu, X.; Xia, S.; Zhang, Z.; Zhang, Y.; Zhao, J.; Ruan, J.; Luo, X.; Lou, X.; Bai, Y. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat. Immunol. 2020, 21, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Masutani, H. Thioredoxin interacting protein in cancer and diabetes. Antioxid. Redox Signal. 2021, 36, 1001–1022. [Google Scholar] [CrossRef] [PubMed]
- Meszaros, M.; Yusenko, M.; Domonkos, L.; Peterfi, L.; Kovacs, G.; Banyai, D. Expression of TXNIP is associated with angiogenesis and postoperative relapse of conventional renal cell carcinoma. Sci. Rep. 2021, 11, 17200. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Dennis, M.; Song, X.; Vadysirisack, D.D.; Salunke, D.; Nash, Z.; Yang, Z.; Liesa, M.; Yoshioka, J.; Matsuzawa, S.A. REDD1/TXNIP pro-oxidant complex regulates ATG4B activity to control stress-induced autophagy and sustain exercise ca-pacity. Nat. Commun. 2015, 6, 7014. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.; Zhou, L.; Liu, H.; Shan, Y.; Zhang, X. MicroRNA-224 promotes pancreatic cancer cell proliferation and migration by targeting the TXNIP-mediated HIF1α pathway. Cell Physiol. Biochem. 2018, 48, 1735–1746. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Y.; Liu, Q.; Tian, N.; Du, P.; Zhu, F.; Han, Y.; Liu, X.; Liu, X.; Peng, X. MondoA-thioredoxin-interacting protein axis maintains regulatory T-cell identity and function in colorectal cancer microenvironment. Gastroenterology 2021, 161, 575–591. [Google Scholar] [CrossRef]
- Wu, H.; Li, H.; Wen, W.; Wang, Y.; Xu, H.; Xu, M.; Frank, J.A.; Wei, W.; Luo, J. MANF protects pancreatic acinar cells against alcohol-induced endoplasmic reticulum stress and cellular injury. J. Hepato-Biliary-Pancreat. Sci. 2021, 28, 883–892. [Google Scholar] [CrossRef]
- Gao, M.; Chen, L.; Yu, H.; Sun, Q.; Kou, J.; Yu, B. Diosgenin down-regulates NF-κB p65/p50 and p38MAPK pathways and attenuates acute lung injury induced by lipopolysaccharide in mice. Int. Immunopharmacol. 2013, 15, 240–245. [Google Scholar] [CrossRef]
- Gong, G.; Qin, Y.; Huang, W. Anti-thrombosis effect of diosgenin extract from Dioscorea zingiberensis C.H. Wright in vitro and in vivo. Phytomedicine 2011, 18, 458–463. [Google Scholar] [CrossRef]
- Danckwardt, S.; Hentze, M.W.; Kulozik, A.E. Pathologies at the nexus of blood coagulation and inflammation: Thrombin in hemostasis, cancer, and beyond. J. Mol. Med. 2013, 91, 1257–1271. [Google Scholar] [CrossRef] [Green Version]
- Ceranowicz, P.; Dembinski, A.; Warzecha, Z.; Dembinski, M.; Cieszkowski, J.; Rembisz, K.; Konturek, S.J.; Kusnierz-Cabala, B.; Tomaszewska, R.; Pawlik, W.W. Protective and therapeutic effect of heparin in acute pancreatitis. J. Physiol. Pharmacol. 2008, 4, 103–125. [Google Scholar]
- Warzecha, Z.; Sendur, P.; Ceranowicz, P.; Dembinski, M.; Cieszkowski, J.; Kusnierz-Cabala, B.; Tomaszewska, R.; Dembinski, A. Pretreatment with low doses of acenocoumarol inhibits the development of acute ischemia/reperfusion-induced pancreatitis. J. Physiol. Pharmacol. 2019, 66, 731–740. [Google Scholar] [CrossRef]
- Warzecha, Z.; Sendur, P.; Ceranowicz, P.; Dembiński, M.; Cieszkowski, J.; Kuśnierz-Cabala, B.; Olszanecki, R.; Tomaszewska, R.; Ambroży, T.; Dembiński, A. Protective effect of pretreatment with acenocoumarol in cerulein-induced acute pancreatitis. Int. J. Mol. Sci. 2016, 17, 1709. [Google Scholar] [CrossRef] [Green Version]
- Warzecha, Z.; Sendur, P.; Ceranowicz, P.; Cieszkowski, J.; Dembiński, M.; Sendur, R.; Bonior, J.; Jaworek, J.; Ambroży, T.; Olszanecki, R.; et al. Therapeutic effect of low doses of acenocoumarol in the course of ischemia/reperfusion-induced acute pancreatitis in rats. Int. J. Mol. Sci. 2017, 18, 882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maduzia, D.; Ceranowicz, P.; Cieszkowski, J.; Gałązka, K.; Kuśnierz-Cabala, B.; Warzecha, Z. Pretreatment with warfarin attenuates the development of ischemia/reperfusion-induced acute pancreatitis in rats. Molecules 2020, 25, 2493. [Google Scholar] [CrossRef]
- Maduzia, D.; Ceranowicz, P.; Cieszkowski, J.; Chmura, A.; Galazka, K.; Kusnierz-Cabala, B.; Warzecha, Z. Administration of warfarin accelerates the recovery in ischemia/reperfusion-induced acute pancreatitis. J. Physiol. Pharmacol. 2020, 71, 417–427. [Google Scholar] [CrossRef]
- Qin, Y.; Wu, X.; Huang, W.; Gong, G.; Li, D.; He, Y.; Zhao, Y. Acute toxicity and sub-chronic toxicity of steroidal saponins from Dioscorea zingiberensis C.H.Wright in rodents. J. Ethnopharmacol. 2009, 126, 543–550. [Google Scholar] [CrossRef]
| Parameter | Score | Pancreatitis Pathology Score |
|---|---|---|
| Edema | 0 | no edema |
| 1 | interlobular edema | |
| 2 | interlobular and moderate intralobular edema | |
| 3 | interlobular edema and severe intralobular edema | |
| Leukocytic infiltration | 0 | absent |
| 1 | scarce perivascular infiltration | |
| 2 | moderate perivascular and scarce diffuse infiltration | |
| 3 | abundant diffuse infiltration | |
| Necrosis | 0 | no necrosis |
| 1 | less than 15% of pancreatic cells involved | |
| 2 | 15–35% of cells involved | |
| 3 | more than 35% of cells involved |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Niu, H.; Wan, C.; Yu, X.; Xin, G.; Zhu, Y.; Wei, Z.; Li, F.; Wang, Y.; Zhang, K.; et al. Drug D, a Diosgenin Derive, Inhibits L-Arginine-Induced Acute Pancreatitis through Meditating GSDMD in the Endoplasmic Reticulum via the TXNIP/HIF-1α Pathway. Nutrients 2022, 14, 2591. https://doi.org/10.3390/nu14132591
Zhang C, Niu H, Wan C, Yu X, Xin G, Zhu Y, Wei Z, Li F, Wang Y, Zhang K, et al. Drug D, a Diosgenin Derive, Inhibits L-Arginine-Induced Acute Pancreatitis through Meditating GSDMD in the Endoplasmic Reticulum via the TXNIP/HIF-1α Pathway. Nutrients. 2022; 14(13):2591. https://doi.org/10.3390/nu14132591
Chicago/Turabian StyleZhang, Cuicui, Hai Niu, Chengyu Wan, Xiuxian Yu, Guang Xin, Yuda Zhu, Zeliang Wei, Fan Li, Yilan Wang, Kun Zhang, and et al. 2022. "Drug D, a Diosgenin Derive, Inhibits L-Arginine-Induced Acute Pancreatitis through Meditating GSDMD in the Endoplasmic Reticulum via the TXNIP/HIF-1α Pathway" Nutrients 14, no. 13: 2591. https://doi.org/10.3390/nu14132591
APA StyleZhang, C., Niu, H., Wan, C., Yu, X., Xin, G., Zhu, Y., Wei, Z., Li, F., Wang, Y., Zhang, K., Li, S., Dong, Y., Li, Y., & Huang, W. (2022). Drug D, a Diosgenin Derive, Inhibits L-Arginine-Induced Acute Pancreatitis through Meditating GSDMD in the Endoplasmic Reticulum via the TXNIP/HIF-1α Pathway. Nutrients, 14(13), 2591. https://doi.org/10.3390/nu14132591





