Abstract
The single nucleotide polymorphisms (SNPs) rs3808607, rs2072183, rs2032582, and rs1761667 are associated with coenzyme Q10 (CoQ10) bioavailability in women after long-term CoQ10 supplementation. However, the beneficial aspects of the association between these SNPs and CoQ10 supplementation remain unknown. We investigated their relationship using the subjective quality of life score SF-36 by reanalyzing previous data from 92 study participants who were receiving ubiquinol (a reduced form of CoQ10) supplementation for 1 year. Two-way repeated-measures analysis of variance revealed a significant interaction between rs1761667 and the SF-36 scores of role physical (p = 0.016) and mental health (p = 0.017) in women. Subgrouping of participants based on the above four SNPs revealed significant interactions between these SNPs and the SF-36 scores of general health (p = 0.045), role emotional (p = 0.008), and mental health (p = 0.019) and increased serum CoQ10 levels (p = 0.008), suggesting that the benefits of CoQ10 supplementation, especially in terms of psychological parameters, are genotype-dependent in women. However, significant interactions were not observed in men. Therefore, inclusion of SNP subgrouping information in clinical trials of CoQ10 supplementation may provide conclusive evidence supporting other beneficial health effects exerted by the association between these SNPs and CoQ10 on women.
1. Introduction
Coenzyme Q10 (CoQ10), which can be synthesized de novo, is a fat-soluble molecule involved in energy production and modulation of the redox state of lipid components in cells and body fluids [1,2,3]. A decrease in bodily CoQ10 levels, owing to aging or age-related neurodegenerative diseases, may lead to mitochondrial dysfunction and increased lipid peroxidation [3]. Therefore, supplementation with CoQ10 can be beneficial for overall health. In animal models, CoQ10 supplementation slowed aging; reduced oxidative damage to proteins, lipids, and DNA [4,5,6]; and improved oxidative stress response to exercise [7,8,9], cognitive function [10], and cognitive performance [11,12]. Consequently, some human interventional studies have indicated that CoQ10 may exert beneficial effects in issues related to aging, age-related deterioration of quality of life (QOL), and degenerative disorders affecting longevity [13,14,15]. However, the benefits of CoQ10 supplementation are still under investigation, probably because of inconsistencies seen among the results of previous studies. Indeed, CoQ10 intervention studies involving patients with Parkinson’s disease, statin-associated myalgia, and obesity, have failed to show any benefits [16,17,18].
Serum CoQ10 levels, which were increased by continuous CoQ10 supplementation, have shown significant variance [14,19]. Such variance, resulting from genetic and dietary factors, may have led to the inconsistencies observed in the studies investigating the beneficial effects of CoQ10 supplementation. To identify factors affecting serum CoQ10 levels, we investigated dietary habits and single nucleotide polymorphisms (SNPs) in CoQ10 and cholesterol metabolism-related genes in the participants of the Ubiquinol Health Examination [14,20]. Participants with higher serum CoQ10 levels tended to consume more eggs and dairy products, although the results failed to indicate a significant difference [21]. The SNPs found to be associated with increased serum CoQ10 levels following 1 year of CoQ10 supplementation in women [22] were rs3808607 in CYP7A1 [23], rs2072183 in NPC1L1 [24,25], rs2032582 in ABCB1 [26], and rs1761667 in CD36 [27]. Furthermore, grouping based on the above-mentioned SNPs helped identify individuals with higher CoQ10 bioavailability following supplementation, who were also likely to exhibit the beneficial effects of CoQ10 supplementation.
As a first step toward studying the role of SNPs in the beneficial effects of CoQ10 supplementation, we reanalyzed their relationships using the findings of the Medical Outcome Study 36-Item Short-Form Health Survey (SF-36, subjective QOL score) [28,29] and the four SNPs identified in the participants of the Ubiquinol Health Examination, held from November 2013 to November 2016 at Kamijima-Cho, Ehime Prefecture, Japan. A previous study had revealed that supplementation with ubiquinol, a reduced form of CoQ10, significantly increased certain SF-36 subscores in women, although not in men [14]. Therefore, in the present study, we performed a two-way repeated-measures analysis of variance (ANOVA) to investigate the possible effects of any interaction between genotypes and changes in SF-36 scores following long-term supplementation with the reduced form of CoQ10.
2. Materials and Methods
2.1. Study Design
Serum CoQ10, total cholesterol (TC), and SF-36 scores were obtained from the “Verification of health enhancement and QOL improvement effect by continuous ubiquinol ingestion (Ubiquinol Health Examination)” study (UMIN000012612) [14,20]. SNP data for rs3808607 (G > T) in CYP7A1, rs2072183 (C > G) in NPC1L1, rs2032582 (G > T) in ABCB1, and rs1761667 (G > A) in CD36 were obtained from the study titled “Relationship between the absorption of ubiquinol supplement and the genetic diversity for participants in the Ubiquinol Health Examination (SNP Study)” [22]. Instead of obtaining informed consent from each participant, we adopted an opt-out recruitment approach, targeting the participants of the two above-mentioned studies. This study was conducted following the guidelines of the Declaration of Helsinki and was approved by the Wayo Women’s University Human Research Ethics Committee (No. 2102, 2102-1, and 2102-2). This study was also registered at the University Medical Information Network Clinical Trials Registry with the title “Investigate the relationship between the individual differences in the effects of continuous ubiquinol (reduced CoQ10) supplementation on improving QOL and cognitive function and the SNPs in CoQ10 metabolism-related genes” (UMIN000045397).
Serum CoQ10 levels and SF-36 scores were recorded at baseline and after 1 year of supplementation with reduced CoQ10, along with the prevalence of the four SNP types in each study participant. The SF-36 score consisted of three aggregate scores (physical component summary: PCS, mental component summary: MCS, and role/social component summary: RCS) based on the eight subscales (physical functioning: PF, role physical: RP, bodily pain: BP, general health: GH, vitality: VT, social functioning: SF, role emotional: RE, and mental health: MH) [28,29]. The participants of the Ubiquinol Health Examination were enrolled every 6 months throughout the study; consequently, the study was divided into five periods. The enrollment periods, as well as the number of participants and the times of determination of serum CoQ10 levels and SF-36 score for each period, are shown (Figure S1). As previously reported, SNPs of all participants were genotyped using blood samples collected in November 2016 [22].
2.2. Participants
Of the 108 participants, consisting of 38 men and 70 women (excluding pregnant and lactating women) aged 20 years or older who were analyzed in the SNP study [22], 11 who joined after May 2016 were excluded (Figure 1) because their dosage of reduced CoQ10 was increased from 100 or 120 mg/day to 150 mg/day from November 2016. Additionally, one person, whose data for 1 year after supplementation were missing, was also excluded. Moreover, four persons, whose increase in serum CoQ10 levels after 1 year was less than 1 µmol/L, were excluded from the analysis, as they seemed not to follow the daily CoQ10 supplement program as described in the previous study [22]. Consequently, data pertaining to 92 participants (31 men, age 64.9 ± 11.3 years; 61 women, age 64.3 ± 10.4 years) were analyzed (Figure 1). A histogram of participants’ age distribution is shown (Figure S2). Although approximately 30% of the male and approximately 40% of the female participants had been treated for dyslipidemia, whether these participants had been treated with statins could not be ascertained.
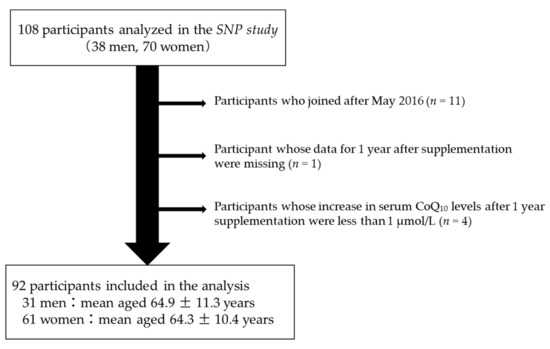
Figure 1.
Flow chart of participant selection. Of the 108 participants analyzed in the SNP study, 16 participants were excluded, resulting in 92 participants being ultimately included in the primary analysis.
2.3. CoQ10 Supplementation
Two forms of reduced CoQ10 supplements (granulated form, 120 mg CoQ10/packet, and soft-encapsulated, 50 mg CoQ10/capsule) were used in the Ubiquinol Health Examination. The participants took either a pack of granular supplements (120 mg CoQ10) or two soft capsules (100 mg CoQ10) every day according to their preferences [14]. Some participants took only granular supplements or only encapsulated supplements, while others took a combination of granular and encapsulated supplements. Participants also took these supplements in the postprandial state (after breakfast or lunch) as described in the previous study [22].
2.4. Data Analysis
IBM SPSS Statistics for Windows, version 28.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses described below. The participants were divided into major homozygote and non-major homozygote (heterozygote and minor homozygote) categories for each SNP. The interaction between the four SNPs (either individually or as a group) and the change in serum CoQ10 levels and SF-36 scores following 1 year of CoQ10 supplementation was investigated using two-way repeated-measures ANOVA. Comparisons between serum CoQ10 levels and SF-36 scores before and after supplementation in a genotype group were made via paired t-tests. Pearson’s chi-square test was used to investigate the presence of any participant’s bias between the CoQ10 supplement form and the SNP genotype. An odds ratio (OR) was calculated to investigate the association between the CoQ10 supplement form and the SNP genotype. The test was also used to investigate the association and to confirm Hardy–Weinberg equilibrium (HWE) of SNP genotypes (Table S1). Statistical significance was set at p < 0.05 for a two-tailed test. All HWE p-values were more than 0.05, demonstrating that all SNPs meet HWE criteria.
3. Results
Participant characteristics, including age, body mass index (BMI), serum TC levels, serum CoQ10 levels, and SF-36 scores, at baseline and 1 year after supplementation, are shown in Table 1. After supplementation, serum CoQ10 levels were increased to 5.35 ± 2.12 and 5.80 ± 1.99 µmol/L in men and women, respectively. With reference to SF-36 scores in women, VT increased significantly (p = 0.007) 1 year after supplementation. By contrast, none of the subscale or summary scores of men differed significantly (Table 1). Therefore, we primarily analyzed the data of female participants. We noted that the results mentioned above were similar to those obtained in the original report [14]. However, the participants of the present study and those of the previous study did not correspond completely, as some patients who participated in the current study did not participate in the previous study, and some who participated in the previous study did not participate in the current study. Approximately 50% of the male and approximately 60% of the female participants in the current study also participated in the previous study.

Table 1.
Changes in the measurements of the participants included in the analysis during the reduced CoQ10 supplementation.
To assess whether each SNP affected an increase in serum CoQ10 and changes in SF-36 scores in women supplemented with CoQ10, we analyzed the interaction effects between the SNPs, serum CoQ10 levels, and SF-36 scores using two-way repeated-measures ANOVA (Table 2, Table 3, Table 4 and Table 5). Female participants were divided into major homozygote and non-major homozygote (heterozygote and minor homozygote) categories for each SNP, following which serum CoQ10 levels and SF-36 scores before and after supplementation were recorded for each group (Table 2, Table 3, Table 4 and Table 5). Because rs3808607 T (minor), rs2072183 C (major), rs2032582 G (major), and rs1761667 A (minor) are associated with the high responder (HR) of the increased CoQ10 after 1 year of supplementation [22], we regarded rs3808607 GT/TT, rs2072183 CC, rs2032582 GG, and rs1761667 GA/AA as the HR-associated genotype groups. The left and right columns in each table indicate HR and the low-responder (LR) of the bioavailability of CoQ10 supplementation-associated genotypes. We also compared serum CoQ10 levels and SF-36 scores before and after supplementation for each group, using a paired t-test, to interpret the effect of each SNP on these parameters when interaction was indicated by the results of the two-way repeated-measures ANOVA. The three SNPs of rs3808607 (G > T) of CYP7A1, rs2072183 (C > G) of NPC1L1, and rs2032582 (G > T) of ABCB1 did not interact with any analytical values upon supplementation (Table 2, Table 3 and Table 4). The SNP rs1761667 (G > A) in CD36 interacted with RP (p = 0.016) and MH (p = 0.017) subscale scores (Table 5). In the HR rs1761667 GA/AA group, the RP (paired t-test, p = 0.003) and MH (paired t-test, p = 0.015) subscale scores were significantly increased following supplementation, but such a change was not observed in the LR rs1761667 GG group. Pearson’s chi-square test (Table S2) did not detect a bias between the CoQ10 supplement form and rs1761667 genotypes. By contrast, the four SNPs did not show any interaction with analytical values upon supplementation in men (Tables S3–S6).

Table 2.
Changes in the measurements of rs3808607 (G > T) in CYP7A1 during the reduced CoQ10 supplementation in women.

Table 3.
Changes in the measurements of rs2072183 (C > G) in NPC1L1 during the reduced CoQ10 supplementation in women.

Table 4.
Changes in the measurements of rs2032582 (G > T) in ABCB1 during the reduced CoQ10 supplementation in women.

Table 5.
Changes in the measurements of rs1761667 (G > A) in CD36 during the reduced CoQ10 supplementation in women.
Next, we divided the women into two groups based on the four SNPs described previously [22]. The participants belonging to group 1 carried four or more of rs3808607 T, rs2072183 C, rs2032582 G, and rs1761667 A alleles, whereas the participants belonging to group 2 carried three or fewer of these alleles. We confirmed no bias between the CoQ10 supplement form and the grouping mentioned above via Pearson’s chi-square test (Table S2E). Next, we investigated whether the grouping interacted with the SF-36 scores upon supplementation (Table 6). Although interaction between the subgrouping and GH, RE, and MH scores were significant (p = 0.045, p = 0.008, and p = 0.019, respectively; Table 6). The subgrouping also interacted with serum CoQ10 levels upon supplementation (p = 0.008, Table 6), demonstrating that CoQ10 bioavailability in group 1 was higher following supplementation. Although the GH, RE, and MH subscale scores of group 1 increased significantly following the 1-year supplementation period (paired t-test, p = 0.042, p = 0.016, and p = 0.009, respectively; Table 6), those of group 2 did not. By contrast, in men, subgrouping based on the above-mentioned alleles did not reveal interactions with any SF-36 subscale or summary score or increased serum CoQ10 levels (Table S7). These results suggested that the grouping based on the four SNPs may be useful for predicting higher CoQ10 bioavailability and certain SF-36 scores, especially the subscales related to psychological parameters, in women. However, interactions between individual SNPs and SF-36 scores were minimal.

Table 6.
Changes in the measurements of Group 1 and Group 2 during the reduced CoQ10 supplementation in women.
4. Discussion
In the current study, we reanalyzed the subjective QOL SF-36 scores of the Ubiquinol Health Examination to investigate whether the four SNPs, rs3808607, rs2072183, rs2032582, and rs1761667, found, respectively, in the genes CYP7A1, NPC1L1, ABCB1, and CD36, which regulate CoQ10 bioavailability, would affect the beneficial effects of CoQ10 supplementation. Acquisition of the SF-36 scores continued after our previous study, in which we showed that psychological QOL had increased following CoQ10 supplementation in women [14]. However, some participants dropped out, and others took part in the study after the results were reported. Therefore, the means and standard deviations of SF-36 scores at baseline, as well as following supplementation, in the current study are not the same as those in the previous study. In addition, although the trend in score changes upon supplementation in women in the present study was similar, only the increase in VT score was statistically significant (Table 1). Consistent with those of the previous report, the scores did not change significantly in men.
In women, the interactions between individual SNPs and serum CoQ10 levels, as well as SF-36 scores, were not strong (Table 2, Table 3, Table 4 and Table 5). The present study found that the four SNPs did not interact with increased serum CoQ10 levels following supplementation (Table 2, Table 3, Table 4 and Table 5), while the interaction between rs1761667 and the RP and MH subscales was significant.
Classification based on combining the four SNPs to predict the HR/LR of the bioavailability of CoQ10 supplementation, which we previously reported, may help find women who may easily benefit from CoQ10 supplementation. The grouping revealed interactions with not only increases in serum CoQ10 levels but also increases in GH, RE, and ME subscales (Table 6). Following supplementation, significant increases in the four above-mentioned SF-36 subscales were observed in the HR-associated allele-rich group 1, but not in those of the HR-associated allele-poor group 2. Furthermore, a significant increase in the RP and VT subscale and the RCS summary score was observed following supplementation only in group 1, although no interaction was observed upon grouping. These results indicate the potential of this classification as a tool that may be used to select women who would benefit from CoQ10 supplementation for health maintenance and promotion. These results suggested that the above-mentioned SNPs may enhance the beneficial effects of CoQ10 supplementation, as indicated by increased SF-36 scores. CYP7A1 and NPC1L1 are involved in cholesterol metabolism. Both the T-allele of rs3808607 and C-allele of rs2072183, associated with the HR phenotype, were found to be involved in the insensitivity of phytosterol-dependent decrease in serum TC levels [30,31]. In addition, the A-allele of rs1761667, associated with the HR phenotype, increased the ratio of people exhibiting high serum cholesterol levels [32]. Although we did not detect the differences in basal serum TC levels between each SNP subgroup, the risk of increased serum total and LDL cholesterol levels may be higher in individuals carrying the above-mentioned HR-associated alleles. This indicates that the beneficial effect of CoQ10 supplementation may be propagated via changes in cholesterol metabolism rather than via the activation of mitochondrial respiratory chains.
As described above, rs3808607, rs2072183, and rs1761667 were associated with increased serum cholesterol levels [30,33,34]. In addition, the mean age of the women included in this study was 64.3 years, suggesting that most were menopausal or postmenopausal. A previous study showed that serum cholesterol and inflammatory substances were higher, and antioxidant activities were lower, in postmenopausal women than in premenopausal women [35]. Furthermore, compared to premenopausal women, postmenopausal women had higher stress and lower QOL scores with more plasma lipid peroxide levels [36]. Considering these reports, it is conceivable that women in group 1, who were prone to increases in serum cholesterol levels, may be more susceptible to oxidative stress, with lower QOL scores related to the psychological parameters, than group 2 women. In addition, excess cholesterol plays a role in the pathogenesis of chronic non-communicable diseases (CNCDs), such as atherosclerosis, diabetes, chronic kidney disease, hepatic disease, Alzheimer’s disease, osteoporosis, and osteoarthritis, at least in part, via mitochondrial dysfunction-induced reactive oxygen species [37]. Such CNCDs and their risk factors may also exert an impact on QOL [38]. Because CoQ10 acts not only to promote ATP synthesis in mitochondria but also to regulate mitochondrial and extramitochondrial redox homeostasis [39], the beneficial effect of CoQ10 supplementation on the SF-36 scores of women may materialize through the buffering of cholesterol-induced oxidative stress. Under these circumstances, the reason for the increase in SF-36 score being more preferentially observed in group 1 than in group 2 women may be attributed to differences in the bioavailability of supplemental CoQ10 and the risk of excess serum cholesterol between these two groups. Baseline serum TC levels of the two groups were not different (Table 6), probably because approximately 40% of female participants had been treated for dyslipidemia.
In contrast to women, the four SNPs, as well as the classification representing the combination of all four SNPs, did not interact with any SF-36 subscales or summary scores in men (Tables S3–S7). None of the SF-36 subscales or summary scores increased upon supplementation in men. One possible reason for us being unable to observe an interaction between the four SNPs and changes in the SF-36 scores in men may be the small sample size; the number of male participants (n = 31) was approximately 50% of that of the female participants (n = 61) in the study. Another reason for the women-specific beneficial effects of CoQ10 supplementation may stem from reduced estrogen level changes associated with menopause, leading to a high risk of excess serum cholesterol accumulation, resulting in CNCDs, such as cardiovascular disease [37,40,41]. Serum cholesterol levels plateau in men >50 years of age [41]. In either case, an allied study with a large sample consisting of a significant number of men may help clarify whether the four SNPs or their classification interact with the changes in SF-36 scores upon CoQ10 supplementation.
This study has some limitations. Firstly, we evaluated only the four SNPs associated with CoQ10 bioavailability found in our previous study [22]. Additional SNPs that are highly suited to interact with increased serum CoQ10 and SF-36 scores may exist. A genome-wide association study of the human SNP array may provide further information regarding other related SNPs. Secondly, this study was a single-arm and open-label study. Follow-up randomized clinical trials focusing on CoQ10 supplementation would be required to enhance confidence in the results. Thirdly, the present study only evaluated a subjective QOL, SF-36. Further studies aimed at determining objective assessments for physical and psychological QOL, as well as at measuring oxidative stress markers and inflammatory markers, may be required to confirm the association between SNPs and the beneficial effects of CoQ10 supplementation.
In addition, whether the classification of participants using a combination of these four SNPs would lead to substantial evidence supporting other beneficial health effects of CoQ10 remains to be investigated. Additional interventional studies combining CoQ10 and SNP genotyping may provide an answer to these issues.
5. Conclusions
This study suggested that, following long-term CoQ10 supplementation, the four SNPs in CYP7A1, NPC1L1, ABCB1, and CD36, which are associated with CoQ10 bioavailability, as well as the classification of participants based on a combination of these four SNPs, may affect an increase in the SF-36 subscales in women, with particular reference to the scales related to psychological parameters. Clinical trials centered on investigating the beneficial effects of CoQ10 supplementation in individuals carrying these SNPs may provide substantial evidence supporting other beneficial health effects of CoQ10, at least in women.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu14132579/s1, Figure S1: Outline of the study. Figure S2: Histogram of participants’ age distribution in men and women. Table S1: The results of Hardy–Weinberg equilibrium (HWE) of SNP genotypes. Table S2A: The odds ratio (OR) and 95% confidence intervals (95%CIs) of GT/TT in rs3808607 (G > T) in CYP7A1 according to CoQ10 supplement forms. Table S2B: The odds ratio (OR) and 95% confidence intervals (95%CIs) of CC in rs2072183 (C > G) in NPC1L1 according to CoQ10 supplement forms. Table S2C: The odds ratio (OR) and 95% confidence intervals (95%CIs) of GG in rs2032582 (G > T) in ABCB1 according to CoQ10 supplement forms. Table S2D: The odds ratio (OR) and 95% confidence intervals (95%CIs) of GA/AA in rs1761667 (G > A) in CD36 according to CoQ10 supplement forms. Table S2E: The odds ratio (OR) and 95% confidence intervals (95%CIs) of group1 according to CoQ10 supplement forms. Table S3: Changes in the measurements of rs3808607 (G > T) in CYP7A1 during the reduced CoQ10 supplementation in men. Table S4: Changes in the measurements of rs2072183 (C > G) in NPC1L1 during the reduced CoQ10 supplementation in men. Table S5: Changes in the measurements of rs2032582 (G > T) in ABCB1 during the reduced CoQ10 supplementation in men. Table S6: Changes in the measurements of rs1761667 (G > A) in CD36 during the reduced CoQ10 supplementation in men. Table S7: Changes in the measurements of Group 1 and Group 2 during the reduced CoQ10 supplementation in men.
Author Contributions
Conceptualization, T.S.; Methodology, M.T., T.K. and T.S.; Validation, M.T., T.K., K.M. and T.S.; Formal analysis, M.T.; Investigation, M.T., T.K. and T.S.; Data Curation, M.T.; Writing—Original Draft Preparation, M.T. and T.S.; Writing—review & editing, M.T., T.K., K.M. and T.S.; Visualization, M.T.; Supervision, T.S.; Project Administration, T.K. and T.S.; Funding Acquisition, T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported in part by JSPS KAKENHI (Grant Numbers JP15K00840 (T.S.) and JP19K11797 (T.S.)). The SNP analyses were performed using a JSPS KAKENHI grant (JP15K00840). Ubiquinol Health Examination was funded by Kaneka Co. (Osaka, Japan) (T.K.). All other expenses for the research, including English proofreading and article processing charges, were made using a JSPS KAKENHI grant (JP19K11797). M.T. was financially supported by a Kazue Suzuki Doctor Courses Scholarship from Wayo Women’s University.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Wayo Women’s University Human Research Ethics Committee (Ethical approval numbers: 2102, 2102-1, and 2102-2, approved on 11 May, 3 September, and 10 December 2021, respectively).
Informed Consent Statement
We used an opt-out approach in this study. Instead of obtaining informed consent, we provided participants with the research information and a chance to decline participation in this study. This information was disclosed on the websites of Dr. Suzuki’s laboratory and the Institute of Community Life Science Co., Ltd. (Matsuyama, Japan) from September to December 2021. Additionally, the information was posted at Kamijima-Cho, the New Ubiquinol Health Examination location, on 13–14 and 20–22 November 2021.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank the staff of the non-profit organization Shima-no-Daigaku and Shinsuke Hata for their valuable contributions to the Ubiquinol Health Examination. We also thank Kaneka Co. for providing SF-36 and serum biochemical data of the participants.
Conflicts of Interest
T.K. is the head of the Institute of Community Life Sciences Co., Ltd. Ubiquinol Health Examination, including serum CoQ10 determination and SF-36 test, was performed by T.K. using R&D expenses of Kaneka Co. based on the agreement between the Institute of Community Life Sciences Co. Ltd. and Kaneka Co. Kaneka Co. was not involved in data analysis, data interpretation, or manuscript writing. M.T., K.M. and T.S. had no personal or financial conflict of interest with Kaneka Co. or the Institute of Community Life Sciences Co. Ltd.
References
- Crane, F.L. Biochemical functions of coenzyme Q10. J. Am. Coll. Nutr. 2001, 20, 591–598. [Google Scholar] [CrossRef]
- Frei, B.; Kim, M.C.; Ames, B.N. Ubiquinol-10 is an effective lipid-soluble antioxidant at physiological concentrations. Proc. Natl. Acad. Sci. USA 1990, 87, 4879–4883. [Google Scholar] [CrossRef]
- Lopez-Lluch, G.; Rodriguez-Aguilera, J.C.; Santos-Ocana, C.; Navas, P. Is coenzyme Q a key factor in aging? Mech. Ageing Dev. 2010, 131, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Sawashita, J.; Kubo, H.; Nishio, S.Y.; Hashimoto, S.; Suzuki, N.; Yoshimura, H.; Tsuruoka, M.; Wang, Y.; Liu, Y.; et al. Ubiquinol-10 supplementation activates mitochondria functions to decelerate senescence in senescence-accelerated mice. Antioxid. Redox Signal. 2014, 20, 2606–2620. [Google Scholar] [CrossRef]
- Yan, J.; Fujii, K.; Yao, J.; Kishida, H.; Hosoe, K.; Sawashita, J.; Takeda, T.; Mori, M.; Higuchi, K. Reduced coenzyme Q10 supplementation decelerates senescence in SAMP1 mice. Exp. Gerontol. 2006, 41, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Varela-Lopez, A.; Ochoa, J.J.; Llamas-Elvira, J.M.; Lopez-Frias, M.; Planells, E.; Ramirez-Tortosa, M.; Ramirez-Tortosa, C.L.; Giampieri, F.; Battino, M.; Quiles, J.L. Age-Related Loss in Bone Mineral Density of Rats Fed Lifelong on a Fish Oil-Based Diet Is Avoided by Coenzyme Q10 Addition. Nutrients 2017, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Andreani, C.; Bartolacci, C.; Guescini, M.; Battistelli, M.; Stocchi, V.; Orlando, F.; Provinciali, M.; Amici, A.; Marchini, C.; Tiano, L.; et al. Combination of Coenzyme Q10 Intake and Moderate Physical Activity Counteracts Mitochondrial Dysfunctions in a SAMP8 Mouse Model. Oxid. Med. Cell. Longev. 2018, 2018, 8936251. [Google Scholar] [CrossRef] [PubMed]
- Belviranli, M.; Okudan, N. Effect of Coenzyme Q10 Alone and in Combination with Exercise Training on Oxidative Stress Biomarkers in Rats. Int. J. Vitam. Nutr. Res. 2018, 88, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Chis, B.A.; Chis, A.F.; Muresan, A.; Fodor, D. Q10 Coenzyme Supplementation can Improve Oxidative Stress Response to Exercise in Metabolic Syndrome in Rats. Int. J. Vitam. Nutr. Res. 2020, 90, 33–41. [Google Scholar] [CrossRef]
- Sandhir, R.; Sethi, N.; Aggarwal, A.; Khera, A. Coenzyme Q10 treatment ameliorates cognitive deficits by modulating mitochondrial functions in surgically induced menopause. Neurochem. Int. 2014, 74, 16–23. [Google Scholar] [CrossRef]
- Dumont, M.; Kipiani, K.; Yu, F.; Wille, E.; Katz, M.; Calingasan, N.Y.; Gouras, G.K.; Lin, M.T.; Beal, M.F. Coenzyme Q10 decreases amyloid pathology and improves behavior in a transgenic mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2011, 27, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Muthukumaran, K.; Kanwar, A.; Vegh, C.; Marginean, A.; Elliott, A.; Guilbeault, N.; Badour, A.; Sikorska, M.; Cohen, J.; Pandey, S. Ubisol-Q10 (a Nanomicellar Water-Soluble Formulation of CoQ10) Treatment Inhibits Alzheimer-Type Behavioral and Pathological Symptoms in a Double Transgenic Mouse (TgAPEswe, PSEN1dE9) Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 61, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, S.; Fujii, K.; Kurihara, T. The effect of the reduced form of coenzyme Q10 (ubiquinol, Kaneka QHTM) on QOL improvement in the elderly. J. Clin. Ther. Med. 2008, 24, 233–238. [Google Scholar]
- Kinoshita, T.; Maruyama, K.; Tanigawa, T. The Effects of Long-Term Ubiquinol Intake on Improving the Quality of Life of Community Residents. Funct. Foods Health Dis. 2016, 6, 16–32. [Google Scholar] [CrossRef]
- Mantle, D.; Hargreaves, I. Coenzyme Q10 and Degenerative Disorders Affecting Longevity: An Overview. Antioxidants 2019, 8, 44. [Google Scholar] [CrossRef]
- Kennedy, C.; Koller, Y.; Surkova, E. Effect of Coenzyme Q10 on statin-associated myalgia and adherence to statin therapy: A systematic review and meta-analysis. Atherosclerosis 2020, 299, 1–8. [Google Scholar] [CrossRef]
- Negida, A.; Menshawy, A.; El Ashal, G.; Elfouly, Y.; Hani, Y.; Hegazy, Y.; El Ghonimy, S.; Fouda, S.; Rashad, Y. Coenzyme Q10 for Patients with Parkinson’s Disease: A Systematic Review and Meta-Analysis. CNS Neurol. Disord. Drug Targets 2016, 15, 45–53. [Google Scholar] [CrossRef]
- Saboori, S.; Rad, E.Y.; Mardani, M.; Khosroshahi, M.Z.; Nouri, Y.; Falahi, E. Effect of Q10 supplementation on body weight and body mass index: A systematic review and meta-analysis of randomized controlled clinical trials. Diabetes Metab. Syndr. 2019, 13, 1179–1185. [Google Scholar] [CrossRef]
- Alehagen, U.; Aaseth, J.; Alexander, J.; Johansson, P.; Larsson, A. Supplemental selenium and coenzyme Q10 reduce glycation along with cardiovascular mortality in an elderly population with low selenium status—A four-year, prospective, randomised, double-blind placebo-controlled trial. J. Trace Elem. Med. Biol. 2020, 61, 126541. [Google Scholar] [CrossRef]
- Kinoshita, T.; Fujii, K. Long-term intake of ubiquinol may improve cognitive performance in community residents. J. Jpn. Assoc. Rural Med. 2019, 68, 8–17. [Google Scholar] [CrossRef][Green Version]
- Takahashi, M.; Kinoshita, T.; Kaneko, T.; Suzuki, T. Investigation of the influence of dietary habits on serum coenzyme Q10 level with long-term CoQ10 supplement intake. J. Wayo Women’s Univ. 2018, 58, 111–118. [Google Scholar] [CrossRef]
- Takahashi, M.; Nagata, M.; Kinoshita, T.; Kaneko, T.; Suzuki, T. CYP7A1, NPC1L1, ABCB1, and CD36 Polymorphisms Are Associated with Increased Serum Coenzyme Q10 after Long-Term Supplementation in Women. Antioxidants 2021, 10, 431. [Google Scholar] [CrossRef] [PubMed]
- Meaney, S. Epigenetic regulation of cholesterol homeostasis. Front. Genet. 2014, 5, 311. [Google Scholar] [CrossRef]
- Sahoo, S.; Aurich, M.K.; Jonsson, J.J.; Thiele, I. Membrane transporters in a human genome-scale metabolic knowledgebase and their implications for disease. Front. Physiol. 2014, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Takekawa, Y.; Sato, Y.; Yamaki, Y.; Imai, M.; Noto, K.; Sumi, M.; Takekuma, Y.; Iseki, K.; Sugawara, M. An Approach to Improve Intestinal Absorption of Poorly Absorbed Water-Insoluble Components via Niemann-Pick C1-Like 1. Biol. Pharm. Bull. 2016, 39, 301–307. [Google Scholar] [CrossRef]
- Itagaki, S.; Ochiai, A.; Kobayashi, M.; Sugawara, M.; Hirano, T.; Iseki, K. Interaction of coenzyme Q10 with the intestinal drug transporter P-glycoprotein. J. Agric. Food Chem. 2008, 56, 6923–6927. [Google Scholar] [CrossRef]
- Anderson, C.M.; Kazantzis, M.; Wang, J.; Venkatraman, S.; Goncalves, R.L.; Quinlan, C.L.; Ng, R.; Jastroch, M.; Benjamin, D.I.; Nie, B.; et al. Dependence of brown adipose tissue function on CD36-mediated coenzyme Q uptake. Cell Rep. 2015, 10, 505–515. [Google Scholar] [CrossRef]
- Fukuhara, S.; Bito, S.; Green, J.; Hsiao, A.; Kurokawa, K. Translation, adaptation, and validation of the SF-36 Health Survey for use in Japan. J. Clin. Epidemiol. 1998, 51, 1037–1044. [Google Scholar] [CrossRef]
- Fukuhara, S.; Ware, J.E., Jr.; Kosinski, M.; Wada, S.; Gandek, B. Psychometric and clinical tests of validity of the Japanese SF-36 Health Survey. J. Clin. Epidemiol. 1998, 51, 1045–1053. [Google Scholar] [CrossRef]
- Granado-Lorencio, F.; de Las Heras, L.; Millan, C.S.; Garcia-Lopez, F.J.; Blanco-Navarro, I.; Perez-Sacristan, B.; Dominguez, G. β-Cryptoxanthin modulates the response to phytosterols in postmenopausal women carrying NPC1L1 L272L and ABCG8 A632 V polymorphisms: An exploratory study. Genes Nutr. 2014, 9, 428. [Google Scholar] [CrossRef]
- MacKay, D.S.; Eck, P.K.; Gebauer, S.K.; Baer, D.J.; Jones, P.J. CYP7A1-rs3808607 and APOE isoform associate with LDL cholesterol lowering after plant sterol consumption in a randomized clinical trial. Am. J. Clin. Nutr. 2015, 102, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Arellano, L.E.; Salgado-Bernabe, A.B.; Guzman-Guzman, I.P.; Salgado-Goytia, L.; Munoz-Valle, J.F.; Parra-Rojas, I. CD36 haplotypes are associated with lipid profile in normal-weight subjects. Lipids Health Dis. 2013, 12, 167. [Google Scholar] [CrossRef] [PubMed]
- Arturo Panduro, O.R. Genetic Variant in the CD36 Gene (rs1761667) is Associated with Higher Fat Intake and High Serum Cholesterol among the Population of West Mexico. J. Nutr. Food Sci. 2015, 5, 353. [Google Scholar] [CrossRef]
- Wang, Y.; Harding, S.V.; Thandapilly, S.J.; Tosh, S.M.; Jones, P.J.H.; Ames, N.P. Barley β-glucan reduces blood cholesterol levels via interrupting bile acid metabolism. Br. J. Nutr. 2017, 118, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Taleb-Belkadi, O.; Chaib, H.; Zemour, L.; Fatah, A.; Chafi, B.; Mekki, K. Lipid profile, inflammation, and oxidative status in peri- and postmenopausal women. Gynecol. Endocrinol. 2016, 32, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, M.A.; Castrejon-Delgado, L.; Zacarias-Flores, M.; Arronte-Rosales, A.; Mendoza-Nunez, V.M. Quality of life among post-menopausal women due to oxidative stress boosted by dysthymia and anxiety. BMC Women’s Health 2017, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, J.; Zhao, K.; Gao, L.; Zhao, J. Cholesterol-induced toxicity: An integrated view of the role of cholesterol in multiple diseases. Cell Metab. 2021, 33, 1911–1925. [Google Scholar] [CrossRef]
- Pequeno, N.P.F.; Cabral, N.L.A.; Marchioni, D.M.; Lima, S.C.V.C.; Lyra, C.O. Quality of life assessment instruments for adults: A systematic review of population-based studies. Health Qual. Life Outcomes 2020, 18, 208. [Google Scholar] [CrossRef]
- Hernandez-Camacho, J.D.; Garcia-Corzo, L.; Fernandez-Ayala, D.J.M.; Navas, P.; Lopez-Lluch, G. Coenzyme Q at the Hinge of Health and Metabolic Diseases. Antioxidants 2021, 10, 1785. [Google Scholar] [CrossRef]
- Kannel, W.B.; Wilson, P.W. Risk factors that attenuate the female coronary disease advantage. Arch. Intern. Med. 1995, 155, 57–61. [Google Scholar] [CrossRef]
- Kreisberg, R.A.; Kasim, S. Cholesterol metabolism and aging. Am. J. Med. 1987, 82, 54–60. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).