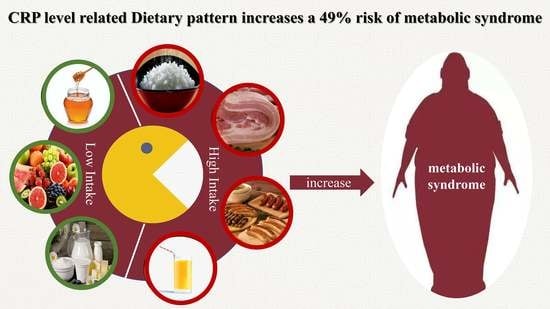
Association between Dietary Patterns Reflecting C-Reactive Protein and Metabolic Syndrome in the Chinese Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. Dietary Assessment
2.3. Covariate Assessment
2.4. Definition of MetS
2.5. Statistical Analysis
3. Results
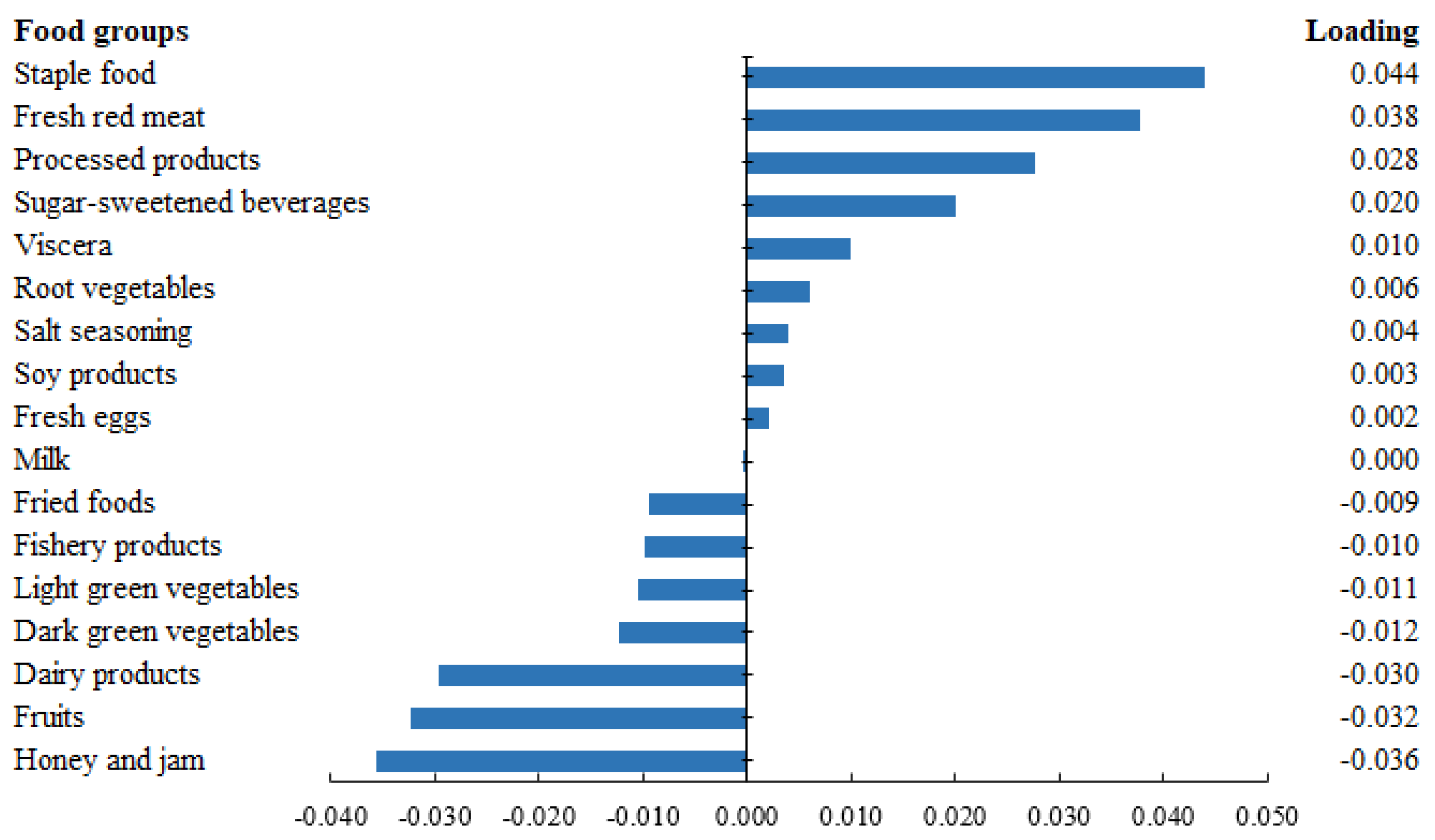
3.1. CRP-Related Dietary Pattern and Its Characteristics
3.2. Association of CRP-Related Dietary Pattern with MetS
4. Discussion
4.1. Comparison with Previous Studies
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aguilar, M.; Bhuket, T.; Torres, S.; Liu, B.; Wong, R.J. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA 2015, 313, 1973–1974. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Yang, S.; Li, S.; Su, M.; Wang, N.; Chen, Y.; Jiang, Q.; Fu, C. Prevalences of metabolic syndrome and its sex-specific association with socioeconomic status in rural China: A cross-sectional study. BMC Public Health 2021, 21, 2033. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, L.; Li, M.; Xu, Y.; Jiang, Y.; Wang, W.; Li, J.; Mi, S.; Zhang, M.; Li, Y.; et al. Metabolic Syndrome Among Adults in China: The 2010 China Noncommunicable Disease Surveillance. J. Clin. Endocrinol. Metab. 2017, 102, 507–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheelock, K.M.; Sinha, M.; Knowler, W.C.; Nelson, R.G.; Fufaa, G.D.; Hanson, R.L. Metabolic Risk Factors and Type 2 Diabetes Incidence in American Indian Children. J. Clin. Endocrinol. Metab. 2016, 101, 1437–1444. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.H.; Chang, H.T.; Tseng, Y.H.; Chen, H.S.; Chiang, S.C.; Chen, T.J.; Hwang, S.J. Changes in metabolic syndrome affect the health-related quality of life of community-dwelling adults. Sci. Rep. 2021, 11, 20267. [Google Scholar] [CrossRef]
- Limon, V.M.; Lee, M.; Gonzalez, B.; Choh, A.C.; Czerwinski, S.A. The impact of metabolic syndrome on mental health-related quality of life and depressive symptoms. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2020, 29, 2063–2072. [Google Scholar] [CrossRef]
- Li, M.C.; Fang, H.Y. Adherence to Daily Food Guides Is Associated with Lower Risk of Metabolic Syndrome: The Nutrition and Health Survey in Taiwan. Nutrients 2020, 12, 2955. [Google Scholar] [CrossRef]
- Shahinfar, H.; Ghanbari, M.; Jalilpiran, Y.; Payande, N.; Shahavandi, M.; Babaei, N.; Djafarian, K.; Clark, C.C.C.; Shab-Bidar, S. Interaction between major dietary patterns and cardiorespiratory fitness on metabolic syndrome in Iranian adults: A cross-sectional study. Nutr. J. 2021, 20, 36. [Google Scholar] [CrossRef]
- Centritto, F.; Iacoviello, L.; di Giuseppe, R.; De Curtis, A.; Costanzo, S.; Zito, F.; Grioni, S.; Sieri, S.; Donati, M.B.; de Gaetano, G.; et al. Dietary patterns, cardiovascular risk factors and C-reactive protein in a healthy Italian population. Nutr. Metab. Cardiovasc. Dis. NMCD 2009, 19, 697–706. [Google Scholar] [CrossRef]
- Pravenec, M.; Kajiya, T.; Zídek, V.; Landa, V.; Mlejnek, P.; Simáková, M.; Silhavý, J.; Malínská, H.; Oliyarnyk, O.; Kazdová, L.; et al. Effects of human C-reactive protein on pathogenesis of features of the metabolic syndrome. Hypertension 2011, 57, 731–737. [Google Scholar] [CrossRef] [Green Version]
- Esposito, K.; Marfella, R.; Ciotola, M.; Di Palo, C.; Giugliano, F.; Giugliano, G.; D’Armiento, M.; D’Andrea, F.; Giugliano, D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA 2004, 292, 1440–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; Macfadyen, J.G.; et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: A prospective study of the JUPITER trial. Lancet 2009, 373, 1175–1182. [Google Scholar] [CrossRef]
- Ruiz-Canela, M.; Bes-Rastrollo, M.; Martínez-González, M.A. The Role of Dietary Inflammatory Index in Cardiovascular Disease, Metabolic Syndrome and Mortality. Int. J. Mol. Sci. 2016, 17, 1285. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K.; Schulze, M.B.; Schienkiewitz, A.; Nöthlings, U.; Boeing, H. Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am. J. Epidemiol. 2004, 159, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Maddock, J.; Ambrosini, G.L.; Griffin, J.L.; West, J.A.; Wong, A.; Hardy, R.; Ray, S. A dietary pattern derived using B-vitamins and its relationship with vascular markers over the life course. Clin. Nutr. 2019, 38, 1464–1473. [Google Scholar] [CrossRef] [Green Version]
- Muga, M.A.; Owili, P.O.; Hsu, C.Y.; Rau, H.H.; Chao, J.C. Association between Dietary Patterns and Cardiovascular Risk Factors among Middle-Aged and Elderly Adults in Taiwan: A Population-Based Study from 2003 to 2012. PLoS ONE 2016, 11, e0157745. [Google Scholar] [CrossRef] [Green Version]
- Joint Committee for Developing Chinese Guidelines on Prevention and Treatment of Dyslipidemia in Adults. Chinese guidelines on prevention and treatment of dyslipidemia in adults. Zhonghua Xin Xue Guan Bing Za Zhi 2007, 35, 390–419. [Google Scholar]
- Neufcourt, L.; Assmann, K.E.; Fezeu, L.K.; Touvier, M.; Graffouillère, L.; Shivappa, N.; Hébert, J.R.; Wirth, M.D.; Hercberg, S.; Galan, P.; et al. Prospective association between the dietary inflammatory index and metabolic syndrome: Findings from the SU.VI.MAX study. Nutr. Metab. Cardiovasc. Dis. NMCD 2015, 25, 988–996. [Google Scholar] [CrossRef]
- Na, W.; Yu, T.Y.; Sohn, C. Development of a food-based index of dietary inflammatory potential for Koreans and its relationship with metabolic syndrome. Nutr. Res. Pract. 2019, 13, 150–158. [Google Scholar] [CrossRef]
- Xue, Q.; Yang, X.; Huang, Y.; Zhu, D.; Wang, Y.; Wen, Y.; Zhao, J.; Liu, Y.; Yang, C.X.; Pan, J.; et al. Association between baseline and changes in high-sensitive C-reactive protein and metabolic syndrome: A nationwide cohort study and meta-analysis. Nutr. Metab. 2022, 19, 2. [Google Scholar] [CrossRef]
- Seah, J.Y.H.; Ong, C.N.; Koh, W.P.; Yuan, J.M.; van Dam, R.M. A Dietary Pattern Derived from Reduced Rank Regression and Fatty Acid Biomarkers Is Associated with Lower Risk of Type 2 Diabetes and Coronary Artery Disease in Chinese Adults. J. Nutr. 2019, 149, 2001–2010. [Google Scholar] [CrossRef]
- Craddock, J.C.; Neale, E.P.; Peoples, G.E.; Probst, Y.C. Vegetarian-Based Dietary Patterns and their Relation with Inflammatory and Immune Biomarkers: A Systematic Review and Meta-Analysis. Adv. Nutr. 2019, 10, 433–451. [Google Scholar] [CrossRef] [PubMed]
- Arouca, A.B.; Meirhaeghe, A.; Dallongeville, J.; Moreno, L.A.; Lourenço, G.J.; Marcos, A.; Huybrechts, I.; Manios, Y.; Lambrinou, C.P.; Gottrand, F.; et al. Interplay between the Mediterranean diet and C-reactive protein genetic polymorphisms towards inflammation in adolescents. Clin. Nutr. 2020, 39, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Arshad, N.; Lin, T.S.; Yahaya, M.F. Stingless Bee Honey Reduces Anxiety and Improves Memory of the Metabolic Disease-induced Rats. CNS Neurol. Disord. Drug Targets 2020, 19, 115–126. [Google Scholar] [CrossRef] [PubMed]
- di Giuseppe, R.; Di Castelnuovo, A.; Melegari, C.; De Lucia, F.; Santimone, I.; Sciarretta, A.; Barisciano, P.; Persichillo, M.; De Curtis, A.; Zito, F.; et al. Typical breakfast food consumption and risk factors for cardiovascular disease in a large sample of Italian adults. Nutr. Metab. Cardiovasc. Dis. NMCD 2012, 22, 347–354. [Google Scholar] [CrossRef]
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for nutrition and health: A review. J. Am. Coll. Nutr. 2008, 27, 677–689. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxidative Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [Green Version]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [Green Version]
- Stephan, Y.; Sutin, A.R.; Terracciano, A. Younger subjective age is associated with lower C-reactive protein among older adults. Brain Behav. Immun. 2015, 43, 33–36. [Google Scholar] [CrossRef]
- Hughes, A.; McMunn, A.; Bartley, M.; Kumari, M. Elevated inflammatory biomarkers during unemployment: Modification by age and country in the UK. J. Epidemiol. Community Health 2015, 69, 673–679. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | Dietary Pattern Scores | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |
| Age (years, ± SD) | 39.8 ± 11.5 | 40.4 ± 11.5 | 39.6 ± 11.2 | 39.2 ± 11.0 | 38.1 ± 10.3 |
| Male (%) | 30.3 | 41.4 | 51.2 | 63.9 | 75.8 |
| Married (%) | 80.3 | 82.9 | 82.9 | 83.1 | 81.9 |
| College and above (%) | 92.4 | 90.6 | 90.5 | 91.2 | 91.2 |
| Annual income (%) | |||||
| <100,000 | 40.0 | 41.2 | 40.4 | 38.5 | 37.6 |
| 100,000–199,999 | 32.0 | 32.3 | 32.8 | 33.0 | 32.7 |
| ≥200,000 | 28.0 | 26.5 | 26.9 | 28.6 | 29.7 |
| Physical leisure activity (MET-h/day, ± SD) * | 4.1 ± 0.5 | 3.5 ± 0.5 | 3.3 ± 0.5 | 3.1 ± 0.5 | 2.8 ± 0.5 |
| Alcohol consumption (%) | |||||
| Never | 78.4 | 74.7 | 70.6 | 64.6 | 57.1 |
| Former drinker | 2.6 | 2.9 | 2.8 | 3.0 | 3.0 |
| Current drinker | 20.0 | 22.4 | 26.4 | 32.4 | 39.9 |
| Smoking (%) | |||||
| Never | 89.2 | 84.8 | 79.9 | 73.3 | 63.5 |
| Former smoker | 3.1 | 3.4 | 3.9 | 4.9 | 5.0 |
| Current smoker | 7.7 | 11.6 | 16.1 | 21.8 | 31.5 |
| Daily energy intake (kcal/day, ± SD) | 1085.8 ± 264.9 | 1066.7 ± 235.1 | 1114.9 ± 242.2 | 1186.7 ± 246.8 | 1350.0 ± 285.2 |
| Family History of HTN (%) | 38.2 | 38.9 | 39.4 | 39.99 | 41.3 |
| Family History of DM (%) | 22.4 | 22.1 | 21.8 | 22.6 | 23.6 |
| OR (95% CI) | Ptrend | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| MetS | ||||||
| Cases (n) | 1374 | 1836 | 2167 | 2558 | 3274 | |
| Model 1 | 1.00 | 1.15 (1.06, 1.24) | 1.23 (1.14, 1.32) | 1.38 (1.28, 1.48) | 1.77 (1.64, 1.90) | <0.001 |
| Model 2 | 1.00 | 1.10 (1.02, 1.19) | 1.14 (1.05, 1.22) | 1.23 (1.15, 1.33) | 1.49 (1.38, 1.61) | <0.001 |
| Abdominal obesity | ||||||
| Cases (n) | 2542 | 3249 | 3668 | 4352 | 5353 | |
| Model 1 | 1.00 | 1.15 (1.08, 1.22) | 1.21 (1.14, 1.28) | 1.33 (1.25, 1.41) | 1.64 (1.55, 1.74) | <0.001 |
| Model 2 | 1.00 | 1.11 (1.05, 1.18) | 1.14 (1.07, 1.21) | 1.22 (1.15, 1.29) | 1.45 (1.36, 1.54) | <0.001 |
| Hyperglycemia | ||||||
| Cases (n) | 1469 | 1871 | 2050 | 2314 | 2517 | |
| Model 1 | 1.00 | 1.18 (1.10, 1.28) | 1.32 (1.22, 1.42) | 1.46 (1.35, 1.58) | 1.72 (1.59, 1.86) | <0.001 |
| Model 2 | 1.00 | 1.16 (1.07, 1.25) | 1.26 (1.17, 1.36) | 1.35 (1.25, 1.46) | 1.52 (1.40, 1.65) | <0.001 |
| High blood pressure | ||||||
| Cases (n) | 2433 | 2933 | 3104 | 3374 | 3651 | |
| Model 1 | 1.00 | 1.11 (1.04, 1.19) | 1.16 (1.09, 1.24) | 1.17 (1.10, 1.24) | 1.28 (1.20, 1.37) | <0.001 |
| Model 2 | 1.00 | 1.09 (1.02, 1.16) | 1.11 (1.04, 1.19) | 1.11 (1.04, 1.18) | 1.19 (1.11, 1.28) | <0.001 |
| Hyperlipidemia | ||||||
| Cases (n) | 7243 | 7548 | 7993 | 8553 | 9515 | |
| Model 1 | 1.00 | 0.98 (0.93, 1.02) | 0.98 (0.94, 1.02) | 1.00 (0.96, 1.05) | 1.09 (1.05, 1.16) | <0.001 |
| Model 2 | 1.00 | 0.96 (0.92, 1.00) | 0.95 (0.91, 0.99) | 0.95 (0.91, 1.00) | 1.00 (0.96, 1.06) | 0.74 |
| OR (95% CI) | Pinteract | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Age (years) | <0.001 | |||||
| <50 | 1.00 | 1.08 (0.98, 1.20) | 1.07 (0.97, 1.18) | 1.19 (1.08, 1.31) | 1.44 (1.31, 1.58) | |
| ≥50 | 1.00 | 1.11 (0.99, 1.25) | 1.27 (1.13, 1.43) | 1.32 (1.17, 1.50) | 1.44 (1.26, 1.65) | |
| Sex | 0.38 | |||||
| Male | 1.00 | 1.03 (0.94, 1.13) | 1.06 (0.97, 1.16) | 1.13 (1.04, 1.23) | 1.34 (1.23, 1.46) | |
| Female | 1.00 | 1.17 (1.01, 1.34) | 1.23 (1.07, 1.43) | 1.42 (1.21, 1.66) | 1.79 (1.49, 2.15) | |
| Alcohol | 0.09 | |||||
| Never | 1.00 | 1.12 (1.01, 1.23) | 1.18 (1.07, 1.31) | 1.22 (1.11, 1.35) | 1.51 (1.36, 1.67) | |
| Ever | 1.00 | 1.06 (0.93, 1.20) | 1.07 (0.94, 1.21) | 1.22 (1.08, 1.37) | 1.45 (1.29, 1.62) | |
| Smoking | 0.46 | |||||
| Never | 1.00 | 1.10 (0.99, 1.21) | 1.19 (1.08, 1.31) | 1.24 (1.13, 1.37) | 1.59 (1.43, 1.77) | |
| Ever | 1.00 | 1.09 (0.96, 1.24) | 1.07 (0.95, 1.21) | 1.21 (1.07, 1.36) | 1.38 (1.23, 1.56) | |
| Physical leisure activity level * | 0.28 | |||||
| Low | 1.00 | 1.19 (1.06, 1.35) | 1.21 (1.07, 1.37) | 1.38 (1.23, 1.56) | 1.64 (1.45, 1.85) | |
| High | 1.00 | 1.03 (0.90, 1.17) | 1.08 (0.96, 1.22) | 1.11 (0.98, 1.26) | 1.34 (1.19, 1.52) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Wen, Q.; Lv, J.; Sun, D.; Ma, Y.; Man, S.; Yin, J.; Tong, M.; Wang, B.; Yu, C.; et al. Association between Dietary Patterns Reflecting C-Reactive Protein and Metabolic Syndrome in the Chinese Population. Nutrients 2022, 14, 2566. https://doi.org/10.3390/nu14132566
Yu H, Wen Q, Lv J, Sun D, Ma Y, Man S, Yin J, Tong M, Wang B, Yu C, et al. Association between Dietary Patterns Reflecting C-Reactive Protein and Metabolic Syndrome in the Chinese Population. Nutrients. 2022; 14(13):2566. https://doi.org/10.3390/nu14132566
Chicago/Turabian StyleYu, Huan, Qiaorui Wen, Jun Lv, Dianjianyi Sun, Yuan Ma, Sailimai Man, Jianchun Yin, Mingkun Tong, Bo Wang, Canqing Yu, and et al. 2022. "Association between Dietary Patterns Reflecting C-Reactive Protein and Metabolic Syndrome in the Chinese Population" Nutrients 14, no. 13: 2566. https://doi.org/10.3390/nu14132566
APA StyleYu, H., Wen, Q., Lv, J., Sun, D., Ma, Y., Man, S., Yin, J., Tong, M., Wang, B., Yu, C., & Li, L. (2022). Association between Dietary Patterns Reflecting C-Reactive Protein and Metabolic Syndrome in the Chinese Population. Nutrients, 14(13), 2566. https://doi.org/10.3390/nu14132566