Isoflavone Protects the Renal Tissue of Diabetic Ovariectomized Rats via PPARγ
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Experimental Design, and Treatments
2.2. Periodic Acid–Schiff Staining and Immunohistochemistry
2.3. Collagen Fiber Density
2.4. Gene Expression Analysis by Reverse-Transcription Quantitative PCR (RT-qPCR)
2.5. Bioinformatic Analysis
2.6. Statistical Analysis
3. Results
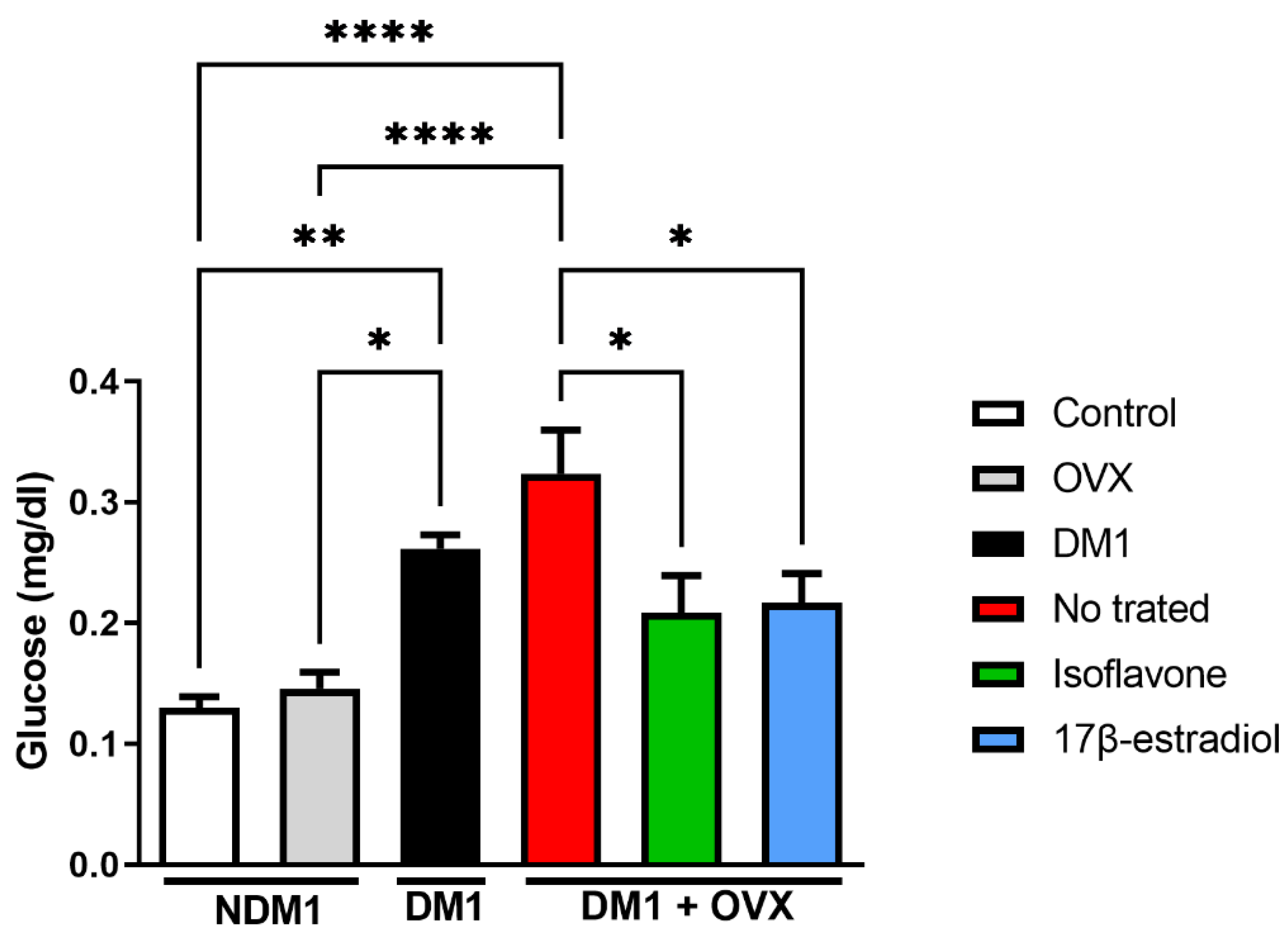
3.1. Glucose Modulation by Isoflavone
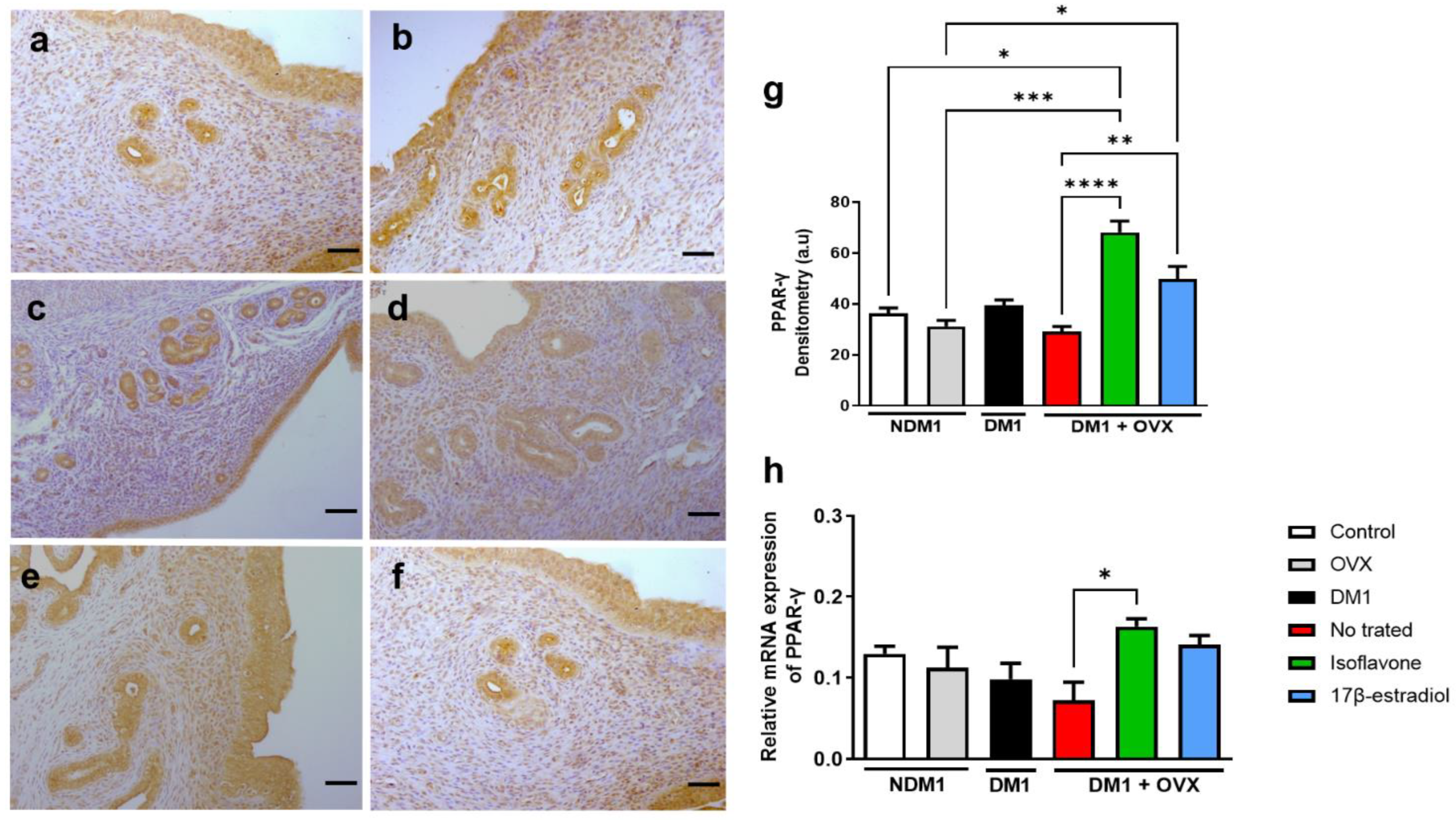
3.2. Expression of PPAR-γ in Isoflavone-Induced Endometrium
3.3. Expression of PPAR-γ Renal Tissue
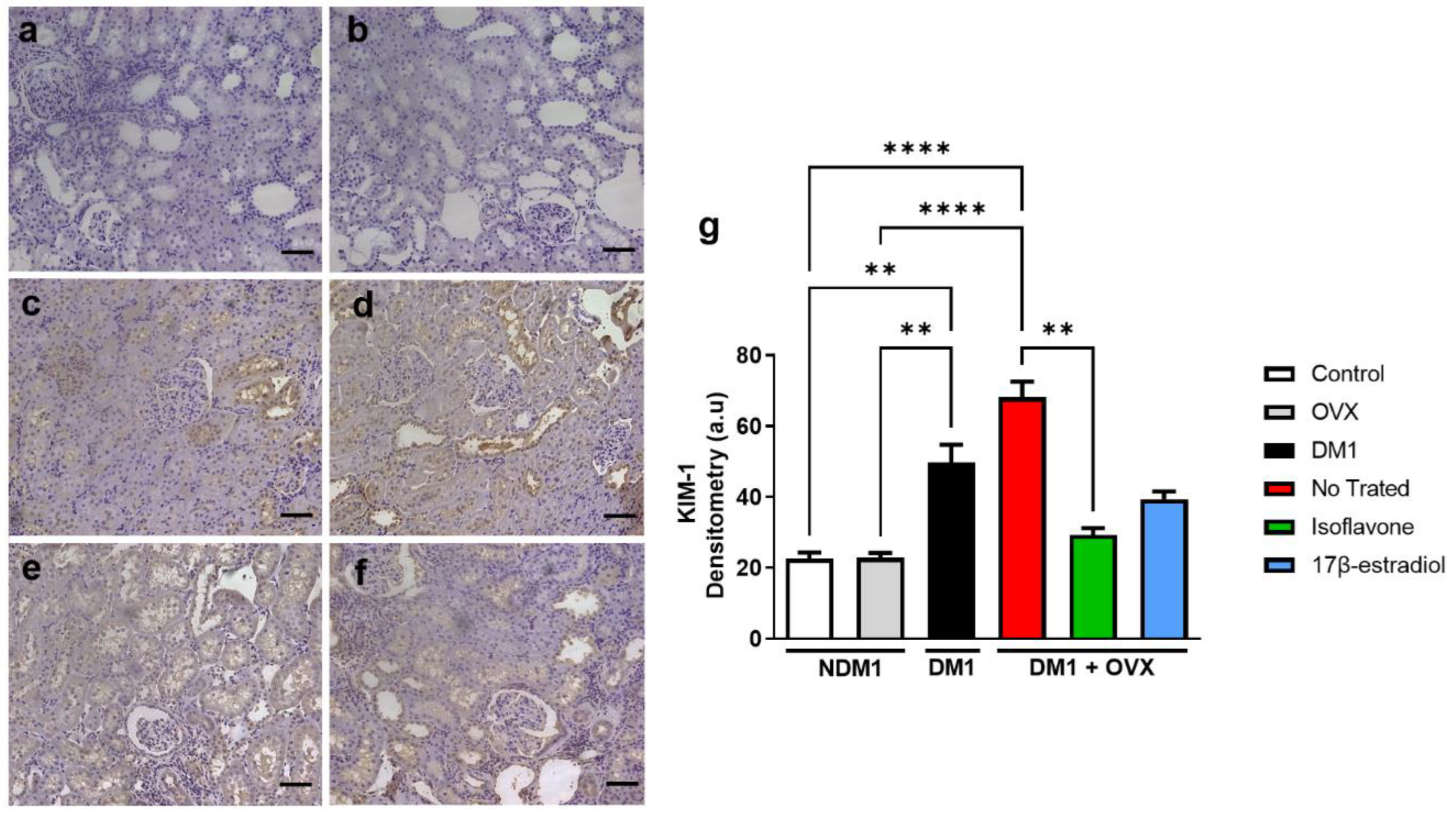
3.4. Assessment of Renal Injury
3.5. Evaluation of Type-I Collagen in Renal Tissue
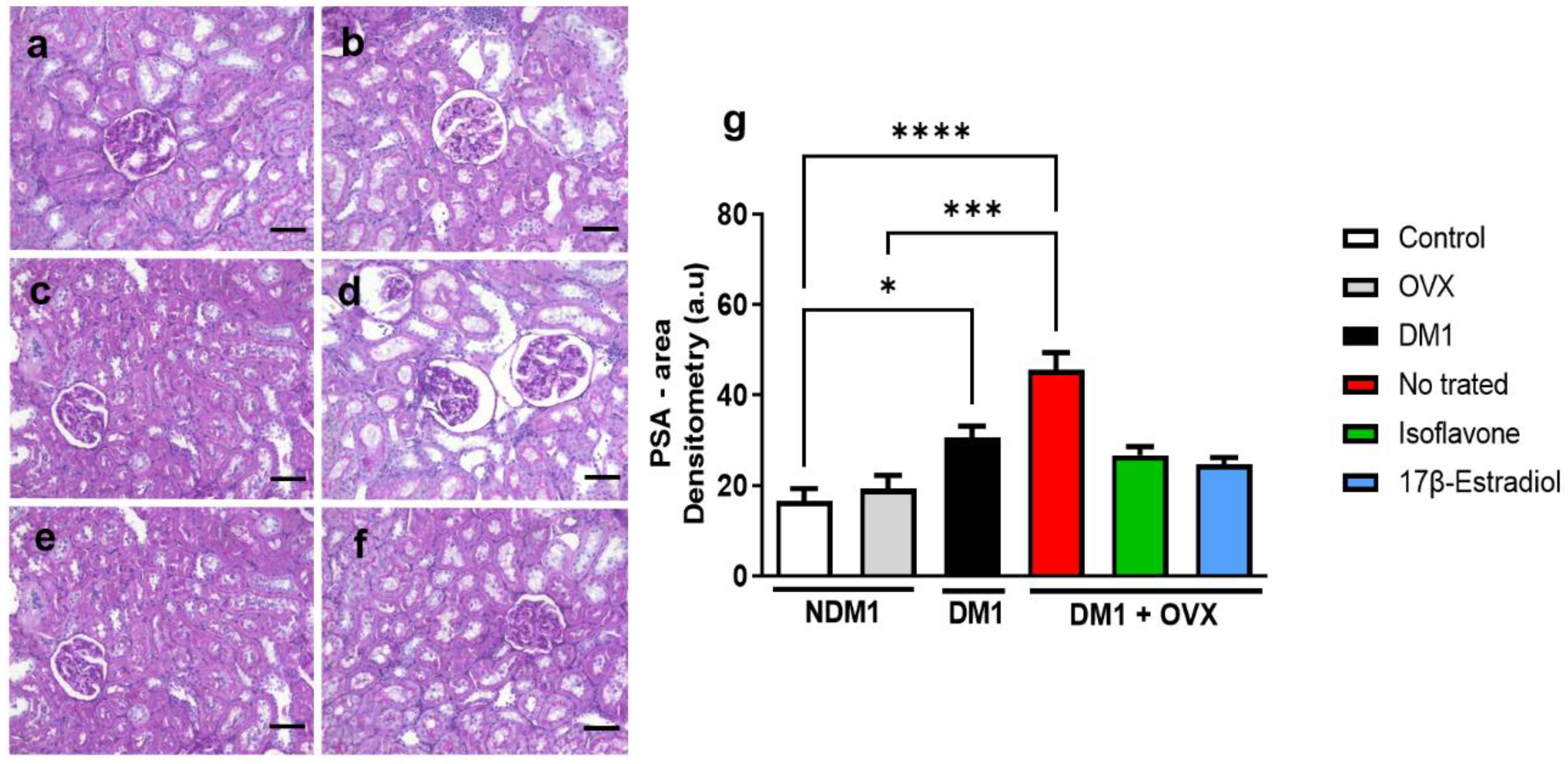
3.6. Renal Glycogen Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahlqvist, E.; Prasad, R.B.; Groop, L. 100 YEARS OF INSULIN: Towards improved precision and a new classification of diabetes mellitus. J. Endocrinol. 2022, 252, R59–R70. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Mao, X.; Zhang, Z.; Wu, H. Classification and Differential Diagnosis of Diabetic Nephropathy. J. Diabetes Res. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Samsu, N. Review Article Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. BioMed Res. Int. 2021, 2021, 1497449. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; James, M.T.; Holroyd-Leduc, J.M.; Wilton, S.B.; Seely, E.W.; Hemmelgarn, B.R.; Tonelli, M.; Wheeler, D.C.; Ahmed, S.B. Estradiol and mortality in women with end-stage kidney disease. Nephrol. Dial. Transplant. 2020, 35, 1965–1972. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.B. Menopause and Chronic Kidney Disease. Semin. Nephrol. 2017, 37, 404–411. [Google Scholar] [CrossRef]
- Farahmand, M.; Tehrani, F.R.; Khalili, D.; Cheraghi, L.; Azizi, F. Endogenous estrogen exposure and chronic kidney disease; a 15-year prospective cohort study. BMC Endocr. Disord. 2021, 21, 1–8. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kasparovska, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Lu, M.-P.; Wang, R.; Song, X.; Chibbar, R.; Wang, X.; Wu, L.; Meng, Q.H. Dietary soy isoflavones increase insulin secretion and prevent the development of diabetic cataracts in streptozotocin-induced diabetic rats. Nutr. Res. 2008, 28, 464–471. [Google Scholar] [CrossRef]
- Woo, H.W.; Kim, M.K.; Lee, Y.-H.; Shin, D.H.; Shin, M.-H.; Choi, B.Y. Sex-specific associations of habitual intake of soy protein and isoflavones with risk of type 2 diabetes. Clin. Nutr. 2021, 40, 127–136. [Google Scholar] [CrossRef]
- Ding, M.; Pan, A.; Manson, J.E.; Willett, W.C.; Malik, V.; Rosner, B.; Giovannucci, E.; Hu, F.B.; Sun, Q. Consumption of soy foods and isoflavones and risk of type 2 diabetes: A pooled analysis of three US cohorts. Eur. J. Clin. Nutr. 2016, 70, 1381–1387. [Google Scholar] [CrossRef]
- Guo, T.L.; Germolec, R.R.; Zheng, J.F.; Kooistra, L.; Auttachoat, W.; Smith, M.J.; White, K.L.; Elmore, S.A. Genistein Protects Female Nonobese Diabetic Mice from Developing Type 1 Diabetes When Fed a Soy- and Alfalfa-free Diet. Toxicol. Pathol. 2015, 43, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Von Hertzen, L.; Forsblom, C.; Stumpf, K.; Pettersson-Fernholm, K.; Adlercreutz, H.; Groop, P.-H. Highly elevated serum phyto-oestrogen concentrations in patients with diabetic nephropathy. J. Intern. Med. 2004, 255, 602–609. [Google Scholar] [CrossRef]
- Janani, C.; Kumari, B.R. PPAR gamma gene—A review. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, E.D.A.; Convento, M.B.; Castino, B.; Leme, A.M.; de Oliveira, A.S.; Aragão, A.; Borges, F.T. Beneficial Effects of Isoflavones in the Kidney of Obese Rats Are Mediated by PPAR-Gamma Expression. Nutrients 2020, 12, 1624. [Google Scholar] [CrossRef]
- Carbonel, A.A.F.; Vieira, M.C.; Simões, R.S.; Lima, P.D.A.; Fuchs, L.F.P.; Girão, E.R.C.; Cicivizzo, G.P.; Sasso, G.R.S.; De Moraes, L.O.C.; Junior, J.M.S.; et al. Isoflavones improve collagen I and glycosaminoglycans and prevent bone loss in type 1 diabetic rats. Climacteric 2019, 23, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Carbonel, A.A.F.; Lima, P.D.A.; Lim, J.J.; Borges, F.T.; Sasso, G.R.D.S.; Fuchs, L.; Simões, R.S.; Baracat, E.C.; Soares, J.M., Jr.; Simões, M.J. Effects of soy isoflavones on the concentration of hyaluronic acid in the vagina of type 1 diabetic rats. Climacteric 2017, 20, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.E.; Sayed, A.M.; El-Bahrawy, A.H.; Omar, Z.M.; Hassanein, E.H. Targeting KEAP1/Nrf2, AKT, and PPAR-γ signals as a potential protective mechanism of diosmin against gentamicin-induced nephrotoxicity. Life Sci. 2021, 275, 119349. [Google Scholar] [CrossRef]
- Rashid, M.; Rahman, A.; Islam, S.; Hossen, A.; Reza, A.S.M.A.; Abu Ahmed, A.M.; Alnajeebi, A.M.; Babteen, N.A.; Khan, M.; Aboelenin, S.M.; et al. Incredible affinity of Kattosh with PPAR-γ receptors attenuates STZ-induced pancreas and kidney lesions evidenced in chemicobiological interactions. J. Cell. Mol. Med. 2022, 26, 3343–3363. [Google Scholar] [CrossRef]
- Patial, V.; Katoch, S.; Chhimwal, J.; Singh, P.P.; Suresh, P.S.; Padwad, Y. Tinospora cordifolia activates PPARγ pathway and mitigates glomerular and tubular cell injury in diabetic kidney disease. Phytomedicine 2021, 91, 153663. [Google Scholar] [CrossRef]
- Santos, M.A.; Florencio-Silva, R.; Teixeira, C.P.; Sasso, G.R.D.S.; Marinho, D.S.; Simões, R.S.; Simões, M.J.; Carbonel, A.F. Effects of early and late treatment with soy isoflavones in the mammary gland of ovariectomized rats. Climacteric 2016, 19, 77–84. [Google Scholar] [CrossRef]
- Sameni, H.R.; Ramhormozi, P.; Bandegi, A.R.; Taherian, A.A.; Mirmohammadkhani, M.; Safari, M. Effects of ethanol extract of propolis on histopathological changes and anti-oxidant defense of kidney in a rat model for type 1 diabetes mellitus. J. Diabetes Investig. 2016, 7, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Silva, R.; Santos, M.A.; De Medeiros, V.P.; Nader, H.; Nonaka, K.O.; Simões, M.J.; Reginato, R.D. Effects of soy isoflavones and mechanical vibration on rat bone tissue. Climacteric 2013, 16, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Koshimizu, J.Y.; Beltrame, F.L.; de Pizzol, J.P., Jr.; Cerri, P.S.; Caneguim, B.H.; Sasso-Cerri, E. NF-kB overexpression and decreased immunoexpression of AR in the muscular layer is related to structural damages and apoptosis in cimetidine-treated rat vas deferens. Reprod. Biol. Endocrinol. 2013, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Singhal, S.; Sens, D.A.; Somji, S.; Davis, B.A.; Guyer, R.; Breen, S.; Kalonick, M.; Garrett, S.H. Elevated glucose represses lysosomal and mTOR-related genes in renal epithelial cells composed of progenitor CD133+ cells. PLoS ONE 2021, 16, e0248241. [Google Scholar] [CrossRef]
- Kuryłowicz, A. The Role of Isoflavones in Type 2 Diabetes Prevention and Treatment—A Narrative Review. Int. J. Mol. Sci. 2021, 22, 218. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Murtaza, G.; Liu, G.; Rahu, N.; Kalhoro, M.S.; Kalhoro, D.H.; O Adebowale, T.; Mazhar, M.U.; Rehman, Z.U.; et al. Flavonoids and type 2 diabetes: Evidence of efficacy in clinical and animal studies and delivery strategies to enhance their therapeutic efficacy. Pharmacol. Res. 2020, 152, 104629. [Google Scholar] [CrossRef]
- Messina, M. Soy foods, isoflavones, and the health of postmenopausal women. Am. J. Clin. Nutr. 2014, 100, 423S–430S. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; Yan, Y.; Liang, J.; Liang, Q.; Lu, Y.; Zhao, L.; Li, H. Preventative effects of resveratrol and estradiol on streptozotocin-induced diabetes in ovariectomized mice and the related mechanisms. PLoS ONE 2018, 13, e0204499. [Google Scholar] [CrossRef]
- Li, W.; Ruan, W.; Peng, Y.; Wang, D. Soy and the risk of type 2 diabetes mellitus: A systematic review and meta-analysis of observational studies. Diabetes Res. Clin. Pr. 2018, 137, 190–199. [Google Scholar] [CrossRef]
- Guo, X.-F.; Ruan, Y.; Li, Z.-H.; Li, D. Flavonoid subclasses and type 2 diabetes mellitus risk: A meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 2850–2862. [Google Scholar] [CrossRef]
- Barańska, A.; Błaszczuk, A.; Polz-Dacewicz, M.; Kanadys, W.; Malm, M.; Janiszewska, M.; Jędrych, M. Effects of Soy Isoflavones on Glycemic Control and Lipid Profile in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 1886. [Google Scholar] [CrossRef]
- He, W.; Barak, Y.; Hevener, A.; Olson, P.; Liao, D.; Le, J.; Nelson, M.; Ong, E.; Olefsky, J.M.; Evans, R.M. Adipose-specific peroxisome proliferator-activated receptor γ knockout causes insulin resistance in fat and liver but not in muscle. Proc. Natl. Acad. Sci. USA 2003, 100, 15712–15717. [Google Scholar] [CrossRef] [PubMed]
- Medjakovic, S.; Mueller, M.; Jungbauer, A. Potential Health-modulating Effects of Isoflavones and Metabolites via Activation of PPAR and AhR. Nutrients 2010, 2, 241–279. [Google Scholar] [CrossRef] [PubMed]
- Calle, P.; Hotter, G. Macrophage Phenotype and Fibrosis in Diabetic Nephropathy. Int. J. Mol. Sci. 2020, 21, 2806. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Huang, L.; Luo, W.; Yu, W.; Hu, X.; Guan, X.; Cai, Y.; Zou, C.; Yin, H.; Xu, Z.; et al. Inhibition of STAT3 in tubular epithelial cells prevents kidney fibrosis and nephropathy in STZ-induced diabetic mice. Cell Death Dis. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Elsherbiny, N.M.; Said, E.; Atef, H.; Zaitone, S.A. Renoprotective effect of calycosin in high fat diet-fed/STZ injected rats: Effect on IL-33/ST2 signaling, oxidative stress and fibrosis suppression. Chem. Interact. 2020, 315, 108897. [Google Scholar] [CrossRef]
- Andugulapati, S.B.; Gourishetti, K.; Tirunavalli, S.K.; Shaikh, T.B.; Sistla, R. Biochanin-A ameliorates pulmonary fibrosis by suppressing the TGF-β mediated EMT, myofibroblasts differentiation and collagen deposition in in vitro and in vivo systems. Phytomedicine 2020, 78, 153298. [Google Scholar] [CrossRef]
- Glastras, S.J.; Chen, H.; Teh, R.; McGrath, R.; Chen, J.; Pollock, C.A.; Wong, M.G.; Saad, S. Mouse Models of Diabetes, Obesity and Related Kidney Disease. PLoS ONE 2016, 11, e0162131. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, F.; Lv, X.; Wang, S.; Liao, X.; Yu, Y.; Dai, Q.; Zhang, Y.; Meng, J.; Hu, G.; et al. Mefunidone ameliorates diabetic kidney disease in STZ and db/db mice. FASEB J. 2021, 35, e21198. [Google Scholar] [CrossRef]
- Mise, K.; Ueno, T.; Hoshino, J.; Hazue, R.; Sumida, K.; Yamanouchi, M.; Hayami, N.; Suwabe, T.; Hiramatsu, R.; Hasegawa, E.; et al. Nodular lesions in diabetic nephropathy: Collagen staining and renal prognosis. Diabetes Res. Clin. Pract. 2017, 127, 187–197. [Google Scholar] [CrossRef][Green Version]


| Gene | Forward | Reverse |
|---|---|---|
| PPAR-γ | GGAGCCTAAGTTTGAGTTTGCTGTG | TGCAGCAGGTTGTCTTGGATG |
| β-actin | GGAGATTACTGCCCTGGCTCCTA | GACTCATCGTACTCCTGCTTGCTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraz Carbonel, A.A.; da Silva, R.A.; de Souza Ferreira, L.P.; Vieira, R.R.; dos Santos Simões, R.; da Silva Sasso, G.R.; de Jesus Simões, M.; Soares Junior, J.M.; Azevedo Lima, P.D.; Borges, F.T. Isoflavone Protects the Renal Tissue of Diabetic Ovariectomized Rats via PPARγ. Nutrients 2022, 14, 2567. https://doi.org/10.3390/nu14132567
Ferraz Carbonel AA, da Silva RA, de Souza Ferreira LP, Vieira RR, dos Santos Simões R, da Silva Sasso GR, de Jesus Simões M, Soares Junior JM, Azevedo Lima PD, Borges FT. Isoflavone Protects the Renal Tissue of Diabetic Ovariectomized Rats via PPARγ. Nutrients. 2022; 14(13):2567. https://doi.org/10.3390/nu14132567
Chicago/Turabian StyleFerraz Carbonel, Adriana Aparecida, Rafael André da Silva, Luiz Philipe de Souza Ferreira, Renata Ramos Vieira, Ricardo dos Santos Simões, Gisela Rodrigues da Silva Sasso, Manuel de Jesus Simões, José Maria Soares Junior, Patrícia Daniele Azevedo Lima, and Fernanda Teixeira Borges. 2022. "Isoflavone Protects the Renal Tissue of Diabetic Ovariectomized Rats via PPARγ" Nutrients 14, no. 13: 2567. https://doi.org/10.3390/nu14132567
APA StyleFerraz Carbonel, A. A., da Silva, R. A., de Souza Ferreira, L. P., Vieira, R. R., dos Santos Simões, R., da Silva Sasso, G. R., de Jesus Simões, M., Soares Junior, J. M., Azevedo Lima, P. D., & Borges, F. T. (2022). Isoflavone Protects the Renal Tissue of Diabetic Ovariectomized Rats via PPARγ. Nutrients, 14(13), 2567. https://doi.org/10.3390/nu14132567







