Early Introduction of Allergenic Foods and the Prevention of Food Allergy
Abstract
:1. Introduction
2. Evolution of the Guidelines for Food Allergy Prevention
| Professional Body | Publication Year | Recommendations |
|---|---|---|
| American Academy of Pediatrics (AAP) [12] | 2019 |
|
| Asia Pacific Association of Pediatric Allergy, Respirology & Immunology (APAPARI) [13] | 2017 |
|
| Australian Society of Clinical Immunology and Allergy (ASCIA) [14] | 2020 |
|
| Canadian Paediatric Society (CPS) [15] | 2021 |
|
| European Academy of Allergy and Immunology (EAACI) [16] | 2020 |
|
| German Society for Allergology and Clinical Immunology (DGAKI) [17] | 2014 |
|
| National Institute of Allergy and Infectious Diseases (NIAID) [18] | 2017 |
|
2.1. Peanut
2.2. Egg
2.3. Milk
2.4. Multiple Foods
3. Study Generalizability
4. Barriers to Adherence
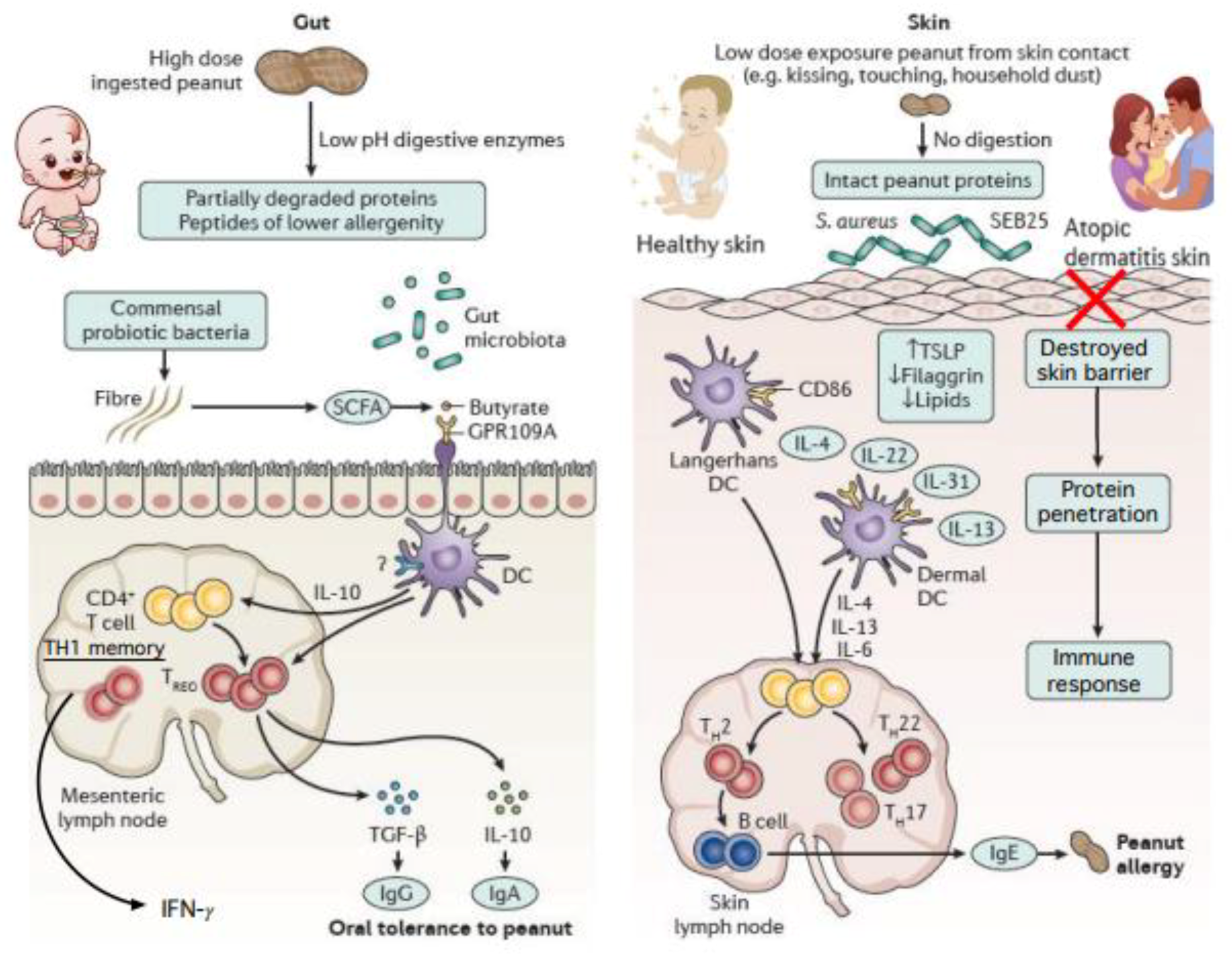
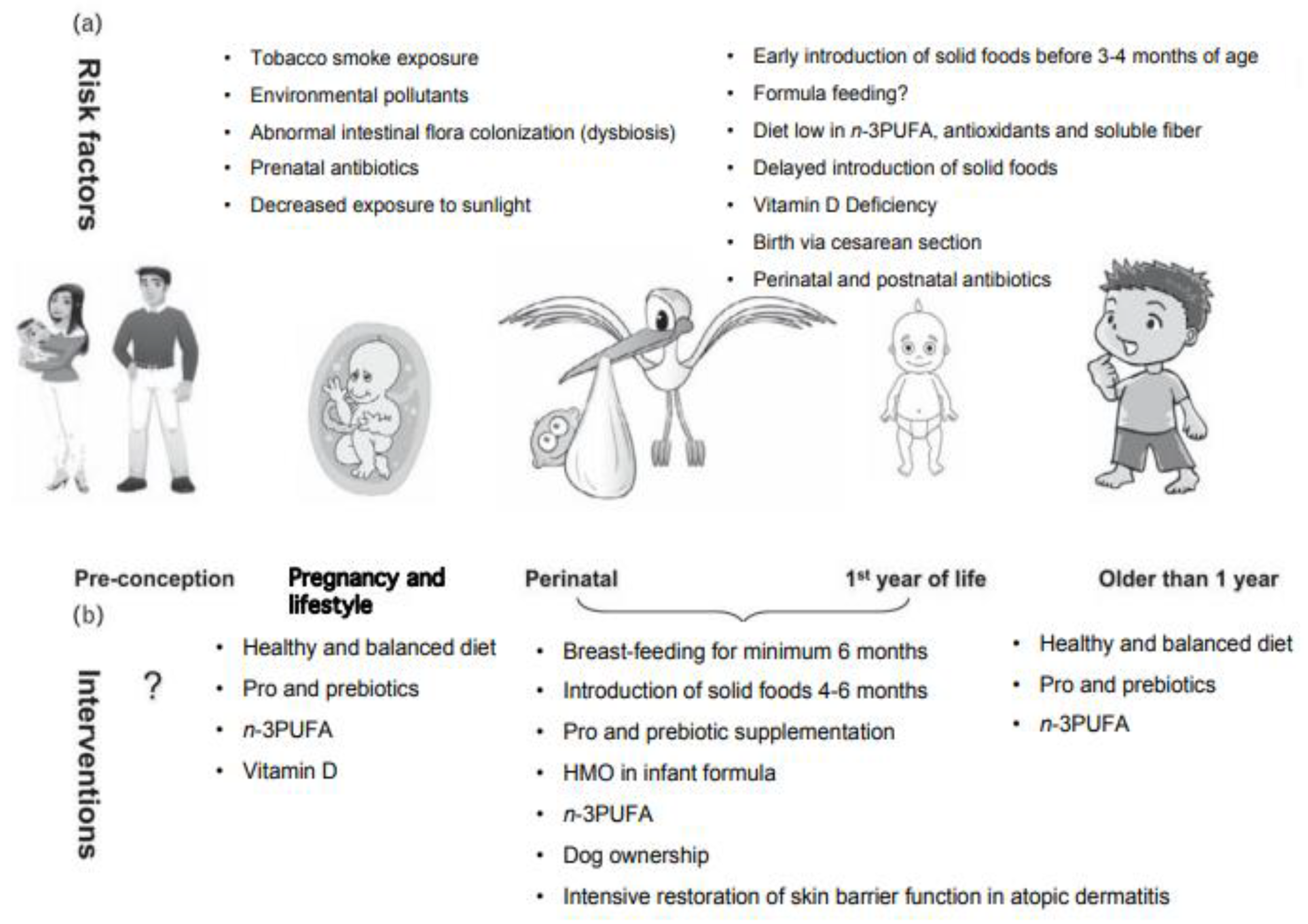
5. Other Directions in Food Allergy Prevention
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sicherer, S.H.; Sampson, H.A. Food Allergy: A Review and Update on Epidemiology, Pathogenesis, Diagnosis, Prevention, and Management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vale, S.L.; Lobb, M.; Netting, M.J.; Murray, K.; Clifford, R.; Campbell, D.E.; Salter, S.M. A Systematic Review of Infant Feeding Food Allergy Prevention Guidelines—Can We AGREE? World Allergy Organ. J. 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Protudjer, J.L.P.; Jansson, S.-A.; Heibert Arnlind, M.; Bengtsson, U.; Kallström-Bengtsson, I.; Marklund, B.; Middelveld, R.; Rentzos, G.; Sundqvist, A.-C.; Åkerström, J.; et al. Household Costs Associated with Objectively Diagnosed Allergy to Staple Foods in Children and Adolescents. J. Allergy Clin. Immunol. Pract. 2015, 3, 68–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.; Holdford, D.; Bilaver, L.; Dyer, A.; Holl, J.L.; Meltzer, D. The Economic Impact of Childhood Food Allergy in the United States. JAMA Pediatr. 2013, 167, 1026–1031. [Google Scholar] [CrossRef] [Green Version]
- Sicherer, S.H.; Noone, S.A.; Muñoz-Furlong, A. The Impact of Childhood Food Allergy on Quality of Life. Ann. Allergy Asthma Immunol. 2001, 87, 461–464. [Google Scholar] [CrossRef]
- de Silva, D.; Halken, S.; Singh, C.; Muraro, A.; Angier, E.; Arasi, S.; Arshad, H.; Beyer, K.; Boyle, R.; du Toit, G.; et al. Preventing Food Allergy in Infancy and Childhood: Systematic Review of Randomised Controlled Trials. Pediatr. Allergy Immunol. 2020, 31, 813–826. [Google Scholar] [CrossRef]
- Kelleher, M.M.; Cro, S.; Cornelius, V.; Carlsen, K.C.L.; Skjerven, H.O.; Rehbinder, E.M.; Lowe, A.J.; Dissanayake, E.; Shimojo, N.; Yonezawa, K.; et al. Skin Care Interventions in Infants for Preventing Eczema and Food Allergy. Cochrane Database Syst. Rev. 2021, 2, CD013534. [Google Scholar] [CrossRef]
- Skin Care and Synbiotics for Prevention of Atopic Dermatitis or Food Allergy in Newborn Infants: A 2 × 2 Factorial, Randomized, Non-Treatment Controlled Trial—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/31394530/ (accessed on 27 April 2022).
- Brough, H.A.; Lanser, B.J.; Sindher, S.B.; Teng, J.M.C.; Leung, D.Y.M.; Venter, C.; Chan, S.M.; Santos, A.F.; Bahnson, H.T.; Guttman-Yassky, E.; et al. Early Intervention and Prevention of Allergic Diseases. Allergy 2022, 77, 416–441. [Google Scholar] [CrossRef]
- Lack, G. Epidemiologic Risks for Food Allergy. J. Allergy Clin. Immunol. 2008, 121, 1331–1336. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Szajewska, H.; Lack, G. Food Allergy and the Gut. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 241–257. [Google Scholar] [CrossRef]
- Greer, F.R.; Sicherer, S.H.; Burks, A.W.; Committee on Nutrition; Section on Allergy and Immunology; Abrams, S.A.; Fuchs, G.J.; Kim, J.H.; Lindsey, C.W.; Magge, S.N.; et al. The Effects of Early Nutritional Interventions on the Development of Atopic Disease in Infants and Children: The Role of Maternal Dietary Restriction, Breastfeeding, Hydrolyzed Formulas, and Timing of Introduction of Allergenic Complementary Foods. Pediatrics 2019, 143, e20190281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tham, E.H.; Shek, L.P.-C.; Van Bever, H.P.; Vichyanond, P.; Ebisawa, M.; Wong, G.W.; Lee, B.W.; the Asia Pacific Association of Pediatric Allergy, R. & I. (APAPARI). Early Introduction of Allergenic Foods for the Prevention of Food Allergy from an Asian Perspective—An Asia Pacific Association of Pediatric Allergy, Respirology & Immunology (APAPARI) Consensus Statement. Pediatr. Allergy Immunol. 2018, 29, 18–27. [Google Scholar] [CrossRef]
- ASCIA Guidelines for Infant Feeding and Allergy Prevention—Australasian Society of Clinical Immunology and Allergy (ASCIA). Available online: https://www.allergy.org.au/hp/papers/infant-feeding-and-allergy-prevention (accessed on 27 April 2022).
- Dietary Exposures and Allergy Prevention in High-Risk Infants|Canadian Paediatric Society. Available online: https://cps.ca/documents/position/dietary-exposures-and-allergy-prevention (accessed on 27 April 2022).
- Halken, S.; Muraro, A.; de Silva, D.; Khaleva, E.; Angier, E.; Arasi, S.; Arshad, H.; Bahnson, H.T.; Beyer, K.; Boyle, R.; et al. EAACI Guideline: Preventing the Development of Food Allergy in Infants and Young Children (2020 Update). Pediatr. Allergy Immunol. 2021, 32, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, T.; Bauer, C.-P.; Beyer, K.; Bufe, A.; Friedrichs, F.; Gieler, U.; Gronke, G.; Hamelmann, E.; Hellermann, M.; Kleinheinz, A.; et al. S3-Guideline on Allergy Prevention: 2014 Update: Guideline of the German Society for Allergology and Clinical Immunology (DGAKI) and the German Society for Pediatric and Adolescent Medicine (DGKJ). Allergo J. Int. 2014, 23, 186–199. [Google Scholar] [CrossRef] [Green Version]
- Togias, A.; Cooper, S.F.; Acebal, M.L.; Assa’ad, A.; Baker, J.R.; Beck, L.A.; Block, J.; Byrd-Bredbenner, C.; Chan, E.S.; Eichenfield, L.F.; et al. Addendum guidelines for the prevention of peanut allergy in the United States: Report of the National Institute of Allergy and Infectious Diseases–sponsored expert panel. World Allergy Organ. J. 2017, 10, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M.; et al. Randomized Trial of Peanut Consumption in Infants at Risk for Peanut Allergy. N. Engl. J. Med. 2015, 372, 803–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feeney, M.; Du Toit, G.; Roberts, G.; Sayre, P.H.; Lawson, K.; Bahnson, H.T.; Sever, M.L.; Radulovic, S.; Plaut, M.; Lack, G.; et al. Impact of Peanut Consumption in the LEAP Study: Feasibility, Growth, and Nutrition. J. Allergy Clin. Immunol. 2016, 138, 1108–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- du Toit, G.; Sayre, P.H.; Roberts, G.; Lawson, K.; Sever, M.L.; Bahnson, H.T.; Fisher, H.R.; Feeney, M.; Radulovic, S.; Basting, M.; et al. Allergen Specificity of Early Peanut Consumption and Effect on Development of Allergic Disease in the Learning Early About Peanut Allergy Study Cohort. J. Allergy Clin. Immunol. 2018, 141, 1343–1353. [Google Scholar] [CrossRef] [Green Version]
- Winters, A.; Bahnson, H.T.; Ruczinski, I.; Boorgula, M.P.; Malley, C.; Keramati, A.R.; Chavan, S.; Larson, D.; Cerosaletti, K.; Sayre, P.H.; et al. The MALT1 Locus and Peanut Avoidance in the Risk for Peanut Allergy. J. Allergy Clin. Immunol. 2019, 143, 2326–2329. [Google Scholar] [CrossRef] [Green Version]
- Kanchan, K.; Grinek, S.; Bahnson, H.T.; Ruczinski, I.; Shankar, G.; Larson, D.; Du Toit, G.; Barnes, K.C.; Sampson, H.A.; Suarez-Farinas, M.; et al. HLA Alleles and Sustained Peanut Consumption Promote IgG4 Responses in Subjects Protected from Peanut Allergy. J. Clin. Investig. 2022, 132, e152070. [Google Scholar] [CrossRef]
- Tsilochristou, O.; du Toit, G.; Sayre, P.H.; Roberts, G.; Lawson, K.; Sever, M.L.; Bahnson, H.T.; Radulovic, S.; Basting, M.; Plaut, M.; et al. Association of Staphylococcus Aureus Colonization with Food Allergy Occurs Independently of Eczema Severity. J. Allergy Clin. Immunol. 2019, 144, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Natsume, O.; Kabashima, S.; Nakazato, J.; Yamamoto-Hanada, K.; Narita, M.; Kondo, M.; Saito, M.; Kishino, A.; Takimoto, T.; Inoue, E.; et al. Two-Step Egg Introduction for Prevention of Egg Allergy in High-Risk Infants with Eczema (PETIT): A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2017, 389, 276–286. [Google Scholar] [CrossRef] [Green Version]
- Palmer, D.J.; Metcalfe, J.; Makrides, M.; Gold, M.S.; Quinn, P.; West, C.E.; Loh, R.; Prescott, S.L. Early Regular Egg Exposure in Infants with Eczema: A Randomized Controlled Trial. J. Allergy Clin. Immunol. 2013, 132, 387–392.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei-Liang Tan, J.; Valerio, C.; Barnes, E.H.; Turner, P.J.; Van Asperen, P.A.; Kakakios, A.M.; Campbell, D.E.; Beating Egg Allergy Trial (BEAT) Study Group. A Randomized Trial of Egg Introduction from 4 Months of Age in Infants at Risk for Egg Allergy. J. Allergy Clin. Immunol. 2017, 139, 1621–1628.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellach, J.; Schwarz, V.; Ahrens, B.; Trendelenburg, V.; Aksünger, Ö.; Kalb, B.; Niggemann, B.; Keil, T.; Beyer, K. Randomized Placebo-Controlled Trial of Hen’s Egg Consumption for Primary Prevention in Infants. J. Allergy Clin. Immunol. 2017, 139, 1591–1599.e2. [Google Scholar] [CrossRef] [Green Version]
- Katz, Y.; Rajuan, N.; Goldberg, M.R.; Eisenberg, E.; Heyman, E.; Cohen, A.; Leshno, M. Early Exposure to Cow’s Milk Protein Is Protective against IgE-Mediated Cow’s Milk Protein Allergy. J. Allergy Clin. Immunol. 2010, 126, 77–82.e1. [Google Scholar] [CrossRef]
- Peters, R.L.; Koplin, J.J.; Dharmage, S.C.; Tang, M.L.K.; McWilliam, V.L.; Gurrin, L.C.; Neeland, M.R.; Lowe, A.J.; Ponsonby, A.-L.; Allen, K.J. Early Exposure to Cow’s Milk Protein Is Associated with a Reduced Risk of Cow’s Milk Allergic Outcomes. J. Allergy Clin. Immunol. Pract. 2019, 7, 462–470.e1. [Google Scholar] [CrossRef]
- Onizawa, Y.; Noguchi, E.; Okada, M.; Sumazaki, R.; Hayashi, D. The Association of the Delayed Introduction of Cow’s Milk with IgE-Mediated Cow’s Milk Allergies. J. Allergy Clin. Immunol. Pract. 2016, 4, 481–488.e2. [Google Scholar] [CrossRef]
- Perkin, M.R.; Logan, K.; Tseng, A.; Raji, B.; Ayis, S.; Peacock, J.; Brough, H.; Marrs, T.; Radulovic, S.; Craven, J.; et al. Randomized Trial of Introduction of Allergenic Foods in Breast-Fed Infants. N. Engl. J. Med. 2016, 374, 1733–1743. [Google Scholar] [CrossRef] [Green Version]
- Logan, K.; Perkin, M.R.; Marrs, T.; Radulovic, S.; Craven, J.; Flohr, C.; Bahnson, H.T.; Lack, G. Early Gluten Introduction and Celiac Disease in the EAT Study. JAMA Pediatr. 2020, 174, 1041–1047. [Google Scholar] [CrossRef]
- Perkin, M.R.; Logan, K.; Bahnson, H.T.; Marrs, T.; Radulovic, S.; Craven, J.; Flohr, C.; Mills, E.N.; Versteeg, S.A.; van Ree, R.; et al. Efficacy of the Enquiring About Tolerance (EAT) Study among Infants at High Risk of Developing Food Allergy. J. Allergy Clin. Immunol. 2019, 144, 1606–1614.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, P.J.; Perkin, M.R. RCT Evidence Suggests That Solids Introduction before Age 6 Months Does Not Adversely Impact Duration of Breastfeeding. Matern Child. Nutr. 2020, 16, e13029. [Google Scholar] [CrossRef] [PubMed]
- Perkin, M.R.; Bahnson, H.T.; Logan, K.; Marrs, T.; Radulovic, S.; Craven, J.; Flohr, C.; Lack, G. Association of Early Introduction of Solids With Infant Sleep. JAMA Pediatr. 2018, 172, e180739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, T.; Fukazawa, M.; Fukuoka, K.; Okasora, T.; Yamada, S.; Kyo, S.; Homan, M.; Miura, T.; Nomura, Y.; Tsuchida, S.; et al. Early Introduction of Very Small Amounts of Multiple Foods to Infants: A Randomized Trial. Allergol. Int. 2022. [Google Scholar] [CrossRef]
- Kalb, B.; Meixner, L.; Trendelenburg, V.; Unterleider, N.; Dobbertin-Welsch, J.; Heller, S.; Dölle-Bierke, S.; Roll, S.; Lau, S.; Lee, Y.-A.; et al. Tolerance Induction through Early Feeding to Prevent Food Allergy in Infants with Eczema (TEFFA): Rationale, Study Design, and Methods of a Randomized Controlled Trial. Trials 2022, 23, 210. [Google Scholar] [CrossRef] [PubMed]
- Krawiec, M.; Fisher, H.R.; Du Toit, G.; Bahnson, H.T.; Lack, G. Overview of Oral Tolerance Induction for Prevention of Food Allergy-Where Are We Now? Allergy 2021, 76, 2684–2698. [Google Scholar] [CrossRef] [PubMed]
- Fisher, H.R.; Du Toit, G.; Bahnson, H.T.; Lack, G. The Challenges of Preventing Food Allergy: Lessons Learned from LEAP and EAT. Ann. Allergy Asthma Immunol. 2018, 121, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Voorheis, P.; Bell, S.; Cornelsen, L.; Quaife, M.; Logan, K.; Marrs, T.; Radulovic, S.; Craven, J.; Flohr, C.; Lack, G.; et al. Challenges Experienced with Early Introduction and Sustained Consumption of Allergenic Foods in the Enquiring About Tolerance (EAT) Study: A Qualitative Analysis. J. Allergy Clin. Immunol. 2019, 144, 1615–1623. [Google Scholar] [CrossRef] [Green Version]
- Perkin, M.R.; Bahnson, H.T.; Logan, K.; Marrs, T.; Radulovic, S.; Knibb, R.; Craven, J.; Flohr, C.; Mills, E.N.; Versteeg, S.A.; et al. Factors Influencing Adherence in a Trial of Early Introduction of Allergenic Food. J. Allergy Clin. Immunol. 2019, 144, 1595–1605. [Google Scholar] [CrossRef] [Green Version]
- Warren, C.; Turner, P.J.; Chinthrajah, R.S.; Gupta, R. Advancing Food Allergy through Epidemiology: Understanding and Addressing Disparities in Food Allergy Management and Outcomes. J. Allergy Clin. Immunol. Pract. 2021, 9, 110–118. [Google Scholar] [CrossRef]
- Home—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 2 May 2022).
- Prescott, S.; Nowak-Węgrzyn, A. Strategies to Prevent or Reduce Allergic Disease. Ann. Nutr. Metab. 2011, 59 (Suppl. S1), 28–42. [Google Scholar] [CrossRef] [Green Version]
- Siriwardhana, N.; Kalupahana, N.S.; Moustaid-Moussa, N. Health benefits of n-3 polyunsaturated fatty acids: Eicosapentaenoic acid and docosahexaenoic acid. Adv. Food Nutr. Res. 2012, 65, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Wiciński, M.; Sawicka, E.; Gębalski, J.; Kubiak, K.; Malinowski, B. Human Milk Oligosaccharides: Health Benefits, Potential Applications in Infant Formulas, and Pharmacology. Nutrients 2020, 12, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trogen, B.; Jacobs, S.; Nowak-Wegrzyn, A. Early Introduction of Allergenic Foods and the Prevention of Food Allergy. Nutrients 2022, 14, 2565. https://doi.org/10.3390/nu14132565
Trogen B, Jacobs S, Nowak-Wegrzyn A. Early Introduction of Allergenic Foods and the Prevention of Food Allergy. Nutrients. 2022; 14(13):2565. https://doi.org/10.3390/nu14132565
Chicago/Turabian StyleTrogen, Brit, Samantha Jacobs, and Anna Nowak-Wegrzyn. 2022. "Early Introduction of Allergenic Foods and the Prevention of Food Allergy" Nutrients 14, no. 13: 2565. https://doi.org/10.3390/nu14132565
APA StyleTrogen, B., Jacobs, S., & Nowak-Wegrzyn, A. (2022). Early Introduction of Allergenic Foods and the Prevention of Food Allergy. Nutrients, 14(13), 2565. https://doi.org/10.3390/nu14132565





