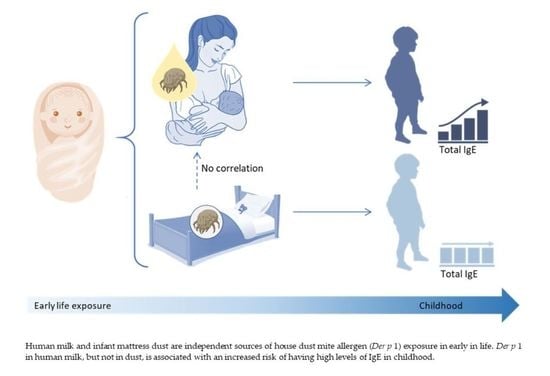
House Dust Mite Exposure through Human Milk and Dust: What Matters for Child Allergy Risk?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Samples Collection and Der p 1 Quantification in Human Milk and Mattress Dust
2.3. Health Outcomes: IgE Levels and Asthma
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Presence of Der p 1 Allergen in Human Milk and Infant Mattress Dust
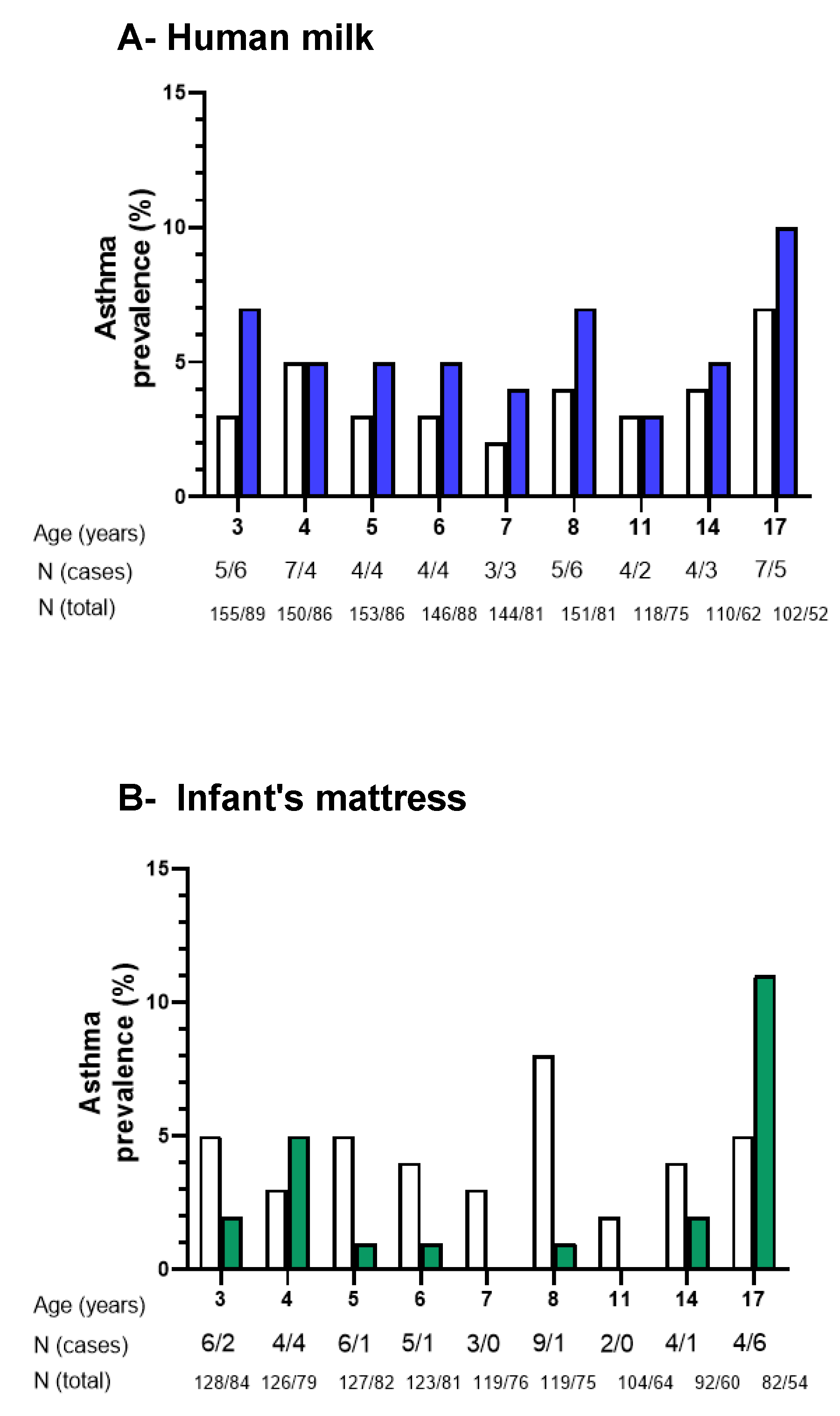
3.3. Associations of Der p 1 Allergen in Human Milk with Child IgE Levels and Asthma
3.4. Associations of Der p 1 Allergen in Mattress Dust with Child IgE Levels and Asthma
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lack, G.; Fox, D.; Northstone, K.; Golding, J. Avon Longitudinal Study of Parents and Children Study Team Factors associated with the development of peanut allergy in childhood. N. Engl. J. Med. 2003, 348, 977–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du Toit, G.; Sampson, H.A.; Plaut, M.; Burks, A.W.; Akdis, C.A.; Lack, G. Food allergy: Update on prevention and tolerance. J. Allergy Clin. Immunol. 2018, 141, 30–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhasselt, V.; Genuneit, J.; Metcalfe, J.R.; Tulic, M.K.; Rekima, A.; Palmer, D.J.; Prescott, S.L. Ovalbumin in breastmilk is associated with a decreased risk of IgE-mediated egg allergy in children. Allergy 2020, 75, 1463–1466. [Google Scholar] [CrossRef]
- Verhasselt, V.; Milcent, V.; Cazareth, J.; Kanda, A.; Fleury, S.; Dombrowicz, D.; Glaichenhaus, N.; Julia, V. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat. Med. 2008, 14, 170–175. [Google Scholar] [CrossRef]
- Serhan, N.; Basso, L.; Sibilano, R.; Petitfils, C.; Meixiong, J.; Bonnart, C.; Reber, L.L.; Marichal, T.; Starkl, P.; Cenac, N.; et al. House dust mites activate nociceptor-mast cell clusters to drive type 2 skin inflammation. Nat. Immunol. 2019, 20, 1435–1443. [Google Scholar] [CrossRef]
- Miller, J.D. The Role of Dust Mites in Allergy. Clin. Rev. Allergy Immunol. 2019, 57, 312–329. [Google Scholar] [CrossRef]
- Baiz, N.; Macchiaverni, P.; Tulic, M.K.; Rekima, A.; Annesi-Maesano, I.; Verhasselt, V.; Group, E.M.-C.C.S. Early oral exposure to house dust mite allergen through breast milk: A potential risk factor for allergic sensitization and respiratory allergies in children. J. Allergy Clin. Immunol. 2017, 139, 369–372.e310. [Google Scholar] [CrossRef] [Green Version]
- Rekima, A.; Bonnart, C.; Macchiaverni, P.; Metcalfe, J.; Tulic, M.K.; Halloin, N.; Rekima, S.; Genuneit, J.; Zanelli, S.; Medeiros, S.; et al. A role for early oral exposure to house dust mite allergens through breastmilk in IgE-mediated food allergy susceptibility. J. Allergy Clin. Immunol. 2020, 145, 1416–1429. [Google Scholar] [CrossRef] [Green Version]
- Gough, L.; Sewell, H.F.; Shakib, F. The proteolytic activity of the major dust mite allergen Der p 1 enhances the IgE antibody response to a bystander antigen. Clin. Exp. Allergy 2001, 31, 1594–1598. [Google Scholar] [CrossRef]
- Macchiaverni, P.; Rekima, A.; Turfkruyer, M.; Mascarell, L.; Airouche, S.; Moingeon, P.; Adel-Patient, K.; Condino-Neto, A.; Annesi-Maesano, I.; Prescott, S.L.; et al. Respiratory allergen from house dust mite is present in human milk and primes for allergic sensitization in a mouse model of asthma. Allergy 2014, 69, 395–398. [Google Scholar] [CrossRef]
- Macchiaverni, P.; Ynoue, L.H.; Arslanian, C.; Verhasselt, V.; Condino-Neto, A. Early Exposure to Respiratory Allergens by Placental Transfer and Breastfeeding. PLoS ONE 2015, 10, e0139064. [Google Scholar] [CrossRef] [PubMed]
- Macchiaverni, P.; Rekima, A.; van den Elsen, L.; Renz, H.; Verhasselt, V. Allergen shedding in human milk: Could it be key for immune system education and allergy prevention? J. Allergy Clin. Immunol. 2021, 148, 679–688. [Google Scholar] [CrossRef]
- Renz, H.; Adkins, B.D.; Bartfeld, S.; Blumberg, R.S.; Farber, D.L.; Garssen, J.; Ghazal, P.; Hackam, D.J.; Marsland, B.J.; McCoy, K.D.; et al. The neonatal window of opportunity-early priming for life. J. Allergy Clin. Immunol. 2018, 141, 1212–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunekreef, B.; Smit, J.; de Jongste, J.; Neijens, H.; Gerritsen, J.; Postma, D.; Aalberse, R.; Koopman, L.; Kerkhof, M.; Wilga, A.; et al. The prevention and incidence of asthma and mite allergy (PIAMA) birth cohort study: Design and first results. Pediatr. Allergy Immunol. 2002, 13, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Wijga, A.; Houwelingen, A.C.; Smit, H.A.; Kerkhof, M.; Vos, A.P.; Neijens, H.J.; Brunekreef, B.; Study, P.B.C. Fatty acids in breast milk of allergic and non-allergic mothers: The PIAMA birth cohort study. Pediatr. Allergy Immunol. 2003, 14, 156–162. [Google Scholar] [CrossRef]
- Tulic, M.K.; Vivinus-Nebot, M.; Rekima, A.; Rabelo Medeiros, S.; Bonnart, C.; Shi, H.; Walker, A.; Dainese, R.; Boyer, J.; Vergnolle, N.; et al. Presence of commensal house dust mite allergen in human gastrointestinal tract: A potential contributor to intestinal barrier dysfunction. Gut 2016, 65, 757–766. [Google Scholar] [CrossRef]
- Van Strien, R.T.; Koopman, L.P.; Kerkhof, M.; Spithoven, J.; de Jongste, J.C.; Gerritsen, J.; Neijens, H.J.; Aalberse, R.C.; Smit, H.A.; Brunekreef, B. Mite and pet allergen levels in homes of children born to allergic and nonallergic parents: The PIAMA study. Environ. Health Perspect. 2002, 110, A693–A698. [Google Scholar] [CrossRef] [Green Version]
- Gehring, U.; de Jongste, J.C.; Kerkhof, M.; Oldewening, M.; Postma, D.; van Strien, R.T.; Wijga, A.H.; Willers, S.M.; Wolse, A.; Gerritsen, J.; et al. The 8-year follow-up of the PIAMA intervention study assessing the effect of mite-impermeable mattress covers. Allergy 2012, 67, 248–256. [Google Scholar] [CrossRef]
- Pinart, M.; Benet, M.; Annesi-Maesano, I.; von Berg, A.; Berdel, D.; Carlsen, K.C.; Carlsen, K.H.; Bindslev-Jensen, C.; Eller, E.; Fantini, M.P.; et al. Comorbidity of eczema, rhinitis, and asthma in IgE-sensitised and non-IgE-sensitised children in MeDALL: A population-based cohort study. Lancet Respir. Med. 2014, 2, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Wijga, A.H.; Kerkhof, M.; Gehring, U.; de Jongste, J.C.; Postma, D.S.; Aalberse, R.C.; Wolse, A.P.; Koppelman, G.H.; van Rossem, L.; Oldenwening, M.; et al. Cohort profile: The prevention and incidence of asthma and mite allergy (PIAMA) birth cohort. Int. J. Epidemiol. 2014, 43, 527–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macchiaverni, P.; Gehring, U.; Rekima, A.; Wijga, A.; Verhasselt, V. House dust mites: Does a clean mattress mean Der p 1-free breastmilk? Pediatr. Allergy Immunol. 2020, 31, 990–993. [Google Scholar] [CrossRef]
- Custovic, A.; Murray, C.S.; Simpson, A. Dust-mite inducing asthma: What advice can be given to patients? Expert Rev. Respir. Med. 2019, 13, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, A.; Robinson, C. Proteolytic, lipidergic and polysaccharide molecular recognition shape innate responses to house dust mite allergens. Allergy 2020, 75, 33–53. [Google Scholar] [CrossRef] [Green Version]
- Dogaru, C.M.; Nyffenegger, D.; Pescatore, A.M.; Spycher, B.D.; Kuehni, C.E. Breastfeeding and childhood asthma: Systematic review and meta-analysis. Am. J. Epidemiol. 2014, 179, 1153–1167. [Google Scholar] [CrossRef] [Green Version]
- Sears, M.R.; Greene, J.M.; Willan, A.R.; Taylor, D.R.; Flannery, E.M.; Cowan, J.O.; Herbison, G.P.; Poulton, R. Long-term relation between breastfeeding and development of atopy and asthma in children and young adults: A longitudinal study. Lancet 2002, 360, 901–907. [Google Scholar] [CrossRef]
- Matheson, M.C.; Erbas, B.; Balasuriya, A.; Jenkins, M.A.; Wharton, C.L.; Tang, M.L.; Abramson, M.J.; Walters, E.H.; Hopper, J.L.; Dharmage, S.C. Breast-feeding and atopic disease: A cohort study from childhood to middle age. J. Allergy Clin. Immunol. 2007, 120, 1051–1057. [Google Scholar] [CrossRef] [Green Version]
- Sunyer, J.; Anto, J.M.; Castellsague, J.; Soriano, J.B.; Roca, J. Total serum IgE is associated with asthma independently of specific IgE levels. The Spanish Group of the European Study of Asthma. Eur. Respir. J. 1996, 9, 1880–1884. [Google Scholar] [CrossRef]
- Sonntag, H.J.; Filippi, S.; Pipis, S.; Custovic, A. Blood Biomarkers of Sensitization and Asthma. Front. Pediatr. 2019, 7, 251. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.; Franca, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef] [Green Version]
- de Silva, D.; Halken, S.; Singh, C.; Muraro, A.; Angier, E.; Arasi, S.; Arshad, H.; Beyer, K.; Boyle, R.; du Toit, G.; et al. Preventing food allergy in infancy and childhood: Systematic review of randomised controlled trials. Pediatr. Allergy Immunol. 2020, 31, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Gungor, D.; Nadaud, P.; LaPergola, C.C.; Dreibelbis, C.; Wong, Y.P.; Terry, N.; Abrams, S.A.; Beker, L.; Jacobovits, T.; Jarvinen, K.M.; et al. Infant milk-feeding practices and food allergies, allergic rhinitis, atopic dermatitis, and asthma throughout the life span: A systematic review. Am. J. Clin. Nutr. 2019, 109, 772S–799S. [Google Scholar] [CrossRef] [PubMed]

| Study Participants | |
|---|---|
| Infant male sex | 126/249 (51%) |
| Maternal asthma, n/N (%) | 40/249 (16%) |
| Maternal allergy to house dust (mites), n/N (%) | 95/244 (39%) |
| Season of birth, n/N (%) | |
| Winter | 85/249 (34%) |
| Spring | 80/249 (32%) |
| Summer | 49/249 (20%) |
| Autumn | 35/249 (14%) |
| Maternal age at birth, mean (SD) | 31.1 (3.8), n = 244 |
| Gestational age at birth, mean (SD) | 40.1 (1.4), n = 249 |
| Infant birth weight (grams), mean (SD) | 3532 (492), n = 249 |
| Caesarian section, n/N (%) | 20/248 (8%) |
| Infant age at human milk collection (days), mean (SD) | 108 (29), n = 245 |
| Duration of breastfeeding (weeks), mean (SD) | 30 (13), n = 249 |
| Pets in the child’s home during 1st year, n/N (%) | 104/248 (42%) |
| Der p 1 Detection | Human Milk (pg/mL) | Dust from Infant’s Mattress (ng/g) |
|---|---|---|
| Number of samples | 218 | 198 |
| Samples with detected Der p 1 (%) | 79 (36) | 81 (41) |
| Median * (concentration range) | 174 [<LOD-1238] | 1165 [<LOD-20,502] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macchiaverni, P.; Gehring, U.; Rekima, A.; Wijga, A.H.; Verhasselt, V. House Dust Mite Exposure through Human Milk and Dust: What Matters for Child Allergy Risk? Nutrients 2022, 14, 2095. https://doi.org/10.3390/nu14102095
Macchiaverni P, Gehring U, Rekima A, Wijga AH, Verhasselt V. House Dust Mite Exposure through Human Milk and Dust: What Matters for Child Allergy Risk? Nutrients. 2022; 14(10):2095. https://doi.org/10.3390/nu14102095
Chicago/Turabian StyleMacchiaverni, Patricia, Ulrike Gehring, Akila Rekima, Alet H. Wijga, and Valerie Verhasselt. 2022. "House Dust Mite Exposure through Human Milk and Dust: What Matters for Child Allergy Risk?" Nutrients 14, no. 10: 2095. https://doi.org/10.3390/nu14102095
APA StyleMacchiaverni, P., Gehring, U., Rekima, A., Wijga, A. H., & Verhasselt, V. (2022). House Dust Mite Exposure through Human Milk and Dust: What Matters for Child Allergy Risk? Nutrients, 14(10), 2095. https://doi.org/10.3390/nu14102095